Abstract
Background
Regorafenib (RGF) is the drug of choice for treating hepatic carcinoma (HCC), but the drug has drawbacks due to resistance and associated adverse effects. Thus, it becomes crucial to understand the causal ‘map’ of the resistance conferred by RGF, so that its clinical potency can be amplified, resulting in enhanced efficacy with reduced adverse effects. Metformin (MTF) has been reported to target NLK (Nemo-like kinase) to inhibit non-small lung cancer cells. Based on the literature, the present investigation was carried out to reveal the effect of RGF and MTF, with an expectation that MTF can synergize therapeutic potential as well reduce chances of resistance.
Material/Methods
Protein expression of hypoxia inducible factors (HIF)-2α, 30 kDa HIV Tat-interacting protein (TIP30), E-cadherin, N-cadherin, and pAMPK were assessed by Western blot analysis. RGF and MTF were exposed to MHCC97H cell and proliferation was quantified by assay of cell viability. Gene silencing and chromatin immunoprecipitation assay were done to reveal the relationship between TIP30 and HIF-2α. The impact of RGF and MTF together on postoperative recurrence and lung metastasis of hepatocellular carcinoma was investigated using tumor engrafted mice after administration of MTF and RGF once daily for 35 days. Immunohistochemistry was used to reveal CD31, Ki67, and TUNEL.
Results
The results suggested MTF-RGF combination lowered expression of HIF-2α gene silencing and suggested increased TIP30 after reduction of HIF-2α. The chromatin immunoprecipitation study indicated that under hypoxia, HIF-2α could bind with TIP30 promoter. Cell number quantification (CCK8), viable cell count, and apoptosis data (using Annexin V-FITC) indicated co-administration of RGF and MTF reduced cell proliferation, encouraging cell apoptosis, and reduced epithelial-mesenchymal transition course. Thus, in orthotopic mice, the RGF-MTF combination exhibited substantial reduction of HCC in lung metastasis and postoperative relapse.
Conclusions
MTF can enhance the potential of RGF and inhibit the recurrence and metastasis of HCC after postoperative liver section by regulating the levels of TIP30 and HIF-2α.
MeSH Keywords: Antineoplastic Agents, Liver Neoplasms, Metformin
Background
Hepatocellular carcinoma (HCC) has been ranked as the fifth most common cancer in males and seventh in females among a broad series of cancers diagnosed globally [1]. HCC removal by surgery has been regarded as a potential alternative to the treatment strategies available [2,3]. However, the surgical procedures may give rise to post-surgical relapse and lung metastasis, which may interfere with further treatment [4,5].
Regorafenib (RGF) is a cogent multikinase inhibitor accepted for the treatment of metastatic colorectal cancer and stromal tumors in the gastrointestinal tract. RGF is known to target selectively not only the kinases in angiogenesis and oncogenesis, but also the tumor microenvironment. Kinases known to be inhibited by RGF include VEGFR, KIT, PDGFR, RET, FGFR, TIE2, DDR2, Trk2A, Eph2A, RAF1, BRAF, SAPK2, and PTK5 [6,7]. The literature suggests that, in preclinical models, RGF interferes with the RAF1 kinase signaling pathway and thereby decreases proliferation of tumor cells. RGF has been linked to reduced proliferation of cells and it triggers apoptosis, thereby suppressing tumor development. Data suggest RGF retards angiogenesis by triggering tyrosine kinase receptor vascular endothelial growth factor (VEGFR2) and platelet-derived growth factor receptor (PDGFR) [8,9]. Despite benefits claimed for enhanced activity of RGF in patients with HCC, the problem of resistance often restricts the decision of the drug to be used alone, which can be successfully overcome by combination therapy. Use of co-administration of drugs may elicit greater activity against cancer, enhance sensitivity towards a single drug, and advance the overall treatment of the disease [10–12].
Environment with reduced oxygen availability, that is a hypoxic surrounding, is known to reduce the extent of resistance offered. Reports suggest availability of HIF (hypoxia inducible factors) is required for the tumor cells to acclimatize with low oxygen levels. Studies on RGF suggests it may cause upregulation of HIF-2α. The resistance is a result of elevated expression of HIF-2α. HIF has been identified to modulate specific signaling pathways known to control self-regeneration of stem cells, and, along with RGF, Notch pathways are known to be a key controller of stemness during hypoxia [13,14].
Reports indicate elevated insensitivity coupled with enhanced metastasis, both lymphatic and distal, after treatment with anti-angiogenic moieties. RGF produces its damaging effect against HCC by suppressing the response of HTATIP2 (HCC suppressor gene) or TIP30. There is no established correlation between HTATIP2 and TIP30 [15].
