Short abstract
Objective
Neural stem cells play an important role in the recovery and regeneration of peripheral nerve injury, and the microRNA-7 (miR-7) regulates differentiation of neural stem cells. This study aimed to explore the role of miR-7 in neural stem cells homing and proliferation and its influence on peripheral nerve injury repair.
Methods
The mice model of peripheral nerve injury was created by segmental sciatic nerve defect (sciatic nerve injury), and neural stem cells treatment was performed with a gelatin hydrogel conduit containing neural stem cells inserted into the sciatic nerve injury mice. The Sciatic Function Index was used to quantify sciatic nerve functional recovery in the mice. The messenger RNA and protein expression were detected by reverse transcription polymerase chain reaction and Western blot, respectively. Luciferase reporter assay was used to confirm the binding between miR-7 and the 3’UTR of cell division cycle protein 42 (cdc42). The neural stem cells migration and proliferation were analyzed by transwell assay and a Cell-LightTM EdU DNA Cell Proliferation kit, respectively.
Results
Neural stem cells treatment significantly promoted nerve repair in sciatic nerve injury mice. MiR-7 expression was decreased in sciatic nerve injury mice with neural stem cells treatment, and miR-7 mimic transfected into neural stem cells suppressed migration and proliferation, while miR-7 inhibitor promoted migration and proliferation. The expression level and effect of cdc42 on neural stem cells migration and proliferation were opposite to miR-7, and the luciferase reporter assay proved that cdc42 was a target of miR-7. Using co-transfection into neural stem cells, we found pcDNA3.1-cdc42 and si-cdc42 could reverse respectively the role of miR-7 mimic and miR-7 inhibitor on neural stem cells migration and proliferation. In addition, miR-7 mimic-transfected neural stem cells could abolish the protective role of neural stem cells on peripheral nerve injury.
Conclusion
MiR-7 inhibited peripheral nerve injury repair by affecting neural stem cells migration and proliferation through cdc42.
Keywords: miRNA-7, peripheral nerve injury, neural stem cell transplantation, cdc42
Introduction
Peripheral nerve injury is a commonly encountered disease, resulting in degeneration of damaged axons and their myelin sheaths distal to the site of the injury. Neural stem cells (NSCs) have considerable capacity for self-renewing and for generating neurons in the mammalian brain. It was reported that NSC transplantation can promote peripheral nerve regeneration, which provides a new means for the treatment of peripheral nerve injury.1 Although the study on NSCs has become a focus in many laboratories, the function regulation mechanisms of NSCs are not fully clear.
Cell division cycle protein 42 (cdc42), a member of the Rho family, has been implicated in the regulation of various cell functions, such as cell morphology, polarity, and cell proliferation.2 A recent study by Han et al.3 reported that the mRNA and protein expression level of cdc42 were both significantly upregulated after sciatic nerve injury (SNI), and cdc42 siRNA remarkably inhibited Schwann cell proliferation and migration, while pcDNA3.1-cdc42 showed a contrary effect. Previous studies reported that cdc42 was involved in mouse neural stem/progenitor cell proliferation,4 embryonic stem cell, and cortical interneuron migration.5,6 These results suggested that cdc42 may be involved in NSCs proliferation and migration alteration.
MicroRNAs (miRNAs) are genomically encoded small RNAs used by organisms to regulate the expression of proteins generated from mRNA transcripts.7 MiR-7 has been characterized as a tumor suppressor in several human cancers and targets a number of proto-oncogenes that contribute to cell proliferation and survival.8 Previous studies have reported that the miR-7 showed conserved expression in neural tissues9 and regulated NSC differentiation.10,11 Fan et al.12 found that miR-7 could enhance subventricular zone neurogenesis by inhibiting the nucleotide-binding oligomerization domain-like receptor family, pyrin domain containing 3 (NLRP3)/Caspase-1 axis in adult NSC. However, there is very little research on the regulation mechanism of miR-7 in NSCs. In addition, with the prediction analysis of TargetScan, we found that miR-7 could bind to cdc42–3’UTR. Therefore, we speculated that miR-7 could regulate NSCs proliferation and migration by targeting cdc42 to influence peripheral nerve injury repair.
Therefore, this article aimed to investigate the expression of miR-7 and cdc42 in injury peripheral nerve mice and NSCs and confirm that miR-7 could regulate NSCs proliferation and migration by targeting cdc42. Moreover, we wanted to demonstrate that miR-7 influence peripheral nerve injury repair by affecting NSCs migration and proliferation through cdc42.
Materials and methods
Surgical procedure and cell transplantation
The mice were randomly divided into five groups (n = 8 in each group): the Sham group, the Sham + NSCs group, the SNI group, the SNI+ Veh (gelatin gel conduit without NSC) group, and the SNI+ NSCs group. The SNI group: Creation of the SNI and cell transplantation were performed as described with a slight modification.13 NSCs were allowed to adhere onto the inner surface of a gelatin hydrogel conduit at 104 cells per tube. Balb/c mice (6∼8 weeks) were anesthetized, and a longitudinal incision was made at the lower limb in parallel with both the extension of the Achilles tendon and gastrocnemius muscle. A segmental sciatic nerve defect was created (the length of the defect was 5 mm). The SNI+ NSCs group: the proximal and distal termini of SNI mice were inserted by a gelatin hydrogel conduit containing NSCs by a manner that the termini were 5 mm apart from each other. The Sham group: mice received the same surgical procedure except for the lack of creation of the nerve defect and transplantation. Every animal experiment was approved by the institutional Animal Experiment Committee, and the care of the animals was in accordance with our institutional guidelines.
