Abstract
Olaratumab (Lartruvo): an innovative treatment for soft tissue sarcoma
INTRODUCTION
Sarcoma comprises about 1% of all adult malignancies and about 15% of all pediatric malignancies, with 7% of pediatric sarcomas characterized specifically as soft tissue sarcoma (STS). Approximately 13,000 newly diagnosed cases of STS and more than 5,000 deaths due to the disease are estimated to occur in the United States in 2018.1
Risk factors associated with STS include prior radiation to the affected area, chemical exposure, and inherited syndromes (e.g., Gardner syndrome, Li-Fraumeni syndrome, neurofibromatosis, retinoblastoma).2,3 Chemicals hypothesized to be associated with STS include chlorophenols, vinyl chloride, arsenic, and phenoxyacetic acids.3
Adult STS is a heterogeneous group of tumors consisting of more than 50 histological subtypes, with about 60% of all cases seen in the extremities and superficial trunk.4 The next most common locations are retroperitoneal and visceral (15%), followed by head and neck (10%).3
First-line treatment for local disease is surgery, followed by radiation. Despite many improvements in control rates, many patients still develop metastatic disease. While local treatment remains the mainstay for limited disease, systemic chemotherapy is used in the treatment of advanced STS. Due to the various histological subtypes, chemotherapeutic regimens yielding the highest response rates should be used.
Several randomized studies have sought to determine whether combination regimens yield benefit over single agents in terms of response rates and overall survival (OS). Doxorubicin is considered to be the most active agent against STS, with a proven response rate of 17% to 21% and OS of 12 to 16.9 months.5–7 After other single agents were compared to doxorubicin and failed to yield any additional benefit, studies aimed to identify combination chemotherapeutic regimens. Despite these efforts, to date no regimen has improved OS beyond doxorubicin monotherapy given at appropriate doses.
Olaratumab (Lartruvo, Eli Lilly) is an immunoglobulin G (Ig) G1 human antibody that received accelerated approval from the Food and Drug Administration (FDA) in 2016 as an innovative treatment for STS. Used in combination with doxorubicin, olaratumab has shown improved OS compared with doxorubicin monotherapy. Olaratumab is indicated for use in combination with doxorubicin to treat adults with STS not amenable to curative treatment with radiotherapy or surgery who have a histological subtype for which an anthracycline-containing regimen is appropriate.8,9
PHARMACOLOGY
Mechanism of Action
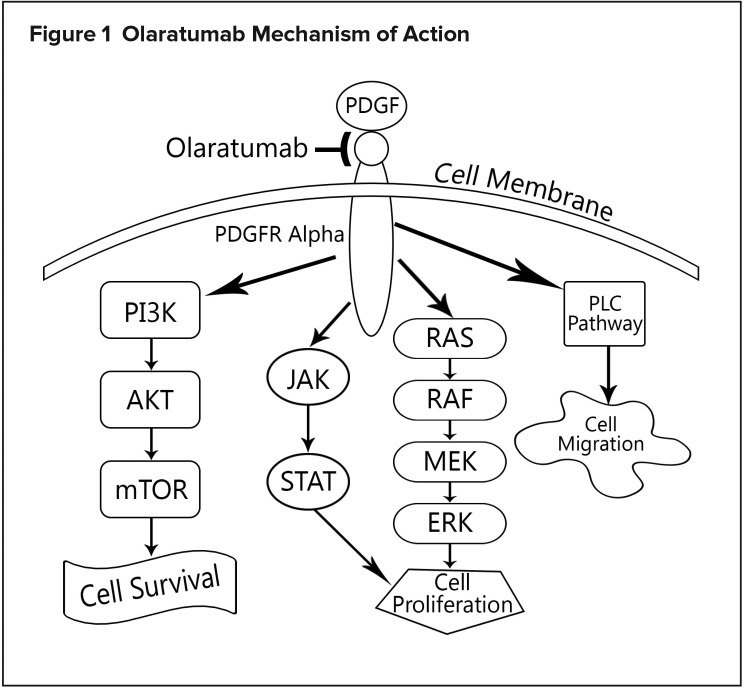
Platelet-derived growth factor alpha (PDGFRα) signaling is involved in multiple processes, such as cell growth, chemotaxis, and mesenchymal stem-cell differentiation. This receptor has been identified as playing a role in STS progression. Olaratumab is an IgG1 human antibody that binds to PDGFRα, preventing binding of receptor-activating ligands that induce pathway signaling.9 Its mechanism of action is depicted in Figure 1.
Figure 1.
