Abstract
Background:
Rapid eye movement (REM) sleep behavior disorder (RBD) and obstructive sleep apnea (OSA) are the most common sleep disorders in Parkinson's disease (PD). The aim of this study was to identify whether RBD could alleviate OSA severity in PD patients and its effect on cognitive impairment.
Methods:
From February 2014 to May 2017, we recruited 174 PD patients from the Second Affiliated Hospital of Soochow University, all of whom underwent polysomnography (PSG). We collected clinical data, PSG results, and compared information between patients with and without RBD or OSA by analysis of covariance. We also investigated the effect of these sleep disorders on cognitive impairment using linear regression.
Results:
We grouped participants as follows: PD only (n = 53), PD + OSA (n = 29), PD + RBD (n = 61), and PD + RBD + OSA (n = 31). Minimum oxygen saturation (SaO2) during whole sleep and in REM sleep was higher in PD + RBD + OSA patients than that in PD + OSA patients. PD + RBD patients had worse Mini-Mental Status Examination and Montreal Cognitive Assessment (MoCA) scores than those in the PD group (P < 0.001), especially in visuospatial/executive, attention, and memory functions. The PD + OSA group performed worse than the PD group in the delayed recall domain. After adjusting for age, sex, body mass index, education, disease severity, and other sleep disorders, MoCA was negatively associated with OSA (β = −0.736, P = 0.043) and RBD (β = −2.575,P < 0.001). The severity of RBD (tonic/phasic electromyography activity) and OSA (apnea-hypopnea index/oxygen desaturation index/minimum SaO2) were also associated with MoCA. The adjusted β values of RBD-related parameters were higher than that for OSA.
Conclusions:
We found that RBD alleviated OSA severity; however, RBD and OSA together exacerbated PD cognitive impairment. Further studies are needed to evaluate whether OSA treatment can improve cognition in PD.
Keywords: Cognitive Dysfunction, Sleep Apnea, Obstructive, Parkinson's Disease, Rapid Eye Movement Sleep Behavior Disorder
摘要
背景:
快速眼动睡眠期行为障碍(Rapid eye movement sleep behavior disorder, RBD)和睡眠呼吸暂停(obstructive sleep apnea, OSA)是帕金森病(Parkinson's disease, PD)患者常见的两种睡眠障碍。本研究旨在研究RBD是否可以改善PD患者OSA严重 程度及两者对PD患者认知功能的影响。
方法:
纳入2014年2月至2017年5月之间就诊于苏州大学附属第二医院的174例PD患者,所有患者均进行多导睡眠监测 (polysomnography, PSG)。评估其临床症状、PSG结果,对PD合并RBD或OSA患者的临床特点及睡眠参数进行对比。另外, 我们还利用线性回归分析研究RBD及OSA对其认知功能障碍的影响。
结果:
所有PD患者分为四组:PD组(不合并RBD及OSA, n = 53),PD + OSA组(PD合并OSA组,n = 29),PD + RBD 组(PD合并RBD, n = 61)及PD + RBD + OSA组 (PD同时合并RBD及OSA, n = 31)。PD + RBD + OSA 组患者夜间最低脉氧及REM期最低脉氧较PD + OSA高。PD + RBD组患者的认知功能较差 (P<0.001),主要表现为视空间/执行功能、注意及记忆功能障碍。PD + OSA组则主要表现为延迟记忆功能障碍。在校正年龄、性别、BMI、教育程度、病程及其他睡眠障碍后,蒙特利尔认知评价量表分数与OSA (β = -0.736, P = 0.043)及RBD (β = -2.575, P <0.001 )呈负相关。且RBD(紧张性及时相性下颏肌电)及OSA(呼吸暂停低通气指数/氧饱和度指数/最低脉氧饱和度)严重程度与MoCA相关。校正β值说明RBD对PD患者认知功能的影响更严重。
结论:
RBD可减轻PD患者OSA严重程度,但合并RBD及OSA加重PD患者认知功能障碍。
INTRODUCTION
Sleep-related problems are common nonmotor symptoms in Parkinson's disease (PD); the sleep-related problems most prevalent in PD are insomnia, rapid eye movement (REM) sleep behavior disorder (RBD), obstructive sleep apnea (OSA), excessive daytime sleepiness, and periodic leg movement syndrome. The prevalence of these disorders ranges between 60% and 98%.[1,2,3] Approximately 30.0–62.5% of PD patients have RBD,[2,4,5] which is characterized by atonia occurring typically in REM sleep. These patients tend to have the akinetic-rigid dominant subtype of PD and exhibit severe cognitive impairment and other nonmotor symptoms.[5]
Epidemiological studies suggested that OSA is another common sleep-related disorder in PD.[6,7] Previous studies reported the frequency of OSA in PD patients between 20% and 66%.[8] Upper airway obstruction plays a critical role in the pathogenesis of OSA.[9] Reduced upper airway dilator muscle activity at sleep results in an obstructive respiratory event. Notably, excessive muscle activity as evaluated by electromyography (EMG) in RBD[10] may contribute to preventing upper airway closure.[11] This phenomenon has been verified in idiopathic RBD patients but remains controversial in PD. Although we found that patients with PD and RBD experienced less OSA symptoms, another study presented the opposite conclusion.[12]
The prevalence of cognitive impairment in PD patients is approximately 30% but increases to 75–90% at 10-year follow-up.[13,14] PD patients with RBD exhibit worse cognition, especially executive/attention and memory functions.[15,16,17] Emerging evidence indicates that, in the healthy population, cognitive impairment is a severe OSA complication.[18] In PD, OSA may be associated with increased nonmotor symptoms, particularly cognitive dysfunction.[19] However, considering RBD may relieve hypoxemia in PD patients with OSA, whether this amelioration protects against cognitive decline is unclear.
As the relationship between OSA and RBD in PD patients remains unclear, we performed extensive clinical evaluations and overnight polysomnography (PSG) to explore whether RBD could alleviate OSA severity in PD patients and investigate the effect of hypoxemia and RBD on cognitive impairment in PD patients.
METHODS
Ethical approval
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Soochow University, and all patients provided written informed consent.
