Abstract
Background:
Polycystic ovary syndrome (PCOS) is a complex endocrine and metabolic disorder, and it's diagnosis is difficult. The aim of this study was to investigate the metabolic profiles of PCOS patients by analyzing urine samples and identify useful biomarkers for diagnosis of PCOS.
Methods:
This study was carried out in the Department of Obstetrics and Gynecology of the Maternal and Child Health Hospital of Hunan Province from December 2014 to July 2016. In this study, the urine samples of 21 women with PCOS and 16 healthy controls were assessed through gas chromatography-mass spectrometry to investigate the urine metabolite characteristics of PCOS and identify useful biomarkers for the diagnosis of this disorder. The Student's t-test and rank sum test were applied to validate the statistical significance of the between the two groups.
Results:
In total, 35 urine metabolites were found to be significantly different between the PCOS patients and the controls. In particular, a significant increase in the levels of lactose (10.01 [0,13.99] mmol/mol creatinine vs. 2.35 [0.16, 3.26] mmol/mol creatinine, P = 0.042), stearic acid (2.35 [1.47, 3.14] mmol/mol creatinine vs. 0.05 [0, 0.14] mmol/mol creatinine, P < 0.001), and palmitic acid (2.13 [1.07, 2.79] mmol/mol creatinine vs. 0 [0, 0] mmol/mol creatinine, P < 0.001) and a decrease in the levels of succinic acid (0 [0, 0] mmol/mol creatinine vs. 38.94 [4.16, 51.30] mmol/mol creatinine, P < 0.001) were found in the PCOS patients compared with the controls. It was possible to cluster the PCOS patients and the healthy controls into two distinct regions based on a principal component analysis model. Of the differentially expressed metabolites, four compounds, including stearic acid, palmitic acid, benzoylglycine, and threonine, were selected as potential biomarkers.
Conclusions:
This study offers new insight into the pathogenesis of PCOS, and the discriminating urine metabolites may provide a prospect for the diagnosis of PCOS.
Keywords: Biomarkers, Gas Chromatography-Mass Spectrometry, Metabolomics, Polycystic Ovary Syndrome, Urine Metabolites
摘要
背景:
多囊卵巢综合征(PCOS)是一种复杂的内分泌代谢疾病,诊断困难。本试验旨在通过分析PCOS患者尿液样本的代谢 谱,来鉴定用于诊断PCOS的生物标志物。
方法:
本研究于2014年12月至2016年7月在湖南省妇幼保健院妇产科进行。本研究采用气相色谱质谱联用技术对21名PCOS女 性和16名健康对照者的尿液进行了检测,以研究其尿代谢物特征并鉴定用于诊断该病的有用的生物标记物。采用两独立样本 t检验及秩和检验比较两组间的差别。
结果:
PCOS患者与对照组共有35种尿代谢物存在显着性差异。与对照相比,PCOS患者中乳糖(10.01 (0.00, 13.99)mmol/mol 肌酐比2.35 (0.16, 3.26)mmol/mol肌酐, P = 0.042),硬脂酸(2.35 (1.47, 3.14)mmol/mol肌酐比0.05 (0.00, 0.14)mmol/mol肌酐, P <0.001),软脂酸(2.13 (1.07, 2.79)mmol/mol肌酐比0.05 (0.00, 0.14)mmol/mol肌酐, P <0.001)等水平显著增加,同时琥 珀酸水平(0.00 (0.00,0.00)mmol/mol肌酐比38.94(4.16, 51.30)mmol/mol肌酐, P <0.001)降低。基于主成分分析模型,可以将 PCOS患者和健康对照聚类为两个不同的区域。在差异表达的代谢物中,硬脂酸,棕榈酸,苯甲酰甘氨酸和苏氨酸四种化合 物为潜在的生物标志物。
结论:
我们的研究结果为PCOS的发病机制提供了新的依据,并且检测出的尿代谢物可能有助于PCOS的诊断。
INTRODUCTION
Polycystic ovary syndrome (PCOS) is one of the most common endocrine and metabolic disorders with a prevalence of approximately 60–70% in Chinese obese women of reproductive age.[1] The common manifestations of PCOS are oligomenorrhea, chronic anovulation, hyperandrogenism, hyperinsulinemia, and polycystic ovaries on ovarian ultrasonography.[2] PCOS increases the risks of type 2 diabetes, cardiovascular disease, endometrial cancer, and ovarian cancer.[3,4] The pathogenesis of PCOS remains unclear, although it is considered to be the result of interaction between genetic, environmental, and lifestyle factors. Although the 2003 Rotterdam criteria provide the most widely used diagnostic standard, the diagnosis of PCOS is difficult because its symptoms are heterogeneous.
