Abstract
Pancreatic cancer is a malignant tumor model with high mortality. Many patients are ineligible for surgical resection once diagnosed; therefore, it is important to explore more safe and effective treatment options. This study was designed to determine the therapeutic effect of reduced graphene oxide combined with the near-infrared laser in animal pancreatic cancer. The results showed that reduced graphene oxide has strong light absorption ability between 600 and 1100 nm detected by spectrophotometer. Experimental results of different concentrations of reduced graphene oxide solution under 980-nm laser irradiation at different powers showed that the enhancement of the photothermal conversion effect of reduced graphene oxide depends on reduced graphene oxide concentration and light dose. In vivo experiments showed that higher laser dose (0.75 W/cm2) combined with higher reduced graphene oxide concentration (2 mg/kg) can achieve higher treatment temperature and slower tumor growth. These results suggest that reduced graphene oxide combined with the 980-nm laser in the treatment of mouse pancreatic cancer can get an ideal thermal killing effect, with the clinical potential of pancreatic cancer treatment.
Keywords: pancreatic cancer, graphene oxide, near-infrared laser, photothermal effects, photothermal therapy
Introduction
The mortality of pancreatic cancer is extremely high, and the incidence rate showed a gradually increasing trend in the recent years.1 In China, the crude incidence rate of pancreatic cancer increased from 12.80/100 000 in 2004 to 15.66/100 000 in 2009. The overall 5-year survival rate was 4.1%, and the median survival time was only 3.9 months.2 Many patients with pancreatic cancer were beyond the chance of surgery at the time of diagnosis. Traditional radiation therapy or chemotherapy cannot often achieve effective therapeutic outcomes and also come with more severe side effects.3-5 Therefore, finding and exploring more safe and effective treatments with fewer side effects have very important clinical value.
As a new method of tumor therapy, photothermal therapy, which uses a laser as a heat source for malignant tumor killing, has received more and more attention.6,7 Photothermal thermotherapy often uses a near-infrared laser for better tissue penetration, but the photothermal effect is still unsatisfactory. For this limitation of laser treatment, researchers had proposed to use light-absorbing materials, such as indocyanine green, gold nanoparticales, carbon nanoparticles, and others, to enhance the light and heat conversion efficiency and improve local tumor thermal damage.8-10
Graphene oxide (GO) has special physical, chemical, and biological characteristics, which have been widely used in the biomedical field.11 The GO carries oxidative functional groups that make it more dispersible in aqueous solutions. In addition, the cytotoxicity of GO is extremely low, and the production cost is not high. These characteristics make the GO to be considered an ideal, highly biocompatible material which was used in the study of related fields.12 It has been found that GO also has a high photothermal conversion capability, especially in the near-infrared wavelength region. The GO can produce a strong absorption and heat transfer effect from light, and this heat could kill tumor cells directly.13
In this study, we used reduced GO (rGO) combined with the near-infrared laser to observe its role in pancreatic cancer treatment.
Materials and Methods
Preparation of GO
One gram expandable graphite flake (XFNANO, China) was ground with NaCl (50 g) for 10 minutes. NaCl was dissolved in water and removed by filtration. The remaining ground expandable graphite flake was stirred in 98% H2SO4 (23 mL) for 8 hours. KMno 4 (3 g) was gradually added while keeping the temperature <20°C. The mixture was then stirred at 35°C to 40°C for 30 minutes and then at 65°C to 80°C for 45 minutes. Next, water (46 mL) was added and the mixture heated at 98°C to 105°C for 30 minutes. The reaction was terminated by addition of distilled water (140 mL) and 30% H2O2 solution (10 mL). The mixture was washed by repeated centrifugation and filtration, first with 5% hydrochloric acid (HCl) aqueous solution and then with distilled water. Water (160 mL) was added to the final product and vortexed well to make a uniform suspension for storage.
Preparation of Nano GO
Sodium hydroxide of 1.2 g was added to 10 mL of GO (1 mg/mL). This solution was sonicated for 3 hours and then concentrated HCl was added until the pH dropped to 1. The solution was then washed 3 times with water and brought to a concentration of 1 mg/mL. Six-armed branched poly-(ethylene glycol) (Laysan Bio, Inc, Arab, AL) with amine termination (6PEG-NH2) was added to the solution at 2 mg/mL and sonicated for 5 minutes.
