Abstract
Building upon studies showing that ischemia/reperfusion-(IR)-injury is complement-dependent, we tested links among complement activation, transplant associated ischemia-reperfusion injury, and murine cardiac allograft rejection. We transplanted BALB/c hearts subjected to 8h cold ischemic storage (CIS) into CTLA4Ig-treated WT or c3−/− B6 recipients. Whereas allografts subjected to 8h CIS rejected in WT recipients with a median survival time (MST) of 37d, identically treated hearts survived >60d in c3−/− mice (p<0.05, n=4–6/group). Mechanistic studies showed recipient C3 deficiency prevented induction of intragraft and serum chemokines/cytokines, and blunted the priming, expansion and graft infiltration of interferon-gamma-(IFNγ)-producing, donor-reactive T cells. MST of hearts subjected to 8h CIS was >60d in mannose binding lectin-(mb1−/−/mbl2−/− recipients, and 42d in factor-B-(cfb)−/− recipients (n=4–6/group, p<0.05, mb1−/−/mbl2−/− vs cfb−/−), implicating the MBL (not alternative) pathway. To pharmacologically target MBL-initiated complement activation, we transplanted BALB/c hearts subjected to 8h CIS into CTLA4Ig-treated WT B6 recipients±C1-inhibitor (C1-INH). Remarkably, peri-transplant administration of C1-INH prolonged graft survival (MST >60d, p<0.05 vs. controls, n=6) and prevented CI-induced increases in donor-reactive IFNγ-producing spleen cells (p<0.05). These new findings link donor IR-injury to T cell-mediated rejection through MBL-initiated, complement activation and support testing C1-INH administration to prevent CTLA4Ig-resistant rejection of deceased donor allografts in human transplant patients.
Introduction
Transplantation is the therapy of choice for patients with end stage organ failure, but long-term allograft survival and function remain suboptimal, particularly for recipients of deceased donor organs whose graft half-lives are significantly shorter than those of living donor transplants (1).
Many studies have shown that ischemia followed by reperfusion of native or transplanted organs initiates reactive oxygen radical-dependent inflammatory responses that mediate organ injury and dysfunction (2–7). While severe IR injury can result in organ failure transient IR injury commonly resolves over days to weeks. Emerging evidence indicates that the poorer long-term survival of deceased donor organs is in part attributable to persistent inflammation initiated by early posttransplant IR injury (8–11). How these early events are linked to subsequent T cell mediated graft injury is not well understood.
In work published since 2014, the Fairchild laboratory has advanced this field through studying a murine model of IR-initiated cardiac allograft rejection in which donor hearts are exposed to 8 h of cold ischemic storage (CIS) prior to transplantation (12). These studies showed that 8 h of allograft CIS triggers CTLA4Ig-resistant, T cell-dependent rejection. Associated mechanistic studies indicated that the IR-injury augments allograft immunogenicity, facilitates activation and amplification of pre-existing (endogenous) donor-reactive pathogenic memory T cells, and enhances rapid donor-reactive memory T cell infiltration into the allograft. Together these events drive immune-mediated graft rejection/failure despite dosing of CTLA4Ig that prevents rejection of allografts derived from the same donor strain but exposed to only 30 min of CIS (12).
Several research groups, using rodent and large animal models, have shown a crucial role for the complement system as a proximal mediator of heart or kidney IR injury (7, 13–16). Evidence indicates IR injury induces neoantigen upregulation on the injured organ that binds collectins, natural autoreactive IgM and/or mannose binding lectin (MBL) to initiate complement activation (4, 14, 16–18). Downstream effectors include the anaphylatoxins C3a and C5a which ligate their respective receptors, C3a receptor (C3aR) and C5a receptor (C5aR), on both graft cells and bone marrow-derived immune cells to mediate inflammation (19–22). While emerging evidence from murine models and humans indicate that heart transplantation initiates antibody dependent complement activation (18), whether and how complement participates in post-transplant, IR-initiated cardiac allograft rejection has not been studied.
Herein we used genetic knockouts and pharmacological inhibitors to test the impact of complement in the pathogenesis of prolonged IR-induced, costimulatory-blockade-resistant cardiac allograft rejection in mice. In addition to providing new mechanistic insight, the data provide preclinical evidence that peri-transplant complement inhibition should be explored as an approach to prevent CTLA4Ig-resistant rejection in human recipients of deceased donor allografts.
Materials and Methods
Animals and procedures
C57BL/6 (CD45.2 and CD45.1, H-2b), mannose binding lectin 1/2 deficient (mbl1−/−/mbl2−/−), and BALB/c (H-2d) mice were purchased from Jackson Laboratory (Bar Harbor, ME) or bred from our in house colony. Congenic c3−/− mice Jackson Laboratory) were backcrossed to B6 45.1. Congenic B6 2C CD8-TCR transgenic mice (reactive to Ld)(23), and B6 factor B deficient (cfb−/−), obtained from M Zhang, SUNY Downstate) were bred from our in house colony. Fate-mapping Rosa26-tomato-Foxp3GFP-ERT2Cre+ developed in the Rudensky lab (24) were re-derived as an in house colony. All animals were maintained in specific pathogen-free conditions and studies were approved by the IACUC at Icahn School of Medicine at Mount Sinai. All transplant studies were performed with WT and KO animals co-housed at Sinai to control for inter-institutional differences in gut microbiomes.
Heterotopic heart transplants were performed as previously described by our laboratories (12, 25). Allografts were subjected to standard (30min) or prolonged (8h) CIS with preservation in ice-cold sterile University of Wisconsin solution. Recipient mice received 250μg CTLA4Ig (Abatacept, Bristol Myers Squibb, NY, NY) i.p. on the day of surgery. For experiments testing C1 inhibitor, recipients were additionally given 0.4 IU/g C1-INH (Berinert, CSL Behring, King of Prussia, PA) i.v. either on days 0 and 1 (peri-transplant) or on days 7 and 8 (delayed).