Participants
From February 2014 to May 2017, we recruited 224 PD patients from the Department of Neurology at the Second Affiliated Hospital of Soochow University. Study participants were diagnosed with PD using the United Kingdom PD Society Brain Bank clinical diagnosis criteria.[20] All the participants had at least 5 years of schooling. We excluded 38 of the recruited PD patients because of problems arising during PSG or the presence of major depression or anxiety as defined by the Diagnostic Statistical Manual-IV criteria. Considering the potential cognitive impairment in patients with hypertension and diabetes, patients with blood pressure either systolic blood pressure >160 mmHg (1 mmHg = 0.133 kPa) or diastolic blood pressure >100 mmHg or diabetes were excluded as well. After excluding the above patients, 174 patients remained.
Clinical assessment
We collected demographic information, past medical history, and medications on all participants. We evaluated motor manifestations of PD patients in the “off” state and cognitive assessment in the “on” state. The clinical manifestations included the Unified Parkinson's Disease Rating Scale, Hoehn and Yahr stage. The Levodopa-equivalent daily dose was calculated according to the method described by Tomlinson et al.[21] We assessed cognition by the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA). We evaluated daytime somnolence and quality of life using the Epworth Sleepiness Scale (ESS) and the PD Questionnaire, respectively.
For analysis, we divided the PD patients into categories based on whether they had RBD or OSA. Table 1 contains data on the comparison of demographic characteristics and PSG results of PD patients with and without RBD. Table 2 contains data on the comparison of demographic characteristics and PSG results of PD patients with and without OSA. Patients were further classified into four groups in Table 3: PD group (PD without OSA or RBD), PD + OSA group (PD patients with OSA only), PD + RBD group (PD patients with RBD only), and PD + RBD + OSA group (PD with both RBD and OSA).
Table 1.
Clinical and polysomnography characteristics in PD patients with and without RBD
| Characteristics | PD without RBD (n = 82) | PD with RBD (n = 92) | Statistics | P |
|---|---|---|---|---|
| Sex (male/female), n | 45/37 | 68/24 | 6.319* | 0.012 |
| Age (years) | 64.0 ± 9.3 | 65.1 ± 5.8 | −0.915† | 0.362 |
| BMI (kg/m2) | 24.1 ± 3.3 | 23.9 ± 2.7 | 0.514† | 0.608 |
| Duration (years) | 3.0 (1.0–5.0) | 3.0 (1.0–5.0) | −1.378‡ | 0.168 |
| Education (years) | 8.8 ± 3.2 | 8.4 ± 3.5 | 0.966† | 0.336 |
| UPDRS III | 23.70 ± 11.54 | 24.33 ± 9.98 | −0.387† | 0.700 |
| H-Y | 2.0 (1.5–2.5) | 2.0 (1.5–2.5) | −0.810‡ | 0.418 |
| LED (mg/d) | 300.0 (75.0–450.0) | 300.0 (100.0–450.0) | −0.534‡ | 0.593 |
| MMSE | 27.01 ± 2.12 | 25.55 ± 2.13 | 4.532† | <0.001 |
| MoCA | 24.61 ± 2.36 | 22.30 ± 2.43 | 6.363† | <0.001 |
| Depression score | 7.50 (4.00–13.00) | 8.00 (4.00–15.00) | −0.314‡ | 0.753 |
| Anxiety score | 6.00 (2.00–10.00) | 7.00 (2.00–9.00) | −0.364‡ | 0.715 |
| ESS | 5.00 (3.00–7.00) | 7.00 (4.00–10.00) | −2.681‡ | 0.007 |
| PDQ | 15.50 (9.00–26.00) | 17.00 (9.00–34.00) | −1.161‡ | 0.246 |
| TST (min) | 341.28 ± 107.91 | 328.65 ± 104.60 | 0.988† | 0.324 |
| SE (%) | 63.01 ± 18.52 | 60.22 ± 17.81 | 1.268† | 0.206 |
| SL (min) | 14.00 (2.50–29.00) | 13.50 (2.50–38.00) | −0.248‡ | 0.804 |
| Awakenings (times) | 22.0 (17.0–31.0) | 22.0 (16.0–29.0) | −0.089‡ | 0.929 |
| NREMS1 (%) | 13.50 (8.80–18.60) | 17.20 (10.00–26.20) | −1.880‡ | 0.060 |
| NREMS2 (%) | 50.57 ± 17.50 | 46.35 ± 16.15 | 2.184† | 0.089 |
| SWS (%) | 16.10 (8.60–24.30) | 16.40 (8.60–26.30) | −0.372‡ | 0.710 |
| REMS (%) | 14.30 (6.30–22.10) | 14.60 (9.70–20.50) | −0.916‡ | 0.360 |
| AHI (/h) | 0.80 (0.00–6.10) | 1.40 (0.00–5.70) | −0.612‡ | 0.541 |
| ODI (/h) | 1.15 (0.00–5.55) | 1.80 (0.20–6.30) | −0.505‡ | 0.613 |
| Min SaO2 (%) | 88.94 ± 6.95 | 90.47 ± 3.35 | −1.283† | 0.201 |
| Min SaO2 in REM (%) | 90.38 ± 6.78 | 92.11 ± 2.76 | −0.307† | 0.023 |
| Min SaO2 in NREM (%) | 90.72 ± 4.73 | 90.74 ± 3.59 | 0.667† | 0.506 |
| Tonic EMG activity (%) | 1.98 (0.61–11.19) | 21.95 (16.52–35.90) | −4.924‡ | <0.001 |
| Phasic EMG activity (%) | 3.05 (0.50–8.33) | 19.42 (15.00–30.42) | −5.344‡ | <0.001 |
Values are n, mean ± SD, or median (IQR). These analyses were performed using *Chi-square test, †independent Student’s t-test, and ‡nonparametric tests. PD: Parkinson’s disease; RBD: Rapid eye movement sleep behavior disorder; LED: Levodopa-equivalent daily dose; BMI: Body mass index; UPDRS: Unified Parkinson’s Disease Rating Scale; H-Y: Hoehn and Yahr stage; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; ESS: Epworth Sleepiness Scale; PDQ: Parkinson’s Disease Questionnaire; TST: Total sleep time; SE: Sleep efficiency; SL: Sleep latency; REMS: Rapid eye movement sleep; NREMS: Non-REMS; SWS: Slow wave sleep; AHI: Apnea-hypopnea index; ODI: Oxygen desaturation index; SaO2: Oxygen saturation; EMG: Electromyography; REM: Rapid eye movement; NREM: Non-REM; SD: Standard deviation; IQR: Interquartile range.
