Abstract
Lower urinary tract function is mainly assessed by means of cystometric bladder function analysis in rodents. Conventional cystometries are usually performed as terminal analysis under urethane anesthesia. It is well known that anesthetic drugs can influence bladder function. Hence, the aim of this technique is to perform cystometric measurements of the urinary bladder and external urethral sphincter in lightly restrained awake rats. For this purpose, a bladder catheter is implanted into the bladder dome. Subsequently, two electrodes are implanted bilateral to the external urethral sphincter and a ground electrode is sutured to a non-responsive skeletal muscle. The bladder catheter and the three electrodes are finally tunneled subcutaneously to the neck region and affixed to a harness. With this technique, the lower urinary tract can be measured at multiple time points in the same animal to assess lower urinary tract function. The main application of this technique is the follow-up of simultaneous urinary bladder and external urethral sphincter function in awake healthy rats and after induction of a disease or injury. Moreover, subsequent lower urinary tract monitoring can be performed during evaluation of the disease/injury and to monitor treatment efficacy.
Keywords: Physiology, Issue 131, Cystometry, rodents, awake, urinary bladder, external urethral sphincter, permanent implantation
Introduction
To analyze urinary storage and voiding function and dysfunction, most studies have used rodent models. Through sequential activation of reflexes, micturition is produced. The coordination of these reflexes is essential for efficient voiding1. Cystometric recording techniques provide valuable tools for analyzing the urinary bladder function under its neural control1.
Most conventional cystometries in rats are done as a single, final analysis in anesthesia, mainly urethane2, and focus on the urinary bladder solely. However, in some pathologies like neurogenic lower urinary tract dysfunction (NLUTD), not only the urinary bladder, but also the bladder outlet, the external urethral sphincter, is dysfunctional3,4. This makes NLUTD difficult to follow-up, if only the bladder is examined in a single cystometric measurement. To get reliable results that are comparable to humans, it is essential to accurately measure both the urinary bladder and the external urethral sphincter function and its interactions2. Furthermore, it is crucial to perform functional analyses in awake rats as anesthesia is very likely to alter bladder function2,5,6. A good cystometric recording in awake animals is the basis for the identification of bladder function and malfunction7.
The small animal cystometry station used (e.g., Catamount cystometry station (CCS)) is a unit to perform cystometric analyses in small awake animals8. By means of a permanent bladder catheter and implanted external urethral sphincter electrodes, repetitive measurements can be performed over a longer time periods2. Thus, the CCS provides a valuable tool for non-neurogenic and NLUTD evaluations in the rodent model, in which the pathomechanisms can change during short- or medium-term follow-up. Additionally, this method includes an artefact-reduced cystometric analysis by using a restrainer to conduct bladder measurements in awake rats.
In this paper, we describe the surgical approach to permanently implant a bladder catheter and external urethral sphincter electrodes, along with cystometric measurements in awake rats.
Protocol
All procedures described here were approved by the Austrian Governmental Ethics committee for animal research (Bundesministerium für Wissenschaft, Forschung und Wirtschaft, WF / V /3b) and were in compliance with the Association for Assessment of Laboratory Animal Care guidelines for animal use. Rats used for this approach were female, 12-week-old Lewis rats. Use sterile instruments throughout the protocol.
1. Material Preparation
- Manufacturing of the Catheter
- Cut the catheter (polyethylene tubing PE-50) in appropriate length (20 to 25 cm) to fit the size of the animal. NOTE: Leave some extra length for tunneling and easier handling.
- Flare one end up with a lighter to get a rounded tip. Check for final appropriate opening and blunt end of the catheter.
- Place a 2-mm long silicone tube over the catheter until it lies just below the flared end.
- Manufacturing of the Electrodes
- Prepare a polytetrafluoroethylene-coated steel wire of 20-25 cm (appropriate length depending on the size of the animal).
- Prepare a silver wire 2 cm in length and twist the ends until a small loop remains. Strip off 2 mm of the Teflon insulation at one end. Solder the twisted silver wire ending to the stripped steel wire.
- Apply conventional nail polish at the coating zone. Prepare 4-mm long polyethylene tubing over the coated region, and seal the end by applying heat to the compressing forceps.
2. Animal Preparation
- Anesthesia
- Use an appropriate anesthesia cocktail approved by the institution for a general anesthesia.