Polymerase chain reaction (PCR)
RNA isolation, cDNA synthesis, reverse transcription, and real-time RT-PCR were performed as described previously (26). Briefly, RNA was isolated from allograft cardiac tissue using Trizol (Life Technologies; CA Ambion). cDNA was reverse-transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, NJ) as per the manufacturer instructions. qPCR was performed with TaqMan primers (Applied Biosystems) and run on the CFX96 Real-Time System (Bio-Rad Laboratories, CA). PCR products were normalized to the 18S control gene and expressed as fold increase over the mean value of WT->WT cardiac isograft samples using the ΔΔCt method.
Cytokine Enzyme linked immunosorbent spot (ELISPOT) assays
ELISPOT assays were performed at 21d post-transplant as previously described (25). Plates were developed and spots were analyzed on the ImmunoSpot Series 3 analyzer (CTL, Shaker Heights OH).
Flow Cytometry
Surface and intracellular staining was performed as previously described (27). Antibodies were purchased from eBioscience, San Diego (Viability, CD45.1, CD45.2), Biolegend, San Diego (CD4, CD8) and anti-1B2 to identify 2C (G Hadley, OSU, Columbus OH). Samples were collected using the FACS Canto II flow cytometer (BD biosciences) and analyzed using FlowJo (Ashland, OR) or Cytobank (Santa Clara, CA) software.
Donor specific antibody (DSA)
Serum from recipient or control mice was diluted with PBS and co-cultured with thymocytes at 4º C. After washing, the thymocytes were stained with fluorescently labeled rat anti-mouse IgG Ab (eBioscience) and analyzed by flow cytometry (25).
2C Adoptive Transfers
B6 2C mice (23) were immunized with 20x106 BALB/c spleen cells. 6 days later we harvested the spleen cells, and isolated CD8+ T cells by negative selection, magnetic bead separation using an Automacs (Miltenyi Biotec, Bergisch Gladback, Germany). The enriched CD8 T cells were phenotyped (CD8+ and 1B2+) by flow cytometry and 5x106 CD8+2C+ cells were injected into congenic WT or C3−/− CD45.1 B6 hosts. The following day the recipient mice underwent heterotopic heart transplant with grafts exposed to 30min or 8h CIS as described above and 48h later the mice were sacrificed and the allografts, spleens, and serum collected for analysis.
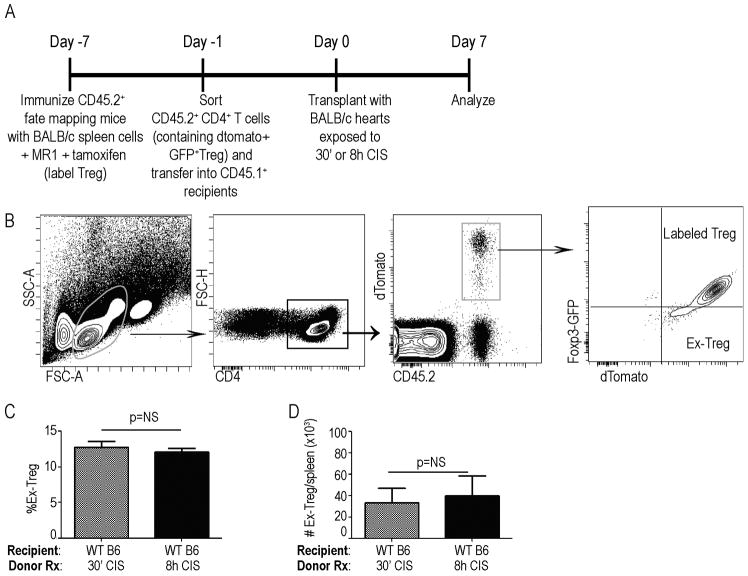
Fate-Mapping Experiments
Rosa26-tomato-Foxp3GFP-ERT2Cre+ fate mapping mice(24) were immunized with 20x106 BALB/c spleen cells and concomitantly received MR1 (anti CD40-L, BioXcell, West Lebanon NH) 1mg i.p. day −7 and a second dose of MR1 250μg day −4 to induce regulatory T cells (Treg). The immunized mice were injected with tamoxifen 75mg/kg dissolved in olive oil i.p. days −7 to −2 to label Treg. Labelled CD4+ T cells were negatively selected (Automacs Miltenyi Biotec) and 8x106 were adoptively transferred into congenic B6 CD45.1 hosts on day −1 followed by heterotopic cardiac transplant on day 0 with WT BALB/c hearts along with CTLA4Ig as described above. Spleen cells from recipient mice were harvested and analyzed by flow cytometry on day 7.
Luminex cytokine assays
Cytokine analysis was performed on serum collected at 48h posttransplant using the Luminex platform and Bio-Plex Pro Mouse Cytokine 23-plex Assay kit (BioRad, Hercules CA) as per manufacturer’s instructions.
Statistics
Statistical analysis was performed using Prism Graphpad software version 5 (La Jolla, CA). Survival statistics were calculated by Mantel Cox log rank test. All other experiments were analyzed using two-tailed student’s t-test with normal distribution, with adjustments for small samples sizes as required. A p-value <0.05 was considered significant.