Table 2.
Clinical and polysomnography characteristics in PD patients with and without OSA
| Characteristics | PD without OSA (n = 114) | PD with OSA (n = 60) | Statistics | P |
|---|---|---|---|---|
| Sex (male/female), n | 72/42 | 41/19 | 0.462* | 0.496 |
| Age (years) | 63.8 ± 7.7 | 66.0 ± 7.5 | −1.844† | 0.067 |
| BMI (kg/m2) | 23.6 ± 2.9 | 24.8 ± 3.1 | −2.541† | 0.012 |
| Duration (years) | 3.0 (1.0–6.0) | 3.0 (2.0–4.0) | −0.367‡ | 0.714 |
| Education (years) | 8.7 ± 3.5 | 8.4 ± 3.0 | 0.673† | 0.502 |
| UPDRS III | 23.54 ± 11.12 | 24.97 ± 9.96 | −0.836† | 0.780 |
| H-Y | 2.0 (1.5–2.5) | 2.0 (1.5–2.5) | −0.279‡ | 0.671 |
| LED (mg/d) | 300.0 (37.5–450.0) | 300.0 (112.5–437.5) | −0.395‡ | 0.693 |
| MMSE | 26.57 ± 2.24 | 25.63 ± 2.14 | 2.664† | 0.008 |
| MoCA | 23.66 ± 2.69 | 22.92 ± 2.56 | 1.758† | 0.081 |
| HRSD | 9.00 (3.00–15.00) | 7.00 (4.00–9.50) | −1.254‡ | 0.210 |
| HAMA | 6.00 (2.00–10.00) | 5.00 (1.50–8.00) | −0.966‡ | 0.334 |
| ESS | 6.00 (3.00–9.00) | 5.50 (3.00–8.50) | −0.550‡ | 0.582 |
| PDQ | 16.00 (8.00–33.00) | 17.00 (13.00–23.25) | −0.921‡ | 0.357 |
| TST (min) | 329.7 ± 106.0 | 338.3 ± 104.0 | −0.514† | 0.608 |
| SE (%) | 60.90 (47.20–74.60) | 67.90 (48.70–76.35) | −0.997‡ | 0.319 |
| SL (min) | 13.25 (1.38–28.63) | 15.75 (5.25–35.88) | −1.286‡ | 0.199 |
| Awakenings (times) | 21.0 (16.0–31.3) | 22.5 (16.3–28.8) | −0.116‡ | 0.908 |
| NREMS1 (%) | 13.25 (6.80–23.05) | 16.50 (11.78–24.53) | −2.045‡ | 0.041 |
| NREMS2 (%) | 47.87 ± 17.98 | 48.33 ± 14.16 | −0.174† | 0.862 |
| SWS (%) | 16.15 (9.50–26.50) | 17.45 (6.60–24.18) | −0.253‡ | 0.800 |
| REMS (%) | 14.50 (8.28–22.83) | 14.20 (7.55–20.10) | −0.667‡ | 0.505 |
| AHI (/h) | 0.00 (0.00–1.23) | 10.20 (5.73–22.08) | −10.772‡ | <0.001 |
| ODI (/h) | 0.35 (0.00–1.50) | 9.00 (4.70–19.70) | −9.220‡ | <0.001 |
| Min SaO2 (%) | 91.83 ± 2.33 | 85.98 ± 6.76 | 6.507† | <0.001 |
| Min SaO2 in REM (%) | 93.17 ± 2.61 | 88.48 ± 5.71 | 6.289† | <0.001 |
| Min SaO2 in NREM (%) | 92.65 ± 1.82 | 87.38 ± 4.25 | 9.169† | <0.001 |
| Tonic EMG activity (%) | 16.09 (6.75–21.34) | 19.72 (1.83–443.67) | −1.268‡ | 0.205 |
| Phasic EMG activity (%) | 11.45 (6.13–17.60) | 16.36 (3.15–39.50) | −0.800‡ | 0.423 |
Values are n, mean ± SD, or median (IQR). These analyses were performed using *Chi-square test, †independent Student’s t-test, and ‡nonparametric tests. PD: Parkinson’s disease; RBD: Rapid eye movement sleep behavior disorder; LED: Levodopa-equivalent daily dose; BMI: Body mass index; UPDRS: Unified Parkinson’s Disease Rating Scale; H-Y: Hoehn and Yahr stage; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; ESS: Epworth Sleepiness Scale; PDQ: Parkinson’s Disease Questionnaire; TST: Total sleep time; SE: Sleep efficiency; SL: Sleep latency; REMS: Rapid eye movement sleep; NREMS: Non-REMS; SWS: Slow wave sleep; AHI: Apnea-hypopnea index; ODI: Oxygen desaturation index; SaO2: Oxygen saturation; EMG: Electromyography; OSA: Obstructive sleep apnea; SD: Standard deviation; IQR: Interquartile range; HRSD: Hamilton Rating Scale for Depression; HAMA: Hamilton Anxiety Rating Scale.
Table 3.
