Abstract
Background
To explore the expression level of retinoic acid induced 14 (RAI14) in gastric cancer (GC) patients and its potentially clinical prognostic value.
Material/Methods
Initially, The Cancer Genome Atlas (TCGA) and Oncomine databases were mined to examine the differential expression levels and clinical prognostic significance of RAI14 mRNA in GC patients. Subsequently, 68 cases of GC and paired adjacent normal tissues were collected retrospectively, and the expression level of RAI14 protein was detected by immunohistochemical staining. In addition, Kaplan-Meier univariate and Cox multivariate survival analyses were used to verify the correlation between RAI14 expression and clinicopathological parameters in GC patients and its clinical prognostic significance.
Results
TCGA and GEO (from Oncomine database) data mining results found that RAI14 mRNA level was remarkably higher in GC than normal gastric tissues (All P<0.05). Besides, immunohistochemical results detected that RAI14 protein level in GC was dramatically higher (P=0.004) compared to that in the matched normal tissues. Moreover, TCGA database and Kaplan-Meier Plotter mining results showed that compared to those with RAI14 low mRNA expression levels, GC patients with RAI14 high mRNA expression levels had remarkably lower time of both overall survival and disease-free survival (All P<0.05). Additionally, based on the immunohistochemical results, Kaplan-Meier univariate and Cox multivariate survival analyses indicated that high expression of RAI14 was the only independent predictor of unfavorable prognosis in patients with gastric cancer (P=0.000).
Conclusions
RAI14 was highly expressed in GC, and the high expression of RAI14 could be an independent predictor of poor prognosis in GC patients.
MeSH Keywords: Bioinformatics, Gastric Cancer, Prognosis, RAI14
Background
Gastric cancer (GC) is one of the most common malignant gastrointestinal cancers in the world [1–5]. GC morbidity and mortality rates both rank the second among malignant tumors [1]. Most of the GC patients have reached the advanced stage when they are diagnosed, and thus have lost the best opportunities for treatment, and the overall prognosis for these patients is still poor [6–8]. In view of this, how to improve GC early diagnosis rate, explore highly sensitive biomarkers, identify new molecular targets, and develop better targeting drugs have become hot topics in the research field of GC.
Retinoic acid induced 14 (RAI14), also named NORPEG, KIAA1334, or RAI13, is a novel protein coding gene first found in human retinal pigment epithelial cells and is induced by all-trans-retinoic acid [9]. Previous evidence revealed that RAI14 was highly expressed in human placenta and testis tissues [10,11] which was closely associated with the cytoskeleton. Recently, some studies have found that RAI14 was abnormally expressed in malignancies, including ovarian cancer [12], prostate cancer [13], gastric cancer [14], and lung adenocarcinoma [15]. The effects of RAI14 in those malignancies were dramatically related to the drug response to chemotherapy and the cancer cell proliferation and invasion. But until now, the relationship between differential expression levels of RAI14 and clinicopathologic parameters of GC patients, and the potentially clinical significance, has not been fully investigated.
Thus, in the present study, The Cancer Genome Atlas (TCGA) and Oncomine databases were first used to compare differential expression levels of RAI14 mRNA between GC and normal gastric tissues. In addition, 68 cases of GC and matched adjacent normal tissues were collected, and immunohistochemistry was performed to validate the bioinformatics results. Moreover, Kaplan-Meier univariate and Cox multivariate survival analyses were done to explore the potentially prognostic significance of RAI14 expression in GC patients.
Material and Methods
Bioinformatics mining methods
TCGA and Oncomine databases were both selected to predict the expression level of RAI14 in GC and normal gastric tissues. In TCGA database (https://cancergenome.nih.gov/), a total of 408 cases of GC and 211 cases of normal gastric tissues containing RAI14 mRNA expression information were downloaded. RStudio-1.1.419 software was performed to analyze the differential expression levels of RAI14 mRNA between the 2 groups and draw the overall survival (OS) and disease-free survival (DFS) curves. In addition, Oncomine database (https://www.oncomine.org) [16] was also selected to search the differential expression levels between GC and normal groups. Additionally, Kaplan-Meier Plotter database (http://kmplot.com/analysis/index.php?p=service&cancer=gastric) [17] was used to draw the OS and DFS curves.