Data in the literature suggests that Metformin, a type 2 anti-diabetic drug, is correlated with lower incidence of cancer in diabetics [16–21]. MTF is known to act on Nemo-like kinase (NLK) and thereby inhibit non-small cell lung cancer (NSCLC) and propagation of cancer at the cellular level [22].
To augment anti-HCC activity, reduce risk of resistance, and recover patient well-being, a combination of RGF and MTF can be administered. The present investigation indicates enhancement of HCC sensitivity to RGF alone and with MTF. The study also found inhibition of recurrence and metastasis of HCC cellular mass after surgery.
Material and Methods
Cell lines and drugs
The HCC cell line (MHCC97H) was procured from the Liver Cancer Institute (Fudan University) and conserved in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, UK), consisting of fetal bovine serum (10%), penicillin (100 units/ml), and streptomycin (100 mg/ml) and then subjected to incubation in a CO2 incubator (5% carbon dioxide diluted in air). RGF (ApexBio Technologies) was dissolved in dimethyl sulfoxide (10 mM) and MTF (Bristol Myers Squibb, China) was solubilized in phosphate-buffered saline (Sigma Aldrich, USA) to achieve a 1-mM final concentration. ShRNA hairpin (Cat. No. CSHCTR001-LVRH1GP) against HIF-2α and lentiviral plasmid overexpress HIF-2α (Control) (Cat. No. EX-M0910-Lv105-5), overexpress control (Cat. No. EX-eGFP-Lv-105), and HIV Lenti-pac™ expression kit (Cat. No. HPK-LvTR-20) were bought from GeneCopoeia, USA.
Cells were subjected to lysis using a mixture containing 25 mM Tris-hydrochloride (pH 7.6), 150 mM sodium chloride, 1 mM EDTA, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and 1% Triton X-100. Protease and phosphatase inhibitor were also included in the above blend. Bradford reagent (Sangon Biotech, Cat. No. B724DB0009) was used to quantify protein concentration in a 20-mg sample volume. Antibodies that were used included anti-HIF2a/EPAS1 rabbit polyclonal antibody (1: 1000, Novus, Cat. No. NB100-122), anti-TIP30 rabbit monoclonal antibody (1: 1000, Abcam, Cat. No. AB1777961), anti-E-Cadherin rabbit polyclonal antibody (1: 1000, Abcam, Cat. No. AB15148), and anti-N-Cadherin rabbit polyclonal antibody (1: 1000), all obtained from Abcam (Cat. No. AB18203).
Orthotopic mouse model (in vivo studies)
BALB/c male mice (nude) weighing 18–20 g and aged 4–6 weeks were obtained from the institutional animal breeding house, with permission from the Institutional Animal Ethics Team (Approval no. KMU/AEC/401/C17/17-18). The mice were housed under standard animal house conditions and were fed with a recommended pelleted diet. A nude animal model was created by injecting a suspension of MHCC97H cells prepared in phosphate-saline buffer (Sigma Aldrich, USA) and 0.2 ml was injected into the left flank. The subcutaneous tumor was removed once it attained a size of around 1 cm diameter. The tumor was sliced carefully and the pieces were further surgically transplanted in all the remaining mice into the left region of the liver under 1% phenobarbital anesthesia. At 14 days after orthotopic implant surgery, another surgery was carried out to remove the tumors from the region where it was implanted earlier. The mice were then randomly split into 4 groups. On the third day after surgical removal of the tumor, the mice were orally administered with normal saline solution (0.9% w/v) (control group), 50 mg/kg RGF, 200 mg/kg MTF, or RGF and MTF combination [23]. The mice were subjected to the treatment for 38 days and euthanized 48 h after the last dosing. Tumor volume (V) was measured with a vernier caliper and the diameter was calculated by the formula: V=1/2 (length × width)2. The lung tissues mass was fixed using a formaldehyde solution (10%) and the hepatocellular carcinoma tissues were fixed in 10% formaldehyde.