Clinical and polysomnography characteristics in PD patients with and without OSA and RBD
| Characteristics | PD (n = 53) | PD + OSA (n = 29) | PD + RBD (n = 61) | PD + RBD + OSA (n = 31) | Statistics | P |
|---|---|---|---|---|---|---|
| Sex (male/female), n | 28/25 | 17/12 | 44/17 | 24/7 | 7.429* | 0.059 |
| Age (years) | 62.8 ± 9.3 | 65.7 ± 8.8 | 64.7 ± 5.9 | 66.4 ± 6.2 | 1.773† | 0.154 |
| BMI (kg/m2) | 23.5 ± 3.0 | 25.3 ± 3.6 | 23.6 ± 2.8 | 24.3 ± 2.4 | 2.748† | 0.045 |
| Duration (years) | 2.0 (1.0–5.5) | 3.0 (1.1–4.5) | 3.0 (1.5–5.0) | 3.0 (2.0–4.0) | 2.316† | 0.509 |
| Education (years) | 9.2 ± 3.4 | 8.4 ± 3.0 | 8.3 ± 3.6 | 8.3 ± 3.2 | 0.724† | 0.539 |
| UPDRS III | 24.71 ± 1.42 | 23.75 ± 1.86 | 22.36 ± 1.29 | 26.53 ± 1.81 | 1.262‡ | 0.289 |
| H-Y | 2.0 (1.5–2.5) | 2.0 (1.5–2.5) | 2.0 (1.5–2.5) | 2.0 (1.5–2.5) | 1.205¦ | 0.752 |
| LED (mg/d) | 310.67 ± 22.67 | 320.05 ± 30.14 | 285.97 ± 20.60 | 303.93 ± 29.92 | 0.358‡ | 0.783 |
| MMSE | 27.42 ± 0.26 | 26.39 ± 0.35|| | 25.73 ± 0.24¶ | 25.20 ± 0.35** | 10.480‡ | <0.001 |
| MoCA | 24.99 ± 0.31 | 24.08 ± 0.41 | 22.37 ± 0.28¶ | 22.27 ± 0.40** | 15.951‡ | <0.001 |
| HRSD | 9.43 ± 0.97 | 7.48 ± 1.28 | 9.94 ± 0.87 | 8.17 ± 1.27 | 1.065‡ | 0.366 |
| HAMA | 6.55 ± 0.77 | 5.40 ± 1.02 | 7.28 ± 0.70 | 5.75 ± 1.02 | 0.988‡ | 0.400 |
| ESS | 6.24 ± 0.62 | 5.74 ± 0.84 | 6.91 ± 0.56 | 7.14 ± 0.81 | 0.668‡ | 0.573 |
| PDQ-39 | 19.64 ± 1.85 | 20.77 ± 2.43 | 20.51 ± 1.66 | 22.97 ± 2.41 | 0.396‡ | 0.756 |
| TST (min) | 329.00 ± 15.20 | 350.50 ± 20.16 | 326.40 ± 13.80 | 336.40 ± 13.80 | 0.359‡ | 0.783 |
| SE (%) | 61.03 ± 2.58 | 64.35 ± 3.43 | 58.74 ± 2.35 | 63.26 ± 3.41 | 0.766‡ | 0.515 |
| SL (min) | 21.14 ± 6.89 | 36.12 ± 9.14 | 37.13 ± 6.26 | 20.45 ± 9.10 | 1.536‡ | 0.207 |
| Awakenings (times) | 24.0 ± 1.7 | 21.3 ± 2.2 | 23.6 ± 1.5 | 25.5 ± 2.2 | 0.624‡ | 0.601 |
| NREMS1 (%) | 18.96 ± 1.81 | 17.19 ± 2.40 | 17.73 ± 1.64 | 22.77 ± 2.38 | 1.238‡ | 0.298 |
| NREMS2 (%) | 50.85 ± 2.34 | 48.30 ± 3.11 | 44.80 ± 2.13 | 48.34 ± 3.09 | 1.209‡ | 0.308 |
| SWS (%) | 15.35 ± 1.76 | 19.15 ± 2.34 | 20.71 ± 1.60 | 17.26 ± 2.32 | 1.781‡ | 0.153 |
| REMS (%) | 14.16 ± 4.86 | 14.16 ± 6.46 | 23.84 ± 4.41 | 14.33 ± 6.41 | 0.995‡ | 0.397 |
| AHI (/h) | 0.20 (0.00–0.80) | 7.90 (5.90–21.15)|| | 0.00 (0.00–1.45) | 10.90 (5.70–23.00)†† | 111.070¦ | <0.001 |
| ODI (/h) | 0.20 (0.00–1.20) | 9.00 (4.03–16.75)|| | 0.40 (0.00–1.80) | 9.00 (6.00–21.40)†† | 85.987¦ | <0.001 |
| Min SaO2 (%) | 92.36 ± 0.62 | 84.25 ± 0.82|| | 91.34 ± 0.56 | 87.48 ± 0.81**,†† | 25.570‡ | <0.001 |
| Min SaO2 in REM (%) | 93.33 ± 0.57 | 86.74 ± 0.76|| | 93.54 ± 0.52 | 90.00 ± 0.75**,†† | 22.856‡ | <0.001 |
| Min SaO2 in NREM (%) | 93.15 ± 0.42 | 87.63 ± 0.56|| | 92.24 ± 0.38 | 87.15 ± 0.55†† | 39.965‡ | <0.001 |
Values are mean ± SD or median (IQR). The P value reflects differences among the four groups. These analyses were performed using *Chi-square test, †one-way analysis of variance, ‡analysis of covariance (adjusted for age, sex, duration, insomnia, and restless leg symptom. MMSE and MoCA were adjusted for education additionally), and §nonparametric tests. ||Significance between PD and PD + OSA groups; ¶Significance between PD and PD + RBD groups; **Significance between PD + OSA and PD + RBD + OSA groups; ††Significance between PD + RBD and PD + RBD + OSA groups. PD: Parkinson’s disease; RBD: Rapid eye movement sleep behavior disorder; LED: Levodopa-equivalent daily dose; BMI: Body mass index; UPDRS: Unified Parkinson’s Disease Rating Scale; H-Y: Hoehn and Yahr stage; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; ESS: Epworth Sleepiness Scale; PDQ: Parkinson’s Disease Questionnaire; TST: Total sleep time; SE: Sleep efficiency; SL: Sleep latency; REMS: Rapid eye movement sleep; NREMS: Non-REMS; SWS: Slow wave sleep; AHI: Apnea-hypopnea index; ODI: Oxygen desaturation index; SaO2: Oxygen saturation; SD: Standard deviation; OSA: Obstructive sleep apnea; IQR: Interquartile range.