Metabolomics is a powerful tool for identifying useful biomarkers in the diagnosis of PCOS and for studying its pathogenesis. Metabolomic studies of PCOS plasma samples have been performed using hydrogen-1 nuclear magnetic resonance,[5,6] gas chromatography-mass spectrometry (GC-MS),[7] and liquid chromatography-mass spectrometry (LC-MS).[8] Multiple metabolic disorders have been observed in PCOS, including the metabolism of steroid hormones, carbohydrates, amino acid, and lipids. Branched-chain amino acids, creatinine, and lipids have been reported as potential biomarkers. However, blood specimen collection is invasive and inconvenient. There have been few metabolomic studies of PCOS urinary samples, and in those that were carried out, the metabolic profile of the PCOS patients’ urine was not clear.
In this study, a metabolomic approach based on GC-MS was used to investigate the metabolic profiles of PCOS patients through analysis of urine samples to identify useful biomarkers for the diagnosis of PCOS.
METHODS
Ethical approval
Ethics committee approval was obtained for this study from the Maternal and Child Health Hospital of Hunan Province, and written informed consent was obtained from all participants.
Participants
The study individuals consisted of 21 patients with PCOS and 16 healthy women as controls. The patients’ PCOS diagnoses were based on the 2003 Rotterdam criteria.[9] Each patient met at least two of the following criteria: (1) oligomenorrhea and/or chronic anovulation; (2) clinical and/or biochemical hyperandrogenism; and (3) polycystic ovary morphology on ultrasound scan following the exclusion of Cushing's syndrome, congenital adrenal hyperplasia, androgen-secreting tumor, and other related disorders. The controls were female members of the staff who volunteered to participate. The specific inclusion criteria for the healthy controls included the following: regular 21–35-day menstrual cycles, no evidence of polycystic ovaries, and no evidence of hyperandrogenism. The exclusion criteria for both groups were the presence of thyroid disease, hematological disease, cardiovascular disease, liver disease, and the use of hormonal medications within the previous three months.
Urine samples for all individuals were collected in untreated vials, mainly in the morning before breakfast during the follicular phase of the menstrual cycle, or amenorrhea. Each urine sample was aliquoted into 1.5 ml Eppendorf tubes and frozen at −80°C immediately after collection until analysis. Serum follicle-stimulating hormone and luteinizing hormone (LH), as well as total testosterone (T), estradiol (E2), and prolactin (PRL) levels, were analyzed using an automated chemiluminescence system.
Gas chromatography-mass spectrometry analysis
The urinary aliquots (100 μl) were thawed at room temperature. To facilitate the removal of interfering urea, the urine samples were first supplemented with 30.0 μl urease (1.2 U/μl) at 37°C for 30 min. Each sample was then spiked with heptadecanoic acid (0.5 mg/ml, 50 μl). The proteins were then precipitated by the addition of 800 μl ethanol and removed after 15 min of centrifugation (3000 ×g). The deproteinized solutions were subsequently supplemented with 40 μl of hydroxylamine hydrochloride (0.04 mol/L) and 60 μl Ba (OH)2 (0.05 mol/L). Then, the solution mixtures were incubated at room temperature for 20 minutes. Once evaporated to dryness, the samples were converted to trimethylsilyl (TMS) derivatives with 100 μl of BSTFA/TMCS (100:1) and were analyzed using an Agilent GC-MS system (7890-5975C; Agilent Technologies, Palo Alto, CA, USA). Following injections in the gas chromatograph system at a split ratio of 50:1, samples were separated using a fused silica HP-5 capillary column. The column temperature was initially set at 60°C for 4 min, then, increased to 320°C at a rate of 6.5°C/min, and finally, maintained for 10 min. The parameters of the mass spectrum were as follows: Mass spectrometry was performed over a mass range of 50–800 m/z. The mass spectrometer was operated in electron-impact (EI) mode at an ionization energy level of 70 eV. The source temperature and interphase temperature were 230°C and 300°C, respectively. We locked heptadecanoic acid at 36 min by means of retention-time locking technology (Agilent) to stabilize the retention times of each peak. The total ion chromatograms (TICs) and fragmentation patterns were acquired using the ChemStation software (Agilent, California, USA). The fragmentation pattern of each compound was compared with a standard mass chromatogram in the National Institute of Standards and Technology (NIST) mass spectra library. The ChemStation software then generated a list of similarities compared to every substance within the NIST library for each peak, and the compound was assigned a name when the peak had a similarity index of more than 70%.
