Abstract
Background:
Research has shown that higher altitude is associated with lower risk of lung cancer and improved survival among patients. The current study assessed the influence of county-level atmospheric pressure (a measure reflecting both altitude and temperature) on age-adjusted lung cancer mortality rates in the contiguous United States, with 2 forms of spatial regression.
Methods:
Ordinary least squares regression and geographically weighted regression models were used to evaluate the impact of climate and other selected variables on lung cancer mortality, based on 2974 counties.
Results:
Atmospheric pressure was significantly positively associated with lung cancer mortality, after controlling for sunlight, precipitation, PM2.5 (µg/m3), current smoker, and other selected variables. Positive county-level β coefficient estimates (P < .05) for atmospheric pressure were observed throughout the United States, higher in the eastern half of the country.
Conclusion:
The spatial regression models showed that atmospheric pressure is positively associated with age-adjusted lung cancer mortality rates, after controlling for other selected variables.
Keywords: altitude, atmospheric pressure, lung cancer mortality, spatial regression, temperature
Introduction
In 2017, lung cancer accounted for an estimated 222 500 of new cases (14% of all cancer cases) and 155 870 of deaths (25% of all cancer deaths) in the United States.1 Tobacco smoking is the leading cause of lung cancer, with men and women who smoke 25 times more likely to develop the disease than nonsmokers.1 Smoking also explains about 80% of deaths from lung cancer, with the percentage even higher for small-cell lung cancer.2 Other risk factors include exposure to second-hand smoke, asbestos, certain metals, radon gas, air pollution, diesel exhaust, and some organic chemicals.1 In addition, research has found that lung cancer risk decreases with higher altitude.3-5 A decreased risk for other forms of cancer has also been associated with higher altitude, which equates to lower atmospheric oxygen concentration, barometric pressure, and natural background radiation.6-10 The association between higher altitude and lower lung cancer risk is stronger than that with other cancers (eg, breast, prostate, or bowels), indicating that the inhalation process plays an important role.3 One study found that, on average, lung cancer decreases by 7.23 per 100 000 cases for every 1000-m rise in altitude, after adjusting for selected environmental correlates of elevation.3
Inspired molecular oxygen (O2) is essential for life. About 21% of the atmosphere consists of O2. Oxygen levels are the same at both high and low altitudes. However, at high altitudes air pressure is much lower, allowing air particles to spread farther apart and making oxygen less accessible. Oxygen tends to easily react with other molecules in a cell, forming a type of unstable molecule called reactive oxygen species (ROS). Under normal conditions, cells control ROS levels. However, under oxidative stress conditions, ROS buildup in cells can contribute to carcinogenesis due to the damage of lipids, DNA, RNA, and cellular protein, as well as inhibit apoptosis and activate proto-oncogenes.11-16
The purpose of the current study was to assess the influence of atmospheric pressure, along with other selected variables (ie, sunlight, precipitation, sex, ethnicity, race, poverty, population density, smoking, obesity, diabetes, and ambient air pollution), on lung cancer mortality using 2 forms of spatial regression. Because ambient air temperature can influence known risk factors (eg, radon gas and ground level ozone) for lung cancer, our analysis used atmospheric pressure (a composite indicator of altitude and temperature). This is the first study to assess whether atmospheric pressure based on population-weighted, county-level altitude and temperature data is associated with lung cancer mortality.
Methods
Cancer Mortality Data
County-level lung cancer mortality rates (age-adjusted using the 2000 US standard population) were obtained from the CDC WONDER database, 1999 to 2010.17 These rates were based on death certificate data from the National Center for Health Statistics (NCHS).18 Annual detailed mortality files from the NCHS are coded according to the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). Counties reporting less than 20 deaths from individual cancers were excluded because of confidentiality and large standard errors. This left 2974 counties for assessment.