Polysomnography
All patients underwent an overnight video-PSG study. Experienced PSG technologists and clinicians scored sleep stages, awakenings, and respiratory-related parameters, including apnea-hypopnea index (AHI), oxygen desaturation index (ODI), and minimum oxygen saturation (SaO2) in REM and non-REM (NREM) sleep according to the American Academy of Sleep Medicine (AASM) guidelines.[22,23] RBD was diagnosed according to the International Classification of Sleep Disorders Third Edition criteria using the PSG and clinical evaluations. The tonic chin EMG activity (tonic density) and the phasic chin EMG density activity (phasic density) were calculated according to a previously published method[24] and AASM criteria. OSA was diagnosed if PSG evaluation yielded an AHI ≥5/h.
Statistical analysis
We used SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA) for the statistical analysis. Data were represented as the mean ± standard deviation (SD) or median (interquartile range). Comparisons were performed using independent Student's t-test or Chi-square test for clinical and PSG variables. Analysis of covariance or logistic regression models were used for adjusted comparisons of continuous and categorical data, respectively. The data were adjusted for age, sex, disease duration, insomnia, and restless leg symptoms. MMSE and MoCA results also were adjusted for education.[25] Linear regression analysis was used to assess for trends in MoCA and index of OSA or RBD. A P < 0.05 was considered statistically significant. A P < 0.013 was considered to meet the threshold for significance in the Bonferroni post hoc test.
RESULTS
Of the 174 PD patients, 113 were men and 61 were women. The mean age of participants was 64.6 ± 7.7 years, and the mean education duration was 8.6 ± 3.4 years. Ninety-two patients (52.3%) had RBD and 60 (34.5%) had OSA.
Clinical and polysomnography characteristics in Parkinson's disease with and without rapid eye movement sleep behavior disorder or obstructive sleep apnea
Table 1 illustrates the clinical characteristics and PSG results from PD patients with and without RBD. We found no significant difference in PD motor symptoms and sleep structure between the two groups. PD patients with RBD performed significantly worse in MMSE (27.01 ± 2.12 vs. 25.55 ± 2.13, t = 4.532, P < 0.001) and MoCA (24.61 ± 2.36 vs. 22.30 ± 2.43, t = 6.363, P < 0.001) than patients without RBD. The median ESS score in PD patients with RBD was higher (7.00 [4.00–10.00] vs. 5.00 [3.00–7.00], U = −2.681, P = 0.007) than that in PD patients without RBD. PD patients with RBD had significantly higher percentages of tonic and phasic EMG activity than patients without RBD. We found no significant difference in AHI, ODI, and SaO2 during the whole sleep period between the two groups. We further explored the difference in minimum SaO2 between REM and NREM sleep. We found that PD patients with RBD had higher minimum SaO2 during REM sleep that those without RBD (90.38 ± 6.78% vs. 92.11 ± 2.76%, t = −0.307, P = 0.023).
Table 2 summarizes the results of our comparison between PD patients with and without OSA. PD patients with OSA had higher AHI and ODI and lower minimum SaO2 compared with those without OSA. Their proportion of time in NREM sleep stage 1 was higher, and they performed worse in MMSE (25.63 ± 2.14 vs. 26.57 ± 2.24, t = 2.664, P = 0.008).
Clinical and polysomnographic characteristics in Parkinson's disease patients with and without obstructive sleep apnea and rapid eye movement sleep behavior disorder
The clinical and PSG characteristics of PD, PD + OSA, PD + RBD, and PD + RBD + OSA patients are presented in Table 3. Comparisons of the clinical symptoms and PSG parameters among the groups were adjusted for age, sex, duration of disease, insomnia, and restless leg symptoms. Comparisons of the MMSE and MoCA scores were also adjusted for education. PD + RBD patients had worse MMSE and MoCA scores than those without. PD + OSA patients performed worse in MMSE than that in PD group as well. Aside from these differences, we found no statistically significant differences in clinical features among the four groups. Obviously, AHI, ODI, and minimum SaO2 in NREM sleep were higher in the PD + OSA and PD + RBD + OSA groups as compared with those in the PD and PD + RBD groups, respectively. Both during whole sleep and in REM sleep, PD + RBD + OSA patients had higher minimum SaO2 than that of PD + OSA patients.
Montreal Cognitive Assessment scores in Parkinson's disease patients with and without obstructive sleep apnea and rapid eye movement sleep behavior disorder
Figure 1 displays the MoCA domain scores and the comparisons of the scores among the four groups. The results were adjusted for age, sex, duration of disease, and education. The PD + RBD and PD + RBD + OSA groups performed worse in visuospatial/executive function, attention, and delayed recall compared with the PD group. However, the PD + RBD + OSA group did not perform significantly worse than the PD + RBD group. Only in the delayed recall domain did the PD + OSA group have worse scores than the PD group have.
Figure 1.
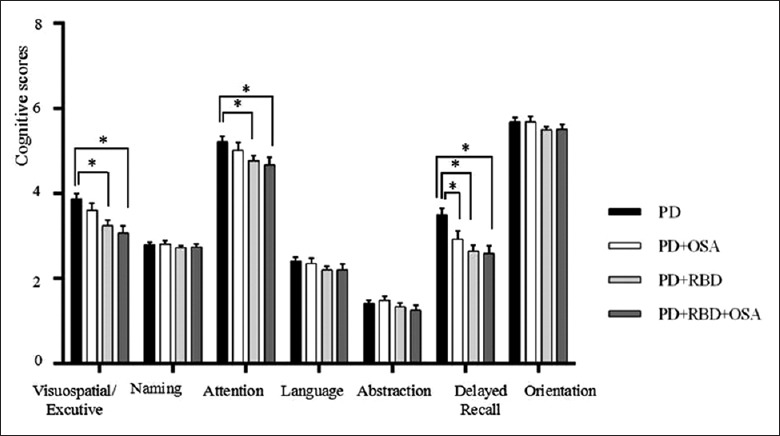
Different domains of MoCA in PD patients with and without OSA or RBD. These analyses were performed using analysis of covariance (adjusted for age, sex, duration of disease, and education) and Bonferroni post hoc test (*P < 0.013). PD: PD without OSA or RBD group, n = 53; PD + OSA: PD patients with OSA only, n = 29; PD + RBD: PD patients with RBD only, n = 61; PD + RBD + OSA: PD patients with both RBD and OSA, n = 31. MoCA: Montreal Cognitive Assessment; OSA: Obstructive sleep apnea; RBD: REM sleep behavior disorder; REM: Rapid eye movement; PD: Parkinson's disease.