Histological study (immunohistochemistry)
Formalin 10% was used to fix the tissue samples of the tumor and lung, followed by implanting in liquid paraffin. The prepared tissue-embedded blocks were sliced into 5-μm-thick sections. The lung tissue slices were stained with hematoxylin and eosin to visualize the metastatic node. Paraffin was completely removed from the tumor tissue slices, which were then rehydrated and further exposed to autoclaving for 5 min to remove antigenic determinants, followed by incubation in 2.5% hydrogen peroxide for 10–12 min for complete inactivation of peroxidase. The specimens were exposed to goat serum (10%) for 1 h and then incubated at 4°C for 18 h with antibody (Ki67 or CD31). On the next day, the slice was exposed to secondary antibody for 1 h. The slice was then colored using the DAB substrate chromogen system. The TUNEL apoptosis detection kit (KeyGenBioTech, China, Cat. No. KGA7022) was used for detection of TUNEL.
Apoptosis assay and cell viability determination
Microplates containing 96 wells were loaded with 4000 MHCC97H cells/well (n=6) along with DMEM (10 ml), FBS (10%), and 400 mM CoCl2 solution and subjected to incubation for 24 h. After 24 h, the cells were treated with RGF and MTF and incubated for 48 h. Later, in each well, 100 ml DMEM and 10 ml CCK8 solutions were added, and after 2 h the absorbance in each plate was measured at λmax450 nm.
To study the effect of RGF and MTF on cell apoptosis, Annexin V and PI (10ml) staining (bought from Annexin V-FITC Apoptosis detection kit, SANGON, Cat. No. BS6336) was applied to the cells and the effects were analyzed using FACS flow cytometry (Becton Dickinson, San Jose, CA).
Chromatin assay
Formaldehyde (1%) was used to fix the MHCC97H cells at room temperature by exposing the cells for 15 min. After this, the cells were subjected to lysis at low temperature (4°C) for 30 min using buffer composed of 50 mM EDTA, 1% sodium dodecyl sulfate, and 50 nM Tris-hydrochloride buffer and a serine protein inhibitor (1 mM phenylmethylsulfonyl fluoride) at low temperature. After lysis of nuclei, a sonicator with a microtip (Misonix Sonicator 3000) was used to break nuclear chromatin into very small fractions. A small quantity of the sample was preserved for PCR study. The samples were subjected to clarification using protein A Sepharose. The purified chromatin aliquots were stored at 4°C with specific rabbit HIF-2α or rabbit antiserum IgG for 14 h, and the resultant complexes were isolated using 20 ml of sepharose at 4°C for 4 h. The complexes were rinsed with a series of buffers in the sequence indicated in Table 1. The resultant complexes were then passed through beads with constant agitation at room temperature using buffer consisting of sodium bicarbonate 0.1 M and sodium dodecyl sulfate 1%. The elutant and the input were maintained for 6 h at 65°C for cross-linking. The precipitated DNA was further exposed to PCR for purification, and the primers we used for PCR resemble the promoter region of TIP30. Densitometry analyses of Western blots were normalized by the loading control and expressed as fold changes with respect to controls.
Table 1.
Buffer compositions used for rinsing chromatin complexes.
| Order of rinsing with buffer | Composition of buffer |
|---|---|
| Buffer mix 1 | Triton X-100 – 1% EDTA – 2 mM Sodium chloride – 150 mM SDS – 0.1% |
| Buffer mix 2 | Triton X-100 – 1% EDTA – 2 mM Sodium chloride – 50 mM Tris-HCl – 20 mM SDS – 0.1% |
| Buffer mix 3 | LiCl – 0.23 mM NP40 – 1% Deoxycholate – 1% EDTA – 1 mM Tris-HCl – 10 mM |
| Buffer mix 4 | Tris-HCl (pH 8.0) – 10 mM EDTA – 1 mM |
Statistical analysis
All statistical analyses were made using SPSS13.0 for Windows software (SPSS Inc., Chicago). The data indicated are expressed as mean ± standard deviation. The t test was used to compare the variables between groups. P value greater than 0.005 was considered as statistically significant.