Health and Socioeconomic Factors
County-level socioeconomic variables for 1999 to 2010 were included: percentage male, caucasian, and Hispanic from the CDC WONDER database17; percentage below the poverty line from the US Census Bureau19; percentage of current smoker from Dwyer-Lindgren et al20; percentage obese and percentage diabetic (2004-2005) from the Behavior Risk Factor Surveillance System21; and population density, calculated using data from the US Census Bureau and CDC WONDER database.17
Climate Factors
Climate factors were atmospheric pressure, average daily sunlight, precipitation, and PM2.5. Weighted county-level altitude was calculated to account for population center residence. This was achieved by using a 30-m elevation data set available through ArcGIS Online from the US Geological Survey.22 Census tract- and county-level shape files were obtained from the US Census Bureau.23 Altitude values were then aggregated to the census tract-level, which approximates neighborhoods. Using census tract population estimates from 2000 and census tract altitude values, we calculated weighted county-level altitude values. Considering the nonlinear relationship between altitude and atmospheric pressure, and the impact of temperature on atmospheric pressure, we derived county atmospheric pressure using the hypsometric atmospheric pressure formula, weighted county elevation, and average daily maximum temperature.24 Average daily maximum temperature was used because there is greater county-to-county variation in maximum than minimum temperature. Average daily sunlight, temperature, and precipitation (1999-2010), and PM2.5 (2003-2006) were obtained from the CDC WONDER database.17
Model Selection
Both ordinary least squares (OLS) regression and geographically weighted regression (GWR) models were computed to evaluate the impact of climate and other selected variables. The GWR is a form of spatial regression that uses a moving bandwidth to calculate regional regression models and is used to determine whether the regression coefficients are consistent across space. These models were estimated using the software packages GeoDa (v 1.12)25 and GWR v.4.09.26 In ArcGIS Pro (v 2.0.1), Moran I identified significant positive spatial autocorrelation in the OLS regression model residuals. There was low multicollinearity for atmospheric pressure coefficients (variation inflation factors ≤2.59).
The presence of spatial autocorrelation was identified (P < .0001), meaning there was a clustering of model residuals. Hence, due to spatial influences, the observations were not considered independent. We used 2 forms of spatial regression to account for this spatial dependence. First, we added a spatial lag to the OLS regression. Spatial weights were calculated using a second-order queen contiguity matrix, and the spatial lag was added using GeoDa. Second, GWR was used to evaluate variation in the association between lung cancer mortality rates, climate, and other selected variables across regions. The use of the spatial lag and GWR produced a better-fitting, model-based lower Akaike information criteria (AIC, a measure of model fit) and larger adjusted R 2 values for the spatial regression models compared to the OLS regression model. The GWR bandwidth (the number of points included in the moving bandwidth) was calculated using a technique called Golden Selection Search, which chooses the optimal bandwidth size for each model according to AIC values.25
Results
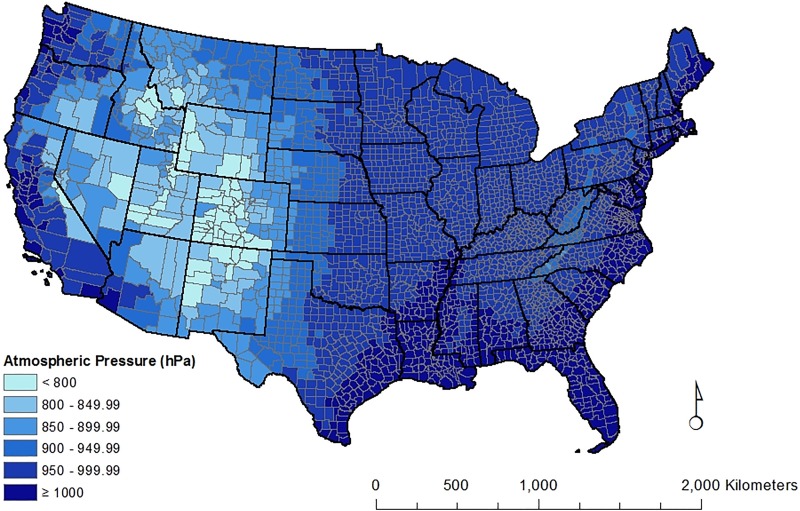
Atmospheric pressure values are presented by county for the contiguous United States (Figure 1). The lowest values are in the Rocky Mountain and plateau region of the United States. The highest values are along the Atlantic and Pacific coastal regions and the Gulf of Mexico. On the state level, mean atmospheric pressure is 960.1 hPa (standard deviation = 54.3), ranging from 809.6 to 1010.4. Eight (16%) states had scores below 900 hPa (Colorado, Utah, Wyoming, New Mexico, Nevada, Idaho, Montana, and Arizona). Seven states (Florida, Delaware, Louisiana, Rhode Island, New Jersey, Mississippi, and Connecticut) and the District of Columbia had scores above 1000 hPa. Based on our calculations, at low altitude and at constant temperature, a decrease in atmospheric pressure of 1 hPa is approximately equal to an 8.33-meter increase in elevation. Because of the nonlinear relationship between atmospheric pressure and altitude, there is a greater pressure change with a fixed altitude change at higher altitudes.