Linear regression analysis of the association between Montreal Cognitive Assessment and obstructive sleep apnea or rapid eye movement sleep behavior disorder
We further investigated the association between MoCA scores and OSA or RBD using linear regression analysis [Table 4]. To reduce the influence of confounding factors such as age, sex, body mass index, education, severity of disease, and other sleep disorders (insomnia and restless legs syndrome), we built multiple models, as a single model, including all the potentially confounding factors, was not feasible because of the correlation between RBD and tonic and phasic EMG activity or between OSA and AHI.
Table 4.
Linear regression models for the association between MoCA scores and characteristics of OSA or RBD
| MoCA linear regression | β | 95% confidence internal | P |
|---|---|---|---|
| OSA | |||
| Unadjusted | −0.722 | −1.544 to 0.101 | 0.085 |
| Model 1 | −0.584 | −1.395 to 0.228 | 0.157 |
| Model 2 | −0.574 | −1.395 to 0.247 | 0.169 |
| Model 3 | −0.736 | −1.447 to −0.025 | 0.043 |
| RBD | |||
| Unadjusted | −2.480 | −3.179 to −1.781 | <0.001 |
| Model 1 | −2.429 | −3.088 to −1.771 | <0.001 |
| Model 2 | −2.445 | −3.106 to −1.784 | <0.001 |
| Model 3 | −2.575 | −3.231 to −1.920 | <0.001 |
| AHI | |||
| Unadjusted | −0.054 | −0.087 to −0.021 | 0.002 |
| Model 1 | −0.046 | −0.079 to −0.012 | 0.008 |
| Model 2 | −0.046 | −0.080 to −0.012 | 0.008 |
| Model 3 | −0.052 | −0.081 to −0.024 | <0.001 |
| ODI | |||
| Unadjusted | −0.061 | −0.094 to −0.028 | <0.001 |
| Model 1 | −0.054 | −0.087 to −0.020 | 0.002 |
| Model 2 | −0.054 | −0.088 to −0.020 | 0.002 |
| Model 3 | −0.060 | −0.089 to −0.031 | <0.001 |
| Tonic EMG activity | |||
| Unadjusted | −0.059 | −0.100 to −0.019 | 0.005 |
| Model 1 | −0.057 | −0.104 to −0.011 | 0.016 |
| Model 2 | −0.059 | −0.105 to −0.012 | 0.016 |
| Model 3 | −0.065 | −0.119 to −0.012 | 0.017 |
| Phasic EMG activity | |||
| Unadjusted | −0.046 | −0.087 to −0.004 | 0.031 |
| Model 1 | −0.043 | −0.088 to 0.001 | 0.057 |
| Model 2 | −0.047 | −0.093 to −0.002 | 0.043 |
| Model 3 | −0.051 | −0.102 to −0.001 | 0.049 |
Model 1: Adjusted for age, duration, education, and BMI; Model 2: Adjusted for age, duration, education, BMI, UPDRS III score, and Levodopa-equivalent daily dosage; Model 3: Adjusted for age, duration, education, BMI, UPDRS III score, Levodopa-equivalent daily dosage, insomnia, restless legs syndrome, RBD, or OSA. MoCA: Montreal Cognitive Assessment; OSA: Obstructive sleep apnea; RBD: Rapid eye movement sleep behavior disorder; AHI: Apnea-hypopnea index; ODI: Oxygen desaturation index; EMG: Electromyography; BMI: Body mass index; UPDRS: Unified Parkinson’s Disease Rating Scale.
Multiple linear regression analysis revealed a significant association between MoCA score and OSA (adjusted for RBD in Model 3; β = −0.736, P = 0.043) and between MoCA score and RBD (adjusted for OSA in Model 3; β = −2.575, P < 0.001). The adjusted β value suggested that for PD patients with RBD, the MoCA score decreased by 2.324. Meanwhile, for PD patients with OSA, the MoCA score decreased by 0.743. To further characterize the relationship between MoCA score and severity of OSA and RBD, we performed linear regression analyses to assess the effect of AHI, ODI, and tonic/phasic EMG activity on MoCA score. The differences we found remained significant after adjustment. The adjusted β value indicated that MoCA score decreased by 0.57 for every 10 unit increase in AHI.
DISCUSSION
In this study, we used PSG to evaluate sleep-related disorders in PD patients. Our results revealed that excessive EMG activity in PD patients with RBD might protect patients against reduced minimum SaO2 occurring in PD patients with OSA, and cognitive performance was worse in PD patients with RBD or OSA than in those patients without those conditions.
In terms of the higher minimum SaO2 in PD patients with RBD, RBD was protective of OSA severity as evaluated by the minimum SaO2. This phenomenon was supported by two of our findings: (1) the minimum SaO2 in REM sleep was significantly higher in PD patients with RBD and (2) PD + RBD + OSA patients had higher minimum SaO2 in both whole sleep and in REM sleep than PD + OSA patients. Our previous research, which found lower AHI and ODI during REM sleep in PD + RBD patients, verifies this result as well.[26] This is consistent with another study which found that PD patients with increased muscle tone during sleep had lower OSA severity.[7] However, another study conducted on 46 PD patients reached the opposite conclusion.[12] This discrepancy could be explained by the relatively small sample size in their study. The possible mechanism for higher minimum SaO2 in PD + RBD patients may be the loss of atonia in upper airway dilator muscles. Another explanation is that drugs that might increase muscle activity could also benefit OSA, such as paroxetine, mirtazapine, and glycinergic antagonists.[27,28] Some investigators obtained similar results, finding that, in idiopathic RBD patients, OSA severity was also alleviated.[10]
OSA and RBD may each play a negative role in the cognitive impairment of PD patients. PD + OSA patients were more likely to have memory dysfunction, while PD + RBD and PD + RBD + OSA patients exhibited impairment in the visuospatial/executive, attention, and memory domains. However, differences between the PD + RBD + OSA and PD + RBD/PD + OSA groups were not statistically significant. Prior research also found executive and attention domain impairment in PD + OSA patients.[29] The use of different tests to evaluate cognitive function could account for this disparity in results. The mechanism for the severe cognitive impairment in PD patients with OSA may include hypoxemia and sleep fragmentation, which may aggravate the dysfunction of the locus coeruleus and multiple other brain areas. On the other hand, for PD patients with RBD, the dysfunction of the pedunculopontine nucleus and cholinergic system plays an important role in their cognitive impairment.[30]
In our study, linear regression models showed that cognitive impairment severity was associated with OSA and RBD. In our participants, MoCA score for PD patients with RBD declined by 2.575 points and by 0.736 points for PD patients with OSA. As the negative effect of RBD on cognition was larger than that of OSA, the higher minimum SaO2 in PD patients with RBD did not counteract the negative effect of RBD on cognitive impairment. Few interventions effectively protect cognition in PD. However, a previous research has explored the association between cognition and RBD or OSA in PD patients.[16,31,32] Our finding that PD patients with RBD or OSA experienced greater cognitive impairment was consistent with previous reports. We also investigated the interaction between RBD and OSA and quantified their effect on cognition. Our study revealed that higher AHI may exacerbate PD cognitive dysfunction. However, whether treatment with continuous positive airway pressure (CPAP) benefits cognition in PD patients remains to be demonstrated.[6,29,33] At present, the longest follow-up duration for CPAP treatment is 3 months, and they found no significant changes in neuropsychological scores after CPAP treatment.[29] The effects of CPAP in PD patients over a longer follow-up period need to be studied.