Electrophysiological studies
The compound muscle action potential (CMAP) of the mice in each group was determined to reflect the number and functional state of excitable cells. After anesthesia was induced, the sciatic nerve was exposed, and the nerve repair site was identified under a surgical microscope. The nerve repair area was insulated from the surrounding muscle with a rubber dam. A bipolar stimulating electrode was placed under the sciatic nerve at a location of 10 mm proximal to the graft site. A recording electrode was placed in the gastrocnemius muscle, and the CMAPs were recorded. The CMAP peak amplitude and the CMAP latency values were calculated.
NSCs isolation and culture
NSCs were isolated from the medial and lateral ganglionic eminences of C57BL/6 mice as described by Addington et al.14 and in accordance with a protocol approved by the Institutional Animal Care and Use Committee at the First Affiliated Hospital of Zhengzhou University. In short, mice were quickly decapitated after anesthesia. The fetus was then extracted from the two uterine horns. Fetal tissue was flushed with cold Leibovitz media (life science and technology, Carlsbad, CA) at the anatomical stages of ganglion uplift. Ganglionic eminence were cleaned before mechanical disinfection by working NSC medium, including 6 ng/ml glucose (Acros Organics, Geel, Belgium), 5 Mm HEPES buffer (Sigma Aldrich, Saint Louis, MO), 62.9 ng/ml progesterone, 9.6 g/ml putrescine, 1.83 g/ml heparin (Sigma, Aldrich), B27 (1X, Life Technologies Growth Supplement), 20 ng/ml epidermal growth factor, 5 ng/ml fibroblast growth factor, 5 g/ml insulin, 5 g/ml transferrin, and 5 ng/ml sodium selenite (sigma Aldrich) in the Dulbecco’s Modified Eagle’s medium (DMEM; Life Technologies) and plated in the humidified incubator at 37°C, 20% O2 and 5% CO2 with a density of 104 cells/ml. NSCs were cultured as non-adherent neurospheres in working NSC medium and passaged by mechanical dissociation.
Estimation of muscle mass
The gastrocnemius muscle was removed from the affected/unaffected limbs of mice in each group and was weighed and calculated. The muscle mass ratio for each mice was calculated using this formula: Muscle mass ratio = (muscle mass of the affected side)/(muscle mass of the unaffected side) × 100%.15
Estimation of myelinated axons
Nerve defect lesions were excised eight weeks after the surgery, the specimens were cross-sectioned at the proximal third of the graft, fixed in 10% buffered formalin solution, and embedded in paraffin.16 Luxol blue dye stained the 5-μm sections, and BZ-9000 (Keyence) with ImageJ software captured fluorescence and optical light microscopic images. Each cross-section with five images was analyzed, and the number of myelinated fibers per nerve was detected to determine the density of the myelinated fibers. At last, ImageJ software was used to calculate the total number of myelinated axons.
Walking track analysis
Six and 12 weeks after the surgery, all mice (n = 8 in each group) were subjected to a walking-track analysis.17 The sciatic functional index (SFI) was calculated using this formula: SFI = 118.9 × ([ETS − NTS]/NTS) − 51.2 × ([EPL − NPL]/NPL) − 7.5, where ETS refers to experimental toe spread; NTS, normal toe spread; EPL, experimental print length; and NPL, normal print length.
Cell transfection
The miR-7 mimic/inhibitor, pcDNA3.1-cdc42, si-cdc42, and their respective control were chemically synthesized or purchased from GenePharma Co. (Shanghai, China) and transfected (100 nM) or co-transfected into NSCs cells using Invitrogen Lipofectamine™ 2000 transfection reagent (Thermo Fisher Scientific). The detailed information regarding miR-7 mimic and miR-7 inhibitor and their controls is listed as follows: miR-7 mimic: (sense: 5′ -UGGAAGACUAGUGAUUUUGUUGU-3′, antisense: 5′ -AACAAAAUCACUAGUCUUCCAUU-3′); the negative control of miR-7 mimic (pre-NC, sense: 5′ -UUCUCCGAACGUGUCACGUTT-3′, antisense: 5′ -ACGUGACACGUUCGGAGAATT-3′); miR-7 inhibitor: (5′ -ACAACAAAAUCACUAGUCUUCCA- 3′), the negative control of miR-7 inhibitor (NC, 5′ -CAGUACUUUUGUG UAGUACA A-3′).
Luciferase reporter assay
Two luciferase reporters containing the wild-type cdc42 (pcDNA-cdc42-WT) or mutant cdc42 (pcDNA-cdc42-MUT) were generated to analyze the interaction between cdc42 and miR-7. Mutant cdc42 contained a mutation site abolishing targeting by miR-7. The luciferase construct along with miR-7 mimic, negative control, miR-7 inhibitor or negative control were transfected into 293A cells. At 48-h post-transfection, luciferase activity assays were performed with the dual-luciferase reporter assay system.
NSCs migration assay
Prior to the migration assay, the NSCs transient transfection was performed for 48 h and incubated with stromal cell derived factor 1a (SDF-1a) for 1 h for migration induction. Migration of NSCs was estimated by transwell-based assay (a 6.5-mm transwell chambers with 8-mm pores). The bottom surface of each membrane was coated with 10 mg/ml fibronectin and then 106 cells/ml NSCs were resuspended in DMEM and placed onto the upper chambers of each transwell and incubated for 1 h. Adding complete medium into the lower chambers, 6 h later, a cotton swab was used to remove the upper surface of each membrane. Cells on the lower surface of each membrane were stained with 0.1% Crystal Violet, imaged, and counted using a DMR inverted microscope (Leica Microsystems, Buffalo Grove, IL).