Figure 1.
Atmospheric pressure and sunlight in the contiguous United States.
Estimates of the association between county-level lung cancer mortality rates and atmospheric pressure and other selected variables are shown in Table 1. Estimated coefficients in the table were simultaneously computed. A spatial lag variable was included in order to account for correlated error terms. The model statistics (adjusted R 2 and AIC) indicate that the variables included in the model do a good job explaining variation in lung cancer mortality rates. County-level, age-adjusted lung cancer mortality rates were significantly positively associated with current smoking (%), atmospheric pressure, and sunlight. Lung cancer mortality rates were significantly negatively associated with being Hispanic (%) or obese (%). In other words, smoking, higher atmospheric pressure, and more sunlight increased the risk of lung cancer, whereas being Hispanic or obese was protective against lung cancer.
Table 1.
Coefficient Estimates Using Ordinary Least Squares Regression.a
| Variable | Estimate | 95% CI | P Value |
|---|---|---|---|
| Spatial lag | 0.5361 | (0.4915, 0.5808) | <.0001 |
| Atmospheric pressure, hPa) | 0.0358 | (0.0267 to 0.0450) | |
| Sun, kJ/m2 | 0.0004 | (0.0001 to 0.0006) | .0043 |
| Precipitation, mm | −0.0213 | (−0.5398 to 0.4972) | .9359 |
| Male, % | −0.0721 | (−0.2162 to 0.0720) | .3268 |
| Hispanic, % | −0.0656 | (−0.1007 to −0.0305) | .0003 |
| Caucasian, % | 0.0001 | (−0.0283 to 0.0285) | .9940 |
| Poverty, % | 0.0691 | (−0.0134 to 0.1516) | .1007 |
| Population density, people per km2 | 0.0000 | (−0.0004 to 0.0004) | .9559 |
| Current smoker, % | 1.5324 | (1.4103 to 1.6545) | <.0001 |
| Obese, % | −0.3579 | (−0.5230 to −0.1928) | <.0001 |
| Diabetic, % | 0.4236 | (−0.0140 to 0.8612) | .05771 |
| PM2.5, µg/m3 | −0.0906 | (−0.2577 to 0.0765) | .28753 |
| Adjusted R 2 = .69 | |||
| AIC = 20582.80 | |||
Abbreviations: AIC, Akaike information criteria; CI, confidence interval.
aCoefficient estimates were simultaneously computed for data from 2974 counties.
The GWR model provided a better fit to the data compared with the original OLS regression model, according to GWR analysis of variance that compares sum of squares (P < .0001). Additionally, the GWR model had a higher adjusted R 2 and lower AIC (Table 2) than the OLS model, after the addition of the spatial lag. The direction of association for each of the coefficient estimates is the same as seen in the previous table, except for precipitation. However, this variable is not statistically significant. Although diabetes (%) was marginally insignificant in the OLS model, the association with lung cancer mortality was greater in the better-fitting GWR model.
Table 2.
Geographically Weighted Regression Model Summary and Coefficient Estimates.a
| Variable | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|
| GWR coefficient estimates | |||||
| Atmospheric pressure, hPa | 0.1498 | 0.0986 | 0.1411 | −0.1717 | 0.4684 |
| Sun, kJ/m2 | 0.0005 | 0.0031 | 0.0005 | −0.0063 | 0.0092 |
| Precipitation, mm | 0.1336 | 3.6609 | 0.1445 | −8.8304 | 10.9696 |
| Male, % | −0.1565 | 0.3598 | −0.1779 | −1.0846 | 1.1535 |
| Hispanic, % | −0.1538 | 0.2070 | −0.1189 | −1.3641 | 0.2968 |
| Caucasian, % | 0.0345 | 0.1535 | 0.0344 | −0.2930 | 0.5820 |
| Poverty, % | 0.1549 | 0.3978 | 0.0539 | −0.7987 | 1.0359 |
| Population density, people per km2 | 0.0050 | 0.0070 | 0.0029 | −0.0195 | 0.0298 |
| Current smoker, % | 1.6636 | 0.4162 | 1.6702 | 0.5672 | 2.5982 |
| Obese, % | −0.0972 | 0.3925 | −0.0876 | −0.9459 | 0.9181 |
| Diabetic, % | 0.7182 | 1.2840 | 0.9072 | −2.7783 | 3.4733 |
| PM2.5, µg/m3 | −0.1721 | 1.2883 | −0.0745 | −4.7130 | 4.0428 |
| Adjusted R 2 = 0.73 | |||||
| AIC = 20342.64 | |||||
| Best bandwidth (counties) = 375 | |||||
Abbreviations: AIC, Akaike information criteria; GWR, geographically weighted regression; SD, standard deviation.
aCoefficient estimates were simultaneously computed for data from 2974 counties.