Each sample was represented by a GC-MS TIC, and the ion peak areas of the compounds were integrated after GC-MS analysis. The peak-area ratio of each compound to creatinine was calculated as the response, and the results were expressed as ratios to the urinary creatinine concentration.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS, Version 20.0, Chicago, IL, USA) was used for data analysis. Data of normal distribution were presented as mean ± standard deviation (SD), and data of unnormal distribution were presented as median and interquartile range. The Student's t-test and rank sum test were applied to validate the statistical significance of the differences between the two groups. A P < 0.05 was considered statistically significant. All the data from the differentially expressed compounds were used to construct PCA models by means of the Mass Profiler Professional software (Agilent, California, USA). The specificity and sensitivity of the compound were assessed using the area under the curve (AUC) of the receiver-operating characteristic (ROC) curves.
RESULTS
Demographic data
The demographic data of women with and without PCOS were analyzed in this study. As expected, we found no differences in terms of mean age (26.0 ± 3.9 years vs. 27.8 ± 2.4 years, t = −1.574, P = 0.13), body mass index (22.39 ± 4.60 kg/m2 vs. 20.64 ± 1.65 kg/m2, t = 1.618, P = 0.12), or waist-hip ratio (0.81 ± 0.06 vs. 0.82 ± 0.05, t = −0.396, P = 0.69) between the women in the PCOS group and those in the control group. However, when compared with the controls, the patients with PCOS presented with increased testosterone (1.61 ± 0.69 nmol/L vs. 0.81 ± 0.29 nmol/L, t = 4.737, P < 0.001), LH (15.68 ± 9.87 U/L vs. 5.40 ± 1.29 U/L, t = 4.720, P < 0.001), and estradiol (177.87 ± 58.45 pmol/L vs. 113.23 ± 47.04 pmol/L, t = 3.617, P = 0.001).
Gas chromatography-mass spectrometry data
Following the normalization of the data using creatinine as an internal standard, 35 urine metabolites relevant to the disease were identified using the Student's t- test with P < 0.05. The name, retention time, regulation, and statistical analysis of the metabolites derived from the non-targeted GC-MS analysis are summarized in[Table 1]. The 35 metabolites were divided into four categories: short-chain fatty acids, free fatty acids, amino acids, and other types that could not be classified clearly into any of the previous three categories. Only succinic acid was significantly down-expressed in the PCOS group compared with the controls, while the remaining 34 urine metabolites were up-expressed in the women with PCOS compared with the controls. A PCA model was constructed using these differentially expressed metabolite intensities as variables. The PCA scores plot shows that the different urine samples were distributed into two different regions [Figure 1].
Table 1.
