Abstract
Osteosarcoma, while rare, is the most common primary bone cancer and accounts for up to 10% of all new pediatric cancer diagnoses annually in the United States. Most commonly, osteosarcoma affects the distal femur and occurs as a high-grade intramedullary (conventional) subtype. Patients with osteosarcoma are treated with a multi-disciplinary team approach.1 Often, an orthopaedic oncologist initiates the workup after making a presumptive diagnosis based on classic clinical and radiographic findings. Advanced imaging and a tissue biopsy are obtained to evaluate the extent of disease and to histologically confirm the diagnosis. Musculoskeletal radiologists and pathologists are key team members who evaluate the imaging and tissue samples to make a definitive diagnosis, establish a prognosis, and help the clinicians develop a treatment plan. Medical/pediatric oncologists are essential team members who provide the appropriate neoadjuvant and adjuvant chemotherapy treatment and assist with long-term surveillance to monitor for local or distant relapse. Orthopaedic oncologists develop and execute a plan for resection of the tumor followed by appropriate reconstruction. The current standard of care for distal femoral osteosarcoma is neoadjuvant chemotherapy followed by limb salvage for the surgically resectable tumor, reconstruction of the bone and soft tissue defect, and adjuvant chemotherapy. The survival for patients with isolated osteosarcoma is approximately 70% and has not substantially improved in over 25 years.2–6
Introduction
Osteosarcoma is the most common primary bone cancer in children and adolescents and accounts for approximately 400-500 new diagnoses annually, representing up to 10% of new cancer diagnoses in that population in the United States.7,8 Osteosarcoma usually involves the metaphyseal aspect of long bones with the distal femur, proximal tibia and proximal humerus being the most common locations. Classic, or conventional, osteosarcoma is the predominant subtype (~75%) and is a high-grade, intramedullary osteogenic neoplasm of mesenchymal origin that frequently penetrates the bone cortex with an associated soft-tissue mass.3,8–12 Approximately 25% of patients have radiographically detectable metastases at the time of diagnosis. Patients without detectable metastases treated with modern cytotoxic chemotherapy and surgical resection have an approximately 70% long-term survival compared to ≤20% for patients with metastatic disease at time of diagnosis or those who develop local disease progression or metastasis over time.8,13,14 The clinical scenario presented in this review is an example of the standard workup, systemic treatment, surgical resection and follow-up. The specific surgical reconstruction performed is but one option amongst many. The specific implant options and outcome data on expandable prostheses are beyond the scope of this review.
Clinical Scenario
A 12-year-old male presented to orthopaedic oncology clinic for evaluation of worsening left knee pain and swelling over the previous 3 months. A left distal femur lesion was identified on standard radiographs obtained in the Emergency Department (ED) four days prior to presentation. The child denied night pain, constitutional symptoms, and neurological symptoms. On physical examination, he was noted to have an antalgic gait, tenderness along the anterolateral distal thigh, decreased knee range of motion (ROM) of 0-120 degrees, and 4/5 quadriceps strength. Standard radiographs of the knee (Figure 1) were concerning for an osteosarcoma of the distal femur. The presumptive diagnosis was discussed with the patient and family. The patient was instructed to protect his weight-bearing on the left until his pain resolved in order to limit the risk of pathologic fracture. Additional imaging studies were obtained, including: standard radiographs and a magnetic resonance imaging (MRI) scan (with and without contrast) of the femur to assess the extent of local disease, a computed tomography (CT) scan of the chest and a whole-body positron emission tomography (PET) scan to assess for lung and distant bony metastases. The patient was referred to interventional radiology for an ultrasound-guided core needle biopsy of the soft-tissue mass. A pediatric oncology referral was made after the result of the biopsy was final to discuss initiation of systemic treatment with chemotherapy. The patient was also referred to physical therapy to maximize function and independence prior to definitive surgical treatment.
Figure 1.
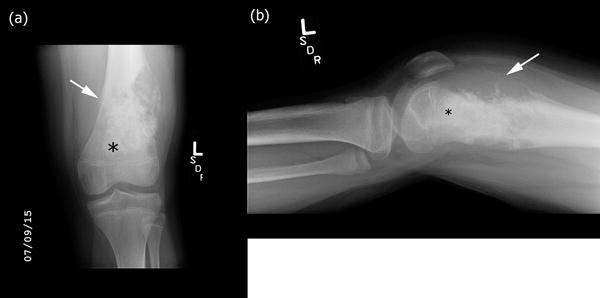
High-grade intramedullary (conventional) osteosarcoma of the distal femur in a 12-year-old boy with knee pain and swelling. (a) Anterior-posterior and (b) lateral radiographs of the left knee demonstrate bone formation (black asterisk) and bone destruction. There is an associated large soft tissue component. The lesion results in periosteal reaction and elevation of the periosteum (Codman triangle) (white arrow). Note that the physes are open.
Team Approach
Orthopaedic Oncology – Initial Evaluation
A detailed history and physical examination in combination with characteristic findings on standard radiographs frequently enable the orthopaedic oncologist to make a presumptive diagnosis of osteosarcoma. The initial pain and swelling are often erroneously attributed to growing pains or trauma in the pediatric population, resulting in a median time from symptom onset to osteosarcoma diagnosis of four months.8 The initial visit with the orthopaedic oncologist sets the stage for the evaluation and treatment the patient will undergo in the following weeks and months, so it is important to develop a trusting relationship with the patient and family. It is essential to provide an overview of the tests that will be performed and the additional members of the team to whom the patient will likely be referred. The patient should then be staged for extent of disease; guidelines for initial evaluation of a suspected distal femur osteosarcoma include plain radiographs and MRI of the femur, CT scan of the chest, and either technetium-99m-methyl diphosphonate (Tc-99m MDP) whole-body bone scan or PET-CT scan.15 Additionally, a bone or soft-tissue biopsy is performed to provide a definitive diagnosis; this is usually performed after MRI so that the imaging can help guide the biopsy location. It can be prior to remaining staging studies to avoid unnecessary radiation exposure in case a benign diagnosis is made. A core needle biopsy is frequently utilized to minimize contamination, although an open incisional biopsy yields more tissue. Biopsy sites should ideally correlate with the planned surgical resection. Surgeon preference dictates whether the biopsy tract is excised at the time of definitive surgery; data is not definitive as to whether resection of the biopsy tract affects recurrence.16 The patient should also be referred to pediatric oncology for evaluation and initiation of appropriate neoadjuvant chemotherapy.