N-(3-Dimethylaminopropyl-N′-ethylcarbodiimide) hydrochloride (EDC; Sigma-Aldrich, St. Louis, MO) was added to the solution (1 mmol) followed by sonication for an additional 1 hour. The GO solution was then centrifuged at 12 000 rpm for 30 minutes in double phosphate-buffered saline to remove any aggregates or multilayered GO sheets. The supernatant was collected after centrifugation and washed 8 times and filtered each time through a 100-kDa molecular weight cutoff (MWCO) centrifuge filter (Millipore) at 4000g.
Preparation of rGO
Reduced GO was synthesized in a manner similar to that of GO. However, before centrifugation at 22 000g, 0.05% v/v of hydrazine monohydrate was added, and the solution is heated to 80°C for 15 minutes. To resuspend the solution, 1000 mg/L of the polymer C18-PMH-mPEG5000 phospholipid-polyethylene glycol (PL-PEG) was added, and another 1 hour of sonication took place. Centrifugation was then done at 22 000g to remove any aggregates or multilayered nano-rGO sheets. The supernatant was collected after centrifugation and washed 8 times with 100-kDa MWCO Millipore centrifuge filter at 4000g.
Characterization of GO
The size of the rGO was measured by dynamic light-scattering instrument. The absorption spectra of rGO, nano GO (NGO), and PL-PEG at 400 to 1100 nm was measured by UV–visible near-infrared spectrophotometer. The measuring peak width is 5 nm, and the measurement speed is 200 nm/min.
The Photothermal Conversion Efficiency of rGO Solution
Reduced GO of 100 μL with a concentration of 50 μg/mL was added in an eppendorf (EP) tube (300 μL) and irradiated by 980-nm laser with differing laser doses of 0.5 to 1.5 W/cm2 for 5 minutes. The temperature was recorded by an infrared thermal imager (FLIR E8; FLIR Systems,Goleta, CA, USA; interval of 30 seconds). Using a micropipette, 100 μL of different concentrations of rGO solution (0-100 μg/mL) was added in the EP tube (300 μL), irradiated for 5 minutes by 980-nm laser at the laser dose of 0.75 W/cm2, and the temperature was recorded using infrared thermography (interval 30 seconds).
Cell Culture
The mouse pancreatic cancer cell Panc02-H7 was cultured in dulbecco's modified eagle medium (DMEM) (Gibco, Gaithersburg, MD; ATCC, Manassas, VA) containing 10% fetal bovine serum (ATCC) and 1% penicillin in an incubator containing 5% CO2 at 37°C.
Tumor Treatment
Animals were housed in the animal facility of the Department of Comparative Medicine at the University of Oklahoma Health Sciences Center (OUHSC). All experiments were conducted in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and approved by the OUHSC Institutional Animal Care and Use Committee.
Six- to 8-week-old female C57BL/6 mice were used. Panc02-H7 pancreatic cancer cells were planted on the back of mice. Tumor treatments were performed when the tumor volume was about 300 mm2. Prior to irradiation, rGO (1-2 mg/kg) was injected directly into the tumors. Two hours later, the 980-nm near-infrared laser (0.5-0.75 W/cm2, 10 minutes) was used. During the treatment, the surface temperature of the tumors was recorded by the infrared thermal imager, and the temperature in the tumor tissue (tumor center and bottom) was detected by thermocouples with needle probes (HYP1-30; Omega Engineering, Stamford, Connecticut). Before the experiment, the thermocouples were calibrated with InstruNET software (Omega Engineering) using freezing and boiling water baths at 0°C and 100°C, respectively. Tumor-bearing mice were killed 10 days after treatment, and the maximum diameter of the tumor was measured by Vernier caliper. After dissection of the mice, the weight of the tumor was measured using an electronic balance scale.
Statistical Analysis
Values are expressed as mean ± standard error of the mean. All of the data were analyzed with 1-way analysis of variance followed by Bonferroni posttest. A P value of <.05 was considered statistically significant.