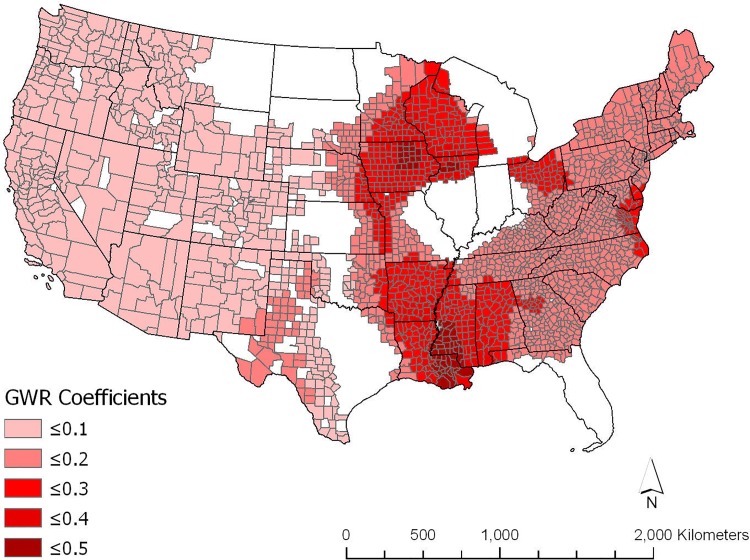
Figure 2 shows the GWR county-level β coefficient estimates (P < .05) of the association between lung cancer mortality rates and atmospheric pressure, adjusted for the other variables shown in Table 2. All significant β coefficients are positive, with the highest levels in the eastern half of the United States, particularly in Wisconsin, Iowa, Arkansas, Mississippi, and Louisiana.
Figure 2.
Significant geographically weighted regression atmospheric pressure coefficients.
Discussion
The purpose of the current study was to assess the relationship between atmospheric pressure and age-adjusted lung cancer mortality rates, using 2 forms of spatial regression. Other selected variables were included in the study. Overall, atmospheric pressure had a significant positive relationship with lung cancer mortality, after adjusting for other selected variables. The positive association observed between atmospheric pressure and lung cancer is consistent with previous studies.3-5 County-level significant positive relationships between lung cancer mortality rates and atmospheric pressure were observed throughout much of the country and were stronger in the eastern half of the contiguous United States.
From the spatial lag model, a 100-hPa decrease (or an increase in altitude by approximately 833 meters) leads to a decrease of 3.58 lung cancer deaths per 100 000, after adjusting for sunlight, precipitation, sex, race, ethnicity, poverty, population density, smoking, obesity, diabetes, and PM2.5 (µg/m3). A previous study observed an average decrease in lung cancer incidence of 7.23 cases per 100 000 for every 1000-meter rise in altitude, after adjusting for selected variables associated with elevation.3 The GWR results showed that the impact of atmospheric pressure on lung cancer mortality may be even greater, especially regionally. In the better-fitting GWR model, we found that the average coefficient for GWR is 0.1498 (median 0.1411), meaning a 100-hPa decrease in atmospheric pressure leads to a decrease of 14.98 lung cancer deaths per 100 000.
One of the primary benefits of GWR is that it allows us to evaluate whether there is any variation in the β coefficients of association across space. The fact that there were no significant negative coefficients for atmospheric pressure indicates consistency in the direction of the association and confirms the results of the spatial lag OLS model. However, the positive county-level association between atmospheric pressure and lung cancer mortality varies considerably by region. The spatial lag in the OLS model was statistically significant. This suggests that other spatially correlated variables (such as diet), which are related to both atmospheric pressure and lung cancer mortality, are unaccounted for in the model. In addition, unaccounted for variables can influence the regional associations between atmospheric pressure and lung cancer mortality.