- Prepare anesthesia cocktail with medetomidine (0.15 mg/kg), midazolam (0.08 mg/kg), and fentanyl (0.01 mg/kg), and inject intramuscularly by a 1 mL syringe with a 27 Gauge needle.
- Surgical Preparation
- Shave the abdomen with an electrical razor, including the genital region and back region at the level of the scapulae.
- Disinfect abdominal and neck regions with 70% ethanol first and then 3 alternating scrubs with povidone-iodine solution.
3. Bladder Catheter Implantation
Perform a low mid-line laparotomy at level of the third and fourth teat (approximately 2 to 2.5 cm in length) by using a scalpel for the skin and a surgical scissor for the abdominal muscle.
Expose the bladder by guiding the abdominal wall in the cranio-caudal direction and fix it in this position by placing the back of a forceps behind the bladder to avoid re-positioning.
Place a purse-string suture around the bladder dome using a 6-0 non-absorbable monofilament suture with a taper-tip needle.
Incise the bladder dome inside the purse-string, either by scalpel tip or 18G needle, to insert the bladder catheter (see Protocol step 1).
Insert the catheter prefilled with the sterile physiological 0.9% sodium chloride solution and carefully retract bladder catheter until flared opening of the catheter is positioned just below bladder dome.
Secure the purse-string suture around the catheter and make a stop-suture around catheter body for further fixation.
Check for leakage via the bladder dome by slowly filling the bladder through the catheter with sterile physiological 0.9% sodium chloride solution.
4. Urethral Sphincter Electrode Implantation
Prepare three electrodes for implantation (see Protocol step 1).
Mark one electrode with a colored permanent felt pen for further identification of the future null electrode. Disinfect electrodes with 70% ethanol. NOTE: The catheters are not suitable for heat and chemical sterilization procedures. Instead cold sterilize the electrodes for 24 h.
Extend the abdominal incision by surgical scissors up to the pubic bone, but do not cut the pubic symphysis.
Identify the urethra and create a blunt pocket by using fine forceps on both sides of the urethra, but avoid trauma of vessels or nerves.
Identify appropriate fat pouches close to the urethra within this window.
Fix electrodes bilaterally to the appropriate fat pouch by using the 6-0 non-absorbable monofilament suture. NOTE: The final position of the electrodes should be bilateral at the mid region of the urethra.
Tie the two electrodes together with 6-0 non-absorbable monofilament suture.
Suture the marked null electrode to the abdominal wall muscle within distance of the urethra. Tie all three electrodes together with a single suture.
5. Tunneling
Make a small skin incision between the scapulae for tunneling.
Tunnel electrode wires to the neck and do a final check for correct placement, and verify the position of electrodes after tunneling.
Tunnel bladder catheter to the scapulae level - pay attention to the bladder while tunneling the catheter to avoid bladder twisting. For this purpose, hold the catheter just before entering the bladder dome to avoid twisting of the bladder.
Close abdominal muscles by continuous or interrupted stitches with a 4-0 polyfilament absorbable suture. Close the skin incision by interrupted mattress sutures.
Stretch the animal to its full length to have maximal distention of the electrode wires and catheter.
Fix catheter and electrode wires by a sunk-in suture to the shoulder muscle.
Close the skin by a single stitch with the 4-0 suture.
6. Harness Fitting
Fit a harness to the animal by pulling the harness over the head of the animal and pull the forelimbs to an end position between the two silicone strips. Check the harness size by the weight of the animal prior to the surgery with the distributor.
Tunnel the bladder catheter to the central hole of the harness and electrodes through the custom-made hole by a drill adjusted to the size of the electrode wires.
Adjust the harness by pulling the silicone strips. Adjust the harness so it is not too loose, but ensure some space remains to maintain the moving ability of the rat.
Use a cable strap to fix silicon strips.
Cut the length of the bladder catheter to 3 cm above the harness, connect to the 23-G stopper, and finally fix it to the harness.
7. Manufacturing of the Electrode Connector
Prepare three small heat shrink tubes (a different color for the null electrode). Prepare two further heat shrink tubes in an appropriate larger size.
Shorten the length of the electrode wires to an optimal length to be able to plug in later to the female plug without being too loose or too short. Strip off the Teflon insulation of the three wires (approximately 2 mm), and twist the steel wires to a string.
Place large heat shrink tubes over all wires, place the small tubes individually for all three wires, and use the colored tube for the null electrode.