Urine metabolites showing statistically significant differences between PCOS patients and controls (mmol/mol creatinine)
| Metabolite | PCOS group (n = 21) | Control group (n = 16) | Z | P |
|---|---|---|---|---|
| Lactose | 10.01 (0, 13.99) | 2.35 (0.16, 3.26) | −2.059 | 0.042 |
| Arabic candy | 0.04 (0, 8.45) | 0.01 (0, 0.81) | −2.029 | 0.017 |
| Stearic acid | 2.35 (1.47, 3.14) | 0.05 (0, 0.14) | −4.577 | <0.001 |
| Palmitic acid | 2.13 (1.07, 2.79) | 0 (0, 0) | −4.654 | <0.001 |
| D-gluconic acid | 141.69 (77.54, 343.61) | 60.96 (43.30, 127.73) | −3.626 | 0.049 |
| 3-hydroxypropionic acid | 562.25 (0, 1005.65) | 169.56 (0, 374.13) | −2.809 | 0.007 |
| Benzoylglycine | 239.99 (16.17, 897.75) | 10.41 (0, 32.61) | −4.407 | <0.001 |
| Ribosinic acid-5 | 2.45 (0.27, 3.77) | 0.72 (0.33, 1.21) | −2.040 | 0.042 |
| Arabinitol | 2.24 (0, 4.18) | 0 (0, 0) | −2.354 | 0.032 |
| Cis-aconitic acid-E | 7.11 (0.16, 9.96) | 1.17 (0.82, 2.10) | −2.594 | 0.016 |
| Fucose | 2.88 (0, 7.36) | 0.32 (0, 0.59) | −2.880 | 0.009 |
| Suberic acid | 10.76 (0, 27.32) | 1.24 (0, 2.15) | −2.110 | 0.002 |
| 3,4,5-hydroxyvaleric acid | 4.08 (0.01, 16.17) | 1.66 (0.76, 2.84) | −2.468 | 0.019 |
| 4-hydroxyphenylacetic acid | 123.83 (80.20, 192.74) | 33.60 (19.75, 59.19) | −3.757 | <0.001 |
| α-ketoglutarate | 605.97 (53.51, 1030.29) | 61.59 (0, 136.38) | −3.314 | 0.001 |
| 3-Hydroxy-3-Methylglutaric acid | 2.42 (0, 6.65) | 0.48 (0.08, 0.89) | −2.195 | 0.009 |
| 2-hydroxyglutaric acid | 5.07 (1.96, 8.37) | 0.52 (0.06, 1.20 ) | −3.255 | 0.001 |
| Threonic acid | 1.84 (1.31, 3.00) | 0.35 (0.24, 0.56) | −3.693 | <0.001 |
| Inosine | 4.87 (2.25, 7.77) | 0.25 (0.20, 0.48) | −3.807 | <0.001 |
| 2,3,4-hydroxybutyric acid | 9.25 (0.14, 13.83) | 1.96 (1.60, 4.25) | −2.687 | 0.005 |
| 5-Oxoproline | 15.39 (6.47, 61.65) | 3.26 (0.51, 6.36) | −3.170 | 0.0001 |
| Erythrocytes-2 | 8.34 (0,12.73) | 2.06 (0, 3.94) | −2.508 | 0.014 |
| Erythrocytes-1 | 3.09 (0,5.46) | 0.46 (0, 1.11) | −3.140 | 0.002 |
| 3,4-hydroxybutyric acid | 7.47 (6.35,13.56) | 0.46 (0, 1.50) | −3.914 | <0.001 |
| Threonine | 4.08 (1.08, 12.48) | 0.02 (0, 0.26) | −4.745 | <0.001 |
| Serine | 5.61 (0, 15.85) | 0 (0, 0) | −4.266 | <0.001 |
| Uracil | 1.70 (0, 23.00) | 0.62 (0, 0.93) | −2.546 | 0.026 |
| Glyceryl acid | 7.31 (4.82, 10.99) | 0.83 (0.50, 1.52) | −3.693 | <0.001 |
| Glycine | 9.10 (5.81, 25.42) | 1.23 (0.58, 2.47) | −3.693 | <0.001 |
| Phosphoethanolamine | 22.53 (15.76, 36.04) | 4.24 (2.41, 5.76) | −3.311 | 0.001 |
| 4-hydroxybutyric acid | 16.70 (10.82, 30.19) | 3.35 (0.40, 5.17) | −3.411 | <0.001 |
| Oxalic acid | 1.88 (0, 2.58) | 0.68 (0, 1.42) | −2.975 | 0.024 |
| Glycolic acid | 36.98 (26.98, 53.55) | 6.20 (3.07, 13.43) | −3.614 | <0.001 |
| 2-hydroxyisobutyric acid | 1.33 (0, 3. 54) | 0 (0,0) | −2.354 | 0.032 |
| Succinic acid | 0 (0, 0) | 38.94 (4.16, 51.30) | −4.553 | <0.001 |
PCOS: Polycystic ovary syndrome.
Figure 1.