Results
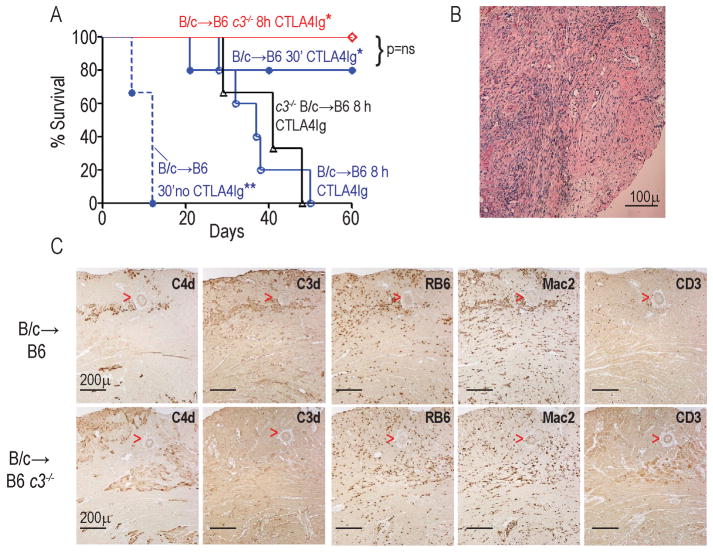
Building upon our previous findings showing that 8 h CIS (but not 4 h or 0.5 h CIS) induced peri-transplant inflammation associated with CTLA4Ig-resistant rejection (12), we tested the effects of 8 h CIS on survival of BALB/c hearts transplanted into B6 recipients [a different strain combination than previously published (12)] given a single dose of CTLA4Ig (Fig 1a). Donor hearts exposed to <30 min of CIS were rejected in untreated recipients with a median survival time (MST) of 10 d, but survived for >60 d in CTLA4Ig-treated hosts. In contrast, donor allografts exposed to 8 h CIS and transplanted in CTLA4Ig-treated hosts were rejected with a MST of 37 d (p<0.05 vs 30 min CIS). At rejection, histological analysis of the allografts exposed to 8 h CIS (in CTLA4Ig-treated recipients) showed diffuse mononuclear cell infiltrates consistent with acute cellular rejection (ACR; Fig 1b). Sera collected at rejection showed low titers of DSA that did not differ among groups (not shown). Together, the data demonstrate that prolonged donor CIS results in delayed, CTLA4Ig-resistant ACR.
Figure 1.
Prolonged donor cold ischemia induces recipient C3-dependent, CTLA4Ig-resistant rejection A. Kaplan-Meier survival curves of WT or c3−/− BALB/c (B/c) hearts subjected to 30′ or 8h CIS and transplanted into WT or c3−/− B6 recipients (n=4–6/group). Recipients were treated with 250ug CTLA4Ig i.p. on day 0 unless indicated otherwise. *p<0.05 vs B/c→B6 8h. Graft survival in all CTLA4Ig-treated groups was longer than in untreated controls. **p<0.05 vs all conditions. B. Representative H&E stained section of B/c heart subjected to 8h CI and transplanted into a CTLA4Ig-treated B6 recipient at cessation of palpable heartbeat, demonstrating diffuse mononuclear cell infiltration consistent with cellular rejection. Scale bar=100 microns. C. Representative serial sections stained for C4d, C3d, RB6 (neutrophils), Mac2 (macrophages), and CD3 (T cells) as indicated from WT B/c hearts subjected to 8h CI 48h after transplantation into WT (upper panels) or c3−/− (lower panels) B6 recipients. Scale bar in all panels = 200 microns. Red arrowheads delineate a blood vessel. Note that the pattern of C3d and C4d staining was similar to that of RB6 and Mac2. The data are representative of allografts from at least 3 different animals per group.
We next tested whether and how complement impacts prolonged CIS-induced, ACR in this model. We stained BALB/c hearts exposed to prolonged CIS and harvested 48 h post-transplant for C3d and C4d. These assays showed positive C3d and C4d staining within the graft parenchyma in regions that co-localized with infiltrating RB6+ neutrophils and Mac2+ macrophages, and without peri- or intravascular staining (Fig 1c). To determine whether recipient vs. donor C3 mechanistically participates in prolonged CIS-induced ACR, we exposed WT or c3−/− BALB/c hearts to 8 h of CIS and transplanted them, respectively, into c3−/− or WT B6 recipients and treated the recipients with a single dose of CTLA4Ig. Survival analyses (Fig 1a) showed that recipient C3 deficiency reversed the effects of 8 h CIS, prolonging graft survival (MST >60d) to that observed in WT recipients of WT allografts exposed to 30 min CIS. In contrast, c3−/− donor allografts exposed to 8 h CIS were rejected (MST>41d) with the same kinetics as WT allografts exposed to 8 h CIS. When we stained additional sets of allografts for complement activation products 48 h post-transplant we did not detect C3d staining in WT grafts exposed to 8 h CIS transplanted into c3−/− recipients (but did detect positive C4d staining, Fig 1c), confirming that the deposited C3d derived from the recipient. Together the data implicate recipient C3 as an essential mediator of CTLA4Ig-resistant ACR in this system.
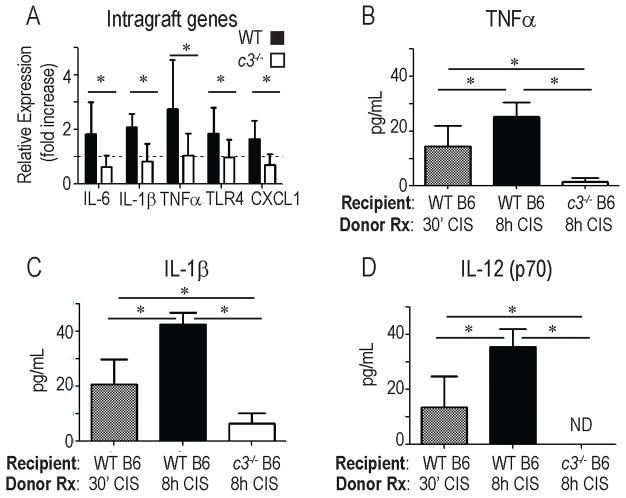
Increasing evidence indicates that early post-transplant inflammation amplifies cellular infiltration into allografts that in turn augments allograft injury (12, 28). At 48 h post-transplant we observed higher expression of IL-6, IL-1β, TNFα, the chemokine CXCL1, and toll-like receptor (TLR)-4 in allografts exposed to 8 h CIS and transplanted into WT recipients (Fig 2a, p<0.05 for each). These changes were abrogated when donor allografts exposed to 8 h CIS were transplanted into c3−/− recipients (Fig 2a). We observed higher serum levels of IL-1, TNFα and IL-12 in WT recipients of donor grafts exposed to prolonged CIS vs. 30 min CIS (p<0.05). Absence of recipient C3 prevented these increases and in fact resulted in serum levels of TNFα, IL-1β and IL-12 that were below those found in the WT B6 recipients of WT BALB/c allografts exposed to 30 min CIS (p<0.05 for each).