GC tissues and relative patients’ clinical information
We retrospectively collected a total of 68 cases of GC and matched adjacent normal tissues from patients who underwent radical gastrectomy in our department from December 2008 to June 2010. All postoperative pathology was found to be gastric adenocarcinoma. The last follow-up time was June 2016. Patients’ detailed clinical information, such as age, gender, tumor location, histologic differentiation, TNM stage, are listed in Table 1. The present study was reviewed and approved by the Ethics Committee of our hospital and informed consent was acquired from each patient.
Table 1.
Relationship between RAI14 levels and clinicopathological parameters of GC patients.
| Clinicopathological parameters | Cases (N) | RAI14 level | χ2 | P value | |
|---|---|---|---|---|---|
| Low | High | ||||
| Age (years) | |||||
| ≤60 | 23 | 13 | 10 | 5.835 | 0.016 |
| >60 | 45 | 12 | 33 | ||
| Gender | |||||
| Male | 53 | 18 | 35 | 0.812 | 0.368 |
| Female | 15 | 7 | 8 | ||
| Tumor location | |||||
| Antrum | 29 | 15 | 14 | 4.867 | 0.027 |
| Other sites | 39 | 10 | 29 | ||
| Tumor size (cm) | |||||
| ≤5 | 35 | 19 | 16 | 9.523 | 0.002 |
| >5 | 33 | 6 | 27 | ||
| Histological differentiation | |||||
| Well/moderate | 35 | 16 | 19 | 2.485 | 0.115 |
| Poor | 33 | 9 | 24 | ||
| T stage | |||||
| T1–2 | 16 | 9 | 7 | 3.417 | 0.065 |
| T3–4 | 52 | 16 | 36 | ||
| N stage | |||||
| N0 | 19 | 11 | 8 | 5.064 | 0.024 |
| N1–3 | 49 | 14 | 35 | ||
| TNM stage | |||||
| I–II | 30 | 16 | 14 | 6.339 | 0.012 |
| III–IV | 38 | 9 | 29 | ||
Immunohistochemistry and determination of the results
According to the protocol instruction, immunohistochemical staining was performed to examine the expression level of RAI14 in GC and matched tissues. RAI14 rabbit monoclonal antibody was purchased from Abcam Corporation (ab137118, Abcam, UK) with a working concentration of 1: 100. The results of immunohistochemical staining were evaluated by 2 pathologists in a double-blind way. The immunoreaction score (IRS) of each slice was calculated referring to the previous reports of literature [18,19]. The range of IRS score was from 0 to 12, which was produced by staining intensity (SI) × percentage of positive cells (PP). High expression of RAI14 was considered as IRS >4, while low expression of RAI14 was considered as IRS ≤4.
Statistical analysis
SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA) was employed to analyze the experimental data. Mean and standard deviation were used to describe quantitative data. TCGA and Oncomine data were analyzed by the independent samples t-test to compare the differential expression levels of RAI14 mRNA between the GC group and the normal gastric tissues group. Chi-square test was performed to analyze the relationship between RAI14 expression level and clinicopathological factors. Kaplan-Meier univariate and Cox multivariate survival analyses were done to investigate the prognostic significance of RAI14 in GC patients. If the P value was less than 0.05, the difference was considered statistically significant.