Solder the electrodes to the male 3-connection-plug. Place the null electrode in the middle, and shrink the three small individual tubes above soldered area.
Shrink the first of the larger tubes at the end of the individual tube endings and the final large tube to the male plug border.
Connect the male plug to the female connector fixed to the harness, and fix with a piece of tape for further safety.
8. Post-surgery Care
Clean surgical areas and disinfect with povidone-iodine.
Place the animal on a heating pad until awake and give 0.9% sodium chloride solution for water substitution, based on the local animal care guidelines during and after surgery.
Administer analgesics (meloxicam 1 mg/kg) and antibiotics (sulfadoxinum 200 mg, trimethoprimum 40 mg, 15 mg/kg) as a combined injection solution subcutaneously. Twice daily, give analgesics (morning and evening), and once per day give antibiotics, for five subsequent days.
Continue with antibiotics at the same dosage over the whole follow-up period at two to three injections per week.
Check daily for appropriate fitting of the harness and perform a surgical field inspection, especially of the neck region. Adjust the harness if it gets too tight by carefully pulling at the silicone strips.
Flush the catheter regularly once per week to avoid a blockade.
9. Preparation for Cystometric Measurement
Perform the first cystometric measurements after six post-operative days. NOTE: Earlier measurements can be influenced by analgesic medication and/or urothelial irritation due to the catheter implantation.
Turn on the main switch, the computer, and the EMG amplifier.
Fill the syringe pump with room temperature warmed 0.9% sodium chloride. Open the three-way connectors one after the other in a descending manner, starting from the pump, and flush the tubes. NOTE: Check carefully for remaining air bubbles, as bubbles will alter the cystometric measurements.
10. Calibration
Start the program uroflowmeter software and go to Calibration.
Close the three-way connector to the pump and animal.
Open the valve to the connected manometer and press zero in the program.
Adjust pressure at the manometer to 100 mmHg, and press 100 mmHg button in the program. Press confirm to save the calibration adjustment. The window will close automatically.
Close the valve to the manometer and open the three-way connectors from the pump to the animal to be ready for the measurement.
11. Animal Database (Animal DB)
To register animals, give the animal a concise ID.
Enter further data, such as date of SCI, start of treatment, date of catheter implantation, experimental group, and the birthdate of the animal.
To finally log the data, press "store record" and save to file.
12. Measurement Settings Prior to Recording
Start the software program by pressing the start button.
Select the animal and press tare scale and zero pressure. NOTE: Pay attention while placing the animal into the restrainer, such that no cable is stuck, as this could lead to a removal of a wire of the EMG cable.
13. Animal Preparation
Remove the catheter plug and disconnect the EMG plug from the harness.
Put the rat in the restrainer. Close the restrainer and lock the harness with a clamp.
Get the catheter and EMG plug out of the restrainer.
Place the restrainer in the catamount unit. Put the tail through the tube and fix the tube with tape to avoid movements.
Connect the male EMG plug to the recording female EMG connector.
Press zero pressure once more and then connect the catheter to the filling/recording tube. Check the pressure in the software. NOTE: Pressure should be positive, around 5-10 cm H2O at baseline. If the pressure is negative, disconnect catheter from the cannula, press zero pressure, and then reconnect the catheter.
14. Recording
If the catheter and EMG cable are connected, press run pump and recording.
Adapt the filling speed to the experimental needs in μL/min.
Note the time the recording starts and the room temperature in the log book.
To stop the cystometric recording, stop the pump by pressing run pump and press recording once again. NOTE: The pump will be off and the green light at the pump is off.
Disconnect the catheter and use the 23G stopper plug to close the catheter end. Disconnect the EMG cable.
Open the front of the restrainer and guide the rat out of the restrainer. NOTE: Handle rat carefully, and monitor the wires to avoid any locking of cables.
Plug the catheter back into the harness. Re-plug the EMG plug to the harness, and put a piece of tape around the plug. NOTE: If the animal has a pathological lower urinary tract condition, express the bladder at the end manually to avoid overdistention of the bladder.
Put the animal back into its home cage.
Close the program by pressing exit.
Clean the restrainer and beaker.
Close the three-way connectors to the pump and the animal outlet.
Shut down the computer, the EMG unit, and the system via the main switch. NOTE: Data is saved in the separate folder "Logged data" with the subfolder file of the measured rat ID. Single recordings are sorted in the single rat ID folders by date.