Figure 2.
Eight hours of donor cold ischemia induces C3-dependent, post-transplant inflammation. A. WT B/c allografts were subjected to 8h CIS and transplanted into WT (filled bars) or c3−/− B6 (open bars) recipients. Graft RNA was isolated 48 h later and gene expression analyzed by qPCR. Results are expressed as fold change relative to WT BALB/c hearts subjected to 30′ CI and transplanted into WT B6 recipients (set at 1, dashed line). *p<0.05 vs. 30′ CI, **p<0.05. n= 6–8 animals/condition performed in triplicate. B–D. Serum cytokine levels (multiplex Luminex analysis) 48 h after transplantation of BALB/c hearts exposed to 30′ or 8 h CI and transplanted into WT B6 or c3−/− B6 recipients. *p<0.05, n= 4–6 animals/condition. ND=none detected
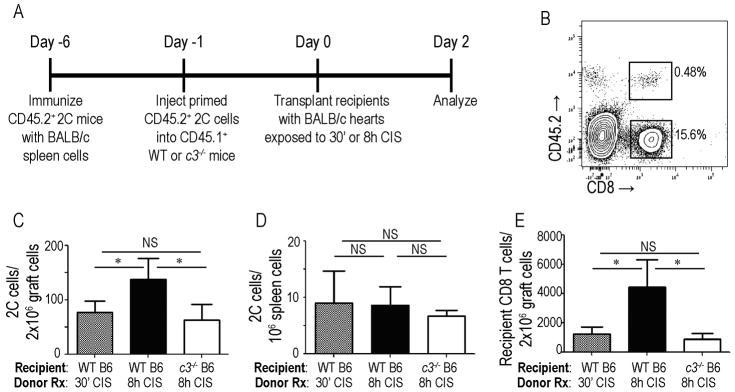
To test the hypothesis that CI-induced complement-dependent allograft inflammation facilitates infiltration of activated T cells into the allografts we transferred primed CD45.2, WT 2C (Ld-reactive) cells into congenic CD45.1 WT or c3−/− hosts (Fig 3a, schematic). We then transplanted WT BALB/c hearts exposed to 30 min or 8 h of CIS into the adoptive hosts and quantified graft-infiltrating cells by flow cytometry 48 h later (Fig 3c–d). These assays showed significantly more CD45.2+CD8+ 2C T cells within allografts exposed to 8 h vs. 30 min CIS (p<0.05, Fig 3c). The increase in graft infiltrating donor-reactive 2C T cells induced by 8 h donor CIS did not occur when the WT allografts were transplanted into c3−/− recipients (Fig 3c). In control analyses we observed similar numbers of 2C T cells in the spleens of all recipients (WT and c3−/− regardless of donor status, Fig 3d), supporting the conclusion that the increased numbers of 2C T cells detected in allografts exposed to 8 h CIS in WT hosts was a result of increased trafficking into the inflamed allograft. Quantification of host-derived CD45.1+CD8+ T cells within the allografts showed a similar pattern: prolonged CIS induced a C3-dependent augmentation of host CD8+ T cell infiltration into the allografts (Fig 3e).
Figure 3.
Cold ischemia-induced graft infiltration of primed CD8+ T cells is recipient C3-dependent. A. Experimental design schematic. B. Representative flow plot of graft infiltrating cells (gated on lymphocytes) 48 h post-transplant demonstrating detection of adoptively transferred CD45.2+CD8+ T cells. C–D. Quantification of CD45.2+ 2C CD8+ T cells within WT BALB/c grafts (C) or in recipient spleens (D) 48 h post-transplant into WT or c3−/− B6 recipients as indicated. (E) Quantification of host CD45.1+CD8+ T cells within graft infiltrating lymphocytes of the same animals. *p<0.05 as compared to BALB/c→ WT B6 8h CI. n= 6 animals/condition. NS: non-significant.
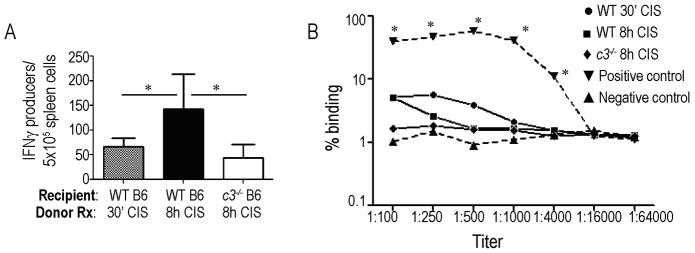
To determine the effects of 8 h donor CIS on induction of adaptive donor-reactive immune responses in WT and c3−/− recipients, we performed additional sets of transplants and sacrificed the animals at 21 d post-transplant for in vitro analyses (Fig 4a). These assays showed higher frequencies of donor-reactive, IFNγ-producing spleen cells by ELISPOT in CTLA4Ig-treated WT recipients of WT grafts exposed to 8 h vs. 30 min CIS. This increase was prevented when WT allografts exposed to 8 h CIS were transplanted into c3−/− recipients (Fig 4a). Serum DSA titers were low in all animals and did not differ among groups (Fig 4b).
Figure 4.
Effects of 8 h donor CIS on post-transplant, donor-reactive immunity. A. Frequencies of donor-reactive IFNγ-producing spleen cells (ELISPOT) 21d post-transplant (WT BALB/c allografts subjected to 30′ or 8h CI transplanted into WT or c3−/− B6 recipients). *p<0.05 vs BALB/c→ WT B6 8h CI. n= 8 animals/condition. B. DSA titers in WT or c3−/− B6 recipients of WT BALB/c allografts subjected to 30′ or 8h CI at 21d post-transplant. (n= 4–8 animals/condition). Serum from non-immunosuppressed WT recipients collected 14d after rejection of WT B/c allografts subjected to 30′ CI served as positive controls. Serum from non-transplanted naïve WT B6 animals served as negative controls. *p<0.05 vs. all other groups. Responses among other groups did not differ (p: non-significant).