Radiology – Diagnostic Imaging
In the setting of a suspected osteosarcoma, the radiologist plays a key role in evaluating the character and extent of local disease and the presence of regional or distant metastases. Radiographs are used for the primary evaluation of all bone lesions. Radiographically, osteosarcomas may be purely osteolytic or osteoblastic, but are usually mixed in appearance. Most conventional osteosarcomas demonstrate amorphous mineralization consistent with new bone production (Figure 1) and can be heterogeneous depending on the exact tumor characteristics. These tumors are usually >5cm at presentation, and radiographs demonstrate cortical destruction and periosteal elevation with soft tissue masses in 80%-90% of cases.3,9–12,17
A contrast enhanced MRI of the femur or thigh is obtained to evaluate the extent of marrow disease, the presence and extent of a soft-tissue component, the presence of skip metastases and the involvement of important neurovascular structures.18,19 MRI is also helpful for identification of necrotic areas to avoid during a needle biopsy so as to improve the yield and utility of the specimen.
Conventional osteosarcomas are isointense to skeletal muscle on T1-weighted images (Figure 2).18,19 High T1-signal intensity areas usually represent areas of hemorrhage within the tumor, while low T1-signal intensity areas usually correspond to areas of bone formation. T2-weighted imaging shows bone marrow and soft tissue edema. Fluid-fluid levels may be seen if there is an associated aneurysmal bone cyst or in telangiectatic osteosarcomas.20 Post-contrast T1-weighted imaging reveals the extent of enhancing intramedullary tumor with areas of non-enhancement and high T2 signal corresponding to areas of necrosis. In this case, there was no involvement of the neurovascular bundle; however, there was abnormal signal extending to the distal physis (Figure 2).
Figure 2.
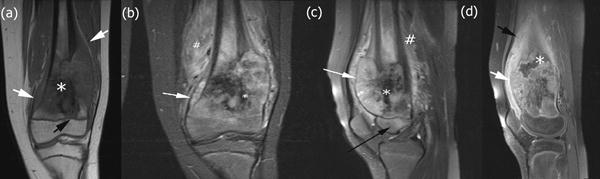
High-grade intramedullary (conventional) osteosarcoma of the distal femur in a 12-year-old boy with knee pain and swelling. (a) Coronal T1-weighted MRI without fat saturation of the left knee. White arrows identify the soft tissue component of the lesion. There is low T1 signal within the lesion corresponding to bone formation (white asterisk). The lesion extends to the physis (black arrow). (b) Coronal short-tau inversion recovery (STIR) MRI of the left knee demonstrates that the soft tissue component of the mass elevates the periosteum (white arrow). There is low STIR signal within the lesion corresponding to bone formation (white asterisk). Extensive peritumoral edema is noted (white hash sign). (c) Sagittal T2-weighted MRI with fat saturation of the left knee showing epiphyseal edema (black arrow). (d) Sagittal T1-weighted MRI of the left knee obtained after intravenous administration of contrast material reveals the extent of enhancing intramedullary and soft tissue components of the lesion (white arrow). There is enhancing peritumoral edema (black arrow). There is an area of necrosis (white asterisk) noted.
Skip metastases are foci of tumor that are distinct and separate from the primary tumor but within the affected bone. Up to 25% of high-grade intramedullary osteosarcomas have skip metastases; the 5-year overall survival in a patient with skip metastases is similar to that of a patient with distal metastases.21
Recent data suggests that PET/CT has increased sensitivity to identify bone metastasis compared to a Tc-99m MDP bone scan.22,23 Whole-body PET/CT performed in this patient showed a hypermetabolic tumor involving the distal femur with no evidence of osseous or pulmonary metastases. PET has been used to assess response to chemotherapy and overall prognosis in small studies.24,25 Chest CT with thin sections (<2mm) is more sensitive and accurate than PET for detection of subcentimeter pulmonary nodules.26 Staging volumetric chest CT showed no evidence of pulmonary metastases in this case.
Pathology – Tissue Diagnosis
The pathologist is engaged in the diagnosis of a patient with osteosarcoma at the time of biopsyin order to confirm the diagnosis suspected on clinical and radiologic grounds, and again at the time of the resection to assess the response to neoadjuvant chemotherapy and status of the surgical margins. The pathologist’s evaluation helps establish a prognosis and guides the oncology team in the subsequent clinical care of the patient.
The diagnosis of osteosarcoma relies almost exclusively on histomorphology on routine hematoxalin and eosin sections. Conventional osteosarcoma is a high-grade sarcoma with variable appearances. There are three major histologic categories of conventional osteosarcoma; osteoblastic, chondroblastic, and fibroblastic. Osteoblastic osteosarcoma consists of malignant osteoblasts with conspicuous bone formation. Chondroblastic osteosarcoma is characterized by cartilaginous matrix, while fibroblastic osteosarcoma has a fibrous matrix. Regardless of the predominant matrix-type, the histologic hallmark of osteosarcoma is the finding of cytologically malignant cells producing bone. Since malignant bone formation may be sparse (e.g. chondroblastic osteosarcoma), adequate sampling at the time of biopsy is required to make an accurate diagnosis.