Representative Results
A schematic showing the process of awake cystometric measurements is presented in Figure 1 and the internal anatomy for bladder catheter implantation is shown in Figure 2. Surgery takes about 2 h. Postoperative analgesia and antibiotics, as described in the protocol, cover pain and infections over five days after surgery. No signs of any pain were noticed thereafter. Twice daily careful inspection of the abdomen, abdominal suture, and the neck suture is necessary to maintain the animal's health. Harness control (position and tightness) should be conducted once daily in the first five days and later on a regular basis. Abdominal sutures can be removed at the 10th post-operative day.
We use soft, non-woody bedding for the first 10 days after surgery to avoid inflammation. Bedding is changed at least twice a week to further lower the risk of inflammation. Animals are kept in single housing, as group housing increases the risk of harness, catheter, or electrode cable biting by cage mates.
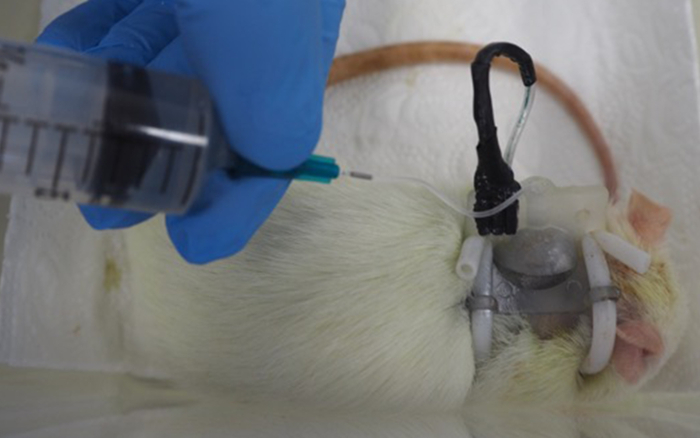
The catheter should be flushed at least once per week, either in the course of a cystometric measurement or by flushing the catheter manually with 1-3 mL of sterile 0.9% sodium chloride at a low infusion speed (Figure 3). Regular antibiotic coverage of the animals further reduces the risk of infections and urinary stone formation. Monitoring fluid uptake is a further major point to prevent urinary stone formation. Citric acid in low concentrations (2-3%) is administered either intravesically via the catheter or into the drinking water to prevent calculi formation.
The success rate of the surgical procedure, as well as maintaining the bladder catheter and electrodes intact, is around 80%. In the remaining 20% of cases, the main problem was detachment of the electrode wires from the plug. Thus, a careful attachment of the electrode wires to the harness is crucial to avoid electrode loss.
Cystometric measurements are usually done until three consecutive voiding cycles are recorded per measurement, which takes between 20 to 40 min, depending on the anxiety and handling status of the rat. The first cystometric measurement is usually done one week after catheter implantation surgery.
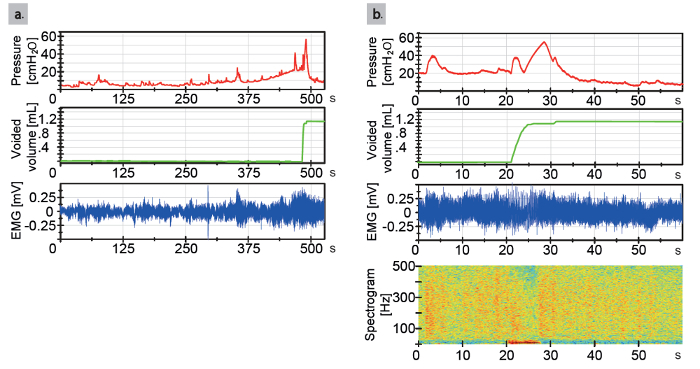
Main read out parameters of the cystometric recording are the baseline pressure, threshold pressure, maximum detrusor pressure, voided volume, average flow, voiding time, average pressure, compliance of the urinary bladder, and simultaneous read out of the external urethral sphincter EMG-activity (Figure 4).
Consecutive cystometric measurements in the follow-up period can be performed for at least four weeks after surgery. If the catheter line is regularly flushed, catheter blockage is no problem. Regular handling and optical control of the rats should be carried out during the whole follow-up period.