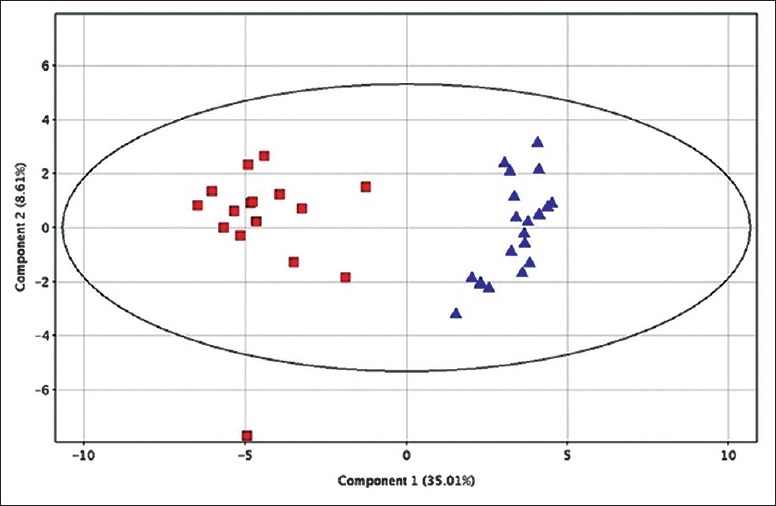
A PCA score plot based on differentially expressed compounds (red squares represent the control group and blue triangles represent the PCOS group). PCA: Principal component analysis; PCOS: Polycystic ovary syndrome.
Investigation of the metabolite biomarkers
To identify useful biomarkers for the diagnosis of PCOS, the specificity and sensitivity of each differentially expressed metabolite were assessed in this study. Among these differentially expressed metabolites, four compounds, including stearic acid, palmitic acid, benzoylglycine, and threonine, were selected as potential biomarkers and are summarized in Table 2.
Table 2.
ROC analysis with AUC, specificity, and sensitivity of each PCOS potential biomarkers
| Parameters | AUC | Best cutoff value (mmol/mol creatinine) | Sensitivity | Specificity |
|---|---|---|---|---|
| Stearic acid | 0.947 | 1.238 | 0.850 | 1.000 |
| Palmitic acid | 0.991 | 1.050 | 0.850 | 1.000 |
| Benzoylglycine | 0.905 | 0.046 | 0.850 | 0.937 |
| Threonine | 0.941 | 0.645 | 0.800 | 1.000 |
ROC: Receiver operating characteristic; AUC: Area under the curve; PCOS: Polycystic ovary syndrome.
DISCUSSION
PCOS is considered to be an endocrine and metabolic syndrome that increases the risk of many diseases.[10,11,12,13] The pathogenesis of PCOS remains unclear, and there is no single diagnostic marker for PCOS.[14] Here, we attempted to explore the metabolic profile of the urine of the PCOS patient group through GC-MS.
The results of our study showed that there were clear differences between the metabolomic profiles of urine in the women with PCOS compared with the controls. The PCA score plot shows an obvious differentiation between the two groups. PCOS has been reported to be associated with impaired lipid metabolism, and a dramatic increase in fatty acids leads to significant lipid profile changes in PCOS patients.[6,15,16,17] Fatty acids are an important energy source in the body and provide energy through β-oxidation. Studies have shown that PCOS is related to a decreased fatty acid oxidation capacity, and as a result, excessive fatty acids accumulate in the body causing lipotoxicity. Increased circulating free fatty acids have many adverse metabolic effects, including dyslipidemia, insulin resistance, type 2 diabetes, and hypertension. In this study, we observed elevated levels of some short-chain fatty acids and free fatty acids in the urine.[18] Apart from lipids, the levels of some amino acids, such as threonine, serine, and glycine, also differed between the PCOS patients and the controls. Amino acids play an important role both as basic substrates and as regulators in many metabolic pathways.[19] Specifically, amino acids and their metabolic intermediates play key roles in anabolic processes and the regulation of the metabolic pathways. Further study of these metabolic pathways using larger populations is needed to understand the pathogenesis of PCOS.
The metabolites found in the urine of the women with PCOS in our study differed from the results of a recently published study on metabolomics and PCOS. The results of that study showed that 59 urine metabolites were found at different concentrations in PCOS patients, including aromatic heteropolycyclic compounds, amino acids and peptides, lipids, and other types. Significant decreases in the levels of most of the aromatic heteropolycyclic compounds and increases in the levels of benzofenap, FMNH2, and 11 metabolites classified into the amino acid and peptide category were observed in the PCOS patients compared with the controls.[20] The differences in the metabolite changes found in both studies may have been the result of different approaches and differential protein binding.