We and others reported that complement activation and inflammation can induce Foxp3 downregulation in regulatory T cells (Treg) thereby limiting Treg suppressive function (26, 29, 30). We tested the effect of prolonged donor CIS on Treg stability through the use of Rosa26-tomato-Foxp3GFP-ERT2-Cre+ fate mapping mice (24). In these animals Foxp3+ cells constitutively express GFP, and tamoxifen treatment induces expression of dTomato under the Foxp3 promoter (GFP+dTomato+). The dTomato expression permits identification of former Treg (ex-Treg) as GFPnegdTomato+. We immunized sets of tamoxifen-treated, Rosa26-tomato-Foxp3GFP-ERT2-Cre+ mice with BALB/c spleen cells along with anti-CD40L mAb (MR1) to induce donor-reactive Tregs (Fig 5a, Schematic). Two weeks later we isolated the CD4+T cells and adoptively transferred equal numbers of Foxp3-GFP+dtomato+ Tregs into congenic WT hosts, followed by transplantation of BALB/c hearts exposed to 30 min or 8 h CIS. When we gated on splenic CD45.2+ (adoptively transferred) CD4+ T cells (Fig 5b) we observed equivalent percentages (Fig 5c) and absolute numbers (Fig 5d) of Foxp3-GFPnegdtomato+ “ex-Treg” in recipients of allografts exposed to 30 min or 8 h of CIS. Together with the effects of prolonged CIS on IFNγ-producing effector T cells detailed above (Fig 4a), the data suggest that prolonged CIS augments expansion of donor-reactive T cells without altering stability of Foxp3 expression in Tregs.
Figure 5.
8 hours of donor cold ischemia does not impact Treg stability in vivo. A. Experimental design schematic. B. Representative gating strategy. C–D. Percentages (C) and total numbers (D) of dTomato+GFPneg “ex-Tregs” in recipients of allografts exposed to 30′ vs 8 h CI. (n=4/condition). NS=nonsignificant.
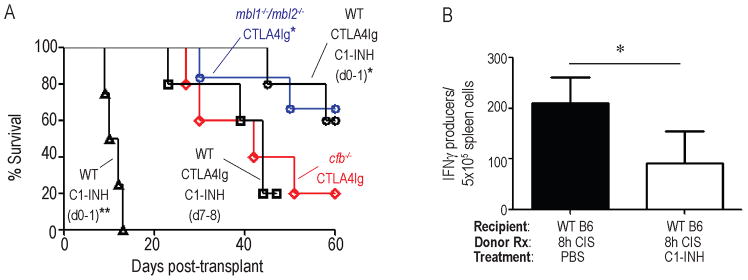
We next attempted to identify proximal initiators and amplifiers of complement activation in this system that could serve as potential therapeutic targets for preventing peri-transplant IR injury and improving graft survival. To this end, we transplanted WT BALB/c hearts exposed to 8 h CIS into cfb−/, (alternative pathway) or mbl1−/−/mbl2−/− B6 recipients. These experiments showed that WT hearts exposed to 8 h CIS were rejected with a MST of >60 d in mbl1−/−/mbl2−/− recipients (Fig 6a), significantly longer than into either WT (MST 37 d, p<0.05 or cfb−/− mice (MST 42 d, p<0.05) and with similar kinetics to WT hearts exposed to 30 min CIS transplanted into WT recipients.
Figure 6.
Absence of recipient MBL or peri-transplant treatment with C1-INH prevents CIS-induced, CTLA4Ig-resistant rejection. A. Kaplan-Meier survival curves of WT BALB/c allografts subjected to 8h CI and transplanted into CTLA4Ig-treated mbl1−/−/mbl2−/− B6, cfb−/− B6, or WT B6+C1-INH (0.4IU/g, d 0–1, or d 7–8), or WT B6 with C1-INH (0.4IU/g, d 0–1) alone. n=4–6/group. p<0.05 vs cfb−/− B6 8h. Statistics calculated by log-rank (Mantel-cox) test. B. Frequencies of donor-reactive IFNγ-producing spleen cells (ELISPOT) 21d post-transplant (WT B/c allografts subjected to 8h CI transplanted into WT B6 recipients ± C1-INH treatment peri-transplant). *p<0.05, n= 4 animals/condition. **p<0.05 vs. all other groups
In an effort to pharmacologically inhibit the prolonged CIS-induced, MBL/complement-initiated ACR we transplanted BALB/c hearts exposed to 8 h CIS into WT recipients treated with CTLA4Ig ± peri-transplant administration of the FDA-approved recombinant human C1-INH [blocks classical, MBL-initiated, and at high doses, alternative complement activation (31–34)]. Remarkably, 2 doses of C1-INH, administered on day 0 and 1 post-transplant (Fig 6a), rescued the prolonged survival of donor allografts exposed to 8 h CIS to MST >60 d. Peri-transplant administration of C1-INH without CTLA4Ig did not prolong allograft survival beyond untreated controls (MST 11d vs 10d, p=ns, Fig 6a). In additional control experiments we transplanted WT BALB/c hearts exposed to 8 h CIS into CTLA4Ig-treated WT B6 recipients and administered the C1-INH on days 7–8 post-transplant, well beyond the time period of reperfusion injury. These allografts were rejected with similar kinetics to PBS control-treated WT allografts exposed to 8 h CIS + CTLA4Ig (MST=42d, p=ns), and significantly faster than those transplanted into recipients treated with CTLA4Ig plus C1-INH on d0–1, p<0.05, Fig 6a).