This study had several limitations. First, the PD + OSA group had a relatively small sample size. Second, we only used MoCA, a multidomain screening tool, to evaluate for cognitive dysfunction. Future studies should employ more detailed neuropsychological tests to assess further domains in which the patient is impaired. Third, we did investigate mild cognitive impairment, which can be a manifestation of numerous complex pathological processes.[34] We plan to evaluate the effect of CPAP on cognitive deficits in PD patients with OSA in futures studies because previous research indicates that cognition partially improves in OSA patients after CPAP treatment.[35]
In conclusion, we found that RBD alleviated OSA severity; however, RBD and OSA together exacerbated PD cognitive impairment. Further studies are needed to evaluate whether OSA treatment can improve cognition in PD.
Financial support and sponsorship
This work was supported by grants from the National Key R & D Program of China (No. 2017YFC0909100), the National Natural Science Foundation of China (No. 91649114), the Jiangsu Provincial Social Development Projects (No. BE2017653), the Jiangsu Provincial Medical Key Discipline Project (No. ZDXKB2016022), the Jiangsu Key Laboratory of Neuropsychiatric Diseases (No. BM2013003), and the Suzhou Clinical Research Center of Neurological Disease (No. Szzx201503). This work was also partly supported by grants from the Suzhou Science and Technology Development Program (No. SYS201624), the Suzhou Youth Technology Project Foundation (No. KJXW2016014), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Ning-Ning Wang
REFERENCES
- 1.Nomura T, Inoue Y, Takigawa H, Nakashima K. Comparison of REM sleep behaviour disorder variables between patients with progressive supranuclear palsy and those with Parkinson's disease. Parkinsonism Relat Disord. 2012;18:394–6. doi: 10.1016/j.parkreldis.2011.10.018. doi: 10.1016/j.parkreldis.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Sobreira-Neto MA, Pena-Pereira MA, Sobreira ES, Chagas MH, Fernandes RM, Tumas V, et al. High frequency of sleep disorders in Parkinson's disease and its relationship with quality of life. Eur Neurol. 2017;78:330–7. doi: 10.1159/000481939. doi: 10.1159/000481939. [DOI] [PubMed] [Google Scholar]
- 3.Pandya M, Kubu CS, Giroux ML. Parkinson disease: Not just a movement disorder. Cleve Clin J Med. 2008;75:856–64. doi: 10.3949/ccjm.75a.07005. doi: 10.3949/ccjm.75a.07005. [DOI] [PubMed] [Google Scholar]
- 4.Iranzo A, Molinuevo JL, Santamaría J, Serradell M, Martí MJ, Valldeoriola F, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: A descriptive study. Lancet Neurol. 2006;5:572–7. doi: 10.1016/S1474-4422(06)70476-8. doi: 10.1016/S1474-4422(06) 70476-8. [DOI] [PubMed] [Google Scholar]
- 5.Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson's disease is associated with specific motor features. J Neurol Neurosurg Psychiatry. 2008;79:1117–21. doi: 10.1136/jnnp.2008.149195. doi: 10.1136/jnnp.2008.149195. [DOI] [PubMed] [Google Scholar]
- 6.Neikrug AB, Liu L, Avanzino JA, Maglione JE, Natarajan L, Bradley L, et al. Continuous positive airway pressure improves sleep and daytime sleepiness in patients with Parkinson disease and sleep apnea. Sleep. 2014;37:177–85. doi: 10.5665/sleep.3332. doi: 10.5665/sleep.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cochen De Cock V, Abouda M, Leu S, Oudiette D, Roze E, Vidailhet M, et al. Is obstructive sleep apnea a problem in Parkinson's disease? Sleep Med. 2010;11:247–52. doi: 10.1016/j.sleep.2009.05.008. doi: 10.1016/j.sleep.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 8.da Silva-Júnior FP, Jr, do Prado GF, Barbosa ER, Tufik S, Togeiro SM. Sleep disordered breathing in Parkinson's disease: A critical appraisal. Sleep Med Rev. 2014;18:173–8. doi: 10.1016/j.smrv.2013.04.005. doi: 10.1016/j.smrv.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J, Zhang J, Lam SP, Li SX, Ho CK, Lam V, et al. Amelioration of obstructive sleep apnea in REM sleep behavior disorder: Implications for the neuromuscular control of OSA. Sleep. 2011;34:909–15. doi: 10.5665/SLEEP.1126. doi: 10.5665/SLEEP.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubin L, Davies RO, Pack AI. Control of upper airway motoneurons during REM sleep. News Physiol Sci. 1998;13:91–7. doi: 10.1152/physiologyonline.1998.13.2.91. doi: 10.1152/physiologyonline.1998.13.2.91. [DOI] [PubMed] [Google Scholar]
- 12.Zhang LY, Liu WY, Kang WY, Yang Q, Wang XY, Ding JQ, et al. Association of rapid eye movement sleep behavior disorder with sleep-disordered breathing in Parkinson's disease. Sleep Med. 2016;20:110–5. doi: 10.1016/j.sleep.2015.12.018. doi: 10.1016/j.sleep.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289:18–22. doi: 10.1016/j.jns.2009.08.034. doi: 10.1016/j.jns.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 14.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: The inevitability of dementia at 20 years. Mov Disord. 2008;23:837–44. doi: 10.1002/mds.21956. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 15.Gagnon JF, Vendette M, Postuma RB, Desjardins C, Massicotte-Marquez J, Panisset M, et al. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson's disease. Ann Neurol. 2009;66:39–47. doi: 10.1002/ana.21680. doi: 10.1002/ana.21680. [DOI] [PubMed] [Google Scholar]
