Abstract
Background:
Parkinson's disease (PD) is a chronic neurodegenerative pathology with unknown etiology. It is characterized clinically by the classic triad that associated tremors, bradykinesia, and rigidity. In Niger, there are no data on PD.
Aims:
We aimed to provide the demographic and clinical profile of PD in patients from Niger to create a database on PD in Niger.
Patients and Methods:
We conducted a retrospective study at the Neurology Outpatient Clinic of the Hôpital National de Niamey (HNN, Niger) over a period of 4.42 years from February 2009 to July 2013 collecting all cases of PD. The demographic and clinical features of all patients were collected and analyzed.
Results:
During the period of the study, 1695 patients consulted at the Neurology Outpatient Clinic of the HNN, among which 76 patients (4.48%) had secondary parkinsonism and 25 patients (1.47%) had features compatible with PD. Only patients with PD were included in this study. The mean age at onset of symptoms was 58 years (range: 42–74 years). The male sex was predominant (60%) with a sex ratio of 1.5. The mean time interval from the onset of symptoms to diagnosis of PD was 1.8 years (range: 1–5 years). The tremor was the most common symptom (84%). Bradykinesia represented 64% of the symptoms and rigidity 20%. At the time of the diagnosis of PD, 8 patients (32%) were in Stage I of the classification of Hoehn and Yahr, 16 patients (64%) in Stage II, and 1 patient (4%) in Stage III. The levodopa/carbidopa combination was the most used antiparkinsonian drug in our patients (88%). The mean time of follow-up of the patients was 2.5 years (range: 1–4.42 years). During the course of the disease, 9 patients (36%) were in Stage II of the classification of Hoehn and Yahr, 13 patients (52%) in Stage III, and 3 patients (12%) in Stage IV.
Conclusion:
Our study provides demographic and clinical data of PD in patients from Niger and shows that the hospital frequency of this disease is low (1.47%). The demographic and clinical features of our patients are similar to those of the patients of the prior studies reported in sub-Saharan Africa.
Keywords: Hospital frequency, Niger, Parkinson's disease
INTRODUCTION
First described in 1817 by James Parkinson,[1] Parkinson's disease (PD) is a chronic neurodegenerative disease with unknown etiology that affects dopaminergic, cholinergic, noradrenergic, and serotoninergic systems. PD represents the second cause of physical disability of neurological origin in the elderly people after stroke. It is characterized clinically by the classic triad that associated tremors, bradykinesia, and rigidity. The diagnosis of PD based on the presence of the classic triad, asymmetry of the signs at onset, absence of atypical features (pyramidal signs, cerebellar and oculomotor disorders, bilateral symptoms at onset, early cognitive impairment, and early dysautonomic disorders), absence of an etiology, and good response to levodopa therapy. PD affects mainly people aged over 65 years in Western countries. The hospital frequency of PD is estimated at 1.47% in Lagos (Nigeria) in 2010[2] and at 6.94% in Kano (Nigeria) in 2012.[3] Naturally, PD progresses in three evolutive clinical stages.[4] The Stage I corresponds to the stage of the first symptoms of the diagnosis of disease, initial treatment, and honeymoon. The Stage II corresponds to the stage of clinical fluctuations and dyskinesias. Finally, the Stage III corresponding to the advanced stage of PD is characterized by cognitive impairment, dysautonomic disorders, sleep disorders, depressive syndrome, postural disorders, freezing, falls, dysarthria, and hypomimia. In the other hand, the nonmotor symptoms may precede the motor symptoms of PD.[5]
This study was designed to provide the demographic and clinical profile of PD in patients from Niger to create a database on PD in Niger.
PATIENTS AND METHODS
We conducted a retrospective study at the Neurology Outpatient Clinic of the Hôpital National de Niamey (HNN) over a period of 4.42 years (4 years and 5 months) from February 2009 to July 2013, collecting all cases of PD. This hospital is the sole largest urban and tertiary care referral center in Niger which had neurologists. During the period of this study (February 2009 to July 2013), Niger had only one neurologist for a population of about 17,129,076 inhabitants in 2012.[6] The HNN covers an area of 23,120.50 m2 and comprises 36 buildings with a bed capacity of 790. This hospital attracts people from all corners of the country to seek medical care [Figure 1].
Figure 1.