NSCs proliferation assay
Prior to the proliferation assay, the NSCs were transient transfected for 48 h and incubated with SDF-1a for 1 h. The Cell-Light™ EdU DNA Cell Proliferation Kit (RiboBio Co., Ltd., China) was used to measure the proliferation of NSCs according to the manufacturer’s instructions. NSCs were suspended with pre-warmed complete medium (37°C), counted, and plated onto 96-well plates coated with 0.01% poly-L-lysine. Then EdU was added into cell culture and incubated for another 24 h, and then the cells were fixed with 4% formaldehyde in PBS for 30 min and nucleus stained with Hoechst 33342. The cell proliferation was analyzed by using images of randomly selected fields obtained on a DMR fluorescence microscope (Leica Microsystems, Bensheim, Germany).
Quantitative reverse transcription polymerase chain reaction analysis
Total RNA was isolated from the harvested proximal stumps of sciatic nerve after injury and the collected NSCs using TRIzol reagent (Invitrogen, Carlsbad, CA). For the miRNA quantification, we used the TaqMan microRNA Reverse Transcription Kit and the TaqMan Universal Master Mix II with the TaqMan MicroRNA Assay of miRNAs (Applied Biosystems, Foster City, CA) to test the miR-7 expression level in the tissues or cells. The cdc42 levels were calculated relative to glyceraldehyde-3-phosphate dehydrogenase (internal control) via the 2−ΔΔCt method using a real-time polymerase chain reaction system according to the manufacturer’s instructions with SYBR® Green Master Mix (Applied Biosystems, Foster City, CA).
Western blotting analysis
The proximal stumps of sciatic nerve were harvested after the injury or the collected NSCs were lysed in protein lysis buffer. Protein samples were separated using sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (PAGE), and then the PAGE was transferred onto polyvinylidene difluoride membranes (Thermo Scientific, Rockford, IL). Primary mouse monoclonal antibodies against Bax (Abcam, Cambridge, MA) and secondary antibody (Sigma, St. Louis, MO) were used in the Western blot. Band intensity was quantified using Quantity One software. The protein expression of Bax was normalized to the β-actin levels.
Overexpression of miR-7 in mice
The mice were randomly divided into three groups (n = 8 in each group): SNI, SNI + NSCs transfected with pre-NC, and SNI + NSCs transfected with miR-7 mimic. The miR-7 mimic or its negative control (pre-NC) was transfected into NSCs by using Invitrogen Lipofectamine™ 2000 transfection reagent (Thermo Fisher Scientific), and the NSCs were then transplanted into mice as described earlier. The number of myelinated fibers per nerve and axons was determined as well as related gene expression.
Statistical analysis
The SPSS 17.0 software (SPSS Inc., Chicago, IL) was applied for statistical analyses. The experiment was randomly divided into groups, in which each group was repeated at least three times, and the analyzers were blinded to the experimental groups. All experiments were repeated three times, and all data were presented as mean ± standard deviation. Differences among groups were analyzed with the one-factor analysis of variance method, and a level of P < 0.05 was considered significant.
Results
NSCs promoted the repair of the injured sciatic nerve
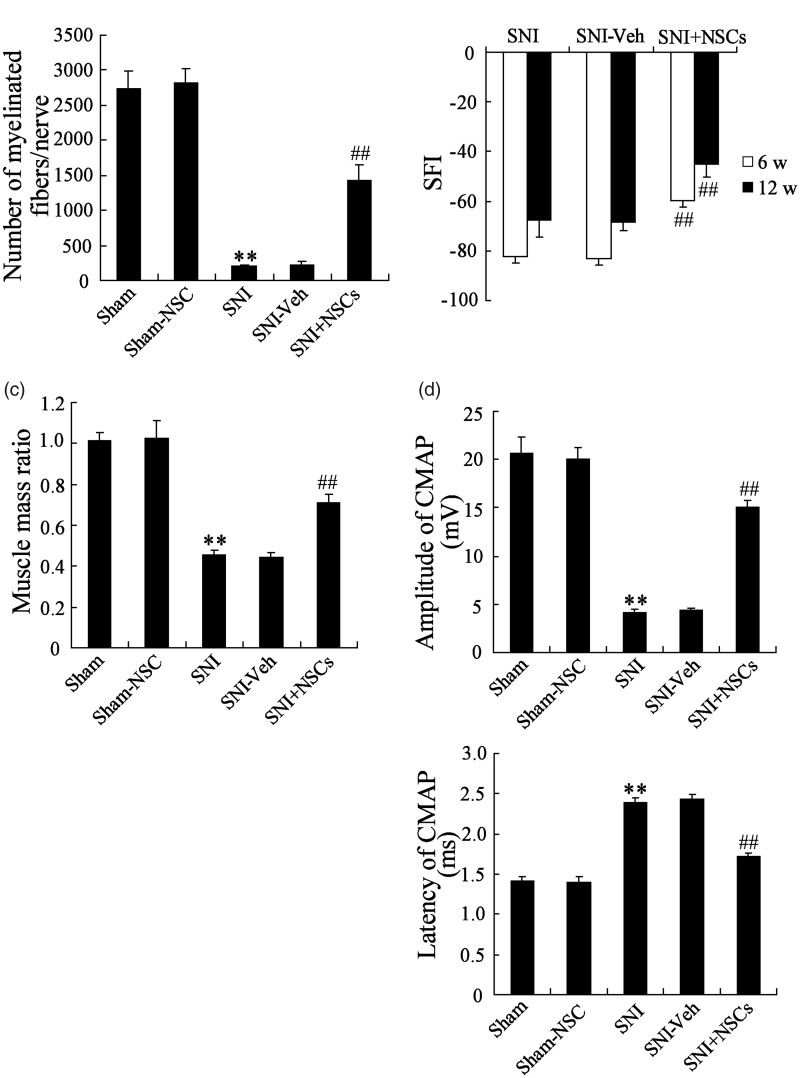
NSCs used in the present study were obtained from mice, and the potential of NSCs to promote the repair of the injured peripheral nerve was examined. NSCs transplantation was performed by a gelatin hydrogel conduit inserted into the created nerve defect lesions in SNI mice. As shown in Figure 1(a), the number of myelinated fibers per nerve in the SNI group was significantly less than in the Sham group, while the number of myelinated fibers/nerve in NSCs-transplanted (SNI + NSCs) group was increased, suggesting that NSCs transplanted obviously promoted myelination of the regenerated nerve. The functional recovery of the injured sciatic nerve was detected as shown in Figure 1(b). The walking track analysis indicated that NSCs transplanted had an obvious improvement in the locomotive performance at 6 and 12 weeks post-injury. The weight of the gastrocnemius muscle in the SNI group was significantly lighted than in the Sham group, while NSCs transplanted increased muscle mass ratio in SNI group, which suggested that NSCs transplanted apparently improved the muscle atrophy (Figure 1(c)). The detection results of amplitude and latency of the CMAP showed that NSCs treatment increased amplitude and decreased latency of CMAP (Figure 1(d)). These results revealed that NSCs transplanted promoted nerve repair after SNI.