- 16.Zhang JR, Chen J, Yang ZJ, Zhang HJ, Fu YT, Shen Y, et al. Rapid eye movement sleep behavior disorder symptoms correlate with domains of cognitive impairment in Parkinson's disease. Chin Med J. 2016;129:379–85. doi: 10.4103/0366-6999.176077. doi: 10.4103/0366-6999.176077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Zhu XY, Zhang XJ, Kuo SH, Ondo WG, Wu YC, et al. Clinical features of Parkinson's disease with and without rapid eye movement sleep behavior disorder. Transl Neurodegener. 2017;6:35. doi: 10.1186/s40035-017-0105-5. doi: 10.1186/s40035-017-0105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan YY, Deng Y, Xie S, Wang ZH, Wang Y, Ren J, et al. Altered Wnt signaling pathway in cognitive impairment caused by chronic intermittent hypoxia: Focus on glycogen synthase kinase-3β and β-catenin. Chin Med J. 2016;129:838–45. doi: 10.4103/0366-6999.178969. doi: 10.4103/0366-6999.178969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaminska M, Lafontaine AL, Kimoff RJ. The interaction between obstructive sleep apnea and Parkinson's disease: Possible mechanisms and implications for cognitive function. Parkinsons Dis. 2015;2015:849472. doi: 10.1155/2015/849472. doi: 10.1155/2015/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE, et al. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–53. doi: 10.1002/mds.23429. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 22.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 24.Montplaisir J, Gagnon JF, Fantini ML, Postuma RB, Dauvilliers Y, Desautels A, et al. Polysomnographic diagnosis of idiopathic REM sleep behavior disorder. Mov Disord. 2010;25:2044–51. doi: 10.1002/mds.23257. doi: 10.1002/mds.23257. [DOI] [PubMed] [Google Scholar]
- 25.Bohnen NI, Kotagal V, Müller ML, Koeppe RA, Scott PJ, Albin RL, et al. Diabetes mellitus is independently associated with more severe cognitive impairment in Parkinson disease. Parkinsonism Relat Disord. 2014;20:1394–8. doi: 10.1016/j.parkreldis.2014.10.008. doi: 10.1016/j.parkreldis.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong Y, Xiong KP, Mao CJ, Shen Y, Hu WD, Huang JY, et al. Clinical manifestations of Parkinson disease and the onset of rapid eye movement sleep behavior disorder. Sleep Med. 2014;15:647–53. doi: 10.1016/j.sleep.2013.12.021. doi: 10.1016/j.sleep.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 27.Kraiczi H, Hedner J, Dahlöf P, Ejnell H, Carlson J. Effect of serotonin uptake inhibition on breathing during sleep and daytime symptoms in obstructive sleep apnea. Sleep. 1999;22:61–7. [PubMed] [Google Scholar]
- 28.Remmers JE, Anch AM, deGroot WJ, Baker JP, Jr, Sauerland EK. Oropharyngeal muscle tone in obstructive sleep apnea before and after strychnine. Sleep. 1980;3:447–53. doi: 10.1093/sleep/3.3-4.447. doi: 10.1093/sleep/3.3-4.447. [DOI] [PubMed] [Google Scholar]
- 29.Terzaghi M, Spelta L, Minafra B, Rustioni V, Zangaglia R, Pacchetti C, et al. Treating sleep apnea in Parkinson's disease with C-PAP: Feasibility concerns and effects on cognition and alertness. Sleep Med. 2017;33:114–8. doi: 10.1016/j.sleep.2017.01.009. doi: 10.1016/j.sleep.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson's disease. Mov Disord. 2011;26:2496–503. doi: 10.1002/mds.23932. doi: 10.1002/mds.23932. [DOI] [PubMed] [Google Scholar]
- 31.Nomura T, Inoue Y, Kagimura T, Nakashima K. Clinical significance of REM sleep behavior disorder in Parkinson's disease. Sleep Med. 2013;14:131–5. doi: 10.1016/j.sleep.2012.10.011. doi: 10.1016/j.sleep.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Mery VP, Gros P, Lafontaine AL, Robinson A, Benedetti A, Kimoff RJ, et al. Reduced cognitive function in patients with Parkinson disease and obstructive sleep apnea. Neurology. 2017;88:1120–8. doi: 10.1212/WNL.0000000000003738. doi: 10.1212/WNL.0000000000003738. [DOI] [PubMed] [Google Scholar]
- 33.Harmell AL, Neikrug AB, Palmer BW, Avanzino JA, Liu L, Maglione JE, et al. Obstructive sleep apnea and cognition in Parkinson's disease. Sleep Med. 2016;21:28–34. doi: 10.1016/j.sleep.2016.01.001. doi: 10.1016/j.sleep.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen MC, Chan LL, Tan LC, Tan EK. Mild cognitive impairment in Parkinson's disease: A distinct clinical entity? Transl Neurodegener. 2017;6:24. doi: 10.1186/s40035-017-0094-4. doi: 10.1186/s40035-017-0094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan YY, Deng Y, Xu X, Liu YP, Liu HG. Effects of continuous positive airway pressure on cognitive deficits in middle-aged patients with obstructive sleep apnea syndrome: A Meta-analysis of randomized controlled trials. Chin Med J. 2015;128:2365–73. doi: 10.4103/0366-6999.163385. doi: 10.4103/0366-6999.163385. [DOI] [PMC free article] [PubMed] [Google Scholar]


