Abstract
Fibroblast growth factor 23 (FGF23)–induced hypophosphatemia is a rare paraneoplastic syndrome of phosphate wasting that, if unrecognized, may cause tumor-induced osteomalacia. It is classically associated with benign mesenchymal tumors but occasionally has been found in patients with other malignancies. Hypophosphatemia has been associated with acute leukemia but has not previously been reported to be due to inappropriate FGF23 secretion. Here, we describe FGF23-induced severe hypophosphatemia and renal phosphate wasting associated with a mixed-phenotype Philadelphia chromosome-like acute leukemia in a previously healthy 22-year-old man. He was found to have low serum 1,25-dihydroxyvitamin D and extremely high FGF23 levels, as well as inappropriate urinary phosphorus excretion. The hypophosphatemia improved with calcitriol and oral phosphate treatment but normalized only during chemotherapy-induced ablation of the blasts. FGF23 levels declined with a reduction in peripheral blast counts. Using real-time reverse transcription polymerase chain reaction, we found that the leukemia cells were the source of FGF23. To our knowledge, this is the first description of FGF23-induced hypophosphatemia associated with acute leukemia. We recommend that the FGF23 paraneoplastic syndrome be considered as a possible etiology of hypophosphatemia in patients with acute leukemia.
Keywords: FGF23, oncogenic osteomalacia, paraneoplastic syndrome, tumor-induced osteomalacia
A young man with refractory mixed-phenotype Philadelphia chromosome-like acute leukemia was found to have phosphaturic hypophosphatemia associated with expression of FGF23 by his leukemia cells.
Fibroblast growth factor 23 (FGF23)–induced hypophosphatemia is a paraneoplastic syndrome of phosphate wasting that leads to fatigue, muscle weakness, bone pain and fractures, commonly denoted tumor-induced osteomalacia (TIO). The syndrome remains poorly recognized, and affected patients may remain undiagnosed for years [1]. Mechanistically, the syndrome is characterized by tumor cell production of the phosphate-regulating hormone FGF23, which is physiologically produced by osteocytes in response to fluctuating serum phosphate and 1,25-dihydroxyvitamin D levels. FGF23 acts by downregulating phosphate transporters in the renal proximal tubule (thus decreasing phosphate reabsorption) and promoting 24α-hydroxylase activity while also downregulating 1α-hydroxylase (thus decreasing 1,25-dihydroxyvitamin D levels and impairing gut phosphate absorption) [2, 3]. TIO has been most commonly associated with benign mesenchymal tumors of the bone and soft tissues [1]. Here, we describe the unique finding of FGF23-induced hypophosphatemia in a patient with acute leukemia.
1. Methods
Plasma FGF23 was measured as a send-out assay to Mayo Medical Laboratories using a commercially available enzyme-linked immunosorbent assay kit with antibodies to the C-terminal portion of the protein (Quidel Corp., San Diego, CA, no. 60-6100, RRID: AB_2722648). Leukocytes were isolated from heparinized peripheral blood by Ficoll gradient centrifugation. Total RNA was isolated using an RNeasy Mini Kit (Qiagen, Inc., Valencia, CA), and reverse transcribed using random hexamer primers and Superscript III reverse transcriptase (Invitrogen, Carlsbad CA). Complementary DNA was analyzed by real-time polymerase chain reaction (PCR) on an Applied Biosystems (Foster City, CA) StepOnePlus instrument.
This study was approved by the Institutional Review Board of the University of Michigan Medical School. Written informed consent was obtained from all subjects.