Figure 1.
NSCs promoted nerve repair after sciatic nerve injury. The mice were randomly divided into five groups (n = 8 in each group). (a) NSCs treatment obviously promoted myelination of the regenerated nerve. (b) NSCs treatment significantly promoted the functional recovery of the injured sciatic nerve. (c) NSCs treatment apparently improved muscle atrophy. (d) NSCs treatment significantly influenced the amplitude and latency of the CMAP. **P < 0.01 vs. Sham group, ##P < 0.01 vs. SNI group.
The miR-7 and cdc24 expression level in injured sciatic nerve of mice
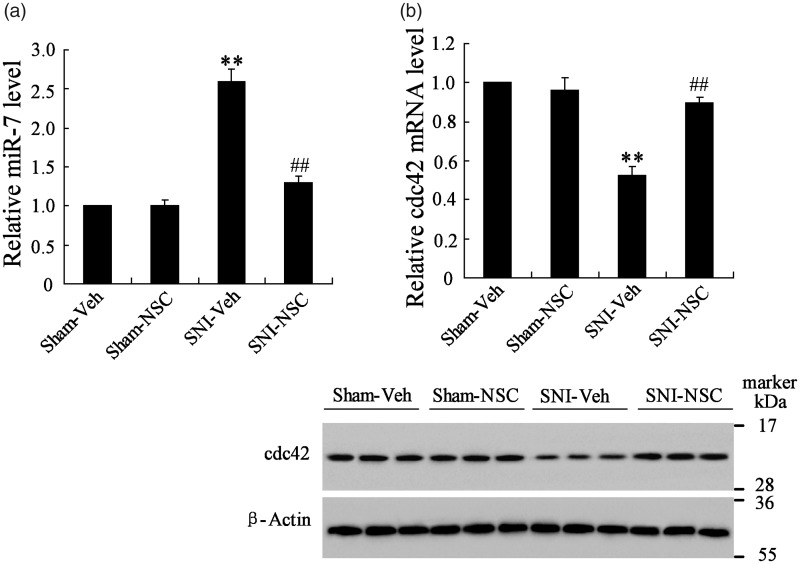
To investigate the expression levels of miR-7 and cdc24 in injured sciatic nerve of mice, the proximal stumps of the injured sciatic nerve tissue were isolated. As shown in Figure 2(a), compared to SNI group, transplantation of NSCs reduced miR-7 expression but increased cdc42 mRNA and protein expression (Figure 2(b)). These data also indicated that the miR-7 expression was the opposite of cdc42 expression in sciatic nerve of mice.
Figure 2.
The expression levels of miR-7 and cdc24 in proximal stumps of the injured sciatic nerve of mice. (a) NSCs treatment decreased miR-7 expression. (b) NSCs treatment increased cdc24 mRNA and protein expression. **P < 0.01 vs. Sham+Veh group, ##P < 0.01 vs. SNI+Veh group.
MiR-7 regulated SDF-1a-induced NSCs migration and proliferation
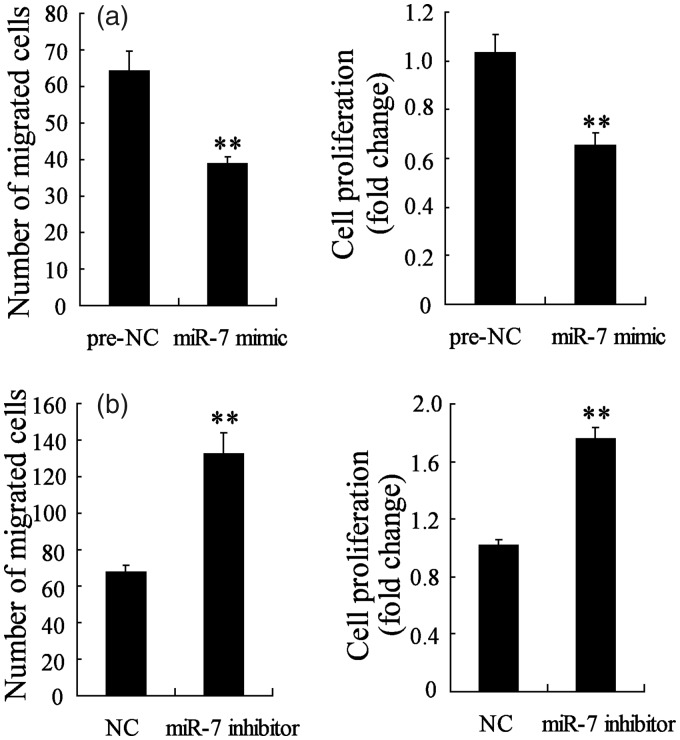
We have demonstrated that miR-7 expression was decreased in NSCs-transplanted SNI mice, so the effect of miR-7 on NSCs migration and proliferation were studied using transfection of miR-7 mimic or inhibitor into NSCs. NSCs express CXC chemokine receptor 4, the cognate receptor for SDF-1a, and exposure of SDF-1a to quiescent NSCs could enhance proliferation and promote migration.18 In this study, prior to the migration and proliferation assay, the NSCs were transient transfected for 48 h and incubated with SDF-1a for 1 h. Figure 3(a) shows that after transfection with miR-7 mimic, NSCs migration and proliferation were both inhibited, suggesting that overexpression of miR-7 suppressed SDF-1a-induced NSCs migration and proliferation. However, NSCs migration and proliferation were both promoted after transfection with miR-7 inhibitor, suggesting that the inhibition of miR-7 promoted SDF-1a-induced NSCs migration and proliferation (Figure 3(b)). These results implied that miR-7 expression could regulate SDF-1a-induced NSCs migration (Supplemental figure) and proliferation.