PCOS is a complex disease with no uniform diagnostic criteria, making it difficult to diagnose.[21] Another study identified two novel potential urinary biomarkers, testosterone-glucuronide and 11α-hydroxyprogesterone, and four candidate urinary biomarkers in PCOS patients using a metabolomic approach.[20] Here, differential metabolites of PCOS were investigated to ascertain the possibility of potential biomarkers for PCOS diagnostics. The classification performance was assessed by AUC of ROC curves performed using the values of these PCOS markers. The specificity and sensitivity values demonstrate the possibility of using these markers for diagnosing PCOS. The largest AUC value (0.991) with high specificity and sensitivity was obtained by the ROC curve of palmitic acid at the cutoff value. Stearic acid, benzoylglycine, and threonine also showed high AUC values with high specificity and sensitivity. These results demonstrate that palmitic acid and three other potential biomarkers may offer good diagnostic value for the diagnosis of PCOS. However, it remains uncertain whether these biomarkers could distinguish polycystic ovary syndrome from other metabolic diseases or divide polycystic ovary syndrome into subgroups. These potential biomarker candidates also confirmed that the pathogenesis of PCOS is closely related to multiple etiologies and pathogeneses. Based on the above findings, further studies should be carried out to verify the changes and the targeted metabolites.
In conclusion, this study evaluated metabolomic profiles in the urine of women with PCOS compared with controls. We found 35 urine metabolites at different concentrations in the PCOS patients. The study revealed four potential urinary biomarkers in PCOS patients. Further research of the differently expressed metabolites is required to understand the mechanism underlying the pathogenesis of PCOS. In addition, further study of the identified biomarkers using a larger sample size is required to assess their usefulness in the diagnosis of PCOS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Ning-Ning Wang
REFERENCES
- 1.Liang P, Xi L, Shi J, Li W, Zhao S, Deng Y, et al. Prevalence of polycystic ovary syndrome in Chinese obese women of reproductive age with or without metabolic syndrome. Fertil Steril. 2017;107:1048–54. doi: 10.1016/j.fertnstert.2016.12.029. doi: 10.1016/j.fertnstert.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan A, Muthusami S. Hormonal alterations in PCOS and its influence on bone metabolism. J Endocrinol. 2017;232:R99–113. doi: 10.1530/JOE-16-0405. doi: 10.1530/JOE-16-0405. [DOI] [PubMed] [Google Scholar]
- 3.Tan J, Wang QY, Feng GM, Li XY, Huang W. Increased risk of psychiatric disorders in women with polycystic ovary syndrome in Southwest China. Chin Med J. 2017;130:262–6. doi: 10.4103/0366-6999.198916. doi: 10.4103/0366-6999.198916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang QY, Song Y, Huang W, Xiao L, Wang QS, Feng GM, et al. Comparison of drospirenone- with cyproterone acetate-containing oral contraceptives, combined with metformin and lifestyle modifications in women with polycystic ovary syndrome and metabolic disorders: A Prospective randomized control trial. Chin Med J. 2016;129:883–90. doi: 10.4103/0366-6999.179783. doi: 10.4103/0366-6999.179783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atiomo W, Daykin CA. Metabolomic biomarkers in women with polycystic ovary syndrome: A pilot study. Mol Hum Reprod. 2012;18:546–53. doi: 10.1093/molehr/gas029. doi: 10.1093/molehr/gas029. [DOI] [PubMed] [Google Scholar]
- 6.RoyChoudhury S, Mishra BP, Khan T, Chattopadhayay R, Lodh I, Datta Ray C, et al. Serum metabolomics of Indian women with polycystic ovary syndrome using 1H NMR coupled with a pattern recognition approach. Mol Biosyst. 2016;12:3407–16. doi: 10.1039/c6mb00420b. doi: 10.1039/c6mb00420b. [DOI] [PubMed] [Google Scholar]