2. Case Report
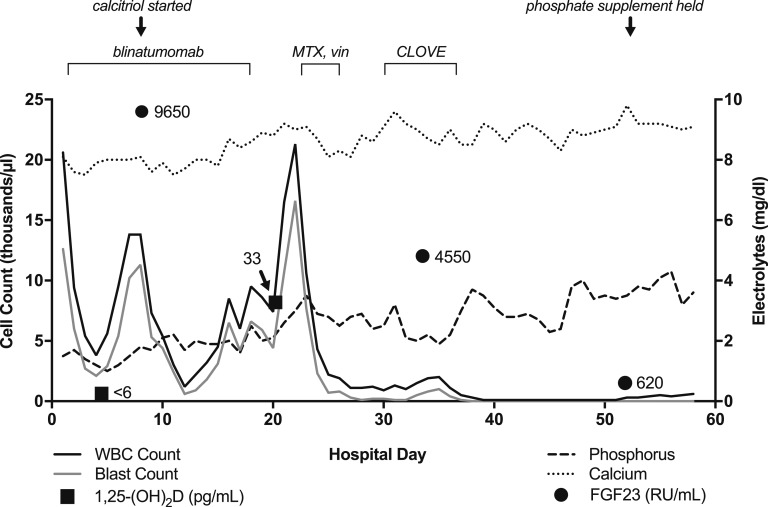
A previously healthy 22-year-old male presented with acute-onset oral mucosal bleeding following a few weeks of progressive dyspnea. Initial evaluation revealed anemia, thrombocytopenia, and blasts in the peripheral blood. A bone marrow aspiration and biopsy were consistent with Philadelphia chromosome-like mixed phenotype acute leukemia, with biphenotypic B/myeloid blasts (referred to as “blasts” herein), given the presence of a P2RY8-CRLF2 fusion [4] (Table 1). He underwent induction chemotherapy with the C8811 protocol (cyclophosphamide, daunorubicin, vincristine, prednisone, and PEG-l-asparaginase) [5] plus rituximab, followed by reinduction therapy with hyperCVAD part B (high-dose methotrexate and high-dose cytarabine) [6] together with ruxolitinib, a dual JAK1/JAK2 inhibitor, given a JAK-1 V658F mutation [7, 8] (Table 1). The endocrinology service was consulted for hypophosphatemia during his third hospitalization, after admission to start blinatumomab for refractory leukemia. His initial serum phosphate level was 1.5 mg/dL, and despite aggressive supplementation with intravenous and oral phosphate (ranging from 30 to 45 mmol IV sodium phosphate plus 32 to 48 mmol oral elemental phosphorus daily), his serum level remained <2 mg/dL. On review of prior admissions, he had hypophosphatemia in the 1- to 2-mg/dL range when his peripheral blast count was elevated and improvement during periods of chemoablation. Other than fatigue, he denied specific symptoms attributable to hypophosphatemia. He had no family history of hypophosphatemia or other disorders of bone or mineral metabolism. Physical exam revealed a tall [height: 77 in (196 cm)], thin young man with splenomegaly but with normal strength and no evidence of acute illness. Laboratory results at the time of our evaluation are shown in Table 2 and are notable for hypophosphatemia, mild hypocalcemia, elevated parathyroid hormone, low 1,25-dihydroxyvitamin D, and dramatically elevated FGF23. The patient did not have baseline laboratory values available for comparison. Calculation of the tubular reabsorption of phosphate should be done on a spot urine after an overnight fast, which was not feasible due to the need for ongoing phosphate supplementation. However, the 24-hour urine phosphate level was near the upper limit of normal despite severe hypophosphatemia. The trends of his leukocyte counts and serum calcium, phosphate, FGF23, and 1,25-dihydroxyvitamin D levels are shown in Fig. 1. Alkaline phosphatase levels remained within normal limits for most of his hospitalization, except for a brief period of mild elevation (all values <170 IU/L) on hospital days 49 to 58, associated with transaminitis following chemotherapy. Renal function remained normal throughout the hospitalization.
Table 1.
Cytogenomic Abnormalities Identified by SNP Microarray Analysis of the Patient’s Bone Marrow Aspirate Upon Diagnosis of Acute Leukemia
| Chromosome Number | Cytoband Start-End | Type | Genomic Coordinate Start-End | Size (Mb) | Genes of Interest |
|---|---|---|---|---|---|
| 4 | q25-q25 | Loss | 109,062,645-109,099,091 | 0.04 | LEF1 |
| 9 | p21.3-p21.3 | Loss | 21,976,837-22,005,382 | 0.03 | CDKN2A, CDKN2B |
| 12 | q12-q12 | Loss | 46,129,241-46,342,788 | 0.21 | ARID2 |
| 13 | q12.2-q12.2 | Loss | 28,684,222-28,780,898 | 0.10 | PAN3 |
| 13 | q31.3-q31.3 | Gain | 90,007,827-91,315,316 | 1.31 | Three non-protein-coding genes |
| 15 | q12-q12 | Loss | 26,036,071-26,113,711 | 0.08 | ATP10A |
| X | p22.33-p22.33 | Loss | 1,324,508-1,651,464 | 0.33 | P2RY8, CRLF2 |