Figure 3.
MiR-7 regulated SDF-1a-induced NSCs migration and proliferation. (a) The NSCs migration and proliferation were both significantly reduced in NSCs transfected with miR-7 mimic. (b) The NSCs migration and proliferation were both obviously increased in NSCs transfected with miR-7 inhibitor. **P < 0.01 vs. NC.
Cdc42 regulated SDF-1a-induced NSCs migration and proliferation
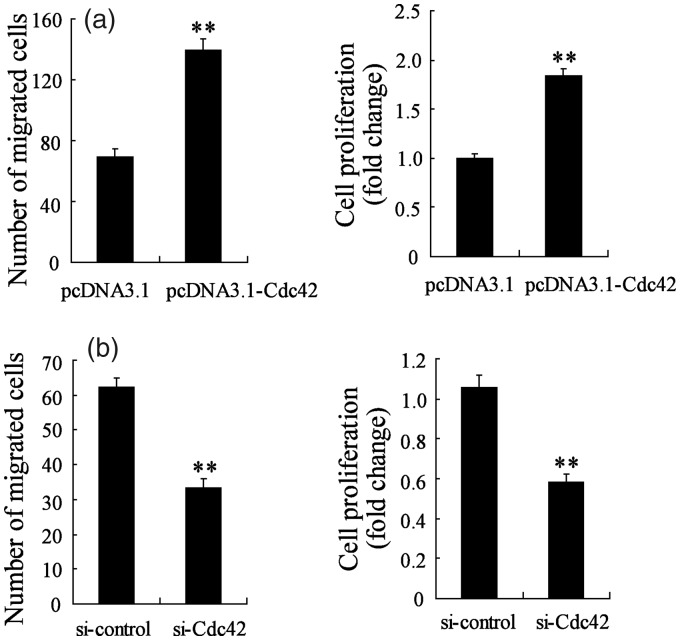
As we have known that cdc42 expression was increased in NSCs-transplanted SNI mice, so the effect of cdc42 on NSCs migration and proliferation were studied using transfection of pcDNA3.1-cdc42 and si-cdc42. Prior to the migration and proliferation assay, the NSCs were transient transfected for 48 h and incubated with SDF-1a for 1 h. Figure 4(a) shows that after transfection with pcDNA3.1-cdc42, NSCs migration and proliferation were promoted, suggesting that overexpression of cdc42 promoted SDF-1a-induced NSCs migration and proliferation. However, NSCs migration and proliferation were reduced after si-cdc42 transfection, suggesting that the inhibition of cdc42 suppressed SDF-1a-induced NSCs migration and proliferation (Figure 4(b)). These results implied that cdc42 expression could regulate SDF-1a-induced NSCs migration and proliferation.
Figure 4.
Cdc42 regulated SDF-1a-induced NSCs migration and proliferation. (a) The NSCs migration and proliferation were both significantly increased in NSCs transfected with pcDNA3.1-Cdc42. (b) The NSCs migration and proliferation were both obviously decreased in NSCs transfected with si-Cdc42. **P < 0.01 vs. pcDNA3.1 or si-control.