Results
High expression of RAI14 in GC
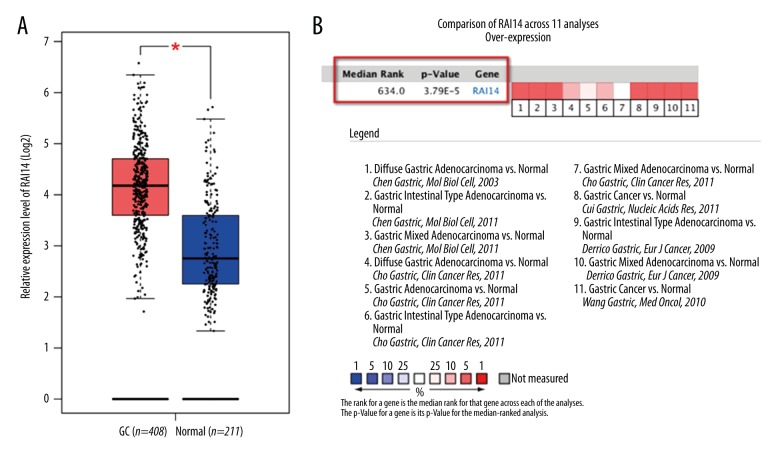
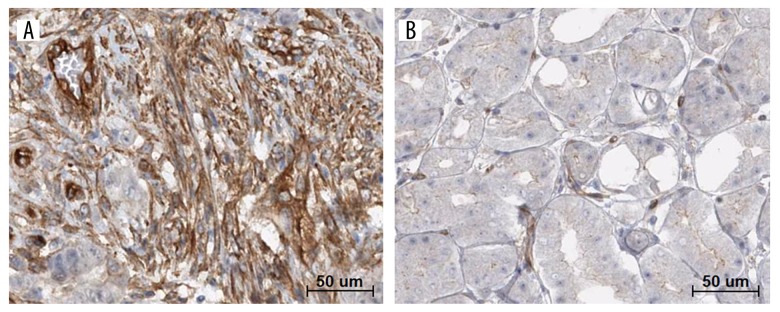
TCGA and GEO (from Oncomine database) data were analyzed by bioinformatics method to examine the differential expression levels of RAI14 mRNA between GC and normal tissues. As shown in Figure 1, RAI14 mRNA expression level was remarkably higher in GC than normal gastric tissues (All P values <0.05). Afterward, to validate the predictive results, immunohistochemistry was performed to detect the RAI14 protein levels in 68 cases of GC and matched normal tissues (Figure 2). The findings indicated that RAI14 was stained mainly located in cytoplasm and cytomembrane of GC cell (Figure 2A). The high positive rate of RAI14 staining in GC was 63.2% (43/68). Compared to that in the matched normal tissues, the expression level of RAI14 protein in GC was dramatically higher (P=0.004, Table 2).
Figure 1.
High expression of RAI14 mRNA in gastric cancer (GC) predicted by TCGA and Oncomine databases. (A) RAI14 mRNA levels of GC versus normal gastric tissue in TCGA database. (B) Meta-analysis of the 11 datasets from 5 studies (DErrico Gastric, 2 datasets, GEO: GSE13911; Chen Gastric, 3 datasets, Reporter ID: IMAGE823850; Wang Gastric, 1 dataset, GEO: GSE19826; Cui Gastric, 1 dataset, GEO: GSE27342; Cho Gastric, 4 datasets, GEO: GSE13861) on RAI14 mRNA levels in GC versus normal gastric tissue searched by Oncomine database.
Figure 2.
Representative immunohistochemical images of RAI14 in 68 cases of paired gastric cancer (GC) and adjacent normal tissues. (A) High expression of RAI14 in GC tissue. (B) Low expression of RAI14 in paired adjacent normal tissue. Bar=50 um.
Table 2.
High expression of RAI14 in 68 cases of GC tissues.
| Tissues | RAI14 level (cases, %) | χ2 | P | |
|---|---|---|---|---|
| Low | High | |||
| Gastric cancer | 25 (36.8) | 43 (63.2) | 8.502 | 0.004 |
| Matched normal tissues | 42 (61.8) | 26 (38.2) | ||
Relationship between RAI14 and clinicopathological parameters of GC patients
Then, we used the chi-square test to detect whether there was a statistically significant difference in the expression of RAI14 in different groups of GC patients based on each parameter. Parameters included age, gender, tumor location, tumor size, histological differentiation, T stage, N stage, and TNM stage. The results are shown in Table 1. The differential expression level of RAI14 protein (low versus high) was dramatically related to the age, tumor location, tumor size, N stage, and TNM stage of GC patients (all P<0.05).