The University of Michigan Clinical Cytogenetics Laboratory performed SNP microarray analysis using the Affymetrix Cytoscan HD platform to identify DNA copy number gains and losses and regions of loss of heterozygosity. The array contains more than 2.6 million copy number markers, including 750,000 SNPs, with a median spacing of 0.88 kb within genes. Gains or losses greater than 35 markers within or including a known clinically significant cancer-related gene, or greater than 1 Mb outside known clinical oncology significant regions, and loss of heterozygosity greater than 10 Mb, are reported. The analysis is based on the GRCh37/hg19 assembly. The results of the standard karyotype analysis were normal in all 20 cells assessed. A loss of 327 kb was noted at Xp22.33 or Yp11.32 involving exon 2 of the P2RY8 gene and at least exons 1 to 3 of the CRLF2 gene, resulting in a P2RY8-CRLF2 fusion, which defines a Philadelphia chromosome-like acute leukemia. FISH analysis of peripheral blood was negative for the mixed-lineage leukemia rearrangement and BCR-ABL1 gene fusion. Peripheral blood cells were also negative for JAK2 exon 12 and V617F mutations (assessed by PCR), and no BCR-ABL fusion products were detected by quantitative real-time RT-PCR of bone marrow aspirate as performed by the University of Michigan Molecular Diagnostics Laboratory. Genoptix commercial next-generation sequencing testing detected a “likely” pathogenic missense c.1972G>T; p.V658F mutation in JAK1, previously found in other patients with hematopoietic neoplasms. It also noted a mutation of uncertain significance in the CDKN2A gene, c. 151-6 delTinsCCAGGGG.
Abbreviations: FISH, fluorescence in situ hybridization; SNP, single nucleotide polymorphism.
Table 2.
Laboratory Values Upon Endocrinologic Evaluation
| Measurement | Values |
|---|---|
| Blood measurement | |
| White blood cells | 5.6 K/µL, with 50.9% blasts |
| Hemoglobin | 8.7 g/dL |
| Platelets | 4 K/µL |
| Serum measurement | |
| Creatinine | 0.91 mg/dL |
| Calcium | 8.0 mg/dL (8.6–10.3) |
| Albumin | 3.8 g/dL |
| Phosphate | 1.0 mg/dL |
| Alkaline phosphatase | 73 IU/L (30–116) |
| PTH | 89 pg/mL (10–65) |
| 25-hydroxyvitamin D | 29 ng/mL (25–100) |
| 1,25-dihydroxyvitamin D | 6 pg/mL (18–78) |
| FGF23 (C-terminal assay) | 9650 RU/mL (<180) |
| 24-h urine measurement | |
| Calcium | 84 mg/d (100–300) |
| Phosphate | 1101 mg/d (400–1200) |
| Creatinine | 2.1 g/d (1.0–1.8) |
| Concurrent serum phosphate | 1.5 mg/dL |
| Concurrent serum calcium | 8.0 mg/dL |
Reference ranges are shown in parentheses. Note that calculation of the tubular reabsorption of phosphate should be done on a spot urine after an overnight fast, which was not feasible due to the need for ongoing phosphate supplementation.
Abbreviation: PTH, parathyroid hormone.
Figure 1.
Relationship between serum calcium and phosphate levels, peripheral white blood cell (WBC) and blast counts, and serum FGF23 levels over the course of hospitalization. White blood cell (solid black line) and blast (solid gray line) counts are plotted on the left axis, and phosphate (dashed line) and calcium (dotted line) levels are plotted on the right axis. The normal range for serum phosphate levels is 2.7 to 4.6 mg/dL and for serum calcium is 8.6 to 10.3 mg/dL. Measured FGF23 levels are plotted as solid circles and 1,25-dihydroxyvitamin D [1,25-(OH)2D] levels as solid squares, with adjacent corresponding values. Serum alkaline phosphatase levels were largely normal throughout the hospitalization (not shown). CLOVE, clofarabine, cyclophosphamide, etoposide; MTX, methotrexate; vin, vincristine.
Given the extremely low activated vitamin D level, the patient was started on calcitriol in addition to substantial oral phosphate supplementation, normalizing serum calcium, improving serum phosphate levels, and allowing for weaning of supplemental oral phosphorus from 48 to 8 mmol daily (Fig. 1). Cinacalcet was briefly used to promote relative hypoparathyroidism [9] but was discontinued with the development of hypercalciuria at 379 mg/d. The patient could not tolerate thiazide use due to low blood pressure. He was subsequently transitioned to a clofarabine, cyclophosphamide, etoposide chemotherapy regimen, which reduced the circulating blast count, coinciding with a reduction in FGF23 and the ability to temporarily discontinue oral phosphate supplementation (Fig. 1). Unfortunately, his leukemia was refractory to subsequent chemotherapy treatments, and he transitioned to hospice care.