To assess the effects of peri-transplant C1-INH on T cell alloimmunity, we transplanted additional sets of WT hearts exposed to 8 h CIS into CTLA4Ig-treated WT recipients, ± C1-INH on days 0–1, and performed donor-reactive ELISPOT assays using recipient spleen cells 21 d later (all grafts beating). These assays showed that peri-transplant treatment with C1-INH fully abrogated the prolonged-CIS-induced increases in donor-reactive, IFNγ-producing spleen cells (Fig 6b).
Discussion
Our results provide new insight into the mechanisms linking CIS-associated IR injury to costimulatory blockade-resistant cardiac allograft rejection. Using a model of prolonged CIS, we demonstrate that CTLA4Ig resistant rejection (Fig 1), early post-transplant intragraft inflammation (Fig 2) and primed CD8+ T cell infiltration into the allograft (Fig 3) are dependent upon recipient C3. As the absence of recipient MBL, but not absence of recipient factor B, rescued prolonged survival despite 8 h CIS (Fig 6), the data support the conclusion that IR-initiated, recipient, MBL-dependent, complement activation is pathogenically linked to delayed, CTLA4Ig-resistant rejection in this system.
We also newly demonstrate that peri-transplant administration of C1-INH rescued prolonged graft survival despite 8 h donor CIS (Fig 6). Together with the observations that delayed administration of C1-INH (d 7–8) was not effective (Fig 6) and the fact that C1-INH has a half-life of ~22 h (35), the data indicate that prolonged CIS leads to CTLA4Ig-resistent rejection through transient complement activation that occurs at the time of reperfusion. While our data cannot distinguish whether the effects of C1-INH are mediated via inhibition of MBL-, classical pathway (C1qrs)-, and/or alternative pathways, the results from the mbl1−/−/mbl2−/− mice imply that the protective effects of C1-INH are likely via inhibiting MBL activation.
A previous publication by others showed that myocardial ischemia-reperfusion injury induces surface alterations on stressed myocardial cells, including exposure of carbohydrate moieties (e.g. L-fucose) that a) bind collectin molecules and activate MASPs, and b) upregulate cytokeratin-1 that can be recognized by MBL (17). Oxidative stress also generates neo-epitopes that can be ligated by naturally occurring IgM that in turn initiate MBL-dependent complement activation (16–18, 36). Staining patterns of C3d/C4d within the allografts at 48 h (Fig 1) suggest complement activation occurs on cells within the parenchyma rather that within blood vessels. The observed co-localization of C3d/C4d with infiltrating neutrophils and macrophages (Fig 1) further suggests the possibility that graft damage by the cellular infiltrates permits MBL to escape from of the vasculature and bind ligands expressed on stressed cells. Deciphering the specific ligands/cells that serve as the target for complement activation and determining the role of natural IgM in this process will require additional experiments, including analyses performed at early post-reperfusion time points (<24h).
Building upon previous studies showing that prolonged CIS augments endogenous memory CD8+ T cells infiltration into allografts (12), our new data show that this process is recipient complement dependent (Fig 4), and additionally show that CIS-driven, complement activation at the time of reperfusion facilitates pathogenic donor-reactive T cell activation/expansion 21 days later (Fig 6). The mechanisms through which peri-transplant complement activation enhances delayed, pathogenic, donor-reactive T cell immunity remain to be fully deciphered. Our previous publications showed that immune cell-derived, alternative-pathway activated complement is crucial for alloreactive T cell immunity (25–27, 29, 37), but we contend that immune cell derived complement is unlikely to account for the effects observed in the CIS system employed in the studies herein. Survival of allografts exposed to 8 h CIS was not impacted by the absence of recipient factor B while C1-INH administration for only 48 h peri-transplant (but not on days 7–8) prevented both T cell expansion and graft rejection. We therefore speculate that the transient, reperfusion-induced complement activation initiates a pro-inflammatory, milieu that has long lasting effects on the development and function of pathogenic donor-reactive T cells capable of mediating rejection. While we and others have demonstrated that complement (29, 30, 38, 39) and pro-inflammatory cytokines, including IL-6 (40), inhibit Treg stability and function, our studies support that the dominant effect of complement in this system is to activate effector T cells (Figs 4, 6) without altering Treg stability (Fig 5). We acknowledge that we cannot exclude the possibility that Foxp3-expressing Tregs are functionally impaired in vivo.
Abatacept, the form of CTLA4Ig used in our studies, contains a series of directed mutations in the hinge region that abrogate complement activation (41), making it unlikely that the improved allograft survival in C3 deficient mice is due to alterations in the pharmacokinetics of CTLA4Ig clearance. Moreover, our finding that transient, peri-transplant administration of C1-INH, which has a half-life of <24h (35), overcomes CTLA4Ig-resistant rejection that occurs ~40 d later, supports a similar conclusion.
Our observation that peri-transplant C1-INH has remarkable, long-term protective effects in recipients given CTLA4Ig (Fig 6) has translational implications, and supports the need for testing this combination of FDA-approved agents in non-human primates and in human kidney or heart transplant recipients. C1-INH is FDA approved for use in angioedema and has been studied in hundreds of subjects with minimal infectious or hematologic complications (e.g. thromboembolism), even with long term use (42–44). It is currently being tested to prevent antibody mediated, complement dependent rejection (NCT02547220 and NCT02134314), and administering it peri-transplant is highly feasible. Specific MBL inhibitors being developed would be intriguing alternatives that would limit off-target effects on infectious immunity and avoid potential complications of blocking the C1q-classical pathway, which has been shown to potentiate faster graft rejection under certain conditions (45). Together the data underscore the need to test the use of C1-INH as an adjunctive induction therapy in recipients of heart transplants (all of which are deceased donors).
In summary, our data show that the inflammation and subsequent delayed T cell mediated, costimulatory blockade resistant cardiac allograft rejection induced by prolonged CIS is recipient MBL/complement dependent. Our data provide further evidence that complement is a critical mediator of not only early IRI in transplantation but also late adaptive immunity and offer a rationale to test the impact of peri-surgical C1-INH therapy in human transplantation on immediate and late transplant outcomes, even in the absence of donor specific alloantibodies.