The neighboring countries of Niger and the different regions of the country which are Agadez, Diffa, Dosso, Maradi, Niamey, Tahoua, Tillabéri, and Zinder
In our patients, the diagnosis of PD was based on the diagnostic criteria of the UK Parkinson's Disease Society Brain Bank.[7] Our diagnostic strategy was first to identify the presence of parkinsonism syndrome (tremor, bradykinesia, rigidity, and postural or gait abnormality) and then to ruling out in a second step other pathologies that could explain the clinical picture by the realization of paraclinical examinations and to confirm the diagnosis of PD by the good improvement of clinical symptoms by levodopa. Patients with parkinsonism for whom the clinical features suggest a secondary etiology or the neuroimaging shows a brain lesion susceptible to give parkinsonism are considered to have secondary parkinsonism.
For each patient, we collected the following information: age at onset of symptoms, sex, medical history, time interval from the onset of symptoms to diagnosis of PD, and clinical signs. All the patients were followed and evaluated throughout the period of the study by a neurologist. For each patient and each follow-up visit, the nonmotor symptoms of PD were systematically searched (sleep disorders, depressive syndrome, pain, visual hallucinations, constipation, urinary tract disorders, and other) and motor symptoms were evaluated.
A total of 1695 patients consulted at the Neurology Outpatients Clinic of the HNN during the period of this study (February 2009 to July 2013). Seventy-six patients (4.48%) had secondary parkinsonism and 25 patients had features compatible with PD (1.47%). Only patients with PD were included in this study.
This study was approved by the Institutional Review Board of the Faculty of Medicine of Abdou Moumouni University of Niamey (Niger).
Patients’ characteristics are presented as percentages for qualitative variables and as mean or median for quantitative variables.
RESULTS
During the period of the study, 1695 patients consulted at the Neurology Outpatient Clinic of the HNN, among which 76 patients (4.48%) had secondary parkinsonism and 25 patients (1.47%) had features compatible with PD. Only patients with PD were included in this study.
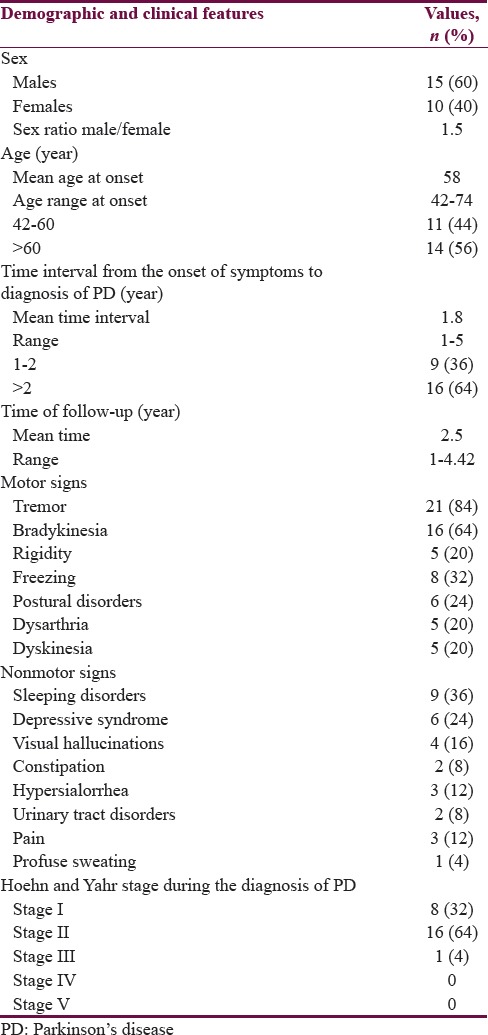
Table 1 summarizes the demographic and clinical features of 25 patients with PD. The male sex was predominant (60%) with a sex ratio of 1.5. The mean age at onset of symptoms was 58 years. Fourteen patients (56%) were older than 60 years at the onset of symptoms. Hypertension was present as a comorbidity in 6 (24%) and diabetes in 3 (12%). No family history of PD was found. The mean time interval from the onset of symptoms to diagnosis of PD of the patients was 1.8 years. Sixteen patients (64%) had consulted 2 years after the onset of symptoms. The tremor was the most common symptom (84%). Bradykinesia represented 64% of the symptoms and rigidity 20%. Tremor was isolated in 7 patients (28%). It was associated with bradykinesia and rigidity in 14 patients (56%). Four patients (16%) had akinetic–rigid syndrome without tremor. At the time of the diagnosis of PD, 8 patients (32%) were in Stage I of the classification of Hoehn and Yahr,[8] 16 patients (64%) in Stage II, and 1 patient (4%) in Stage III.
Table 1.