The biopsy specimen in this case contains haphazardly deposited bone in small, irregular spicules and short, interlacing cords (Figure 3a, yellow arrowheads). The cellular component consists of polymorphous, large malignant cells bearing a superficial resemblance to osteoblasts but with clear features of malignancy. These cells contain enlarged, irregular nuclei with fine, even chromatin and nucleoli and a moderate amount of pale, eosinophilic cytoplasm. Mitotic forms are frequent (Figure 3a, white arrow) and include atypical forms. Individual cells undergoing degeneration and cell death are also apparent (Figure 3a, gray arrow). No ancillary immunohistochemical studies were necessary for diagnosis. The histologic features led to a diagnosis of high-grade conventional osteosarcoma, osteoblastic type. Molecular and cytogenetic tests were not necessary for diagnosis in this case but, if performed, would show a highly abnormal karyotype with extensive structural abnormalities and numerous chromosomal gains and losses.
Figure 3.
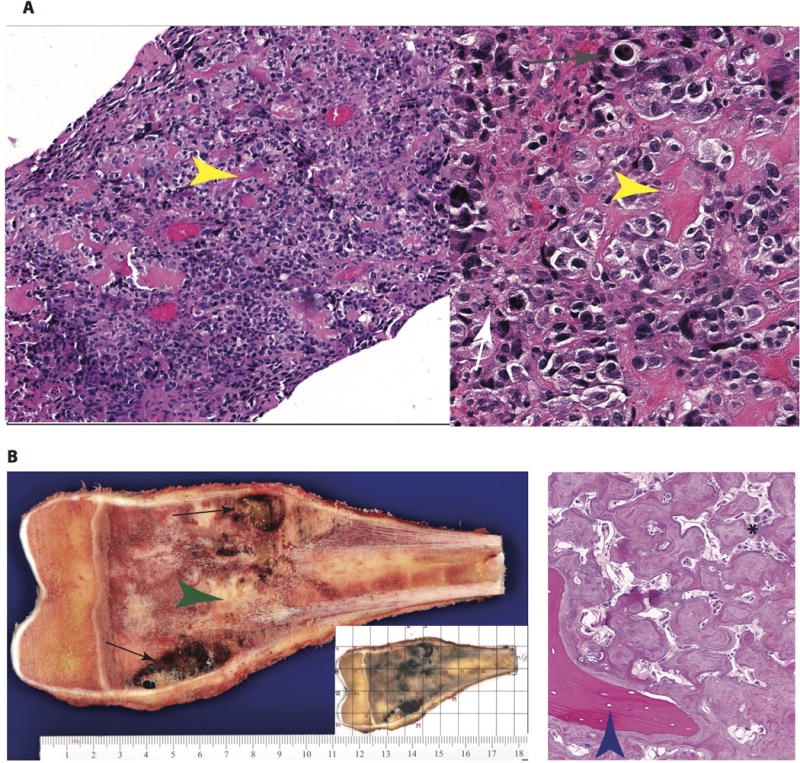
Pathology of a high-grade intramedullary (conventional) osteosarcoma. (a) At low-(100x, left) and intermediate (200x, right) magnification, the diagnostic biopsy reveals a cellular malignancy with conspicuous malignant osteoid formation (yellow arrow). The cellular component is composed of pleomorphic cells with osteoblast-like morphology. Increased mitotic activity (white arrow) and individual cell necrosis (gray arrow) are evidence of rapid cell turnover. (b) Gross images of the resected specimen (left) reveal an expansile mass centered in the medullary cavity with areas of hemorrhage and cystic degeneration (black arrows) and neoplastic bone formation (green arrowhead). In routine processing of these specimens, a complete cross section is blocked out (grid, small inset) and submitted for histologic processing to assess treatment response. The histologic findings in the resection specimen (right) are typical of treated osteosarcoma. The cellularity is markedly diminished, with necrosis and neoplastic bone remaining. The marbled, irregular seams of osteoid have a paucicellular stroma and are distinct in appearance from the native, lamellar bone (blue arrowhead).
Pediatric Oncology – Neoadjuvant Therapy
Chemotherapy is critical to achieve a cure for patients with osteosarcoma, even in those with localized disease, due to the presence of micro-metastases not visible even with modern staging techniques. The longstanding backbone of chemotherapy for the pediatric and adolescent patient includes cisplatin, doxorubicin and high-dose methotrexate with citrovorum rescue. Successful completion of therapy yields a long-term cure in approximately 70% of patients.8,13 Efforts to intensify therapy have, to date, not yielded an improved outcome.27
A port is placed prior to chemotherapy initiation in order to facilitate the frequency and volume of blood draws and drug/fluid infusions. Patients are monitored closely for chemotherapy-related side effects/complications, and dose adjustments are made if needed. Short-term chemotherapy-related toxicity includes hair loss, myelosuppression, chemotherapy-induced nausea and vomiting, and mucositis. The placement of a feeding tube is sometimes necessary to combat the decreased appetite and malnutrition secondary to nausea and vomiting. Long-term toxicity includes hearing loss (cisplatin), cardiac toxicity (doxorubicin), and renal dysfunction (cisplatin). Chemotherapy-induced sterility is a known risk; sperm/oocyte banking is routinely discussed prior to the initiation of chemotherapy.
Commonly, patients receive eleven weeks of neoadjuvant chemotherapy, followed by surgical local control. Chemotherapy begins with cisplatin and doxorubicin, followed by a two week period to recover from the acute toxicity. Subsequently, patients receive weekly high-dose methotrexate for two weeks followed by another round of cisplatin and doxorubicin. This sequence repeats itself until surgery. After neoadjuvant therapy, this patient was noted to have a decrease in soft tissue fullness around the left knee and resolution of pain; while potentially reassuring, there is no data to suggest that “favorable” clinical signs correlate with improved histologic treatment response or a better prognosis. Post-operatively, patients receive eighteen weeks of adjuvant chemotherapy, using the same drugs and cycle timing. There has been no compelling data to suggest that changing the chemotherapy drugs in a patient with <90% tumor necrosis at resection improves prognosis.