Acknowledgments
The work was supported by R21 AI 11769501 (awarded to PSH and MZ), R01 AI 071185 (PSH), R01 AI40459 (RLF) and P01 AI087506 (RLF and WMB). NC is supported by T32 (5T32DK007757). The authors thank Peter Boros for support of the microsurgery core at the Icahn School of Medicine at Mount Sinai.
Abbreviations
- C1-INH
C1-inhibitor
- CIS
cold ischemic storage
- ELISPOT
Enzyme-Linked ImmunoSpot
- IFNγ
interferon-gamma
- IR
ischemia–reperfusion
- MBL
mannose-binding lectin
- MST
median survival time
- PCR
polymerase chain reaction
- TLR
Toll-like receptor
- Treg
regulatory T cell
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Hart A, Smith JM, Skeans MA, Gustafson SK, Stewart DE, Cherikh WS, et al. OPTN/SRTR Annual Data Report 2014: Kidney. Am J Transplant. 2016 doi: 10.1111/ajt.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorsuch WB, Chrysanthou E, Schwaeble WJ, Stahl GL. The Complement System in Ischemia-Reperfusion Injuries. Immunobiology. 2012;217:1026–33. doi: 10.1016/j.imbio.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavlov VI, Tan YS, McClure EE, La Bonte LR, Zou C, Gorsuch WB, et al. Human mannose-binding lectin inhibitor prevents myocardial injury and arterial thrombogenesis in a novel animal model. Am J Pathol. 2015 Feb;185(2):347–55. doi: 10.1016/j.ajpath.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, et al. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol. 2005 Jul 1;175(1):541–6. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- 5.Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, et al. Predominant role for C5b-9 in renal ischemia/reperfusion injury. Journal of Clinical Investigation. 2000;105(10):1363–71. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwaeble WJ, Lynch NJ, Clark JE, Marber M, Samani NJ, Ali YM, et al. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7523–8. doi: 10.1073/pnas.1101748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thurman J, Ljubanovic D, Edelstein C, Gilkeson G, Holers V. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. Journal of Immunology. 2003;170(3):1517–23. doi: 10.4049/jimmunol.170.3.1517. [DOI] [PubMed] [Google Scholar]
- 8.Gourishankar S, Hunsicker LG, Jhangri GS, Cockfield SM, Halloran PF. The Stability of the Glomerular Filtration Rate after Renal Transplantation Is Improving. Journal of the American Society of Nephrology. 2003;14:2387–94. doi: 10.1097/01.asn.0000085019.95339.f0. [DOI] [PubMed] [Google Scholar]
- 9.Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002;62(1):311–8. doi: 10.1046/j.1523-1755.2002.00424.x. [DOI] [PubMed] [Google Scholar]
- 10.Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb S, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Heart Transplantation Report--2015; Focus theme: Early Graft Failure. The Journal of Heart and Lung Transplantation. 2015;34(10):1244–54. doi: 10.1016/j.healun.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Libby P, Pober JS. Chronic Rejection. Immunity. 2001;14(4):387–97. doi: 10.1016/s1074-7613(01)00119-4. [DOI] [PubMed] [Google Scholar]
- 12.Su CA, Iida S, Abe T, Fairchild RL. Endogenous memory CD8 T cells directly mediate cardiac allograft rejection. Am J Transplant. 2014 Mar;14(3):568–79. doi: 10.1111/ajt.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurman JM, Ljubanovic D, Royer PA, Kraus DM, Molina H, Barry NP, et al. Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/reperfusion. J Clin Invest. 2006 Feb;116(2):357–68. doi: 10.1172/JCI24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan JE, Montalto MC, Stahl GL. Inhibition of mannose-binding lectin reduces postischemic myocardial reperfusion injury. Circulation. 2001;104:1413–8. doi: 10.1161/hc3601.095578. [DOI] [PubMed] [Google Scholar]
- 15.Riedemann NC, Ward PA. Complement in ischemia reperfusion injury. Am J Pathol. 2003 Feb;162(2):363–7. doi: 10.1016/S0002-9440(10)63830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrar CA, Tran D, Li K, Wu W, Peng Q, Schwaeble W, et al. Collectin-11 detects stress-induced L-fucose pattern to trigger renal epithelial injury. Journal of Clinical Investigation. 2016;126(5):1911–25. doi: 10.1172/JCI83000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collard CD, Montalto MC, Reenstra WR, Buras JA, Stahl GL. Endothelial Oxidative Stress Activates the Lectin Complement Pathway. Am J Pathol. 2001;159(3):1045–54. doi: 10.1016/S0002-9440(10)61779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkinson C, Qiao F, Yang X, Zhu P, Reaves N, Kulik L, et al. Targeting pathogenic postischemic self-recognition by natural IgM to protect against posttransplantation cardiac reperfusion injury. Circulation. 2015 Mar 31;131(13):1171–80. doi: 10.1161/CIRCULATIONAHA.114.010482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng Q, Li K, Smyth LA, Xing G, Wang N, Meader L, et al. C3a and C5a promote renal ischemia-reperfusion injury. J Am Soc Nephrol. 2012 Sep;23(9):1474–85. doi: 10.1681/ASN.2011111072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou W, Medof ME, Heeger PS, Sacks S. Graft-derived complement as a mediator of transplant injury. Curr Opin Immunol. 2007 Oct;19(5):569–76. doi: 10.1016/j.coi.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Thurman JM, Royer PA, Ljubanovic D, Dursun B, Lenderink AM, Edelstein CL, et al. Treatment with an inhibitory monoclonal antibody to mouse factor B protects mice from induction of apoptosis and renal ischemia/reperfusion injury. J Am Soc Nephrol. 2006 Mar;17(3):707–15. doi: 10.1681/ASN.2005070698. [DOI] [PubMed] [Google Scholar]