Prognostic significance of RAI14 expression in GC patients
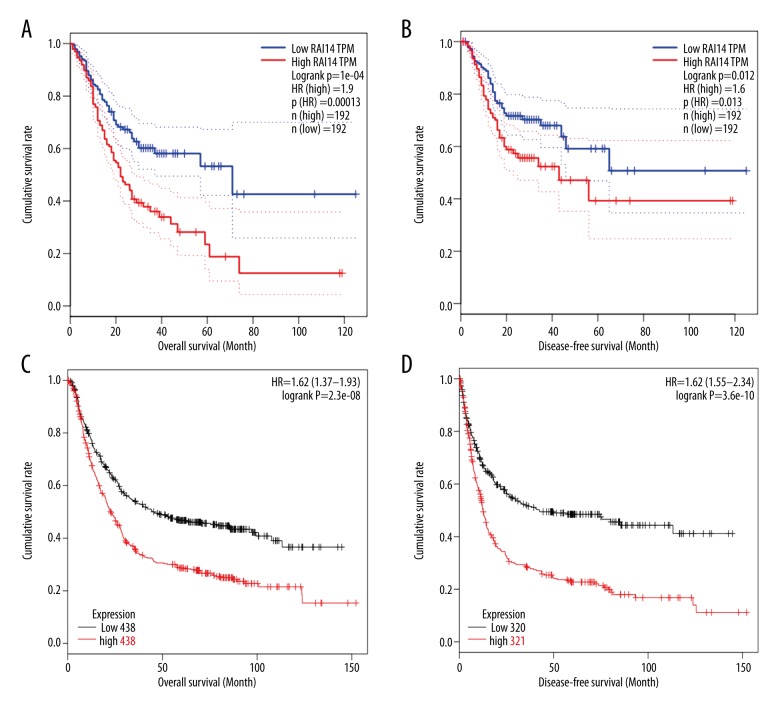
Finally, the potentially prognostic significance of RAI14 expression levels in GC patients was investigated. First, through mining the TCGA database and Kaplan-Meier Plotter, we found that compared to those with RAI14 low mRNA expression levels, GC patients with RAI14 high mRNA expression levels had remarkably lower time for both OS and DFS (all P<0.05, Figure 3). Subsequently, to verify the predictive results, we performed a statistical analysis of the real results of immunohistochemical staining and found that GC patients with high expression of RAI14 protein had dramatically lower OS than those with low expression of RAI14 protein (P=0.000, Figure 4). In addition, Kaplan-Meier univariate and Cox multivariate survival analyses were performed included the RAI14 expression levels and patients’ clinicopathological parameters. Kaplan-Meier survival analysis found RAI14 differential expression levels, age, tumor location, tumor size, histological differentiation, T stage, N stage, and TNM stage were the important parameters affecting the survival time of GC patients (Table 3). Furthermore, Cox multivariate survival analysis were done, including those 8 significantly statistic parameters, and indicated that high expression of RAI14 was the only independent predictor of unfavorable prognosis in patients with gastric cancer (P=0.000, Table 4).
Figure 3.
Kaplan-Meier analysis of overall survival (OS) and disease-free survival (DFS) curves of gastric cancer (GC) patients based on RAI14 mRNA expression (low versus high). (A) Relationship between RAI14 mRNA expression level and OS of GC patients based on TCGA data. (B) Relationship between RAI14 mRNA expression level and DFS of GC patients based on TCGA data. (C) Relationship between RAI14 mRNA expression level and OS of GC patients based on GEO data (analyzed in Kaplan-Meier Plotter website). (D) Relationship between RAI14 mRNA expression level and DFS of GC patients based on GEO data (analyzed in Kaplan-Meier Plotter website).
Figure 4.