Results
Regorafenib tumor cell resistance was reversed with Metformin
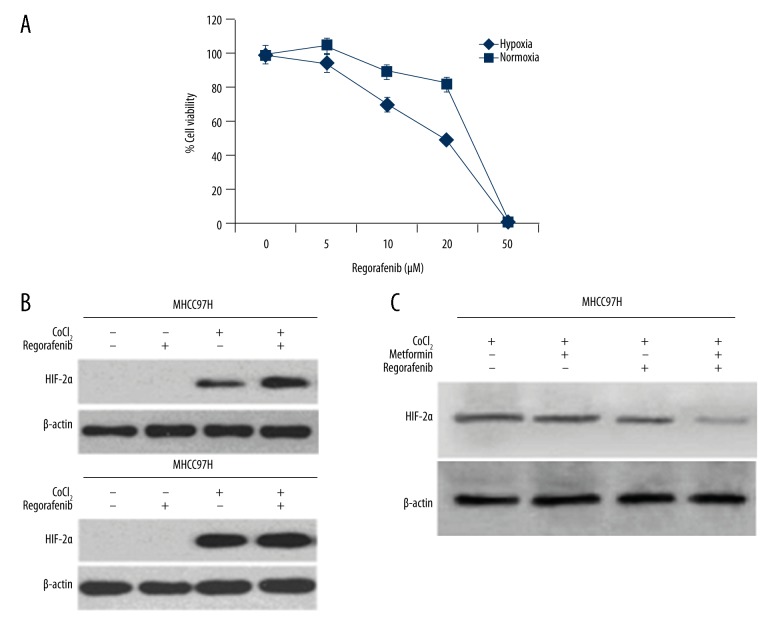
MHCC97H tumor cell lines were incubated with variable concentrations of RGF under hypoxic environment for 24 h. The hypoxic cells, as well as normal cell growth, were suppressed linearly with incremental doses of RGF, but hypoxic cells showed resistance with increasing doses of RGF (Figure 1A). MTF had negligible effect on HIF-2α expression, which was indicated more in cells with hypoxia (Figure 1B). The results clearly indicate that MTF and RGF together suppress HIF-2α activity to a larger extent and thereby could restore sensitivity of hypoxic cells to RGF (Figure 1C).
Figure 1.
Enhancement of hypoxic tumor sensitivity of Regorafenib with MTF. (A) Comparative resistance of hypoxic tumor cells towards Regorafenib. CoCl2 (400 μM)-treated MHCC97H cells incubated with MTF and Regorafenib for 48 h. Percent cell viability was analyzed using CCK8 method in comparison to control. Effect of Metformin and Regorafenib alone and in combination was evaluated on HIF-2α. (B) MTF indicated weak effect on expression of HIF-2α protein, whereas Regorafenib enhanced expression of HIF-2α, after treatment with CoCl2. (C) Remarkable reduction in HIF-2α activity was seen after CoCl2, treatment followed by MTF and Regorafenib combination.
Regulation of TIP30 through HIF-2α in HCC cell line under hypoxia
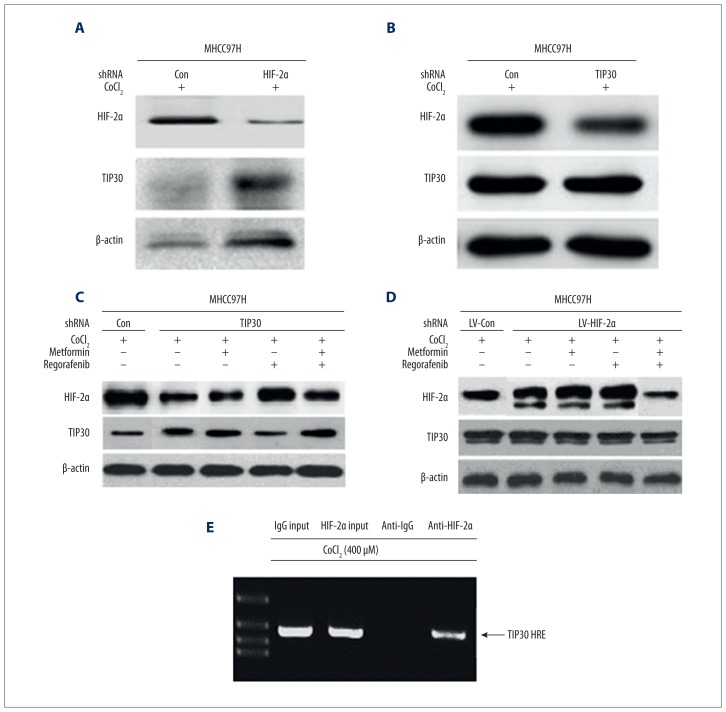
Infection with lentivirus was used to stabilize MHCC97H cells and reduce expression of HIF-2α. Reports in the literature reveal that suppression of TIP30 can result in prolonged EGFR signaling. The Western blot results in the present investigation revealed that suppression of HIF-2α enhanced TIP30 activity in MHCC97H and Hep3B cell cultures and reduced the activity levels of EGFR and cascading molecules such as ERK and phosphorylated AKT. Active suppression of HIF-2α can increase activity of TIP30 [24,25].