Radiology – Treatment Effect Imaging
The radiographic changes with treatment in this case can be seen in Figures 4 and 5. Bone tumors often demonstrate increased ossification and decreased size of the soft-tissue mass after chemotherapy due to bone remodeling and healing (as seen in Figure 4).28 Necrosis, hemorrhage and cystic change can be seen in response to chemotherapy (as seen in Figure 5). After neoadjuvant chemotherapy, a new MRI of the area of tumor is obtained to assess response and to aid operative planning. This patient’s lesion showed increased necrosis, decreased enhancement, decreased size of the associated soft tissue mass, and decreased peritumoral soft-tissue and bone marrow edema (Figure 5). There was no evidence of involvement of the neurovascular bundle.
Figure 4.
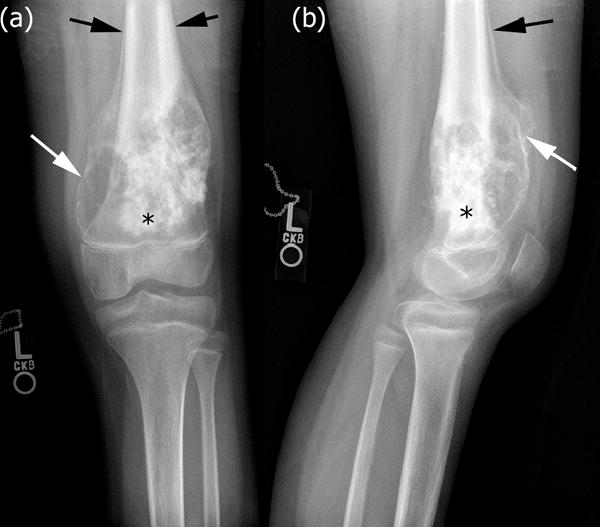
High-grade intramedullary (conventional) osteosarcoma of the left distal femur in a 12-year-old boy after neoadjuvant chemotherapy. (a) Anterior-posterior and (b) lateral radiographs of the left knee demonstrate extensive new bone formation (black asterisk). There is periosteal reaction (black arrows). Note interval increase in periosteal reaction as well as development of mineralization of the periosteum surrounding the soft tissue mass that often occurs with treatment (white arrow).
Figure 5.
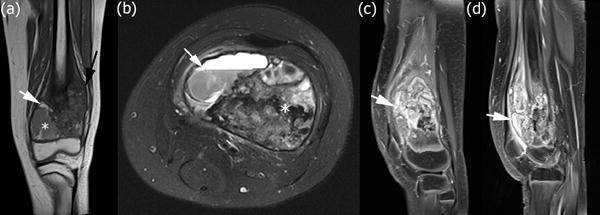
High-grade intramedullary (conventional) osteosarcoma of the left distal femur in a 12-year-old boy with distal femoral osteosarcoma after neoadjuvant chemotherapy. (a) Coronal T1-weighted MRI without fat saturation of the left knee. The lesion shows interval decrease in size of the soft tissue mass (black arrow). There are now new foci of increased T1 signal within the lesion consistent with hemorrhage (white arrow). An area of necrosis with hemorrhage and cystic change is seen in the medial metaphysis (white asterisk). (b) Axial T2-weighted MRI with fat saturation illustrating an area of necrosis with hemorrhage and cystic change with a fluid-fluid level (white arrow). There remains unchanged low T2 signal within the lesion corresponding to malignant bone formation (white asterisk). (c) Sagittal T1-weighted MRI of the left knee obtained after intravenous administration of contrast material reveals interval decrease in size of the soft tissue mass with decreased enhancing peritumoral edema and internal necrosis within the tumor after chemotherapy. (d) Sagittal T1-weighted MRI of the left knee obtained after intravenous administration of contrast material shows internal cystic change within the tumor.
Orthopaedic Oncology – Preoperative Consultation
After neoadjuvant chemotherapy and repeat imaging (standard radiographs and MRI with and without contrast of the affected region), the orthopaedic oncologist meets with the patient for a pre-operative evaluation to discuss surgical resection of the tumor. After neoadjuvant chemotherapy, this patient was pain free and able to ambulate unassisted. He had a firm, nontender left distal thigh mass. His physical exam was notable for decreased knee ROM of 0-105 degrees likely due to deconditioning, as well as decreased strength in his quadriceps (3/5) and hip flexors (4/5).
There are several considerations that impact the planned surgical resection and reconstruction for distal femur osteosarcoma. The patient’s age and projected remaining growth are considered to best optimize function and leg length. The response to chemotherapy and assessment of tumor proximity to the femoral/popliteal neurovascular bundle, the epiphyseal plate, and the adjacent joint are critical. Amputation should be performed if a functional and disease-free limb cannot be achieved otherwise. Limb salvage is possible for treatment of distal femoral osteosarcoma in 90% of cases and is usually not precluded by a pathologic fracture.29 Limb salvage is associated with faster return to ambulation and allows for more efficient ambulation and a more “normal” appearance. There is, however, no overall difference in patient satisfaction or overall survival between limb salvage and amputation.30–32 Limb salvage has an increased incidence of complications and subsequent surgery.31,32
The decision was made to proceed with a limb salvage surgery in this case. For reconstruction of the bone defect, there are different options available depending on the exact location and extent of disease, including: tumor endoprosthesis (expandable vs non-expandable), intercalary graft with supplemental fixation, allograft prosthesis composite (APC), osteoarticular allograft, and rotationplasty. In patients less than 8-years old, an expandable endoprosthesis may be considered; however, due to higher complication rates and greater potential for limb length discrepancy (due to greater skeletal immaturity at time of surgery), a rotationplasty should also be considered. In patients aged 10 to 12 years, expandable prostheses are sometimes considered due to increased need to account for skeletal growth and the complications related to the implant are less in this age group than in younger patients. In patients older than 12 years a traditional modular distal femoral endoprosthesis with or without contralateral epiphysiodesis is often used, as these patients (especially females) are closer to final skeletal maturity. Surgeons can use standard growth charts or bone age films to predict final leg lengths at skeletal maturity, and these tools are helpful in determining whether to use an expandable prosthesis in a young patient. The distal femur + proximal tibia physes can be expected to grow approximately 1.5cm-2.0cm annually in the final years of skeletal growth.33,34 Models for LLD, such as those by Paley35 and Moseley,36 can provide an estimated final LLD.