Olaratumab Mechanism of Action
Pharmacokinetics
At steady state, the volume of distribution of olaratumab is 7.7 L. The half-life is approximately 11 days (range, 6–24 days), and the mean clearance is 0.56 L per day.9
CLINICAL STUDIES
Phase 1b/2 Trial
Trial 1, a phase 1b/2 study by Tap and colleagues, compared olaratumab in combination with doxorubicin with doxorubicin monotherapy in STS treatment. The primary endpoint for the open-label phase 1b portion was establishing safety, while the primary endpoint in the randomized phase 2 trial was progression-free survival (PFS). Secondary endpoints included OS, objective response rate (ORR), safety, and pharmacokinetics.10
Eligible patients were 18 years of age or older and had a histologically confirmed diagnosis of locally advanced metastatic STS not previously treated with an anthracycline, available tumor tissue to determine PDGFRα expression, and an Eastern Cooperative Oncology Group performance status of 0 to 2. Exclusion criteria included confirmed diagnosis of Kaposi’s sarcoma, untreated central nervous system metastases, therapy with any drug targeting PDGFR, previous anthracycline treatment, prior radiation therapy to the mediastinal or pericardial area, concurrent anticancer therapy, use of an investigational agent within four weeks of study entry, a history of specified cardiac problems, human immunodeficiency virus, pregnancy, and current lactation.10
In phase 2, 131 patients were randomized 1:1 to receive treatment with intravenous (IV) olaratumab 15 mg/kg on days 1 and 8 plus doxorubicin 75 mg/m2 on day 1 of each 21-day cycle or doxorubicin alone on the same dosing schedule. Each group was treated for up to eight cycles, after which patients in the olaratumab/doxorubicin group (n = 64) were allowed to continue receiving olaratumab on days 1 and 8 of each 21-day cycle until disease progression or unacceptable toxicity. Patients in the doxorubicin-only group (n = 65) were observed after completion of the first eight cycles and were allowed to receive olaratumab as monotherapy if there was documented disease progression.10
The protocol-defined significance level was set at 0.1999. Survival was assessed every two months until study completion, resulting in a median PFS of 6.6 months in the olaratumab/doxorubicin group and 4.1 months in the doxorubicin-only group (P = 0.0615). In a blinded independent retrospective review, median PFS was 8.2 months in the olaratumab/doxorubicin group and 4.4 months in the doxorubicin-only group (P = 0.1208).10
Median OS was 26.5 months in the olaratumab/doxorubicin group and 14.7 months in the doxorubicin-only group (P = 0.0003); the results were consistent across all subgroups. The ORRs for olaratumab/doxorubicin and doxorubicin alone were reported as 18.2% and 11.9%, respectively (P = 0.3421), but were changed after independent assessment to 18.2% and 7.5% (P = 0.0740).10
The most common adverse events (AEs) of any grade that occurred in the olaratumab/doxorubicin group were nausea, fatigue, neutropenia, and mucositis; the most common in the doxorubicin-only group were fatigue, nausea, alopecia, and neutropenia. The most common AE leading to discontinuation of doxorubicin was a decrease in left ventricular ejection fraction, which occurred in three of 64 patients (5%) treated with olaratumab plus doxorubicin and four of 64 patients (6%) who received doxorubicin monotherapy. Discontinuation of olaratumab primarily was attributed to infusion-related reactions, which occurred in two of 64 patients (3%).10
ANNOUNCE Trial
The ongoing phase 3 ANNOUNCE trial is enrolling an estimated 460 patients to assess a primary outcome of OS for doxorubicin plus olaratumab compared with doxorubicin plus placebo in participants with advanced or metastatic STS. The experimental arm will receive 75 mg/m2 of IV doxorubicin on day 1 of each 21-day cycle for eight cycles, plus 20 mg/kg of IV olaratumab on days 1 and 8 of cycle 1 and 15 mg/kg of IV olaratumab on days 1 and 8 of cycles 2 to 8. After completion of cycle 8, participants will receive 15 mg/kg of olaratumab on days 1 and 8 of each 21-day cycle until disease progression or discontinuation. The control arm will receive 75 mg/m2 of IV doxorubicin on day 1 of each 21-day cycle for up to eight cycles, plus placebo on days 1 and 8 of each cycle. After cycle 8, the patient will receive placebo on days 1 and 8 of each cycle until disease progression or discontinuation.11