To analyze the binding capacity of HIF-2α and TIP30 promoter, CHIP (chromatin immunoprecipitation) technique was employed. The procedure included fixing of MHCC97H cells previously incubated for 6–8 h in cobalt(II) chloride solution (400 mM) with formaldehyde, and then isolation of nuclei and subjecting them to sonication. The lysed fractions of chromatin were treated with specific antibodies against anti-IgG and anti-HIF-2α. Amplification of 214-bp segment of TIP30 was carried out using the DNA extracted using the above technique. The technique indicated amplification of 214-bp, observed in DNA collected from samples treated with HIF-2α, resembling hypoxic condition, whereas no amplification was indicated in non-specific anti-IgG samples. The above results reveal that in the hypoxic environment, there is binding of HIF-2α with TIP30 promoter (Figure 2).
Figure 2.
In HCC cell lines, HIF-2α regulated TIP30 at the protein level. To achieve enhanced activity of HIF-2α in HCC cell lines, lentivirus was used and the HIF-2α – TIP30 correlation was studied using Western blot analysis. (A) Inhibition of HIF-2α suppressed TIP30 activity. (B) Suppression of TIP30 activity had no effect on HIF-2α activity. (C, D) MTF and regorafenib together potentiated reduction in HIF-2α activity and promoted TIP30 activity. (E) HCC cell lines were treated with CoCl2 for 6 h and were immunoprecipitated with anti-HIF-2α or anti-IgG. Further purified DNA with input DNA (1%) was subjected to PCR of 214-bp fragment surrounding TIP30-HRE area.
EMT suppression and apoptosis induction in hypoxia with co-administration of Metformin and Regorafenib
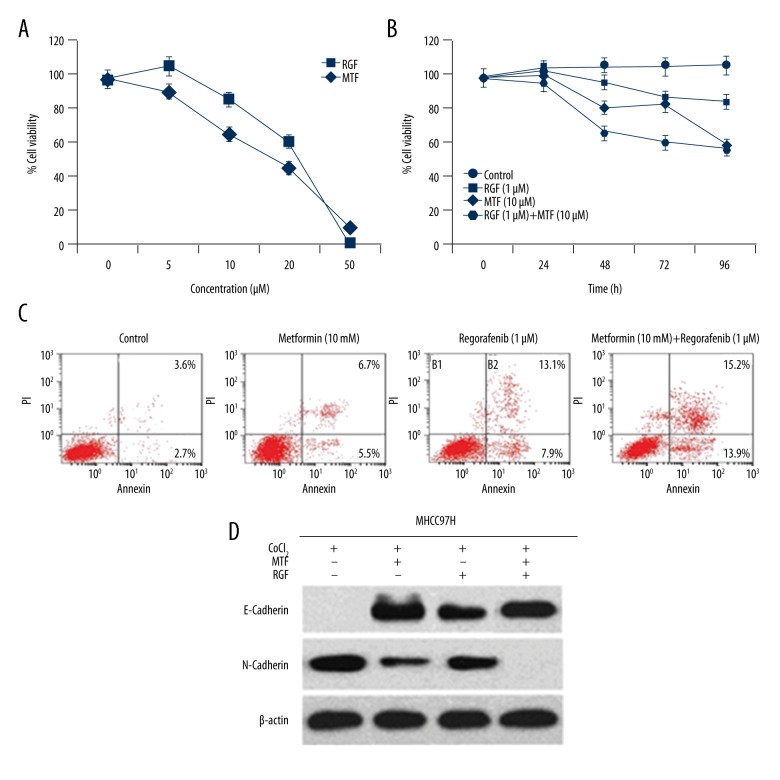
To study whether MTF could make tumor cells sensitive to RGF, cell viability was assessed using a cell counting kit (CCK8). The cells were exposed to different concentrations of MTF and RGF for 48 h and effects were analyzed after various lengths of time. Results indicated linearity in a dose-time relationship in terms of declining effects on MHCC97H proliferation after administration of RGF and MTF alone, and the combination of MTF-RGF exhibited significant suppression of MHCC97H cells (Figure 3A, 3B).
Figure 3.
Enhanced HCC cell line apoptosis and suppression of epithelial-mesenchymal transition were seen after co-administration of MTF and regorafenib. (A) After 48 h of CoCl2 treatment, cells were cultured with MTF and regorafenib. Results indicated reduced growth of MHCC97H cells proportional to dose. (B) Treatment of MHCC97H cells with MTF and regorafenib at various time intervals, indicating time-dependent effect with combination of MTF and regorafenib. (C) Approximately 5×105 cells in 6-well plates were detected using flow cytometry 48 h after treatment with MTF-RGF combination. MTF and regorafenib combination remarkably produced apoptosis of MHCC97H cells. (D) EMT proteins were analyzed using Western blot after treatment with MTF and regorafenib, indicating regorafenib provoked EMT whereas the combination with MTF suppressed the EMT process in vitro.