Limb salvage procedures require detailed discussion of the risks and benefits to the planned procedure. Pediatric patients and their families must be informed of potential risks, including: tumor recurrence, infection, wound complications, delayed healing, injury to surrounding neurovascular structures, implant failure, joint stiffness, limb length inequality, and the possible need for additional procedures. Limb salvage with endoprosthetic reconstruction in adolescents incur the same potential complications as joint replacement in older patients, but there is also an increased risk of infection in tumor cases due to chemotherapy-induced immunosuppresion, an increased risk of prosthetic loosening over time, potential expansion mechanism failure, and a risk for limb length discrepancy as the child grows; patients often will require additional surgeries to address either short- or long-term complications related to the endoprosthesis. Patients and families must also understand that activity restrictions to avoid regular impact loading on the extremity are often recommended after endoprosthetic reconstruction due to the altered anatomy and concerns for implant longevity in a younger patient.
Orthopaedic Oncology – Operative Management
En bloc resection of the distal femur and associated soft-tissue mass was performed with the goal of adequate bone and soft tissue margins based on post-chemotherapy imaging. There is no consensus as to what constitutes a wide resection; however, it is clear that negative resection margins allow a higher chance of local control. Intraoperative frozen sections were sent from the adjacent marrow cavity to demonstrate negative bone margins prior to proceeding with reconstruction.
In this case, the distal femoral reconstruction utilized a cemented modular rotating-hinge expandable distal femur endoprosthesis (Figure 6). The implant inserted was 10mm longer than the resection specimen which allowed acute lengthening of the limb within the limits of sciatic nerve tolerance. Cement creates immediate fixation and allows for early weight-bearing but does not allow for bony ingrowth. A longer stem minimizes lateral bending stress and a larger stem decreases the risk of aseptic loosening. The modularity of the device allows for appropriate re-approximation of the correct limb length. The rotating hinge component reduces the torsional and shear stresses transferred to the cemented stem.
Figure 6.
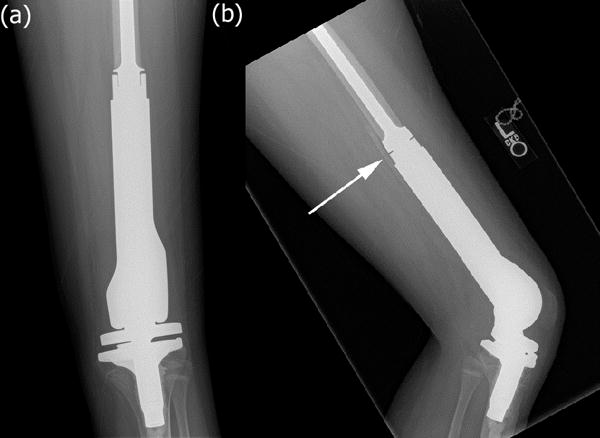
High-grade intramedullary (conventional) osteosarcoma of the distal femur in a 12-year-old boy one year after chemotherapy and surgical resection. (a) Anterior-posterior radiograph of the left knee demonstrates interval resection of the distal femur and reconstruction with a cemented, mobile-bearing, modular, expandable distal femur endoprosthesis. (b) Cross-table lateral radiograph of the left knee demonstrates heterotopic ossification posterior to the femur at the bone prosthesis interface (white arrow).
Modular rotating-hinge endoprostheses have become the standard of care in this setting owing to increased performance; however stem fixation remains controversial. Stem fixation can be achieved via cement fixation, uncemented press-fit fixation or through end compression-based press-fit fixation. There are theoretical advantages and disadvantages to each type of stem fixation with clinical evidence that demonstrates good outcomes for each fixation type. There is no definitive evidence that supports one type of stem fixation over the others.37 Cemented modular rotating-hinge distal femoral endoprostheses have reported long-term survival of approximately 77% at 10-years to 50% at 25-years.38 Early failures are often related to infection, tumor recurrence or mechanical complications, whereas late failures are related to implant fatigue or aseptic loosening.37,39–41
Orthopaedic Oncology – Rehabilitation
Postoperatively in this patient, the goals were immediate weight-bearing and maximum knee ROM. After a 3-5 day inpatient stay, patients are transitioned to home or a rehabilitation facility. In the immediate post-operative period, regaining motion (flexion and extension) is critical. Physical therapists work with the patient daily and the nurses and ancillary staff encourage ambulation and adherence to the protocol. In a 30-year study of cemented modular rotating-hinge distal femoral endoprosthesis, patients achieved a mean flexion of 110°, with a mean extensor lag of 6.9° and passive extension to 1.3°. Flexion contractures were present in 4.3% of patients with a mean of 10°.38
In this case, active knee ROM was 15°–95° with 3/5 strength in the quadriceps and hip flexors two weeks postoperatively. At one-year follow up, the patient was noted to have active knee ROM from 0°–130° and full 5/5 strength in all muscle groups.
Pathology – Surgical Specimen
The pathologist reviews gross and histologic findings in the resection to determine the biologic extent of the tumor, the completeness of the resection and the percentage of tumor necrosis. The gross resection is carefully inspected and the distance of tumor to all margins is documented both on the gross specimen and on the representative histologic sections. A complete slab of the tumor at the point of its greatest cross-sectional area is also processed for histologic examination to determine the amount of remaining viable tumor (Figure 3b, lower right, inset).