Demographic and clinical features of the 25 patients
The levodopa/carbidopa combination was the most used antiparkinsonian drug (APD) in our patients (88%). Only 3 patients (12%) had received trihexyphenidyl. The mean time of follow-up of the patients was 2.5 years.
During the course of the disease, the nonmotor signs found in our patients were sleep disorders in 9 patients (36%), depressive syndrome in 6 patients (24%), visual hallucinations in 4 patients (16%), and dysautonomic disorders in 11 patients (44%) [Table 1]. The motor signs associated were freezing with or without falls in 8 patients (32%), postural disorders in 6 patients (24%), clinical fluctuations and dyskinesias in 5 patients (20%), and dysarthria in 5 patients (20%). Nine patients (36%) were in Stage II of the classification of Hoehn and Yahr,[8] 13 patients (52%) in Stage III, and 3 patient (12%) in Stage IV [Table 2].
Table 2.
Different stages of the disease during the course

DISCUSSION
In this retrospective study of 25 patients with PD, collected in the Neurology Outpatient Clinic of the HNN over a period of 4.42 years from February 2009 to July 2013, we described the demographic and clinical profile of the first documented cases of PD in Niger. No study has been published previously by Niger on PD to report on the demographic and clinical features of this disease in Niger. So that, the present study shows not only a predominance of male sex (60%) but also a predominance of the disease in the people aged over 60 years (56%). The clinical presentation was mainly characterized by tremors (84%).
The hospital frequency of PD in the present study was 1.47% that appeared to be low. Okubadejo et al.[2] reported in 2010 in Lagos a hospital frequency of 1.47% which is similar to our findings. Femi et al.[3] reported in 2012 in Kano as far as they are concerned a hospital frequency of 6.94%. The low hospital frequency of PD in the societies from Niger could be explained by the fact that this disease is underdiagnosed. In addition, all the major clinical features of PD, such as tremors, slowing of movements, and postural or gait abnormality, are considered in our societies as features of normal physiological aging. Thus, few of the elderly people consult for these signs, and therefore, the diagnosis cannot be made; hence, the low hospital frequency observed in this study.
The mean age at onset of symptoms in the present study was 58 years. Similar findings had been reported by some authors in sub-Saharan Africa: 61.5 years by Okubadejo et al.[2] and 58.2 years by Femi et al.[3] Taba and Asser[9] reported in Estonia in 2002 a mean age at onset of symptoms of PD of 66.9 years and Osaki et al.[10] reported as far as they are concerned in Japan in 2011 a mean age at onset of symptoms of 67 years. Our study shows a predominance not only of people aged over 60 years at onset of symptoms (56%) but also of male sex (60%). This predominance of male sex has been reported in several studies in sub-Saharan Africa[2,3,11] and many other Asian[12,13] and Western studies.[14,15] An Indian study had shown that male sex is a risk factor of PD.[13] However, some Japanese studies have reported a predominance of the female sex in the PD.[10,16]
In our study, the mean time interval from the onset of symptoms to diagnosis of PD of the patients was 1.8 years. Similar findings had been reported in Lagos by Okubadejo et al.[2] in 2010 (2.05 years). Femi et al.[3] reported as far as they are concerned in 2012 in Kano a mean time interval from the onset of symptoms to diagnosis of PD of 3.6 years. This elongation of the mean time interval from the onset of symptoms to diagnosis of PD of the patients in the present study could be explained by two reasons. First, the early manifestations of PD are neglected by the patients in our societies because they are considered to be related to normal physiological aging. Second, some patients consult first at the onset of symptoms in the traditional practitioners before consulting in the neurologist.
Tremor is the most common symptom in PD and it is unilateral at the onset of the disease. In the present study, only 4 patients (16%) had no tremor at the time of the diagnosis of PD. Femi et al.[3] in 2012 and Khealani and Baig[12] in 2006 report similar findings, respectively, 12.5% and 17.5%.
The natural course of PD is characterized by the occurrence of motor and nonmotor disorders despite a well-conducted treatment.[4] In the present study, sleep disorders and dysautonomic disorders were the most common nonmotor signs of PD. Femi et al.[3] report also a predominance of sleep disorders and dysautonomic disorders in their study. We did not find cases of the patients with cognitive impairment in the present study, likely due to a short period of follow-up of the patients (4.42 years), because cognitive impairment are quite common in the advanced stage of PD.