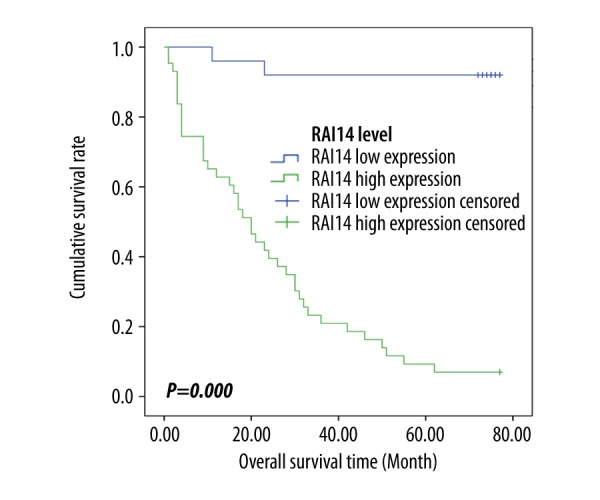
Kaplan-Meier analysis of overall survival curve of gastric cancer patients based on RAI14 protein expression (low vs. high) using the immunohistochemical data.
Table 3.
Kaplan-Meier univariate survival analysis of RAI14 and other clinicopathological parameters in GC patients.
| Clinicopathological parameters | Mean survival time (months) | 95% CI | P value |
|---|---|---|---|
| RAI14 expression | |||
| Low | 72.200 | 65.785–78.615 | 0.000 |
| High | 24.535 | 18.136–30.933 | |
| Age (years) | |||
| ≤60 | 52.174 | 39.883–64.465 | 0.041 |
| >60 | 36.889 | 28.403–45.375 | |
| Gender | |||
| Male | 41.264 | 33.351–49.177 | 0.653 |
| Female | 44.867 | 28.144–61.590 | |
| Tumor location | |||
| Antrum | 50.414 | 39.171–61.656 | 0.030 |
| Other sites | 35.846 | 26.981–44.711 | |
| Tumor size (cm) | |||
| ≤5 | 56.371 | 46.725–66.018 | 0.000 |
| >5 | 26.879 | 18.939–34.818 | |
| Histological differentiation | |||
| Well/moderate | 50.886 | 40.947–60.825 | 0.015 |
| Poor | 32.697 | 23.269–42.125 | |
| T stage | |||
| T1–2 | 63.235 | 52.112–76.356 | 0.004 |
| T3–4 | 35.173 | 27.318–43.028 | |
| N stage | |||
| N0 | 60.684 | 49.052–72.316 | 0.002 |
| N1–3 | 34.837 | 26.791–42.882 | |
| TNM stage | |||
| I–II | 59.067 | 49.598–68.536 | 0.000 |
| III–IV | 28.632 | 20.350–36.914 | |
Table 4.
Cox multivariate analysis of RAI14 and other clinicopathological parameters in GC patients.