- 22.Zhou W, Patel H, Li K, Peng Q, Villiers M, Sacks S. Macrophages from C3-deficient mice have impaired potency to stimulate alloreactive T cells. Blood. 2006;107:2461–9. doi: 10.1182/blood-2005-08-3144. [DOI] [PubMed] [Google Scholar]
- 23.Wu LC, Tuot DS, Lyons DS, Garcia KC, Davis MM. Two-step binding mechanism for T-cell receptro recognition of peptide-MHC. Nature. 2002;418:552–6. doi: 10.1038/nature00920. [DOI] [PubMed] [Google Scholar]
- 24.Rubtsov YP, Niec R, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–71. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raedler H, Vieyra MB, Leisman S, Lakhani P, Kwan W, Yang M, et al. Anti-complement component C5 mAb synergizes with CTLA4Ig to inhibit alloreactive T cells and prolong cardiac allograft survival in mice. Am J Transplant. 2011 Jul;11(7):1397–406. doi: 10.1111/j.1600-6143.2011.03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008 Mar;28(3):425–35. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raedler H, Yang M, Lalli P, Medof M, Heeger P. Primed CD8(+) T-cell responses to allogeneic endothelial cells are controlled by local complement activation. Am J Transplant. 2009;9(8):1784–95. doi: 10.1111/j.1600-6143.2009.02723.x. [DOI] [PubMed] [Google Scholar]
- 28.El-Sawy T, Miura M, Fairchild R. Early T Cell Response to Allografts Occuring Prior to Alloantigen Priming Up-Regulates Innate-Mediated Inflammation and Graft Necrosis. Am J Pathol. 2004;165(1):147–57. doi: 10.1016/s0002-9440(10)63283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwan WH, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013 Feb 11;210(2):257–68. doi: 10.1084/jem.20121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng Q, Li K, Patel H, Sacks S, WZ Dendritic Cell Synthesis of C3 is Required for Full T Cell Activation and Development of a Th1 Phenotype. J Immunol. 2006;176(6):3330–41. doi: 10.4049/jimmunol.176.6.3330. [DOI] [PubMed] [Google Scholar]
- 31.Jiang H, Wagner E, Zhang H, Frank M. Complement 1 inhibitor is a regulator of the alternative complement pathway. J Exp Med. 2001;194(11):1609–16. doi: 10.1084/jem.194.11.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karpel-Massler G, Fleming SD, Kirschfink M, Tsokos GC. Human C1 esterase inhibitor attenuates murine mesenteric ischemia/reperfusion induced local organ injury. J Surg Res. 2003;115(2):247–56. doi: 10.1016/s0022-4804(03)00192-6. [DOI] [PubMed] [Google Scholar]
- 33.Matsushita M, Thiel S, Jensenius JCTI, Fujita T. Proteolytic Activities of Two Types of Mannose-Binding Lectin-Associated Serine Protease. Journal of Immunology. 2000;165(5):2637–42. doi: 10.4049/jimmunol.165.5.2637. [DOI] [PubMed] [Google Scholar]
- 34.Lu F, Fernandes SM, Davis AE. The effect of C1 inhibitor on myocardial ischemia and reperfusion injury. Cardiovascular Pathology. 2013;22(1):75–80. doi: 10.1016/j.carpath.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chrvala CA, Caspi A. C1 Esterase Inhibitor (Human) PT. 2010;35:2–17. [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M, Kazue T, Alicot E, Vorup-Jensen T, Kessler B, Thiel S, et al. Activation of the Lectin Pathway by Natural IgM in a Model of Ischemia/Reperfusion Injury. Journal of Immunology. 2006;177(7):4727–34. doi: 10.4049/jimmunol.177.7.4727. [DOI] [PubMed] [Google Scholar]
- 37.Vieyra M, Leisman S, Raedler H, Kwan WH, Yang M, Strainic MG, et al. Complement regulates CD4 T-cell help to CD8 T cells required for murine allograft rejection. Am J Pathol. 2011;179(2):766–74. doi: 10.1016/j.ajpath.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Touw W, Cravedi P, Kwan W, Paz-Artal E, Merad M, Heeger PS. Cutting edge: Receptors for C3a and C5a modulate stability of alloantigen-reactive induced regulatory T cells. J Immunol. 2013;190:5921–5. doi: 10.4049/jimmunol.1300847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absent C3a and C5a receptor signaling into CD4+ T cells enables auto-inductive TGF-B1 signaling and induction of Foxp3+ T regulatory cells. Nat Immunol. 2013;14:162–71. doi: 10.1038/ni.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 41.Davis PM, Abraham R, Xu L, Nadler SG, Suchard SJ. Abatacept binds to the Fc receptor CD64 but does not mediate complement-dependent cytotoxicity or antibody-dependent cellular cytotoxicity. J Rheumatol. 2007;34:2204–10. [PubMed] [Google Scholar]
- 42.Craig T, Levy R, Wasserman R, Bewtra A, Hurewitz D, Obtułowicz K, et al. Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol. 2009;124(4):801–8. doi: 10.1016/j.jaci.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Craig T, Bewtra A, Bahna S, Hurewitz D, Schneider L, Levy R, et al. C1 esterase inhibitor concentrate in 1085 Hereditary Angioedema attacks--final results of the I.M.P.A.C.T.2 study. Allergy. 2011;66(12):1604–11. doi: 10.1111/j.1398-9995.2011.02702.x. [DOI] [PubMed] [Google Scholar]
- 44.Berger M, Baldwin WM, Jordan SC. Potential Roles for C1 Inhibitor in Transplantation. Transplantation. 2016;100(7):1415–24. doi: 10.1097/TP.0000000000000995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Csencsits K, Burrell BE, Lu G, Eichwald EJ, Stahel GL, Bishop DK. The Classical Complement Pathway in Transplantation: Unanticipated Protective Effects of C1q and Role in Inductive Antibody Therapy. Am J Transplant. 2008;8(8):1622–30. doi: 10.1111/j.1600-6143.2008.02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]