If the catheter is kinked or blocked, the intravesical pressure will increase linearly up to very high pressures (above 100 cm H2O). In this case, filling should be stopped and the visible catheter end should be checked for kinking. If no kinking is seen, the catheter should be checked for a blocked outlet. For this purpose, the catheter can be flushed manually via the catheter. If fluid is not easily flowing into the bladder, pulling back and forward lightly can be tried. For one last attempt, an acidic flushing solution (citric acid 2-3%) can be used to try to clear the obstructed region within the catheter. This solution might have a higher chance to dissolve the blockade, yet, the bladder will be irritated after successful flushing and consecutive measurements should only be performed two days after flushing with acidic solution. If no fluid can be flushed into the bladder, the catheter is permanently blocked and no further measurements are possible, and the animal is lost for follow-up.
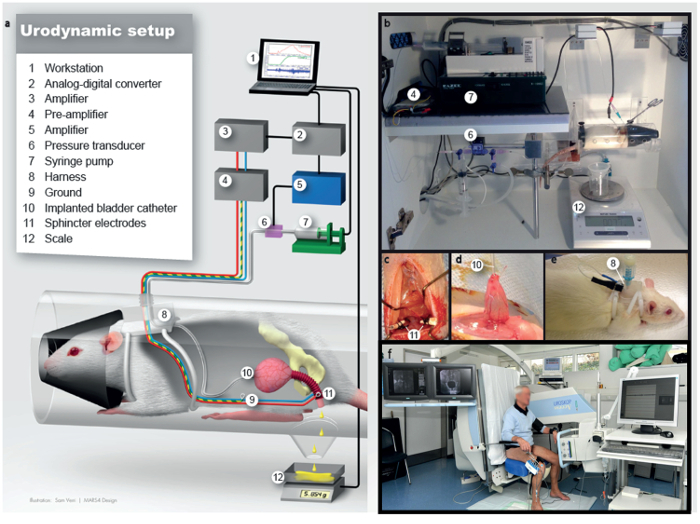
Figure 1: Schematic drawing of the cystometric measurements in awake rats. This figure has been adapted from2. (a) Illustration of the urodynamic setup. (b) Lab station for urodynamic examination. (c) Implantation of the external urethral sphincter electromyography electrodes lateral to urethra, intraoperative view. (d) View of the bladder dome at the moment of bladder catheter implantation, intraoperative view. (e) After implantation of electrodes and catheter, the rat will be fitted with a harness to safely store plugs and connectors. (f) Human urodynamics. Numbers in b-e relate to the legend in a. Please click here to view a larger version of this figure.
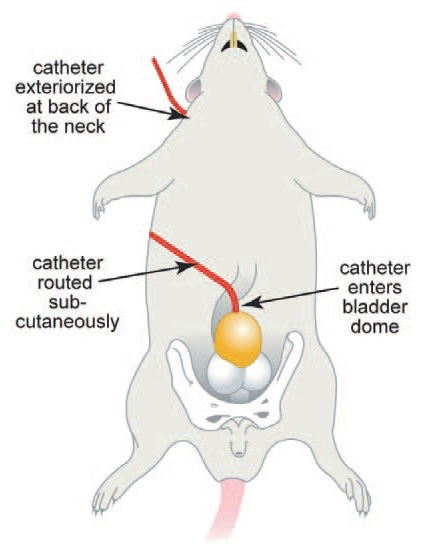
Figure 2: Internal anatomy of the rat for bladder catheter implantation. This figure has been modified from8.
Figure 3: Flushing of the catheter line of a rat. Please click here to view a larger version of this figure.
Figure 4: Urodynamic tracings in an animal 12 day after catheter implantation. (a) Representative urodynamic tracing from a naïve rat. On top is shown the bladder pressure tracing, in the middle the secreted urine weight tracing, and on the bottom the external urethral sphincter EMG tracing. (b) Zoom window from a naïve animal of 60 s, taken from (a). An important remark is that there is less external urethral sphincter EMG activity during voiding than before and after voiding. On top is shown the bladder pressure tracing, in the middle the secreted urine weight tracing, and on the bottom the external urethral sphincter EMG tracing. At the bottom, a heat plot is shown with time matched frequency spectrogram (corresponding to frequency at the current time point). Red represents a high power and blue represents low power. Please click here to view a larger version of this figure.
Discussion
This protocol describes the surgical procedure of a permanent catheter and urethral sphincter electrodes implantation and the cystometric recording technique in awake, lightly restrained rats including both the simultaneous analysis of the urinary bladder and external urethral sphincter.