Atmospheric pressure coefficients in those areas with the highest β coefficients can exceed 0.4. This means that in those areas an increase of 3 hPa (or about 25 meters in attitude) would lead to a decrease in 1.2 cancer deaths per 100 000. This is of particular importance because day-to-day variations in atmospheric pressure may exacerbate the physiological effects of lung cancer and, subsequently, lead to an increase in lung cancer mortality. Additional research is needed to examine the impact of seasonal and daily changes in atmospheric pressure on lung cancer mortality.
Higher altitude is correlated with greater Vitamin D synthesis from sunlight. Higher altitude is related to less atmospheric filters of ultraviolet radiation. Ultraviolet radiation levels increase from 10% to 12% for each 1000 m\ in altitude.27 Greater natural radiation from cosmic rays at higher altitudes increases vitamin D levels produced by the skin.28 Vitamin D synthesis from sunlight is also greater at lower latitudes because the zenith angle is smaller. Vitamin D is important because it modulates cell growth and stabilizes chromosomal structure, thus guarding against chromosomal aberrations and the formation of cancer.29,30 Nevertheless, sunlight had a small positive effect on lung cancer mortality. The positive effect was unexpected, based on previous studies,31-33 possibly occurring because of an unaccounted confounding variable. It is important to note that observed positive correlation is between sunlight and lung cancer mortality and not vitamin D concentration. Sunlight may have an effect, such as with temperature, that is independent of sun-synthesized vitamin D. One study involving cardiovascular disease showed that sunlight has an impact on the body independent of serum vitamin D concentration.34 Therefore, we may not fully understand the impact of sunlight on the body independent of vitamin D concentrations. Additional research is needed to fully examine the impact of sunlight on lung cancer mortality.
The hormetic model states that low doses of radiation reduce cancer risk, whereas high doses above a certain threshold increase cancer risk.35-37 Ecologic studies have shown an association between natural background radiation and lower cancer mortality, which supports the possibility of radiation hormesis.6-8,38 Although the study results are consistent with the possibility of radiation hormesis, the ecologic data limit drawing definitive conclusions.
The relatively large, positive association between smoking and lung cancer mortality is expected. Smoking has been an established cause of lung cancer since the 1964 Surgeon General’s Report.39 Thus, it is important to note that with this variable in the model, atmospheric pressure continues to have a positive association with lung cancer mortality.
Obesity was negatively associated with lung cancer mortality, which is consistent with 2 previous meta-analyses that found that overweight and obesity were protective against lung cancer.40,41 Studies have also shown that lung cancer-induced cachexia may lead to decreases in body weight.42,43 However, another meta-analysis found that abdominal obesity was positively associated with the development of lung cancer.44 Yet another study found no association between body mass index and lung cancer risk, regardless of smoking status.45
Diabetes was independently associated with an increased risk of lung cancer mortality. This result is consistent with previous research.46 Elevation and temperature both affect blood glucose,47 so we included this variable in the model.
Hispanics had a lower rate of lung cancer mortality. The lower mortality is despite Hispanic patients tending to be diagnosed at later stage of disease and having higher levels of obesity and diabetes than non-Hispanic whites.48 Although we adjust for obesity and diabetes in our models, we did not take into account the relatively low rates of lung cancer incidence among Hispanics.49 The comparatively low incidence of lung cancer among Hispanics likely explains this result.
The present study has 2 primary limitations. First, it is an ecological study and, therefore, does not account for individual-level exposure to atmospheric pressure, sunlight, precipitation, and so on. Second, data are aggregated over a 12-year period, so the results do not capture daily and yearly changes in atmospheric pressure, sunlight, precipitation, pollution levels, and human migration.
Conclusion
This study shows that atmospheric pressure is positively associated with age-adjusted lung cancer mortality rates, after adjusting for other selected variables. This association appears to be greater for mortality than has been observed in previous studies involving incidence. Conclusions cannot be made from our results about the role of sunlight and sunlight-synthesized vitamin D, as they relate to lung cancer mortality rates. Air pollution was not significant in the models. This may be because of the large variation in air pollution within a county and because air pollution is associated with other variables in the models (ie, sunlight, temperature, and diabetes). Because of the influence of atmospheric pressure on lung cancer, individuals at high risk of lung cancer or patients with lung cancer may benefit from living at higher altitude.