- 7.Zhang XJ, Huang LL, Su H, Chen YX, Huang J, He C, et al. Characterizing plasma phospholipid fatty acid profiles of polycystic ovary syndrome patients with and without insulin resistance using GC-MS and chemometrics approach. J Pharm Biomed Anal. 2014;95:85–92. doi: 10.1016/j.jpba.2014.02.014. doi: 10.1016/j.jpba.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Haoula Z, Ravipati S, Stekel DJ, Ortori CA, Hodgman C, Daykin C, et al. Lipidomic analysis of plasma samples from women with polycystic ovary syndrome. Metabolomics. 2015;11:657–66. doi: 10.1007/s11306-014-0726-y. doi: 10.1007/s11306-014-0726-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao X, Xu F, Qi B, Hao S, Li Y, Li Y, et al. Serum metabolomics study of polycystic ovary syndrome based on liquid chromatography-mass spectrometry. J Proteome Res. 2014;13:1101–11. doi: 10.1021/pr401130w. doi: 10.1021/pr401130w. [DOI] [PubMed] [Google Scholar]
- 10.Soares EM, Azevedo GD, Gadelha RG, Lemos TM, Maranhão TM. Prevalence of the metabolic syndrome and its components in Brazilian women with polycystic ovary syndrome. Fertil Steril. 2008;89:649–55. doi: 10.1016/j.fertnstert.2007.03.081. doi: 10.1016/j.fertnstert.2007.03.081. [DOI] [PubMed] [Google Scholar]
- 11.De Leo V, Musacchio MC, Palermo V, Di Sabatino A, Morgante G, Petraglia F, et al. Polycystic ovary syndrome and metabolic comorbidities: Therapeutic options. Drugs Today (Barc) 2009;45:763–75. doi: 10.1358/dot.2009.45.10.1429463. doi: 1396674/dot.2009.45.10.1429463. [DOI] [PubMed] [Google Scholar]
- 12.Orio F, Muscogiuri G, Nese C, Palomba S, Savastano S, Tafuri D, et al. Obesity, type 2 diabetes mellitus and cardiovascular disease risk: An uptodate in the management of polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2016;207:214–9. doi: 10.1016/j.ejogrb.2016.08.026. doi: 10.1016/j.ejogrb.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 13.Blay SL, Aguiar JV, Passos IC. Polycystic ovary syndrome and mental disorders: A systematic review and exploratory meta-analysis. Neuropsychiatr Dis Treat. 2016;12:2895–903. doi: 10.2147/NDT.S91700. doi: 10.2147/NDT.S91700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Göbl CS, Ott J, Bozkurt L, Feichtinger M, Rehmann V, Cserjan A, et al. To assess the association between glucose metabolism and ectopic lipid content in different clinical classifications of PCOS. PLoS One. 2016;11:e0160571. doi: 10.1371/journal.pone.0160571. doi: 10.1371/journal.pone.0160571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun L, Hu W, Liu Q, Hao Q, Sun B, Zhang Q, et al. Metabonomics reveals plasma metabolic changes and inflammatory marker in polycystic ovary syndrome patients. J Proteome Res. 2012;11:2937–46. doi: 10.1021/pr3000317. doi: 10.1021/pr3000317. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Fu L, Li R, Wang LN, Yang Y, Liu NN, et al. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: Plasma metabolomics analysis. BMC Med. 2012;10:153. doi: 10.1186/1741-7015-10-153. doi: 10.1186/1741-7015-10- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whigham LD, Butz DE, Dashti H, Tonelli M, Johnson LK, Cook ME, et al. Metabolic evidence of diminished lipid oxidation in women with polycystic ovary syndrome. Curr Metabolomics. 2014;2:269–78. doi: 10.2174/2213235X01666131203230512. doi: 10.2174/2213235X01666131203230512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muneyyirci-Delale O, Kaplan J, Joulak I, Yang L, Von Gizycki H, Nacharaju VL, et al. Serum free fatty acid levels in PCOS patients treated with glucophage, magnesium oxide and spironolactone. Gynecol Endocrinol. 2013;29:474–7. doi: 10.3109/09513590.2013.769515. doi: 10.3109/09513590.2013.769515. [DOI] [PubMed] [Google Scholar]
- 19.Wu G. Amino acids: Metabolism, functions, and nutrition. Amino Acids. 2009;37:1–7. doi: 10.1007/s00726-009-0269-0. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Wang S, Tan S, Wen M, Qian Y, Zeng X, et al. Detection of urine metabolites in polycystic ovary syndrome by UPLC triple-TOF-MS. Clin Chim Acta. 2015;448:39–47. doi: 10.1016/j.cca.2015.06.008. doi: 10.1016/j.cca.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Bachelot A. Polycystic ovarian syndrome: Clinical and biological diagnosis. Ann Biol Clin (Paris) 2016;74:661–7. doi: 10.1684/abc.2016.1184. doi: 10.1684/abc.2016.1184. [DOI] [PubMed] [Google Scholar]