In this case, the gross specimen consisted of a solid continuous tumor mass centered within the metaphyseal-diaphyseal junction and extending beyond the confines of the native cortex. There were extensive areas of necrosis and cystic degeneration (Figure 3b, black arrows). The tumor was separated from the proximal resection margin by a segment of viable, normal bone. There was firm, gritty material throughout the tumor consistent with neoplastic bone (Figure 3b, green arrowhead). On histologic sections, the cellularity seen in the biopsy specimen was nearly gone. The tumor was more than 95% non-viable, with extensive mottled, irregular, neoplastic bone left behind as the malignant population died away. The appearance of this abnormal bone was distinct from the residual areas of normal, mature, lamellar bone of the medullary cavity (Figure 3b, blue arrowhead).
Pediatric Oncology – Adjuvant Therapy & Surveillance
Adjuvant chemotherapy begins approximately 2 weeks postoperatively after the incision has healed to minimize chemotherapy-related wound complications. Surgical complications such as wound dehiscence or infection can delay the resumption of adjuvant therapy. Surgical margins and histologic response following chemotherapy are important prognostic factors in osteosarcoma; >90% necrosis in the surgical specimen portends a favorable prognosis, while authors have reported <50% survival for patients with a poor histological response (<90% necrosis).42,43 The addition of etoposide and ifosfamide in the adjuvant setting for patients with a poor histologic response has been evaluated and does not improve overall or event-free survival.44 Inadequate surgical margins lead to a higher risk of local recurrence which is associated with poor prognosis.
Following completion of chemotherapy, patients are monitored closely for local recurrence, metastatic disease, and end-organ toxicity. The lungs are the most common site of osteosarcoma metastasis. Isolated pulmonary metastases can sometimes be treated with surgery alone. Thus, chest CTs are obtained every three months for the first two years off therapy. After that, surveillance occurs every six months until five years post-therapy completion. In the absence of clinical symptoms, imaging for distant disease ceases at the five-year mark, and patients are transitioned to an oncology survivorship clinic. There is no standard regimen for disseminated relapse, and the prognosis for this population is poor.
Radiology – Post-operative Surveillance
Clinical exam and plain radiographs are used for assessment of local recurrence and to assess hardware integrity and alignment. Local recurrence is often noted by increased sclerosis, periosteal reaction, or development of an ossified soft tissue mass.
In this case, routine thin-section chest CT scans are used for surveillance. While chest CTs are more sensitive than chest radiographs for the detection of subcentimeter pulmonary nodules, it is unclear whether this increased sensitivity translates into better clinical outcomes. PET-CT is not routinely used for surveillance due to the relatively high radiation dose.
Orthopaedic Oncology – Post-operative Follow-up
Following distal femoral resection with endoprosthetic reconstruction, patients should be evaluated clinically for assessment of limb function and for tumor surveillance approximately every three months for the first two years and then every six months to one year thereafter. Evaluation of the endoprosthesis can be accomplished via standard radiographs and clinical exam.
Due to this patient’s relative skeletal immaturity and the decision to utilize an expandable endoprosthesis, the patient’s leg lengths are evaluated at regular intervals with lower extremity scanograms. If a LLD is identified and is clinically significant, appropriate treatment should be initiated. For a small (<2cm) LLD, a shoe lift is most appropriate. For a LLD >2cm, surgical management should be considered. Surgical options include lengthening of the expandable prosthesis and/or contralateral epiphysiodesis. Considerations include the overall LLD, remaining growth estimate, and the remaining expandable length of the prosthesis. Long-term survival for distal femoral endoprosthesis has been reported between 20%-70%.39,40,45 There is no long-term data on newer generations of expandable endoprostheses. There is data that expandable prostheses have high complication rates related to aseptic loosening, infection and implant failure.46–48 Patients with certain expandable prostheses may require subsequent procedures to expand the implant, and it may be necessary to exchange the expandable prosthesis for a more durable implant at or near skeletal maturity.41
Conclusion
This case demonstrates the common presentation, evaluation and management of conventional osteosarcoma of the distal femur in a 12-year old boy. The team approach to successful management requires cohesive, coordinated care delivered by members of orthopaedic oncology, pediatric oncology, radiology, pathology, and physical therapy, in addition to the nurses and ancillary staff who help throughout the process. Following initial diagnostic and prognostic workup, this patient underwent a standard course of neoadjuvant chemotherapy. The patient then proceeded with a limb salvage resection and reconstruction using an expandable distal femoral endoprosthesis. Pathologic analysis of the surgical specimen confirmed good therapeutic response and adequate surgical margins, so the patient underwent standard adjuvant chemotherapy. He is currently 18 months out from surgery and undergoing active postoperative surveillance with an excellent functional outcome. He does have a limb length difference of approximately 0.5cm and is being monitored to determine if and when his endoprosthesis will be expanded.
Acknowledgments
Source of Funding:
There was no external source of funding.