Footnotes
Authors’ Note: All authors listed have made substantial, direct, and intellectual contribution to the work and approved it for publication.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. American Cancer Society. (2017). Cancer Facts & Figures 2017. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. Accessed October 23, 2017.
- 2. American Cancer Society. (2016). Small Cell Lung Cancer Risk Factors. https://www.cancer.org/cancer/small-cell-lung-cancer/causes-risks-prevention/risk-factors.html. Accessed October 23, 2017.
- 3. Simeonov KP, Himmelstein D. Lung cancer incidence decreases with elevation: evidence for oxygen as an inhaled carcinogen. PeerJ. 2015;3:e705 https://peerj.com/articles/705/. Accessed October 23, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bidoli E, Franceschi S, Dal Maso L, Guarneri S, Barbone F. Cancer mortality by urbanization and altitude in a limited area in Northeastern Italy. Rev Epidemiol Sante Publique. 1993;41(5):374–382. [PubMed] [Google Scholar]
- 5. Amsel J, Waterbor JW, Oler J, Rosenwaike I, Marshall K. Relationship of site-specific cancer mortality rates to altitude. Carcinogenesis. 1982;3(5):461–465. [DOI] [PubMed] [Google Scholar]
- 6. Hart J. Cancer mortality in six lowest versus six highest elevation jurisdictions in the US. Dose Response. 2011;9(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hart J, Hyun S. Cancer mortality, state mean elevations, and other selected predictors. Dose Response 2012;10(1):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hart J. Land elevation and cancer mortality in US. Cities and counties using median elevations derived from geographic information systems. Dose Response. 2013;11(1):41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burtscher M. Effects of living at higher altitudes on mortality: a narrative review. Aging Dis. 2014;5(4):274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinberg CR, Brown KG, Hoel DG. Altitude, radiation, and mortality from cancer and heart disease. Radiat Res. 1987;112:381–390. [PubMed] [Google Scholar]
- 11. Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996. 313(1): 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidate DNM damage: mechanisms, mutation, and disease. FASEB J. 2003;17(10):1195–11214. [DOI] [PubMed] [Google Scholar]
- 14. Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Ref. 2008;11(1):1–15. [DOI] [PubMed] [Google Scholar]
- 15. Ames B.N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983;221:1256–64. [DOI] [PubMed] [Google Scholar]
- 16. Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011; 7(8):504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. CDC Wonder; 2017. https://wonder.cdc.gov/ Accessed September 11, 2017.
- 18. Centers for Disease Control and Prevention; 2017. Data Access - Compressed Mortality File. https://www.cdc.gov/nchs/data_access/cmf.htm. Accessed September 11, 2017.
- 19. US Census Buraeu. Model-based Small Area Income & Poverty Estimates (SAIPE) for School Districts, Counties, and States; 2017. https://www.census.gov/did/www/saipe/
- 20. Dwyer-Lindgren L, Mokdad A, Srebotnjak T, Flaxman A, Hansen G, Murray C. Cigarette smoking prevalence in US counties: 1996-2012, Popul Health Metr. 2014;12(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention. (2017). County Data Indicator. https://www.cdc.gov/diabetes/data/countydata/countydataindicators.html. Accessed September 18, 2017.
- 22. Esri. Ground Surface Elevation – 30 m. (2017). http://www.arcgis.com/home/item.html?id=0383ba18906149e3bd2a0975a0afdb8e. Accessed September 18, 2017.
- 23. US Census Bureau. (2017) TIGER/Line Shapefiles and TIGER/Line Files. https://www.census.gov/geo/maps-data/data/tiger-line.html. Accessed September 18, 2017.
- 24. Keisan Online Calculator. (2017). Atmospheric pressure from altitude calculator. http://keisan.casio.com/exec/system/1224579725. Accessed September 18, 2017.
- 25. GeoDa Software (1.12); 2017. http://geodacenter.github.io/download.html. Accessed September 18, 2017.
- 26. Maynooth University. GWR4 for Windows; 2017. http://gwr.maynoothuniversity.ie/gwr4-software/. Accessed September 18, 2017.
- 27. World Health Organization. (2017). Ultraviolet radiation and health; 2017. http://www.who.int/uv/uv_and_health/en/. Accessed September 18, 2017.