Critical steps during surgery are the careful implantation of the bladder catheter, avoiding leakage and extensive manipulation. Moreover, a precise implantation of the electrodes bilateral to the urethral external sphincter is crucial for a sound measurement of the external urethral sphincter. A close inspection of the surgical fields after implantation is also essential to maintaining the animal's health. Antibiotic coverage during follow-up helps in preventing infections along the catheter line, as well as the occurrence of urinary tract infections.
During cystometric measurement, a handled rat will be calmer and more relaxed than a rat which was not handled previously. Thus, the cystometric recording might differ in its outcome. Moreover, the restrainer offers the rat a confining space with a dark front area to feel more comfortable and thus, reduces stress levels. In other published awake cystometric measurements, rats can freely move in the measuring cage. However, this bears a higher artefact risk during the measurement and might increase the time of recording, and also the stress level in the animal. In healthy rats, the optimal cystometric measurement can be replicated at multiple measuring time points during follow-up. During the cystometric measurement, problems that commonly occur are kinked catheters or a mistake in the step-wise conduction of the protocol. If a technical or software error occurs, a re-initialization of the cystometric measurement and stepwise repeat of the protocol is highly recommended for troubleshooting.
Limitations of this technique are the inter-animal variability of the cystometric recordings, structural changes in the urinary bladder tissue due to the implanted catheter, which might hamper histological or molecular biological examinations of this tissue, and the single housing of the animals during the follow-up period. Furthermore, this technique has only been tested in female rats, the applicability and outcomes for male rats have not yet been examined.
The main advantage of this technique is the simultaneous measurement of the urinary bladder and the external urethral sphincter, as well as the awake measurement setting. Thus, a more translational examination of the lower urinary tract in awake animals is available, in comparison to the single, terminal cystometric analysis in anesthesia5,6,9,10. Moreover, with this approach, the progression of a lower urinary tract dysfunction or pathology can be followed in the same animal over time, as well as treatment successes. Especially, NLUTD can be examined in a time course, which was not possible to a full extent with the common cystometric technique2.
In conclusion, the presented surgery and cystometric measurement are used for multiple, artefact-reduced analyses of the lower urinary tract, including the interaction of the urinary bladder and the external urethral sphincter in awake rats.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors do not have any acknowledgements.
References
- Andersson KE, Soler R, Fullhase C. Rodent models for urodynamic investigation. Neurourol Urodyn. 2011;30:636–646. doi: 10.1002/nau.21108. [DOI] [PubMed] [Google Scholar]
- Schneider MP, et al. A novel urodynamic model for lower urinary tract assessment in awake rats. BJU Int. 2015;115(Suppl 6):8–15. doi: 10.1111/bju.13039. [DOI] [PubMed] [Google Scholar]
- Cruz CD, Cruz F. Spinal cord injury and bladder dysfunction: new ideas about an old problem. Scientific World Journal. 2011;11:214–234. doi: 10.1100/tsw.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp Neurol. 2012;235:123–132. doi: 10.1016/j.expneurol.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura S, Downie JW. Effect of anesthetics on reflex micturition in the chronic cannula-implanted rat. Neurourol Urodyn. 2000;19:87–99. doi: 10.1002/(sici)1520-6777(2000)19:1<87::aid-nau9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Cannon TW, Damaser MS. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci. 2001;69:1193–1202. doi: 10.1016/s0024-3205(01)01182-1. [DOI] [PubMed] [Google Scholar]
- Patra PB, Thorneloe KS. Enhanced sensitivity to afferent stimulation and impact of overactive bladder therapies in the conscious, spontaneously hypertensive rat. J Pharmacol Exp Ther. 2011;338:392–399. doi: 10.1124/jpet.111.180885. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Meredith AL. Diurnal variation in urodynamics of rat. PloS One. 2010;5:e12298. doi: 10.1371/journal.pone.0012298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Havton LA. Differential effects of urethane and isoflurane on external urethral sphincter electromyography and cystometry in rats. Am J Physiol Renal Physiol. 2008;295:F1248–F1253. doi: 10.1152/ajprenal.90259.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Durant PA, Brent CR. Micturition in rats: a chronic model for study of bladder function and effect of anesthetics. Am J Physiol. 1986;251:R1177–R1185. doi: 10.1152/ajpregu.1986.251.6.R1177. [DOI] [PubMed] [Google Scholar]