| Covariates | HR | 95% CI for HR | P value |
|---|---|---|---|
| RAI14 expression level (low vs. high) | 30.840 | 6.488–146.601 | 0.000 |
| Age (≤60 vs. >60 years) | 1.560 | 0.696–3.495 | 0.280 |
| Tumor location (antrum vs. other sites) | 1.193 | 0.572–2.487 | 0.637 |
| Tumor size (≤5 vs. >5 cm) | 1.265 | 0.534–2.997 | 0.594 |
| Histological differentiation (well/moderate vs. poor) | 1.184 | 0.560–2.505 | 0.658 |
| T stage (T1–2 vs. T3–4) | 2.623 | 0.648–10.626 | 0.177 |
| N stage (N0 vs. N1–3) | 1.708 | 0.542–5.386 | 0.361 |
| TNM stage (I–II vs. III–IV) | 1.668 | 0.578–4.816 | 0.344 |
Discussion
RAI14 was initially found in human retinal pigment epithelial cells induced by all-trans-retinoic acid [9]. Subsequent researches showed that RAI14 was closely associated with the cytoskeleton and highly expressed in human placenta and testis tissues [10,11]. Recent studies have suggested that RAI14 might play a key role in the process of the drug response to chemotherapy and cancer cell proliferation and invasion in some kind of malignancies [12–15,20]. For instance, Hsu et al. [20] have performed genome-wide analysis to identify a set of 8 genes (EGFR, ITGA3, MYLK, RAI14, AHNAK, GLS, IL32 and NNMT) significantly associated with the chemotherapeutic drugs including paclitaxel, docetaxel, erlotinib, everolimus, and dasatinib. Hawkins et al. [12] have found that NR2F2 could regulate the expression of NEK2, RAI14, and multiple other genes involved in the cell cycle of ovarian cancer. Paez et al. [13] have demonstrated that RAI14 was involved in the regulation of cell cytoskeletal of prostate cancer. Additionally, Zhou et al. [14] used an integrative approach to identify genes related to drug response in GC and the results also showed that knockdown of RAI14 could decrease the cell proliferation of GC. However, the phenomenon of inconsistency was found in lung adenocarcinoma. Yuan et al. [15] have found that RAI14 was overexpressed in A549 and 31 out of 71 patients and high expression of RAI14 could inhibit cell proliferation of lung adenocarcinoma. Until now, the expression levels of RAI14 and its potentially clinical significance in GC patients have not been fully explored.
In the present study, we first examined the differential expression levels of RAI14 in GC compared to normal gastric tissues. Bioinformatics prediction results based on the TCGA and GEO data showed that RAI14 mRNA level was significantly overexpressed in GC tissues than that in normal gastric tissues. Subsequently, our immunohistochemical results validated the predictive findings. The high positive rate of RAI14 staining in GC was 63.2% (43 out of 68). Compared to that in the matched normal tissues, the expression level of RAI14 protein in GC was dramatically higher. All these results suggested that RAI14 gene might act as an oncogene in promoting GC occurrence and development.
Then, the potentially prognostic significance of RAI14 expression levels in GC patients was investigated. First, results of chi-square test showed that differential expression level of RAI14 protein was dramatically related to the age, tumor location, tumor size, N stage, and TNM stage of GC patients. Second, through mining the TCGA database and Kaplan-Meier Plotter online website, we found that compared to those with RAI14 low mRNA expression levels, GC patients with RAI14 high mRNA expression levels had remarkably lower time for both OS and DFS. Moreover, based on the real results of immunohistochemical staining, Kaplan-Meier univariate and Cox multivariate survival analyses were performed including the RAI14 expression levels and patients’ clinicopathological parameters. Kaplan-Meier survival analysis found RAI14 differential expression levels, age, tumor location, tumor size, histological differentiation, T stage, N stage, and TNM stage were the important parameters affecting the survival time of GC patients. Further, Cox multivariate survival analysis were done and indicated that high expression of RAI14 was the only independent predictor of unfavorable prognosis in GC patients. All these findings suggested that the high expression of RAI14 could be used as an independent predictor to indicate the poor prognosis of GC patients and RAI14 might be one of the key target genes involved in the growth and metastasis of GC.
Admittedly, there were some limitations in our present study. First, there were only 68 cases of GC tissues collected in this study, which was a small sample size. Therefore, a larger sample size is needed for future validation. Second, some clinical information that was missing for the GC patients, such as DFS, tumor biomarkers CEA and CA199 data, might influence the depth and breadth of statistical analysis. Third, this study focused on the expression and prognostic significance of RAI14 in GC, but its detailed molecular mechanism was not further explored. All these limitations should be improved and investigated in future studies.
Conclusions
Our study demonstrated that RAI14 gene was highly expressed in GC and high expression of RAI14 could serve as an independent parameter to indicate the unfavorable prognosis of GC patients. RAI14 may be a potential new therapeutic target for GC in the future.