- 28. Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007;22(suppl 2):V28–V33. [DOI] [PubMed] [Google Scholar]
- 29. Hayes DP. Cancer protection related to solar ultraviolet radiation, altitude and vitamin D. Med Hypotheses. 2010;75(4):378–382. [DOI] [PubMed] [Google Scholar]
- 30. Schumacker PT. Reactive oxygen species in cancer: a dance with the devil. Elsevier. 2015;27(2):156–157. [DOI] [PubMed] [Google Scholar]
- 31. Norton R, O’Connel MA. Vitamin D: potential in the prevention and treatment of lung cancer. Anticancer Res. 2012;32(1):211–221. [PubMed] [Google Scholar]
- 32. Zhang L, Want S, Che X, Li X. Vitamin D and lung cancer risk: a comprehensive review and meta-analysis. Cell Physiol Biochem. 2015;36:299–305. [DOI] [PubMed] [Google Scholar]
- 33. Kikkinen A, Knekt P, Heliovaara M, et al. Vitamin D status and the risk of lung cancer: a cohort study in Finland. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3274–3278. [DOI] [PubMed] [Google Scholar]
- 34. Weller R. Sunlight has cardiovascular benefits independently of vitamin D. Blood Purification. 2016;41(1-3):130–134. [DOI] [PubMed] [Google Scholar]
- 35. Vaiserman AM. Radiation hormesis: historical perspective and implications for low-dose cancer risk assessment. Dose Response. 2010;8(2):172–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scott BR, Di Palma J. Sparsely ionizing diagnostic and natural background radiations are likely preventing cancer and other genomic-instability-associated diseases. Dose Response. 2006;5(3):230–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scott BR. Low-dose radiation stimulated natural chemical and biological protection against lung cancer. Dose Response. 2008;6(3):299–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lehrer S, Rosenzweig KE. Lung cancer hormesis in high impact states where nuclear testing occurred. Clin Lung Cancer. 2015;16(2):152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. U.S. Public Health Service U.S. Surgeon General’s Advisory Committee on Smoking and Health. Smoking and Health. U.S Public Health Service, Washington, DC; 1964. [Google Scholar]
- 40. Yang Y, Dong J, Sun K, et al. Obesity and incidence of lung cancer: a meta-analysis. Int J Cancer. 2013;132(5):1162–9. [DOI] [PubMed] [Google Scholar]
- 41. Guo L, Liu S, Zhang S, Chen Q, Zhang M, Quan P, Lu J, Sun X. A meta-analysis of body mass index and the risk of lung cancer in the Chinese population. Zhonghua Yu Fang Yi Xue Za Zhi. 2015;49(7):649–53. [PubMed] [Google Scholar]
- 42. Del Ferrara C, Grant M, Koczywas M, Dorr-Uyemura LA. Management of anorexia-cachexia in late stage lung cancer patients. J Hosp Palliat Nurs. 2012;14(6):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jatoi A. Pharmacologic therapy for the cancer anorexia/weight loss syndrome: a data-driven, practical approach. J Support Oncol. 2006;4(10):499–502. [PubMed] [Google Scholar]
- 44. Khemayanto H, Xuan D, Guochong C, Minhua S, Bimin S. Abdominal obesity and lung cancer risk: systematic review and meta-analysis of prospective studies. Nutrients. 2016;8(12):810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Patel AV, Carter BD, Stevens VL, Gaudet MM, Campbell PT, Gapstur SM. The relationship between physical activity, obesity, and lung cancer risk by smoking status in a large prospective cohort of US adults. Cancer Causes Control. 2017;28(12):1357–1368. [DOI] [PubMed] [Google Scholar]
- 46. Lee JY, Jeon I, Lee JM, Yoon JM, Park SM. Diabetes mellitus as an independent risk factor for lung cancer: a meta-analysis of observational studies. Eur J Cancer. 2013;49(10):2411–2423. [DOI] [PubMed] [Google Scholar]
- 47. Fink KS, Christensen DB, Ellsworth A. Effect of high altitude on blood glucose meter performance. Diabetes Technol Ther. 2002;4(5):627–635. [DOI] [PubMed] [Google Scholar]
- 48. Siegel RL, Fedewa SA, Miller KD, et al. Cancer statistics for Hispanics/Latinos. CA Cancer J Clin. 2015;65:457–480. [DOI] [PubMed] [Google Scholar]
- 49. Centers for Disease Control and Prevention. Lung Cancer Rates by Race and Ethnicity; 2017. https://www.cdc.gov/cancer/lung/statistics/race.htm. Accessed October 23, 2017.