References
- 1.Wittig JC, Bickels J, Priebat D, et al. Osteosarcoma: a multidisciplinary approach to diagnosis and treatment. Am Fam Physician. 2002;65(6):1123–1132. [PubMed] [Google Scholar]
- 2.Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006;32(6):423–436. doi: 10.1016/j.ctrv.2006.05.005.. [DOI] [PubMed] [Google Scholar]
- 3.Dahlin DC, Coventry MB. Osteogenic sarcoma. A study of six hundred cases. J Bone Joint Surg Am. 1967;49(1):101–110. [PubMed] [Google Scholar]
- 4.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol Off J Am Soc Clin Oncol. 2002;20(3):776–790. doi: 10.1200/jco.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 5.Bacci G, Ferrari S, Bertoni F, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the istituto ortopedico rizzoli according to the istituto ortopedico rizzoli/osteosarcoma-2 protocol: an updated report. J Clin Oncol Off J Am Soc Clin Oncol. 2000;18(24):4016–4027. doi: 10.1200/jco.2000.18.24.4016. [DOI] [PubMed] [Google Scholar]
- 6.Varan A, Yazici N, Aksoy C, et al. Treatment results of pediatric osteosarcoma: twenty-year experience. J Pediatr Orthop. 2007;27(2):241–246. [PubMed] [Google Scholar]
- 7.Arndt CA, Crist WM. Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999;341(5):342–352. doi: 10.1056/NEJM199907293410507. [DOI] [PubMed] [Google Scholar]
- 8.Chou AJ, Malek F. Orthopaedic Knowledge Update: Musculoskeletal Tumors. 3rd. Rosemont, Illinois: American Academy of Orthopaedic Surgeons; 2014. Osteosarcoma of bone. [Google Scholar]
- 9.Huvos. Bone Tumors: Diagnosis, Treatment, and Prognosis. Philadelphia, PA: Saunders; 1991. Osteogenic Sarcoma; pp. 85–156. [Google Scholar]
- 10.Mirra JM. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Philadelphia, PA: Lea & Febiger; 1989. Osseous Tumors of Intramedullary Origin; pp. 248–438. [Google Scholar]
- 11.Resnick D, Kyriakos M, Greenway GD. Diagnosis of Bone and Joint Disorders. 3rd. Philadelphia, PA: Saunders; 1995. Tumor like diseases of bone: imaging and pathology of specific lesions; pp. 3662–3697. [Google Scholar]
- 12.Murphey MD, Robbin MR, McRae GA, Flemming DJ, Temple HT, Kransdorf MJ. The many faces of osteosarcoma. Radiogr Rev Publ Radiol Soc N Am Inc. 1997;17(5):1205–1231. doi: 10.1148/radiographics.17.5.9308111. [DOI] [PubMed] [Google Scholar]
- 13.Chou AJ, Merola PR, Wexler LH, et al. Treatment of osteosarcoma at first recurrence after contemporary therapy: the Memorial Sloan-Kettering Cancer Center experience. Cancer. 2005;104(10):2214–2221. doi: 10.1002/cncr.21417. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi A, Lewis VO, Satcher RL, Moon BS, Lin PP. What Are the Factors That Affect Survival and Relapse After Local Recurrence of Osteosarcoma? Clin Orthop. 2014;472(10):3188–3195. doi: 10.1007/s11999-014-3759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biermann JS, Adkins DR, Agulnik M, et al. Bone cancer. J Natl Compr Cancer Netw JNCCN. 2013;11(6):688–723. doi: 10.6004/jnccn.2013.0088. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira MP, de A Lima PM, da Silva HJ, de Mello RJV. Neoplasm seeding in biopsy tract of the musculoskeletal system. A systematic review. Acta Ortop Bras. 2014;22(2):106–110. doi: 10.1590/1413-78522014220200422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Praemer A, Furner S, Ricc DP. Musculoskeletal Conditions in the United States. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1992. Neoplasms of bone and connective tissue. [Google Scholar]
- 18.Redmond OM, Stack JP, Dervan PA, Hurson BJ, Carney DN, Ennis JT. Osteosarcoma: use of MR imaging and MR spectroscopy in clinical decision making. Radiology. 1989;172(3):811–815. doi: 10.1148/radiology.172.3.2772193. [DOI] [PubMed] [Google Scholar]
- 19.Schima W, Amann G, Stiglbauer R, et al. Preoperative staging of osteosarcoma: efficacy of MR imaging in detecting joint involvement. AJR Am J Roentgenol. 1994;163(5):1171–1175. doi: 10.2214/ajr.163.5.7976895. [DOI] [PubMed] [Google Scholar]
- 20.Gao Z-H, Yin J-Q, Liu D-W, Meng Q-F, Li J-P. Preoperative easily misdiagnosed telangiectatic osteosarcoma: clinical-radiologic-pathologic correlations. Cancer Imaging Off Publ Int Cancer Imaging Soc. 2013;13(4):520–526. doi: 10.1102/1470-7330.2013.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enneking WF, Kagan A. The implications of “skip” metastases in osteosarcoma. Clin Orthop. 1975;(111):33–41. doi: 10.1097/00003086-197509000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Kneisl JS, Patt JC, Johnson JC, Zuger JH. Is PET useful in detecting occult nonpulmonary metastases in pediatric bone sarcomas? Clin Orthop. 2006;450:101–104. doi: 10.1097/01.blo.0000229329.06406.00. [DOI] [PubMed] [Google Scholar]
- 23.Hurley C, McCarville MB, Shulkin BL, et al. Comparison of (18) F-FDG-PET-CT and Bone Scintigraphy for Evaluation of Osseous Metastases in Newly Diagnosed and Recurrent Osteosarcoma. Pediatr Blood Cancer. 2016;63(8):1381–1386. doi: 10.1002/pbc.26014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheon GJ, Kim MS, Lee JA, et al. Prediction model of chemotherapy response in osteosarcoma by 18F-FDG PET and MRI. J Nucl Med Off Publ Soc Nucl Med. 2009;50(9):1435–1440. doi: 10.2967/jnumed.109.063602. [DOI] [PubMed] [Google Scholar]
- 25.Im HJ, Kim TS, Park S-Y, et al. Prediction of tumour necrosis fractions using metabolic and volumetric 18F-FDG PET/CT indices, after one course and at the completion of neoadjuvant chemotherapy, in children and young adults with osteosarcoma. Eur J Nucl Med Mol Imaging. 2012;39(1):39–49. doi: 10.1007/s00259-011-1936-4. [DOI] [PubMed] [Google Scholar]