Inclusion and exclusion criteria for this study are similar to those in the phase 1b/2 trial. However, this study also specifies that patients must have a left ventricular ejection fraction of at least 50% and excludes patients with gastrointestinal stromal tumors (GISTs), as well as Kaposi’s sarcoma. The study is expected to conclude in February 2019.11
ANNOUNCE-2 Trial
Olaratumab is also being studied in other combinations. ANNOUNCE-2 is an open-label phase 1b and randomized, double-blind phase 2 study evaluating gemcitabine and docetaxel with or without olaratumab for the treatment of advanced STS. This trial is expected to enroll 211 patients, with the primary endpoint for phase 1b being identification of an olaratumab dose for phase 2, and the phase 2 primary endpoint being OS. In the dose-escalation phase, patients will receive IV olaratumab 15 mg/kg or 20 mg/kg on days 1 and 8, IV gemcitabine on days 1 and 8, and IV docetaxel on day 8 of each 21-day cycle. In phase 2, the experimental group will receive the same regimen using the olaratumab dose determined in phase 1b. The placebo comparator arm will receive placebo on days 1 and 8, gemcitabine IV on days 1 and 8, and docetaxel IV on day 8 of each 21-day cycle.12
Patients in this trial are precluded from having received more than two prior lines of systemic therapy (not including neo-adjuvant and adjuvant therapies). Patients diagnosed with GISTs or Kaposi’s sarcoma will be excluded. This study is expected to be completed in August 2020.12
Trials in Other Malignancies
Olaratumab has been studied in other malignancies. In a phase 1 trial, 16 Japanese patients with advanced solid tumors, including colorectal, gastric, gastrointestinal stroma, head and neck, and sarcoma, were split into three cohorts. Cohort 1 (n = 3) received IV olaratumab 10 mg/kg on days 1 and 8 every three weeks, cohort 2 (n = 7) received IV olaratumab 20 mg/kg every two weeks, and cohort 3 (n = 6) received IV olaratumab 15 mg/kg on days 1 and 8 every three weeks. The primary objectives were to establish olaratumab’s safety and pharmaco kinetic profile. The disease control rates for cohorts 1, 2, and 3 were 66.7%, 42.9%, and 33.3%, respectively, while the median duration of disease stabilization was 2.8 months, 2.8 months, and 4.9 months, respectively. The half-life of olaratumab ranged from 4.42 to 9.38 days following the first dose and 4.06 to 8.83 days after multiple doses. The most common olaratumab-related AEs were proteinuria (25%) and increased aspartate aminotransferase (12.5%). These effects were consistent across all three cohorts, indicating that they were not dose related.13
An ongoing phase 1, open-label, dose-escalation study is comparing olaratumab monotherapy with olaratumab in combination with doxorubicin, vincristine/irinotecan, or high-dose ifosfamide in pediatric patients with relapsed or refractory solid tumors. The estimated completion date is June 2019.14
Olaratumab is also being studied in Japanese patients with advanced cancer. The study is separated into Part A and Part B. Part A has two cohorts; cohort 1 is to receive IV olaratumab on days 1 and 8 plus 25 mg/m2 of IV doxorubicin on days 1, 2, and 3 of each 21-day cycle. Cohort 2 is to receive IV olaratumab on days 1 and 8 plus 75 mg/m2 of IV doxorubicin on day 1 of every 21-day cycle. Part B consists of one cohort that is to receive IV olaratumab as monotherapy on days 1 and 8 of each 21-day cycle. Part A is evaluating the number of participants with serious AEs related to the study drug, while Part B will evaluate concentrations of olaratumab. The estimated study completion at the time of writing was March 2018; data were not yet published.15
ADVERSE DRUG REACTIONS
The AEs reported here relate to Trial 1, the phase 1b/2 study comparing olaratumab plus doxorubicin with doxorubicin alone. The median exposure to olaratumab was six months. AEs leading to treatment discontinuation occurred in five patients (8%) in the olaratumab/doxorubicin group; the most common was infusion-related reactions in two patients (3%). Infusion-related reactions of any grade occurred in eight patients (13%) in the olaratumab/doxorubicin group and none in the doxorubicin-only group. The most common AEs of any grade occurring in 20% or more of patients in the olaratumab/doxorubicin group were nausea, fatigue, neutro penia, mucositis, and alopecia (Table 1). The most common AEs of grade 3–4 occurring in 5% or more of patients in the olaratumab/doxorubicin group were fatigue (9%), neutropenia (53%), febrile neutropenia (13%), and infections and infestations (8%).10
Table 1.