The difficulties encountered in the management of our patients are the limited access to some APDs and the poor drug compliance of the patients occasioned by poverty. The APDs available in Niger during the period of the study were levodopa/carbidopa, trihexyphenidyl, and bromocriptine. The management of clinical fluctuations has been a real challenge in our patients due to the unavailability of some APDs in Niger. Only two patients had ordered the pramipexole from the Maghreb. Some patients should have been proposed to the surgical treatment for PD (deep-brain stimulation), but unfortunately, this surgery is not available in Niger. In addition, these patients do not have financial funding to be referred abroad to a specialized center for surgery of PD.
Our study has some limitations. First, the study did not assess the predisposing factors to the PD in Niger. Second, the study did not compare the features of the patients from urban populations and patients from rural populations to assess some risk factors for PD between these populations. Third, the genetic study was not performed in our study due to the lack of laboratory of genetic study in Niger, and the limited financial means of the patients to send their blood samples to other countries for the genetic tests.
CONCLUSION
Our study provides demographic and clinical data of PD in patients from Niger and shows that the hospital frequency of this disease is low (1.47%) because underdiagnosed in Niger. The demographic and clinical features of our patients are similar to those of the patients of the prior studies reported in sub-Saharan Africa. However, limited access to APDs, poor drug compliance occasioned by poverty, and insufficient number of experts in Niger are a real problem in the management of these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Parkinson J. An Essay on the Shaking Palsy. London: Sherwood, Nelly and Jones; 1817. [Google Scholar]
- 2.Okubadejo NU, Ojo OO, Oshinaike OO. Clinical profile of parkinsonism and Parkinson's disease in Lagos, Southwestern Nigeria. BMC Neurol. 2010;10:1. doi: 10.1186/1471-2377-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Femi OL, Ibrahim A, Aliyu S. Clinical profile of parkinsonian disorders in the tropics: Experience at Kano, Northwestern Nigeria. J Neurosci Rural Pract. 2012;3:237–41. doi: 10.4103/0976-3147.102589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziégler M. Advanced Parkinson's disease. Psychol NeuroPsychiatr Vieil. 2006;4:S5–10. [PubMed] [Google Scholar]
- 5.Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–34. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 6.Calverton, Maryland, USA: NIS and ICF International; 2013. National Institute of Statistic (NIS) and ICF International. Demographic and Health Survey and Multiple Indicators of Niger 2012. [Google Scholar]
- 7.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoehn MM, Yahr MD. Parkinsonism: Onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 9.Taba P, Asser T. Prevalence of Parkinson's disease in Estonia. Acta Neurol Scand. 2002;106:276–81. doi: 10.1034/j.1600-0404.2002.01286.x. [DOI] [PubMed] [Google Scholar]
- 10.Osaki Y, Morita Y, Kuwahara T, Miyano I, Doi Y. Prevalence of Parkinson's disease and atypical Parkinsonian syndromes in a rural Japanese district. Acta Neurol Scand. 2011;124:182–7. doi: 10.1111/j.1600-0404.2010.01442.x. [DOI] [PubMed] [Google Scholar]
- 11.Schoenberg BS, Osuntokun BO, Adeuja AO, Bademosi O, Nottidge V, Anderson DW, et al. Comparison of the prevalence of Parkinson's disease in black populations in the rural United States and in rural Nigeria: Door-to-door community studies. Neurology. 1988;38:645–6. doi: 10.1212/wnl.38.4.645. [DOI] [PubMed] [Google Scholar]
- 12.Khealani BA, Baig SM. Clinical spectrum of Parkinson's disease from Pakistan. Singapore Med J. 2006;47:1075–9. [PubMed] [Google Scholar]
- 13.Behari M, Srivastava AK, Das RR, Pandey RM. Risk factors of Parkinson's disease in Indian patients. J Neurol Sci. 2001;190:49–55. doi: 10.1016/s0022-510x(01)00578-0. [DOI] [PubMed] [Google Scholar]
- 14.Tanner CM, Goldman SM. Epidemiology of Parkinson's disease. Neurol Clin. 1996;14:317–35. doi: 10.1016/S0733-8619(05)70259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wickremaratchi MM, Perera D, O’Loghlen C, Sastry D, Morgan E, Jones A, et al. Prevalence and age of onset of Parkinson's disease in cardiff: A community based cross sectional study and meta-analysis. J Neurol Neurosurg Psychiatry. 2009;80:805–7. doi: 10.1136/jnnp.2008.162222. [DOI] [PubMed] [Google Scholar]
- 16.Kimura H, Kurimura M, Wada M, Kawanami T, Kurita K, Suzuki Y, et al. Female preponderance of Parkinson's disease in Japan. Neuroepidemiology. 2002;21:292–6. doi: 10.1159/000065527. [DOI] [PubMed] [Google Scholar]