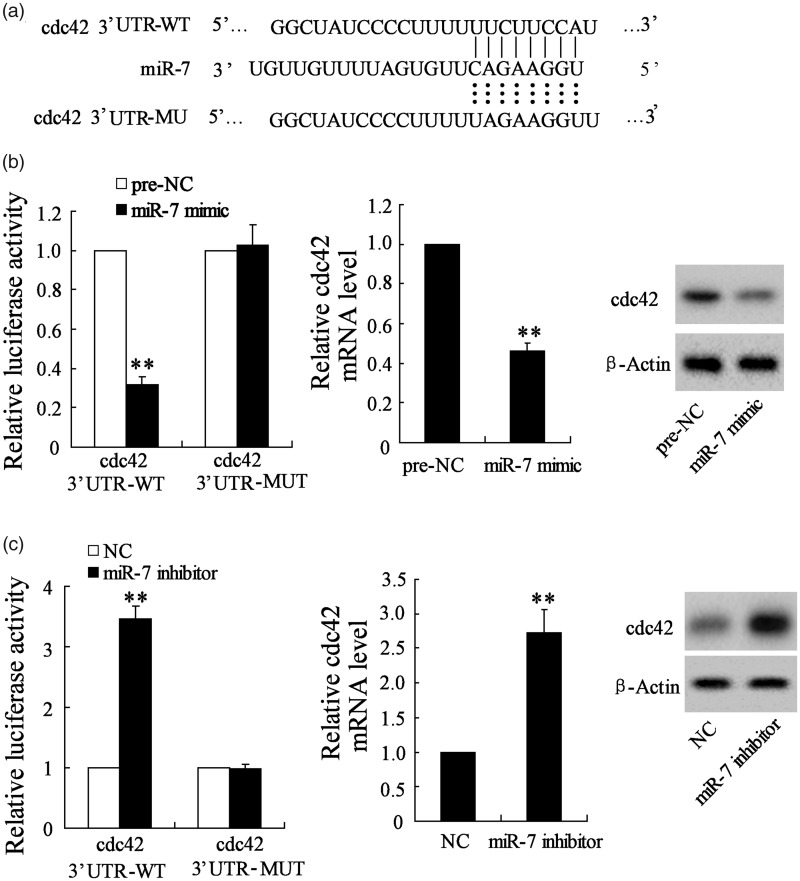
Cdc42 was a target of miR-7
To verify the relationship between miR-7 and cdc42, the luciferase reporter assay was used in this study. Figure 5(a) shows the sequences of the miR-7–binding sites in cdc42–3’UTR, which were predicted by TargetScan, and mutation design of the putative binding site in cdc42–3’UTR for miR-7. As shown in Figure 5(b), the miR-7 mimic reduced the luciferase activity in NSCs transfected with WT-cdc42, while had no impact in NSCs transfected with MUT-cdc42. Meanwhile, the mRNA and protein expression levels of cdc42 were significantly decreased in NSCs after transfection of miR-7 mimic. In addition, miR-7 inhibitor enhanced the luciferase activity in NSCs transfected with WT-cdc42, while had no impact in NSCs transfected with MUT-cdc42. Meanwhile, the mRNA and protein expression levels of cdc42 were increased in NSCs after transfection of miR-7 inhibitor (Figure 5(c)). These data demonstrated that cdc42 was a target of miR-7.
Figure 5.
Cdc42 was a target of miR-7. (a) The sequences of the miR-7–binding sites in cdc42 3’UTR were predicted by TargetScan, and mutation design of the putative binding site in cdc42 3’UTR for miR-7. (b) The miR-7 mimic reduced the luciferase activity in NSCs transfected with WT-cdc42, while had no impact in NSCs transfected with MUT-cdc42. Meanwhile, the mRNA and protein expression levels of cdc42 were significantly decreased in NSCs after transfection of miR-7 mimic. (c) The miR-7 inhibitor enhanced the luciferase activity in NSCs transfected with WT-cdc42, while had no impact in NSCs transfected with MUT-cdc42. Meanwhile, the mRNA and protein expression levels of cdc42 were significantly increased in the NSCs after transfection of miR-7 inhibitor. *P < 0.05 vs. NC.
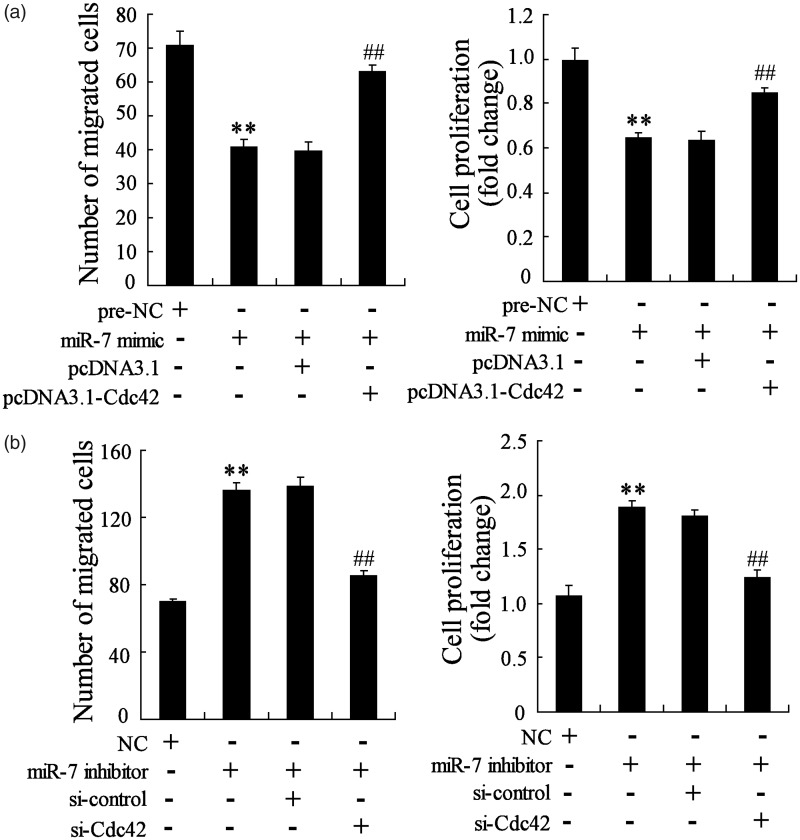
MiR-7 regulated SDF-1a-induced NSCs migration and proliferation by cdc42
Based on the above results, to confirm that miR-7 regulated SDF-1a-induced NSCs migration and proliferation by cdc42, the NSCs were co-transfected with miR-7 mimic and pcDNA3.1-cdc42 or miR-7 inhibitor and si-cdc42. Prior to the migration and proliferation assay, the NSCs were transient transfected for 48 h and incubated with SDF-1a for 1 h. As shown in Figure 6(a), the NSCs migration and proliferation were reduced in miR-7 mimic-transfected NSCs, which was reversed by pcDNA3.1-cdc42 co-transfection with miR-7 mimic into NSCs. Meanwhile, the NSCs migration and proliferation were promoted in miR-7 inhibitor-transfected NSCs, while si-cdc42 co-transfection with miR-7 inhibitor into NSCs reversed the increased migration and proliferation (Figure 6(b)). These data proved that miR-7 regulated SDF-1a-induced NSCs migration and proliferation by cdc42.