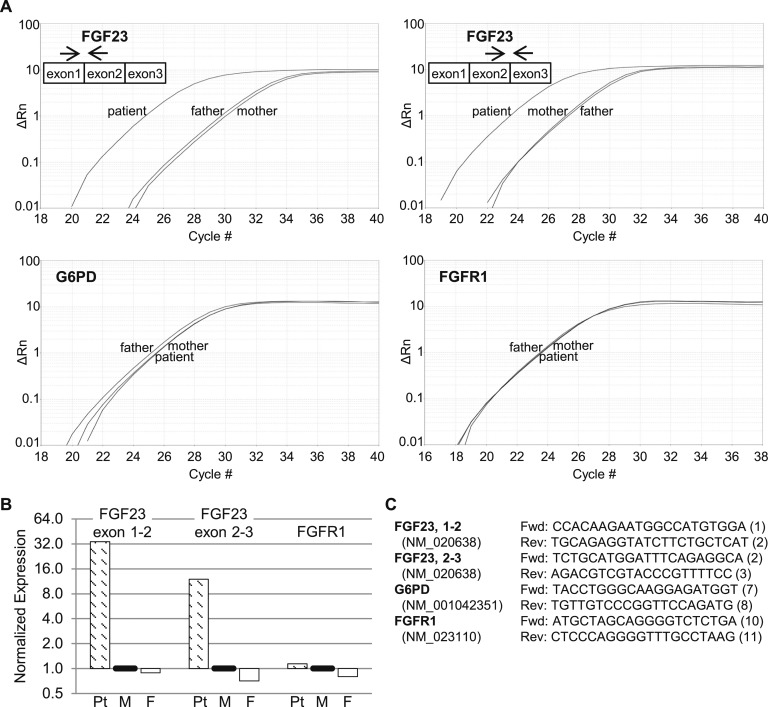
As the onset of hypophosphatemia presumably coincided with development of leukemia in this previously healthy patient, we questioned whether his leukemic cells were the source of FGF23. Therefore, we isolated peripheral leukocyte RNA from the patient and both parents to assess FGF23 expression. Using real-time reverse transcription-polymerase chain reaction (RT-PCR), we found that FGF23 messenger RNA (mRNA) was elevated >10-fold in the patient (Fig. 2). In contrast, there was no difference in the expression of glucose-6-phosphate dehydrogenase mRNA, assessed as a control. Classical cases of TIO due to inappropriate FGF23 secretion from benign mesenchymal tumors often are associated with FN1-FGFR1 gene fusions that result in high-level expression of the FGFR1 kinase domain, or (more rarely) FN1-FGF1 gene fusions that result in increased fibroblast growth factor 1 (FGF1) expression [10]. However, FGFR1 mRNA expression using kinase domain primers was not increased in our patient (Fig. 2), and FGF1 mRNA was expressed at negligible levels (real-time PCR cycle threshold >35 cycles; data not shown).
Figure 2.
Isolated white blood cells from the patient demonstrate increased expression of FGF23 mRNA compared with both parents. (A) Gene expression was measured by real-time RT-PCR in samples collected on hospital day 27. The y-axes of the amplification plots (ΔRn) represent fluorescence of the reporter signal normalized to that of the passive reference dye, minus the baseline, and the x-axes represent PCR cycle number. The locations of FGF23 primers are shown schematically in each panel. (B) Relative levels of the FGF23 and FGFR1 amplicons in the patient (Pt), mother (M), and father (F), normalized to glucose-6-phosphate dehydrogenase (G6PD) and relative to the mother (defined as 1). (C) Forward (Fwd) and reverse (Rev) primers for each gene, with the targeted exon shown in parentheses after the sequence.
3. Discussion
TIO is an uncommon paraneoplastic syndrome characterized by phosphate wasting attributed to increased circulating FGF23. The syndrome is classically associated with benign mesenchymal tumors; however, recent case reports have associated this syndrome with malignancies, including anaplastic thyroid, colon, and lung carcinomas and diffuse large B cell non-Hodgkin lymphoma [11, 12]. One study evaluated FGF23 concentrations in various plasma cell dyscrasias after finding elevated circulating FGF23 in one patient with chronic lymphocytic leukemia and hypophosphatemia, but the authors paradoxically found a weakly positive correlation of FGF23 with serum phosphate in their cohort [13]. Another study reported TIO-like syndromes in patients with plasma cell disorders causing light-chain nephropathy and renal phosphate wasting, but FGF23 was undiscovered at the time [14].