Results
Characterization of rGO
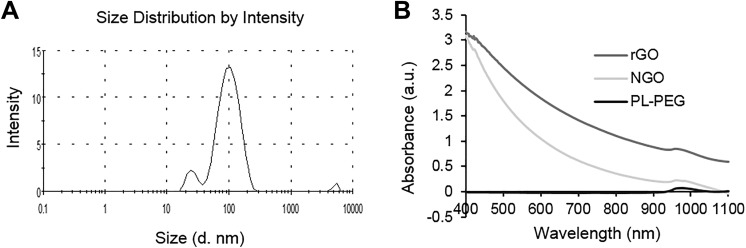
Graphene is a 2-dimensional crystal nanomaterial with sp2 mixed monatomic layers composed of honeycomb crystal lattice. In order to understand the rGO size feature, we used dynamic light scatterometer to detect rGO, and the results show that rGO has a maximum diameter peak at about 100 nm (Figure 1A). We used the spectrophotometer to measure the light absorption of rGO and NGO with PL-PEG as a control. The results showed that rGO and NGO have light absorption ability between 600 and 1100 nm, and rGO has higher light absorption capacity in this wavelength range compared to NGO (Figure 1B).
Figure 1.
Characteristic of reduced graphene oxide (rGO). A, The size distribution of rGO detected by dynamic light scattering. B, Absorption spectra of nano GO (NGO; 100 μg/mL) and rGO (100 μg/mL).
Analysis of rGO’s Photothermal Conversion Effect
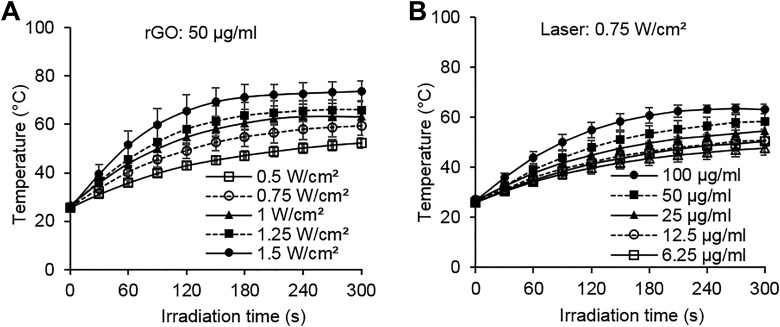
In order to confirm the photothermal conversion characteristics of rGO, we examined the temperature changes in rGO solution with different concentrations under laser irradiation at different powers. The same concentration of rGO solution (50 μg/mL) was able to obtain varying degrees of temperature growth under different light doses (Figure 2A), while at the same light dose (0.75 W/cm2) different rGO concentrations can also cause different temperature increases (Figure 2B). These results showed that the photothermal conversion effect of rGO depends on the rGO concentration and light dose.
Figure 2.
The photothermal conversion effect of rGO under laser irradiation. A, Temperature increase in reduced graphene oxide (rGO) solution (50 µg/mL) during 980-nm laser irradiation with different doses (n = 3). B, Temperature increase in rGO solution at different concentrations during 980-nm laser irradiation (0.75 W/cm2; n = 3).
In Vivo Experiment of Tumor Therapy
In order to study the effect of different laser doses combined with different rGO doses on tumor killing, we used the mouse pancreatic tumor model for treatment. The temperature of the surface, center, and bottom of the tumors was measured simultaneously using the infrared thermal imager and thermocouple probes.