Tumor cell apoptosis effect was screened using flow cytometry. The least significant cell apoptotic effects were found with RGF and MTF alone, whereas RGF-MTF combination revealed exceptional tumor cell apoptosis. Reports in the literature indicate that EMT (epithelial-mesenchymal transition) and decline of hepatic carcinoma cells is a result of reduced expression of TIP30 [26]. Thus, tumor and metastasis are both significantly affected by EMT [27]. The results obtained in the present study are in accordance with the literature, showing that the combination of RGF and MTF can potentiate reduction in HCC metastasis, and that MTF may amplify tumor cell sensitivity to RGF (Figure 3C, 3D).
Co-administration of Metformin and Regorafenib inhibits postoperative revival of HCC and lung metastasis in orthotopic animal models
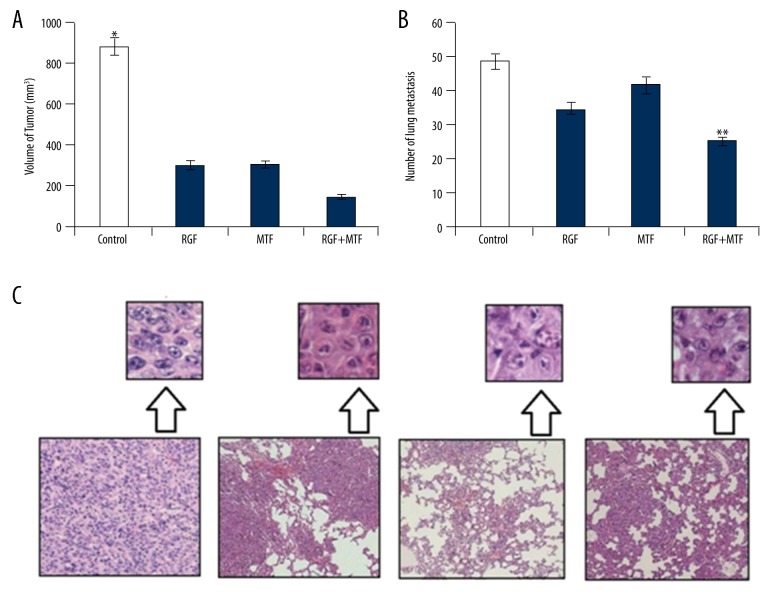
The present study aimed to demonstrate the effect of MTF and RGF on HCC developed in liver tissues in experimental animals. Two weeks after orthotropic tumors were grafted in mouse models, the lobe was dissected from the respective area. Four groups of mice were used for further studies 3 days after tumor removal: (1) a group orally administered with normal saline solution (control group), (2) a group administered 50 mg/kg RGF and another with 200 mg/kg MTF, (3) and a group administered MTF and RGF together. The results in the control group (administered normal saline solution) indicated dramatic tumor growth by 37 days after initiating treatment (Figure 4A). Tumor size was comparatively smaller in the group treated with RGF and MTF together. Subsequent reduction in tumor growth relapse was seen with the RGF-MTF combination compared to the control group, as well as in the groups administered with RGF and MTF alone. Tumor samples were treated with 10% formaldehyde and fixed in paraffin blocks, from which tissue slices (5-mm) were stained with hematoxylin and eosin and subjected to microscopic visualization. The density of nodules due to metastasis were remarkably lower in the animal group treated with RGF-MTF combination, in comparison with RGF and MTF administered individually (Figure 4B, 4C).
Figure 4.
Tumor growth and its relapse were suppressed after administration with MTF and Regorafenib. (A) Tumor size (mm3) was measured after administration of MTF and Regorafenib alone and in combination. (B) Mean number of relapsing lung tumor nodules. (C) Detection of lung tumor nodules after HE staining (100×). * P<0.05; ** P<0.01.