Adverse Events of Any Grade in Phase 1b/2 Clinical Trial10
| Adverse Event | Treatment Regimen | |
|---|---|---|
| Olaratumab/Doxorubicin* (n = 64) | Doxorubicin Alone (n = 65) | |
| Nausea | 47 (73%) | 34 (52%) |
| Fatigue | 44 (69%) | 45 (69%) |
| Musculoskeletal pain | 41 (64%) | 16 (25%) |
| Neutropenia | 37 (58%) | 23 (35%) |
| Mucositis | 34 (53%) | 23 (35%) |
| Alopecia | 33 (52%) | 26 (40%) |
| Vomiting | 29 (45%) | 12 (18%) |
| Infections and infestations | 27 (42%) | 27 (42%) |
| Anemia | 26 (41%) | 24 (37%) |
| Leucopenia | 26 (41%) | 12 (19%) |
| Diarrhea | 22 (34%) | 15 (23%) |
| Constipation | 22 (34%) | 21 (32%) |
| Decreased appetite | 20 (31%) | 13 (20%) |
| Abdominal pain | 15 (23%) | 9 (14%) |
| Pyrexia | 15 (23%) | 12 (19%) |
| Cardiac dysfunction | 15 (23%) | 11 (17%) |
The most common AEs of any grade occurring in 20% or more of patients in the olaratumab/doxorubicin group.
DOSAGE AND ADMINISTRATION
The FDA-approved dosing for STS patients is 15 mg/kg of IV olaratumab on days 1 and 8 every three weeks, in combination with doxorubicin, for eight cycles. Subsequently, olaratumab should be continued as monotherapy until disease progression or unacceptable toxicity. Premedication with diphen-hydramine (25–50 g IV) and dexamethasone (10–20 mg IV) should be given on day 1 of cycle 1 prior to administration of olaratumab.9
WARNINGS AND PRECAUTIONS
Patients should be monitored during the administration of olaratumab for infusion-related reactions, including flushing, shortness of breath, bronchospasm, and fever/chills, and, in severe incidences, severe hypotension, anaphylactic shock, or cardiac arrest. Of 485 patients in clinical trials who received at least one dose of olaratumab, 70 (14%) experienced an infusion-related reaction. Of these 70, 68 (97%) experienced their occurrence during the first or second cycle, 11 (2.3%) experienced a grade 3 or worse reaction, and one patient died (0.2%).9
Dose Modifications
Olaratumab should be permanently discontinued if a patient experiences grade 3 or 4 infusion-related reactions. If the patient experiences grade 1 or 2 infusion-related reactions, olaratumab should be held until resolution and then restarted at 50% of the initial infusion rate. In the case of neutropenic fever/infection or grade 4 neutropenia lasting longer than one week, olaratumab should be discontinued until the absolute neutrophil count is at least 1,000/mcL and then resumed at a permanently reduced dose of 12 mg/kg.9
Use in Specific Populations
Pregnancy and Lactation
Based on animal tests and pharmacology, olaratumab can cause fetal harm, although there are no definitive data about its use in pregnancy. Women of reproductive age are advised to use contraception to prevent pregnancy for the duration of treatment and for three months following the last dose.9
There are no data regarding the presence of olaratumab in breast milk, its effects on milk production, or on the breastfed infant. Due to the potential risks associated with olaratumab, mothers should be advised not to breastfeed while on olaratumab and for three months following the last dose.9
Pediatric and Geriatric Patients
The safety and effectiveness of olaratumab in the pediatric population have yet to be established. Clinical studies have not included a sufficient number of patients 65 years of age or older to determine a difference in response compared with younger patients.9
Renal and Hepatic Impairment
There is no clinically important effect on olaratumab in patients with mild-to-moderate renal impairment (creatinine clearance, 30–89 mL/min) or mild-to-moderate hepatic impairment. The clinical effects of severe renal or hepatic impairment on olaratumab are unknown.9
Drug–Drug Interactions
Olaratumab has not been evaluated in formal drug interaction studies. There were no clinically relevant changes in patient exposure when olaratumab 15 mg/kg and doxorubicin 75 mg/m2 were administered together in patients with solid tumors.9
COST
Olaratumab is supplied as a sterile, preservative-free, clear to slightly opalescent, and colorless to slightly yellow solution.9 It is available in 19-mL and 50-mL vials containing 10 mg/1 mL with average wholesale prices of $1,098 and $2,889, respectively.16 Patients unable to afford therapy may be eligible for financial assistance through Lilly PatientOne, a reimbursement program for qualifying Lilly Oncology products.17
P&T COMMITTEE CONSIDERATIONS
Olaratumab is the first agent approved for use in combination with doxorubicin that has shown an OS improvement in STS patients. With its unique mechanism of action and acceptable toxicity profile, olaratumab is a novel treatment option for STS. When adding it to their formularies, hospitals are encouraged to consider restricting its use to hematology/oncology prescribing for indications approved by the FDA.