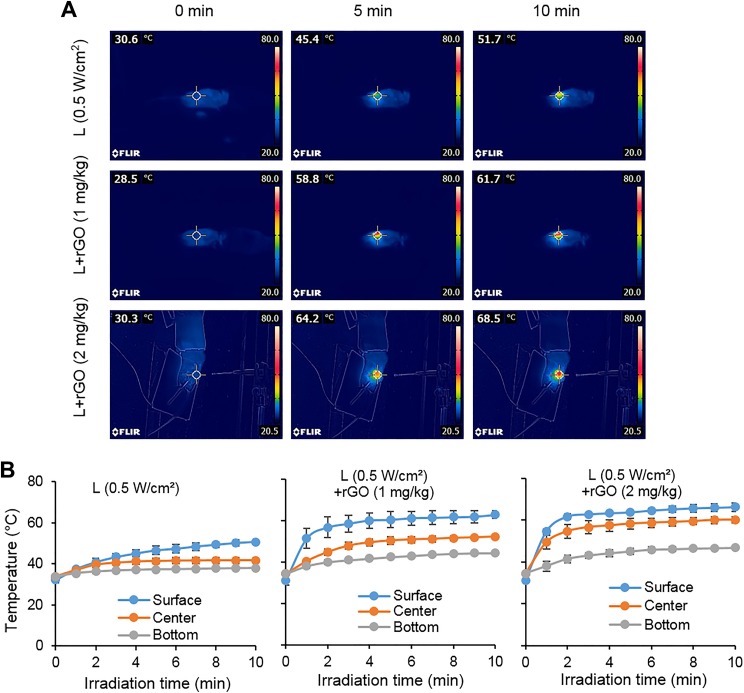
A laser power density of 0.5 W/cm2 combined with rGO treatment can obtain a higher tumor surface temperature than laser irradiation alone. Compared to the results of 1 mg/kg rGO group, 2 mg/kg rGO has a higher temperature rise to about 68°C (Figure 3A). The temperatures of the center and bottom of the tumors showed that the temperature exhibited a gradient distribution in the tumor and that the temperature at the center of the tumor and the bottom of the tumor also resulted in a higher temperature rise in the 2 mg/kg rGO group (Figure 3B).
Figure 3.
Temperature increase in tumor tissue during laser irradiation (0.5 W/cm2) with or without reduced graphene oxide (rGO). A, Thermographic images of mice under laser irradiation with intratumoral rGO injection at different concentrations. Bottom images show the thermocouple detection with inserted needle probes in tumor tissue during laser irradiation. B, Plots of temperature increase at different positions in tumor tissue during laser irradiation by a 980-nm laser with rGO at different concentrations (n = 3).
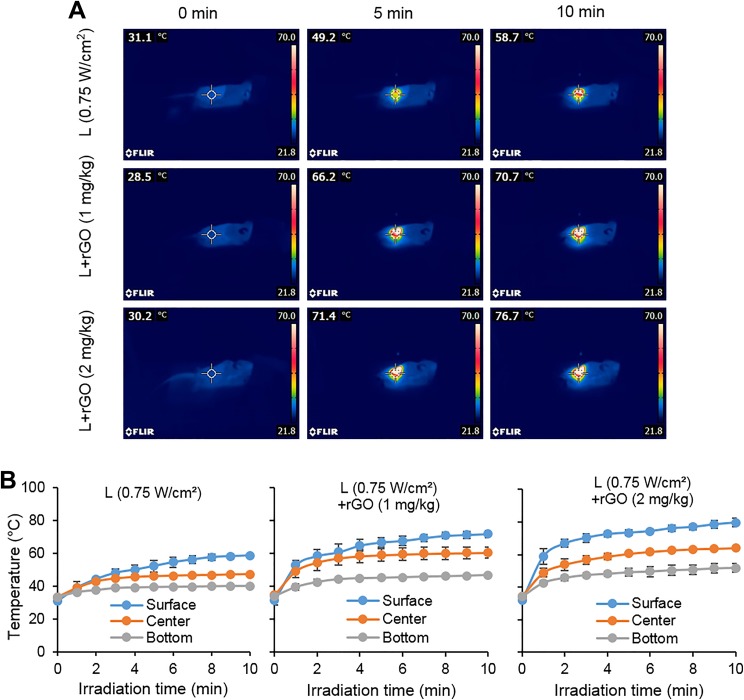
A higher treatment temperature was obtained at a higher laser dose of 0.75 W/cm2. Similar to the previous results, the use of higher dose of rGO can result in higher treatment temperature increases. The surface temperature of the laser combined with 2 mg/kg rGO group was increased by about 76°C after 10 minutes irradiation (Figure 4A). Tumor surface, center, and bottom temperature measurements confirm the characteristics of the temperature gradient distribution in the tumor (Figure 4B).
Figure 4.
Temperature increase in tumor tissue during laser irradiation (0.75 W/cm2) with or without reduced graphene oxide (rGO). A, Thermographic images of mice under laser irradiation with intratumoral rGO injection at different concentrations. B, Plots of temperature increase at different positions in tumor tissue during laser irradiation by a 980-nm laser with rGO at different concentrations (n = 3).