To our knowledge, this is the first description of FGF23-induced hypophosphatemia associated with acute leukemia. Hypophosphatemia in acute leukemia is not uncommon, but has previously been attributed to increased uptake of phosphate by actively proliferating blasts or certain chemotherapies; multiple cases are referenced by Soyoral et al. [15]. FGF23 excess was suspected in our case given that the patient demonstrated hypophosphatemia on initial presentation, was otherwise healthy with good nutrition, and had inappropriately high urinary phosphate excretion and low serum 1,25-dihydroxyvitamin D levels. We observed that FGF23 levels were positively correlated with circulating blast counts and that FGF23 mRNA was highly expressed in the patient’s circulating leukocytes compared with those of either parent. It is possible that not all circulating FGF23 was of the active (intact) form, as the clinical assay also detects inactive (cleaved) FGF23 using a C-terminus antibody. The presence of inactive FGF23 fragments could have contributed to the relatively modest relationship between measured FGF23 and phosphate concentrations. Given the acute nature of his leukemia and hypophosphatemia, he was unlikely to have had time to develop clinically meaningful osteomalacia, as suggested by his lack of bone symptoms and normal alkaline phosphatase levels. The detectable FGF23 level observed during a period of neutropenia likely reflected persistent leukemia in the bone marrow.
The FGFR1 catalytic domain is frequently overexpressed as a fusion protein in phosphaturic mesenchymal tumors and is hypothesized to underlie the induction of FGF23 in those cases [10]. Similarly, FGF1 overexpression as a fusion protein (or perhaps by other mechanisms) has been described in TIO [10]. However, overexpression of neither FGFR1 nor FGF1 was found in our patient. The specific mechanism leading to increased FGF23 expression by our patient’s leukemic cells remains unclear.
The medical management of TIO associated with unresectable tumors is challenging and involves aggressive oral phosphate supplementation and replacement of 1,25-dihydroxyvitamin D [1]. Cinacalcet has been used to reduce oral phosphate supplement needs [9], but the resulting hypercalciuria and risk of nephrolithiasis was deemed unacceptable in the setting of ongoing cytopenias and transfusion dependence. In the absence of effective treatments for his leukemia, definitive therapy for this hypophosphatemia syndrome was unable to be achieved.
Acknowledgments
We thank Dr. Gregory Clines (University of Michigan) and Dr. Michael Collins (National Institutes of Health) for their assistance with management of hypophosphatemia and helpful comments, Dr. Sami Malek and Patricia Tamsen for isolation of peripheral white blood cells, Jingcheng Yu for the RT-PCR analysis, and Timothy Muth and Carole Ramm for assisting with patient consent. We regret that we were unable to cite all relevant case reports due to space limitations.
Financial Support: This work was supported in part by National Institutes of Health Grant 5T32DK007245 (to R.B.R.).
Author Contributions: R.B.R., D.B., and R.J.K. cared for the patient. R.J.K. directed the real-time RT-PCR studies. R.B.R. drafted the manuscript, which was edited by D.B. and R.J.K.
Disclosure Summary:
The authors have nothing to disclose.