Olaratumab has an added benefit of being used in the outpatient setting, potentially reducing the number of hospital admissions due to treatment requirements.
CONCLUSION
Olaratumab in combination with doxorubicin received accelerated FDA approval based on its OS benefit in a phase 1b/2 trial, and continued approval may be contingent on results of confirmatory trials. Doxorubicin has been studied with many other therapeutic agents in STS, but none has previously shown improvement in OS. Due to this novel improvement, olaratumab is being studied in other malignancies. With its unique mechanism of action and acceptable toxicity profile, this breakthrough treatment option has the potential to change the treatment paradigm of STS.
Footnotes
Disclosure: The authors report no commercial or financial interests in regard to this article.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2017;687:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Penel N, Grosjean J, Robin YM, et al. Frequency of certain established risk factors in soft tissue sarcomas in adults: a prospective descriptive study of 658 cases. Sarcoma. 2008;2008:459386. doi: 10.1155/2008/459386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pisters PWT, Weiss M, Maki R, Raut CP. Soft tissue sarcomas. Jun 2, 2016. [Accessed March 20, 2018]. Available at: www.cancernetwork.com/cancer-management/soft-tissue-sarcomas.
- 4.Fletcher CDM, Unni KK, Mertens F, editors. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002. pp. 12–18. [Google Scholar]
- 5.Ryan CW, Merimsky O, Agulnik M, et al. PICASSO III: A phase III, placebo-controlled study of doxorubicin with or without palifosfamide in patients with metastatic soft tissue sarcoma. J Clin Onc. 2016;34:3898–3905. doi: 10.1200/JCO.2016.67.6684. [DOI] [PubMed] [Google Scholar]
- 6.Borden EC, Amato DA, Edmonson JH, et al. Randomized comparison of doxorubicin and vindesine to doxorubicin for patients with metastatic soft-tissue sarcomas. Cancer. 1990;66:862–867. doi: 10.1002/1097-0142(19900901)66:5<862::aid-cncr2820660509>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 7.Santoro A, Tursz T, Mouridsen H, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1995;13:1537–1545. doi: 10.1200/JCO.1995.13.7.1537. [DOI] [PubMed] [Google Scholar]
- 8.Food and Drug Administration. Olaratumab (Lartruvo) Oct 20, 2016. [Accessed March 1, 2018]. Available at: www.fda.gov/Drugs/Information-OnDrugs/ApprovedDrugs/ucm526087.htm.
- 9.Lartruvo (olaratumab) prescribing information. Indianapolis, Indiana: Eli Lilly and Company; Oct, 2016. [Google Scholar]
- 10.Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomized phase 2 trial. Lancet. 2016;388:488–497. doi: 10.1016/S0140-6736(16)30587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ClinicalTrials.gov. A study of doxorubicin plus olaratumab (LY3012207) in participants with advanced or metastatic soft tissue sarcoma (ANNOUNCE). NCT02451943. May, 2015. [Accessed April 17, 2017]. Available at: https://clinicaltrials.gov/ct2/show/NCT02451943.
- 12.ClinicalTrials.gov. A study of olaratumab (LY3012207) in participants with advanced soft tissue sarcoma. NCT02659020. Mar, 2017. [Accessed April 17, 2017]. Available at: https://clinicaltrials.gov/ct2/show/NCT02659020?term==olaratumab&rank==4.
- 13.Doi T, Ma Y, Dontabhaktuni A, et al. Phase I study of olaratumab in Japanese patients with advanced solid tumors. Cancer Sci. 2014;105:862–869. doi: 10.1111/cas.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ClinicalTrials.gov. A study of olaratumab alone and in combination with standard chemotherapies in children with cancer. NCT02677116. Apr, 2017. [Accessed April 17, 2017]. Available at: https://clinicaltrials.gov/ct2/show/NCT02677116.
- 15.ClinicalTrials.gov. A study of olaratumab in Japanese participants with advanced cancer. Feb, 2017. [Accessed March 20, 2018]. NCT02377752. Available at: https://clinicaltrials.gov/ct2/show/NCT02377752.
- 16.Red Book Online. Ann Harbor, Michigan: Truven Health Analytics; [Accessed March 20, 2018]. [Google Scholar]
- 17.Eli Lilly and Company. Financial assistance for cancer patients. May, 2017. [Accessed May 25, 2017]. Available at: http://lillypatientone.com/financial-assistance-for-cancer-patients.html.