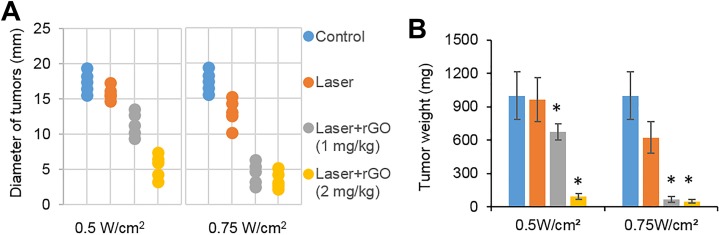
At 10 days after treatment, the tumor size and weight of the tumor-bearing mice were measured. The results showed that the tumor size and weight of the laser combined with rGO treatment group were significantly lower than those of the control group and the laser-only group under the laser dose of 0.5 and 0.75 W/cm2, respectively. Among these treatment groups, mice with the irradiation dose of 0.75 W/cm2 combined with the 2 mg/kg rGO treatment group received the highest inhibition of tumor growth (Figure 5).
Figure 5.
Therapeutic effects of rGO on tumors under laser irradiation by a 980-nm laser. Diameter (A) and weight (B) of tumors 10 days after indicated treatment (n = 5; *P < .05).
Discussion
The clinical treatment for pancreatic cancer is difficult, and the mortality is very high.14 Because of the biological characteristics of pancreatic cancer, the clinical effect of radiation therapy and chemotherapy is usually poor, and because of the high side effects, patient intolerance, and other reasons, the traditional treatments had many limitations in clinical use.15,16 Therefore, it is of great clinical significance to find a new, low-toxicity, and low-risk treatment strategy for advanced pancreatic cancer. The interventional ablation treatments for unresectable malignancies by high temperature, freezing, and other physical methods have received more and more attention.17,18
Laser therapy is one of the means of interventional ablation because it can achieve the effect of killing the tumor by irradiation, without damaging the integrity of the tumor. However, due to the limited penetration of the laser to the tissue, the use of photosensitizers in combination is often required.19 The GO has a good photothermal conversion effect and, when combined with laser treatment, can effectively produce overheating effect, which leads to tumor killing. Therefore, GO is considered to be efficient as the photosensitizer.20 Studies have shown that the size, shape, and other physical properties of graphene could play important roles in the toxicity, distribution, and excretion. Thus, different size graphene materials may have different effects on the therapeutic effect.21,22 The results of this study show that rGO has higher light absorption capability than NGO, so rGO has shown higher clinical application potential.
The results of photothermal experiments showed that rGO has a good photothermal conversion effect and can effectively produce an overheating effect when used in conjunction with laser. In addition, it was found that an increased laser dose used with a higher rGO concentration produces an increase in temperature. In vivo experiments showed that, compared to the laser treatment alone, rGO combined with laser treatment can form an ideal photothermal reaction, resulting in higher thermal effects. The temperature characteristics of gradient distribution suggest that when laser treatment is used, full consideration should be given to the distribution of temperature in the tumor and control the distribution of heat to achieve the better tumor-killing effect.
In summary, in vitro results showed that rGO is an ideal photosensitizing material, and the photothermal conversion effect of rGO increases with the increase in rGO concentration and laser dose. In vivo experiments showed that 980-nm laser combined with rGO can achieve higher therapeutic temperature and better therapeutic effect than laser alone and has the potential for clinical application.
Acknowledgments
The authors want acknowledge the contribution of Elivia Layton in this manuscript.
Abbreviations
- GO
graphene oxide
- NGO
nano graphene oxide
- OUHSC
University of Oklahoma Health Sciences Center
- rGO
reduced graphene oxide.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research is supported by grants from the Oklahoma Center for Advancement of Science and Technology (HR16-085), and from National Natural Science Foundation of China (No. 81571726/ 61675043).