Acknowledgement
We sincerely thank Dr. Hang-Cheng Zhou and Dr. Ao Xu (2 pathologists, Department of Pathology, The First Affiliated Hospital of University of Science and Technology of China, Hefei, China) for their kind help in pathology.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Sitarz R, Skierucha M, Mielko J, et al. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–48. doi: 10.2147/CMAR.S149619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song Z, Wu Y, Yang J, et al. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39 doi: 10.1177/1010428317714626. 1010428317714626. [DOI] [PubMed] [Google Scholar]
- 4.Chon SH, Berlth F, Plum PS, et al. Gastric cancer treatment in the world: Germany. Transl Gastroenterol Hepatol. 2017;2:53. doi: 10.21037/tgh.2017.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee HS, Kim WH, Kwak Y, et al. Molecular testing for gastrointestinal cancer. J Pathol Transl Med. 2017;51:103–21. doi: 10.4132/jptm.2017.01.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li B, Liu HY, Guo SH, et al. Detection of microsatellite instability in gastric cancer and dysplasia tissues. Int J Clin Exp Med. 2015;8:21442–47. [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Z, Wang Q, Yu X, et al. Risk factors associated with splenic hilar lymph node metastasis in patients with advanced gastric cancer in northwest China. Int J Clin Exp Med. 2015;8:21358–64. [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H, Wang W, Tong S, et al. Nucleostemin regulates proliferation and migration of gastric cancer and correlates with its malignancy. Int J Clin Exp Med. 2015;8:17634–43. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Kutty RK, Kutty G, Samuel W, et al. Molecular characterization and developmental expression of NORPEG, a novel gene induced by retinoic acid. J Biol Chem. 2001;276:2831–40. doi: 10.1074/jbc.M007421200. [DOI] [PubMed] [Google Scholar]
- 10.Peng YF, Mandai K, Sakisaka T, et al. Ankycorbin: A novel actin cytoskeleton-associated protein. Genes Cells. 2000;5:1001–8. doi: 10.1046/j.1365-2443.2000.00381.x. [DOI] [PubMed] [Google Scholar]
- 11.Yuan W, Zheng Y, Huo R, et al. Expression of a novel alternative transcript of the novel retinal pigment epithelial cell gene NORPEG in human testes. Asian J Androl. 2005;7:277–88. doi: 10.1111/j.1745-7262.2005.00040.x. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins SM, Loomans HA, Wan YW, et al. Expression and functional pathway analysis of nuclear receptor NR2F2 in ovarian cancer. J Clin Endocrinol Metab. 2013;98:E1152–62. doi: 10.1210/jc.2013-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paez AV, Pallavicini C, Schuster F, et al. Heme oxygenase-1 in the forefront of a multi-molecular network that governs cell-cell contacts and filopodia-induced zippering in prostate cancer. Cell Death Dis. 2016;7:e2570. doi: 10.1038/cddis.2016.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Yong WP, Yap CS, et al. An integrative approach identified genes associated with drug response in gastric cancer. Carcinogenesis. 2015;36:441–51. doi: 10.1093/carcin/bgv014. [DOI] [PubMed] [Google Scholar]
- 15.Yuan C, Hu H, Kuang M, et al. Super enhancer associated RAI14 is a new potential biomarker in lung adenocarcinoma. Oncotarget. 2017;8:105251–61. doi: 10.18632/oncotarget.22165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: A cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szász AM, Lánczky A, Nagy Á, et al. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016;7:49322–33. doi: 10.18632/oncotarget.10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu PL, He YF, Yao HH, et al. Martrilin-3 (MATN3) Overexpression in Gastric Adenocarcinoma and its Prognostic Significance. Med Sci Monit. 2018;24:348–55. doi: 10.12659/MSM.908447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu DD, Li PC, He YF, et al. Overexpression of coiled-coil domain-containing protein 34 (CCDC34) and its correlation with angiogenesis in esophageal squamous cell carcinoma. Med Sci Monit. 2018;24:698–705. doi: 10.12659/MSM.908335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu YC, Chen HY, Yuan S, et al. Genome-wide analysis of three-way interplay among gene expression, cancer cell invasion and anti-cancer compound sensitivity. BMC Med. 2013;11:106. doi: 10.1186/1741-7015-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]