- 26.Gerth HU, Juergens KU, Dirksen U, Gerss J, Schober O, Franzius C. Significant benefit of multimodal imaging: PET/CT compared with PET alone in staging and follow-up of patients with Ewing tumors. J Nucl Med Off Publ Soc Nucl Med. 2007;48(12):1932–1939. doi: 10.2967/jnumed.107.045286. [DOI] [PubMed] [Google Scholar]
- 27.Eselgrim M, Grunert H, Kühne T, et al. Dose intensity of chemotherapy for osteosarcoma and outcome in the Cooperative Osteosarcoma Study Group (COSS) trials. Pediatr Blood Cancer. 2006;47(1):42–50. doi: 10.1002/pbc.20608. [DOI] [PubMed] [Google Scholar]
- 28.Ecklund K. Orthopaedic Knowledge Update: Musculoskeletal Tumors. 3rd. Rosemont, Illinois: American Academy of Orthopaedic Surgeons; 2014. Imaging of musculoskeletal tumors: updates and current practice; pp. 13–22. [Google Scholar]
- 29.Ferguson PC, McLaughlin CE, Griffin AM, Bell RS, Deheshi BM, Wunder JS. Clinical and functional outcomes of patients with a pathologic fracture in high-grade osteosarcoma. J Surg Oncol. 2010;102(2):120–124. doi: 10.1002/jso.21542. [DOI] [PubMed] [Google Scholar]
- 30.Rougraff BT, Simon MA, Kneisl JS, Greenberg DB, Mankin HJ. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur. A long-term oncological, functional, and quality-of-life study. J Bone Joint Surg Am. 1994;76(5):649–656. doi: 10.2106/00004623-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Malek F, Somerson JS, Mitchel S, Williams RP. Does limb-salvage surgery offer patients better quality of life and functional capacity than amputation? Clin Orthop. 2012;470(7):2000–2006. doi: 10.1007/s11999-012-2271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ottaviani G, Robert RS, Huh WW, Jaffe N. Functional, psychosocial and professional outcomes in long-term survivors of lower-extremity osteosarcomas: amputation versus limb salvage. Cancer Treat Res. 2009;152:421–436. doi: 10.1007/978-1-4419-0284-9_23. [DOI] [PubMed] [Google Scholar]
- 33.Anderson M, Green WT, Messner MB. Growth and predictions of growth in the lower extremities. J Bone Joint Surg Am. 1963;45-A:1–14. [PubMed] [Google Scholar]
- 34.Green WT, Anderson M. Skeletal age and the control of bone growth. Instr Course Lect. 1960;17:199–217. [PubMed] [Google Scholar]
- 35.Paley D, Bhave A, Herzenberg JE, Bowen JR. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82-A(10):1432–1446. doi: 10.2106/00004623-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Moseley CF. A straight-line graph for leg-length discrepancies. J Bone Joint Surg Am. 1977;59(2):174–179. [PubMed] [Google Scholar]
- 37.Pazionis T, Ghert M. Orthopaedic Knowledge Update: Musculoskeletal Tumors. 3rd. Rosemont, Illinois: American Academy of Orthopaedic Surgeons; 2014. Distal femoral resection and reconstruction; pp. 389–398. [Google Scholar]
- 38.Schwartz AJ, Kabo JM, Eilber FC, Eilber FR, Eckardt JJ. Cemented distal femoral endoprostheses for musculoskeletal tumor: improved survival of modular versus custom implants. Clin Orthop. 2010;468(8):2198–2210. doi: 10.1007/s11999-009-1197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myers GJC, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumours: long-term results. J Bone Joint Surg Br. 2007;89(4):521–526. doi: 10.1302/0301-620X.89B4.18631. [DOI] [PubMed] [Google Scholar]
- 40.Grimer RJ, Aydin BK, Wafa H, et al. Very long-term outcomes after endoprosthetic replacement for malignant tumours of bone. Bone Jt J. 2016;98-B(6):857–864. doi: 10.1302/0301-620X.98B6.37417. [DOI] [PubMed] [Google Scholar]
- 41.Gupta A, Meswania J, Pollock R, et al. Non-invasive distal femoral expandable endoprosthesis for limb-salvage surgery in paediatric tumours. J Bone Joint Surg Br. 2006;88(5):649–654. doi: 10.1302/0301-620X.88B5.17098. [DOI] [PubMed] [Google Scholar]
- 42.Picci P, Bacci G, Campanacci M, et al. Histologic evaluation of necrosis in osteosarcoma induced by chemotherapy. Regional mapping of viable and nonviable tumor. Cancer. 1985;56(7):1515–1521. doi: 10.1002/1097-0142(19851001)56:7<1515::aid-cncr2820560707>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 43.Raymond AK, Chawla SP, Carrasco CH, et al. Osteosarcoma chemotherapy effect: a prognostic factor. Semin Diagn Pathol. 1987;4(3):212–236. [PubMed] [Google Scholar]
- 44.Marina NM, Smeland S, Bielack SS, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol. 2016;17(10):1396–1408. doi: 10.1016/S1470-2045(16)30214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wirganowicz PZ, Eckardt JJ, Dorey FJ, Eilber FR, Kabo JM. Etiology and results of tumor endoprosthesis revision surgery in 64 patients. Clin Orthop. 1999;(358):64–74. [PubMed] [Google Scholar]
- 46.Cipriano CA, Gruzinova IS, Frank RM, Gitelis S, Virkus WW. Frequent complications and severe bone loss associated with the repiphysis expandable distal femoral prosthesis. Clin Orthop. 2015;473(3):831–838. doi: 10.1007/s11999-014-3564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henderson ER, Pepper AM, Marulanda G, Binitie OT, Cheong D, Letson GD. Outcome of lower-limb preservation with an expandable endoprosthesis after bone tumor resection in children. J Bone Joint Surg Am. 2012;94(6):537–547. doi: 10.2106/JBJS.I.01575. [DOI] [PubMed] [Google Scholar]
- 48.Benevenia J, Patterson F, Beebe K, et al. Results of 20 consecutive patients treated with the Repiphysis expandable prosthesis for primary malignant bone. SpringerPlus. 2015;4:793. doi: 10.1186/s40064-015-1582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