Figure 6.
MiR-7 controlled SDF-1a-induced NSCs migration and proliferation by cdc42. (a) The NSCs migration and proliferation were both significantly decreased in NSCs transfected with miR-7 mimic, while pcDNA3.1-Cdc42 co-transfection with miR-7 mimic reversed the decreased NSCs migration and proliferation. (b) The NSCs migration and proliferation were both obviously increased in NSCs transfected with miR-7 inhibitor, while si-Cdc42 co-transfection with miR-7 inhibitor reversed the increased NSCs migration and proliferation. **P < 0.01 vs. NC, ##P < 0.01 vs. miR-7mimic+pcDNA3.1 or miR-7 inhibitor+ si-control.
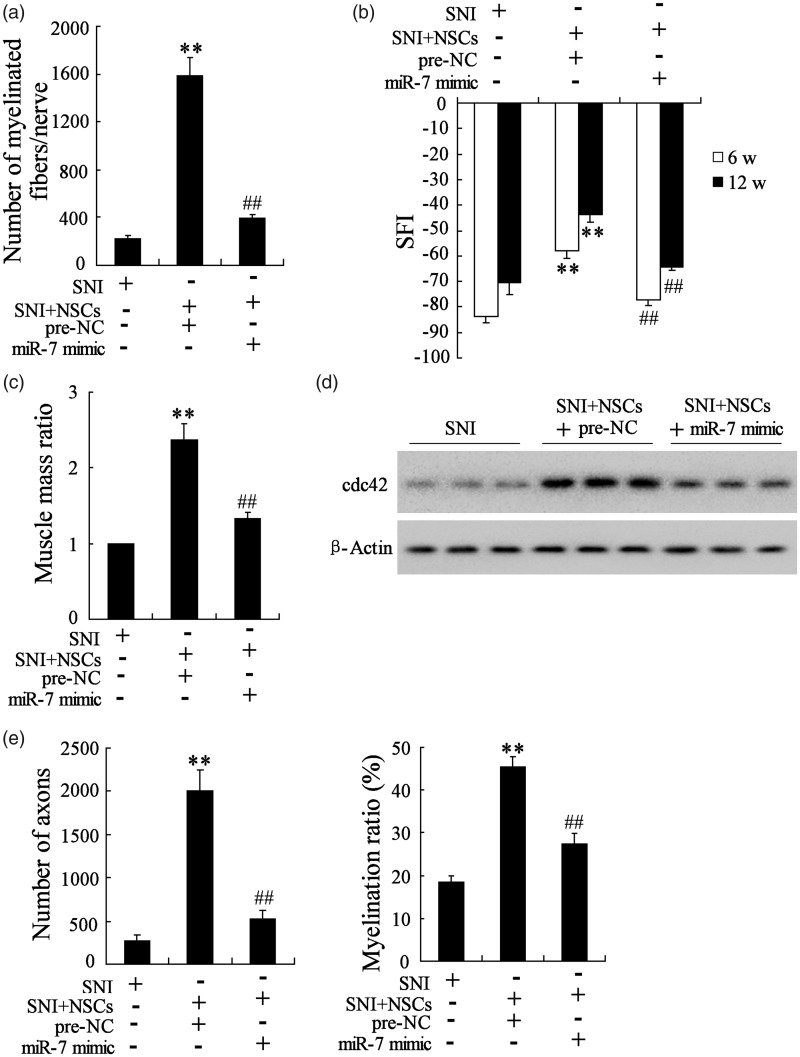
Overexpression of miR-7 abolished the protective role of NSCs on peripheral nerve injury
To explore the effect of miR-7 on peripheral nerve injury, the SNI mice received transplantation of miR-7 mimic-transfected NSCs, and then the number of myelinated fibers per nerve was calculated, and the SFI and muscle mass ratio after NSCs transplantation were also evaluated. As shown in Figure 7(a), the number of myelinated fibers/nerve was significantly greater in the pre-NC-SNI + NSCs group than in the SNI group. Transplantation of MiR-7 mimic-transfected NSCs inhibited the myelination of the regenerated nerve, presenting a decreased number of myelinated fibers/nerve and suggesting that overexpression of miR-7 abolished the promotive role of NSCs on myelination of the regenerated nerve. Compared to SNI group, the walking track analysis indicated that the functional recovery of the injured sciatic nerve in transplantation of pre-NC-transfected NSCs group had an obvious improvement of the locomotive performance at 6 and 12 weeks post-injury. While transplantation of miR-7 mimic-transfected NSCs obviously inhibited functional recovery of the injured sciatic nerve, presenting a decreased SFI in Figure 7(b) and suggesting that overexpression of miR-7 abolished the promotive role of NSCs on functional recovery of the injured sciatic nerve. Comparing to SNI group, the muscle atrophy in transplantation of pre-NC-transfected NSCs group had an obvious improvement, while transplantation of miR-7 mimic-transfected NSCs aggravated muscle atrophy, presenting a lighter muscle mass ratio in Figure 7(c) and suggesting that overexpression of miR-7 abolished the improving role of NSCs on muscle atrophy. Lastly, we also found that the miR-7 mimic abolished the effect of NSCs on axon number (Figure 7(e)). These results revealed that overexpression of miR-7 abolished the protective role of NSCs on peripheral nerve injury.
Figure 7.