Metformin and Regorafenib in combination stimulates in vivo apoptosis
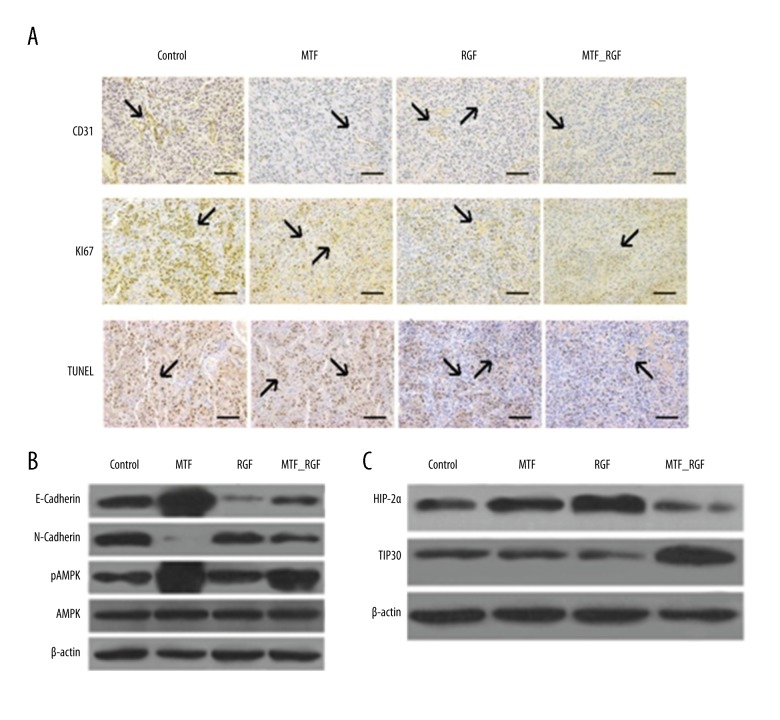
Ki67-positive cells were in the following order: control group >RGF >MTF >RGF-MTF combination. Co-administration of RGF and MTF remarkably reduced tumor angiogenesis. Fewer apoptotic tumor cells (TUNEL-positive) were found in the control group and significantly more were found in the RGF and MTF mono-therapy animal group (Figure 5A). The group subjected to combination therapy had more TUNEL-positive cells.
Figure 5.
Co-administration of MTF and regorafenib reduced angiogenesis, cell growth and EMT and stimulates apoptosis in vivo. Antibodies of Ki67, CD31, and TUNEL were used to stain tumor sections and images were magnified at ×200. (A) Co-administration of MTF and regorafenib potentiated reduction of Ki67 and CD31 activity but enhanced TUNEL expression. (B) MTF-regorafenib combination reduced EMT effect, and pAMPK activity was enhanced in MTF alone and in combination with Regorafenib. (C) Combination of MTF and regorafenib inhibited HIF-2α activity revealed through Western blot analysis.
Western blot analysis was used to determine proteins associated with EMT from tumor cells. Co-administration of RGF and MTF suppressed the EMT process by enhancement of E-cadherin and suppression of N-cadherin. The results show that combination therapy with RGF and MTF not only reduced HIF-2α effects, but also enhanced expression of TIP30, indicating relevance with in vitro results (Figure 5B, 5C).
Discussion
Recurrence and re-growth of the tumor cells after surgical removal is a major issue that may have reduced the effectiveness of HCC treatment [4,5]. RGF is widely used for the treatment of HCC, but its efficacy has been compromised owing to increasing resistance. Hence, there is a serious need to enhance the sensitivity of HCC to RGF.
HIF-2α is known to be a key player in tumors, coupled with hypoxia, which is a ubiquitous phenomenon prevalent in tumors. Prior research showed that HIF-2 α is linked with tumor growth, invasion, and relapse [28,29]. The present investigation indicates that MTF could possibly induce sensitization of hypoxic hepatic carcinoma cells to RGF by reduction in HIF-2α activity. Data in the literature suggest reduced TIP30 is connected with EMT, and the present research suggests that RGF enhances EMT by reducing TIP30 activity [26]. The present research also emphasized that increased HIF-2α activity led to lowering of TIP30 effect and helped the EMT process. In a hypoxic environment, amplification of 214-bp spectra in in vitro CHIP analysis was seen in anti-HIF-2α antibodies. This study had confirmed the involvement of TIP30, as an exclusive HIF-2α genetic fragment, in controlling tumor relapse and subsequent growth.
The present investigation indicates that co-administration of MTF and RGF reduced HIF-2α activity to a broader extent, revealing MTF can enhance sensitivity of HCC cells to RGF. To study the effect of MTF and RGF alone and in combination on tumor growth and its relapse, we used an orthotopic mouse model grafted with tumor cells.
The tumor tissues were surgically removed and sliced into 2-mm3 sections and were grafted in situ. Every possible care in selection of uniform tumors was exercised, but owing to irregular and uncoordinated growth, the grafting had deviations. MTF and RGF remarkably suppressed relapse and further growth in hepatic carcinoma cells. Such activity before acquiring resistance has been indicated in hepatic carcinoma cells, and the present investigation indicated combined therapy with MTF synergistically enhanced activity of RGF alone.