Glossary
Abbreviations:
- FGF1
fibroblast growth factor 1
- FGF23
fibroblast growth factor 23
- FGFR1
fibroblast growth factor receptor 1
- mRNA
messenger RNA
- PCR
polymerase chain reaction
- RT-PCR
reverse transcription-polymerase chain reaction
- TIO
tumor-induced osteomalacia
References and Notes
- 1. Chong WH, Molinolo AA, Chen CC, Collins MT. Tumor-induced osteomalacia. Endocr Relat Cancer. 2011;18(3):R53–R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jan de Beur SM. Tumor-induced osteomalacia. JAMA. 2005;294(10):1260–1267. [DOI] [PubMed] [Google Scholar]
- 3. Minisola S, Peacock M, Fukumoto S, Cipriani C, Pepe J, Tella SH, Collins MT. Tumour-induced osteomalacia. Nat Rev Dis Primers. 2017;3:17044. [DOI] [PubMed] [Google Scholar]
- 4. Choi SM, Frederiksen JK, Ross CW, Bixby DL, Shao L. Philadelphia chromosome-like mixed-phenotype acute leukemia demonstrating P2RY8-CRLF2 fusion and JAK1 mutation. Am J Clin Pathol. 2017;148(6):523–528. [DOI] [PubMed] [Google Scholar]
- 5. Larson RA, Dodge RK, Burns CP, Lee EJ, Stone RM, Schulman P, Duggan D, Davey FR, Sobol RE, Frankel SR. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood. 1995;85(8):2025–2037. [PubMed] [Google Scholar]
- 6. Kantarjian HM, O’Brien S, Smith TL, Cortes J, Giles FJ, Beran M, Pierce S, Huh Y, Andreeff M, Koller C, Ha CS, Keating MJ, Murphy S, Freireich EJ. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18(3):547–561. [DOI] [PubMed] [Google Scholar]
- 7. Quintás-Cardama A, Vaddi K, Liu P, Manshouri T, Li J, Scherle PA, Caulder E, Wen X, Li Y, Waeltz P, Rupar M, Burn T, Lo Y, Kelley J, Covington M, Shepard S, Rodgers JD, Haley P, Kantarjian H, Fridman JS, Verstovsek S. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115(15):3109–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang Y-L, Pei D, McCastlain K, Ding L, Lu C, Song G, Ma J, Becksfort J, Rusch M, Chen S-C, Easton J, Cheng J, Boggs K, Santiago-Morales N, Iacobucci I, Fulton RS, Wen J, Valentine M, Cheng C, Paugh SW, Devidas M, Chen I-M, Reshmi S, Smith A, Hedlund E, Gupta P, Nagahawatte P, Wu G, Chen X, Yergeau D, Vadodaria B, Mulder H, Winick NJ, Larsen EC, Carroll WL, Heerema NA, Carroll AJ, Grayson G, Tasian SK, Moore AS, Keller F, Frei-Jones M, Whitlock JA, Raetz EA, White DL, Hughes TP, Guidry Auvil JM, Smith MA, Marcucci G, Bloomfield CD, Mrózek K, Kohlschmidt J, Stock W, Kornblau SM, Konopleva M, Paietta E, Pui C-H, Jeha S, Relling MV, Evans WE, Gerhard DS, Gastier-Foster JM, Mardis E, Wilson RK, Loh ML, Downing JR, Hunger SP, Willman CL, Zhang J, Mullighan CG. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371(11):1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geller JL, Khosravi A, Kelly MH, Riminucci M, Adams JS, Collins MT. Cinacalcet in the management of tumor-induced osteomalacia. J Bone Miner Res. 2007;22(6):931–937. [DOI] [PubMed] [Google Scholar]
- 10. Lee J-C, Su S-Y, Changou CA, Yang R-S, Tsai K-S, Collins MT, Orwoll ES, Lin C-Y, Chen S-H, Shih S-R, Lee C-H, Oda Y, Billings SD, Li C-F, Nielsen GP, Konishi E, Petersson F, Carpenter TO, Sittampalam K, Huang H-Y, Folpe AL. Characterization of FN1-FGFR1 and novel FN1-FGF1 fusion genes in a large series of phosphaturic mesenchymal tumors. Mod Pathol. 2016;29(11):1335–1346. [DOI] [PubMed] [Google Scholar]
- 11. Abate EG, Bernet V, Cortese C, Garner HW. Tumor induced osteomalacia secondary to anaplastic thyroid carcinoma: a case report and review of the literature. Bone Rep. 2016;5:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elderman JH, Wabbijn M, de Jongh F. Hypophosphataemia due to FGF-23 producing B cell non-Hodgkin’s lymphoma. BMJ Case Rep. 2016;2016:bcr2015213954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stewart I, Roddie C, Gill A, Clarkson A, Mirams M, Coyle L, Ward C, Clifton-Bligh P, Robinson BG, Mason RS, Clifton-Bligh RJ. Elevated serum FGF23 concentrations in plasma cell dyscrasias. Bone. 2006;39(2):369–376. [DOI] [PubMed] [Google Scholar]
- 14. Rao DS, Parfitt AM, Villanueva AR, Dorman PJ, Kleerekoper M. Hypophosphatemic osteomalacia and adult Fanconi syndrome due to light-chain nephropathy. Another form of oncogenous osteomalacia. Am J Med. 1987;82(2):333–338. [DOI] [PubMed] [Google Scholar]
- 15. Soyoral Y, Aslan M, Ebinc S, Dirik Y, Demir C. Life-threatening hypophosphatemia and/or phosphate depletion in a patient with acute lymphoblastic leukemia: a rare case report. Am J Emerg Med. 2014;32(11):1437.e3–1437.e5. [DOI] [PubMed] [Google Scholar]