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2. Luo J, Xiao L, Wu C, Zheng Y, Zhao N. The incidence and survival rate of population-based pancreatic cancer patients: Shanghai Cancer Registry 2004-2009. PLoS One. 2013;8(10):e76052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rossi ML, Rehman AA, Gondi CS. Therapeutic options for the management of pancreatic cancer. World J Gastroenterol. 2014;20(32):11142–11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ghosn M, Kourie HR, El Karak F, Hanna C, Antoun J, Nasr D. Optimum chemotherapy in the management of metastatic pancreatic cancer. World J Gastroenterol. 2014;20(9):2352–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Z, Ren ZG, Ma NY, et al. Intensity modulated radiotherapy for locally advanced and metastatic pancreatic cancer: a mono-institutional retrospective analysis. Radiat Oncol. 2015;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crochet JJ, Gnyawali SC, Chen Y, Lemley EC, Wang LV, Chen WR. Temperature distribution in selective laser-tissue interaction. J Biomed Opt. 2006;11(3):34031. [DOI] [PubMed] [Google Scholar]
- 7. Chen WR, Korbelik M, Bartels KE, Liu H, Sun J, Nordquist RE. Enhancement of laser cancer treatment by a chitosan-derived immunoadjuvant. Photochem Photobiol. 2005;81(1):190–195. [DOI] [PubMed] [Google Scholar]
- 8. Zhou F, Xing D, Ou Z, Wu B, Resasco DE, Chen WR. Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. J Biomed Opt. 2009;14(2):021009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng X, Xing D, Zhou F, Wu B, Chen WR. Indocyanine green-containing nanostructure as near infrared dual-functional targeting probes for optical imaging and photothermal therapy. Mol Pharm. 2011;8(2):447–456. [DOI] [PubMed] [Google Scholar]
- 10. Hainfeld JF, Lin L, Slatkin DN, et al. Gold nanoparticle hyperthermia reduces radiotherapy dose. Nanomedicine. 2014;10(8):1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geim AK, Novoselov KS. The rise of graphene. Nat Mater. 2007;6(3):183–191. [DOI] [PubMed] [Google Scholar]
- 12. Bitounis D, Ali-Boucetta H, Hong BH, Min DH, Kostarelos K. Prospects and challenges of graphene in biomedical applications. Adv Mater. 2013;25(16):2258–2268. [DOI] [PubMed] [Google Scholar]
- 13. Yang K, Zhang S, Zhang G, Lee ST, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10(9):3318–3323. [DOI] [PubMed] [Google Scholar]
- 14. Mohammed S, Van Buren G, Fisher WE. Pancreatic cancer: advances in treatment. World J Gastroenterol. 2014;20(28):9354–9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sugawara A, Kunieda E. Effect of adjuvant radiotherapy on survival in resected pancreatic cancer: a propensity score surveillance, epidemiology, and end results database analysis. J Surg Oncol. 2014;110(8):960–966. [DOI] [PubMed] [Google Scholar]
- 16. Gurusamy KS, Kumar S, Davidson BR, Fusai G. Resection versus other treatments for locally advanced pancreatic cancer. Cochrane Database Syst Rev. 2014;(2):CD010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fegrachi S, Besselink MG, van Santvoort HC, Molenaar IQ. Radiofrequency ablation for unresectable locally advanced pancreatic cancer: a systematic review. HPB (Oxford). 2014;16(2):119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song ZG, Hao JH, Gao S, Gao CT, Tang Y, Liu JC. The outcome of cryoablation in treating advanced pancreatic cancer: a comparison with palliative bypass surgery alone. J Dig Dis. 2014;15(10):561–569. [DOI] [PubMed] [Google Scholar]
- 19. Chen WR, Adams RL, Carubelli R, Nordquist RE. Laser-photosensitizer assisted immunotherapy: a novel modality for cancer treatment. Cancer Lett. 1997;115(1):25–30. [DOI] [PubMed] [Google Scholar]
- 20. Sahu A, Choi WI, Lee JH, Tae G. Graphene oxide mediated delivery of methylene blue for combined photodynamic and photothermal therapy. Biomaterials. 2013;34(26):6239–6248. [DOI] [PubMed] [Google Scholar]
- 21. Yang Y, Rigdon W, Huang X, Li X. Enhancing graphene reinforcing potential in composites by hydrogen passivation induced dispersion. Sci Rep. 2013;3:2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akhavan O, Ghaderi E, Akhavan A. Size-dependent genotoxicity of graphene nanoplatelets in human stem cells. Biomaterials. 2012;33(32):8017–8025. [DOI] [PubMed] [Google Scholar]