The enhanced sensitivity of hepatic carcinoma cells with co-administration of MTF and RGF demands further clinical investigation, which could lead to enhanced effectiveness in growth and relapse of tumor after surgical removal.
Conclusions
The findings of the present research indicate regorafenib alone enhances HIF-2α activity and reduces TIP30 activity, which subsequently encourages activity against HCC and elevates metastasis. Co-therapy of regorafenib and Metformin potentially stimulates sensitivity of HCC cells towards regorafenib and reduces relapse after surgical removal of the tumor.
Footnotes
Source of support: Departmental sources
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Qi X, Wang D, Su C, et al. Hepatic resection versus transarterial chemoembolization for the initial treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Oncotarget. 2015;6(21):18715–33. doi: 10.18632/oncotarget.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai EC, Fan ST, Lo CM, et al. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg. 1995;221(3):291–98. doi: 10.1097/00000658-199503000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen WT, Chau GY, Lui WY, et al. Recurrent hepatocellular carcinoma after hepatic resection: Prognostic factors and long-term outcome. Eur J SurgOncol. 2004;30(4):414–20. doi: 10.1016/j.ejso.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: Risk factors, treatment, and outcomes. Surgery. 2007;141(3):330–39. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–55. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 9.Abou-Elkacem L, Arns S, Brix G, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther. 2013;12:1322–31. doi: 10.1158/1535-7163.MCT-12-1162. [DOI] [PubMed] [Google Scholar]
- 10.Bozic I, Reiter JG, Allen B, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747. doi: 10.7554/eLife.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang QW, Cheng KJ, Mei XL, et al. Synergistic anticancer effects of triptolide and celastrol, two main compounds from thunder god vine. Oncotarget. 2015;6:32790–804. doi: 10.18632/oncotarget.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diyabalanage HV, Granda ML, Hooker JM. Combination therapy: Histone deacetylase inhibitors and platinum-based chemotherapeutics for cancer. Cancer Lett. 2013;329:1–8. doi: 10.1016/j.canlet.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–61. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Mazumdar J, O’Brien WT, Johnson RS, et al. O2 regulates stem cells through Wnt/b-catenin signalling. Nat Cell Biol. 2010;12:1007–13. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Libby G, Donnelly LA, Donnan PT, et al. New users of metformin are at low risk of incident cancer: A cohort study among people with type 2 diabetes. Diabetes Care. 2009;32(9):1620–25. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kisfalvi K, Eibl G, Sinnett-Smith J, Rozengurt E. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009;69(16):6539–45. doi: 10.1158/0008-5472.CAN-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu B, Fan Z, Edgerton SM, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8(13):2031–40. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- 20.Kisfalvi K, Moro A, Sinnett-Smith J, et al. Metformin inhibits the growth of human pancreatic cancer xenografts. Pancreas. 2013;42(5):781–85. doi: 10.1097/MPA.0b013e31827aec40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donadon V, Balbi M, Mas MD, et al. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30(5):750–58. doi: 10.1111/j.1478-3231.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 22.Dong S, Zeng L, Liu Z, et al. NLK functions to maintain proliferation and stemness of NSCLC and is a target of metformin. J Hematol Oncol. 2015;8(1):120. doi: 10.1186/s13045-015-0203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67(14):6745–52. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 24.Li A, Zhang C, Gao S, et al. TIP30 loss enhances cytoplasmic and nuclear EGFR signaling and promotes lung adenocarcinogenesis in mice. Oncogene. 2013;32(18):2273–81. doi: 10.1038/onc.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang C, Mori M, Gao S, et al. Tip30 deletion in MMTV-Neu mice leads to enhanced EGFR signaling and development of estrogen receptor-positive and progesterone receptor-negative mammary tumors. Cancer Res. 2010;70(24):10224–33. doi: 10.1158/0008-5472.CAN-10-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu M, Yin F, Fan X, et al. Decreased TIP30 promotes Snail-mediated epithelial-mesenchymal transition and tumor-initiating properties in hepatocellular carcinoma. Oncogene. 2015;34(11):1420–31. doi: 10.1038/onc.2014.73. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Weinberg RA. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–29. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Patel SA, Simon MC. Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ. 2008;15(4):628–34. doi: 10.1038/cdd.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordan JD, Bertout JA, Hu CJ, et al. HIF-2αlpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11(4):335–47. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]