Overexpression of miR-7 abolished the protective role of NSCs on peripheral nerve injury. The mice were randomly divided into three groups (n = 8 in each group): the SNI group, the SNI+ pre-NC-transfected NSCs group, the SNI+ miR-7 mimic-transfected NSCs group. (a) MiR-7 mimic abolished the promotive role of NSCs on myelination of the regenerated nerve. (b) MiR-7 mimic abolished the promotive role of NSCs on functional recovery of the injured sciatic nerve. (c) MiR-7 mimic abolished the improving role of NSCs on muscle atrophy. (d) MiR-7 mimic decreased cdc42 expression in vivo. (e) MiR-7 mimic abolished the effect of NSCs on axon number. **P < 0.01 vs. SNI, ##P < 0.01 vs. SNI+ pre-NC-NSCs.
In addition, the cdc42 protein was also analyzed by Western blot. As shown in Figure 7(d), compared to SNI group, the cdc42 expression was increased in transplantation of pre-NC-transfected NSCs group, and it was reversed in transplantation of miR-7 mimic-transfected NSCs group, suggesting overexpression miR-7 decreased cdc42 expression in vivo.
Discussion
Peripheral nerve injuries are common, and there is no easily available formula for successful treatment. In recent years, cell transplantation approaches are being used in experimental models of peripheral nerve injury to enhance nerve regeneration. Transplantation of Schwann cells,19 olfactory ensheathing cells,20 embryonic spinal cord-derived cells,21 nestin-positive hair follicle pluripotent stem cells,22 and so on were reported to be beneficial to the regenerative process because they could secrete chemokines and arrange along with the long axis of the nerve fibers to guide extension of regenerative axon to target structure. NSCs are a distinct group of cells present in the embryonic and adult mammalian central nervous system (CNS) that can self-renew and give rise to the major cell types of the CNS, such as neurons, astrocytes, and oligodendrocytes.23 NSCs transplantation could promote nerve regeneration in acute peripheral nerve traction injury as shown by a prolonged increase of nerve T1 and T2 values.1 In this study, we transplanted NSCs within a gelatin hydrogel conduit into segmental nerve defect lesions surgically created at the sciatic nerve of SNI mice, and the results showed the promotive and improving role of NSCs on myelination of the regenerated nerve, functional recovery of the injured sciatic nerve, and muscle atrophy (Figure 1).
A number of miRNAs have been found in the mammalian CNS and peripheral nerve system, including the brain, spinal cord, and dorsal root ganglion, where they are involved in neuro-development and neurological diseases. MiR-7 was a conserved gene in neural tissues, regulated NSC differentiation.9–11 In this study, the miR-7 expression was upregulated in proximal stumps of the injured sciatic nerve tissue, while after transplantation of NSCs, miR-7 expression was significantly reduced (Figure 2(a)). In addition, the effect of miR-7 on NSCs migration and proliferation was also investigated. As shown in Figure 3, NSCs transfected with miR-7 mimic had a significantly decreased migration and proliferation, while NSCs transfected with miR-7 inhibitor had a significantly increased migration and proliferation, which is the first to demonstrate that miR-7 could regulate SDF-1a-induced NSCs migration (Supplemental figure) and proliferation. An in vivo experiment was carried out by transplantation of miR-7 mimic-transfected NSCs into SNI mice, and the result proved that miR-7 mimic abolished the promotive and improving role of NSCs on myelination of the regenerated nerve, functional recovery of the injured sciatic nerve, and muscle atrophy (Figure 7).
Previous study has reported that miR-7 could enhance subventricular zone neurogenesis by targeting NLRP3 in adult NSC.12 In our study, the cdc42 mRNA and protein expression in proximal stumps of the injured sciatic nerve tissue were detected by reverse transcription polymerase chain reaction and Western blot, respectively, and the results showed that after transplantation of NSCs, cdc42 mRNA and protein expression were significantly increased in proximal stumps of the injured sciatic nerve tissue (Figure 2(b)). A recent study by Han et al.3 reported that comparing to the control Sham, the cdc42 mRNA and protein expression level were both significantly upregulated after SNI. We believed that the increased cdc42 in this case was protective, so the cdc42 expression was further promoted after NSCs treatment. Moreover, cdc42 also could regulate SDF-1a-induced NSCs migration and proliferation, but it was the opposite of miR-7 (Figure 4). With the prediction analysis of TargetScan and then verification test by the luciferase reporter assay, we confirmed that cdc42 was a target of miR-7 (Figure 5). Next, in vitro knockdown or overexpression rescue experiments showed that pcDNA3.1-cdc42 and si-cdc42 could reverse the effect of miR-7 mimic and inhibitor on NSCs migration and proliferation, respectively, suggesting that miR-7 regulated SDF-1a-induced NSCs migration and proliferation by cdc42 (Figure 6).
In conclusion, it is revealed that miR-7 in the NSCs was a metastasis- and proliferation-suppressing miRNA, a potential inhibitor of recovery from peripheral nerve injury. Furthermore, our data suggested that miR-7 regulated NSCs migration and proliferation by targeting cdc42. The findings from our study demonstrated that miR-7 influenced peripheral nerve injury repair through affecting NSCs migration and proliferation by target cdc42.
Supplemental Material
Supplemental material, Supplementary Figure for MiR-7 inhibited peripheral nerve injury repair by affecting neural stem cells migration and proliferation through cdc42 by Nan Zhou, Shuang Hao, Zongqiang Huang, Weiwei Wang, Penghui Yan, Wei Zhou, Qihang Zhu and Xiaokang Liu in Molecular Pain
Acknowledgments
We would like to thank Dr Wei Zhou for his kind assistance in the language embellishment of this manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Nos. 81601082, 81371363, and 81771329) and the Youth Foundation of the First Affiliated Hospital of Zhengzhou University.
Supplemental Material
Supplementary material is available for this article online.
References
- 1.Xue D. Transplanted neural stem cells promote nerve regeneration in acute peripheral nerve traction injury: assessment using MRI. AJR Am J Roentgenol 2011; 196: 1381–1387. [DOI] [PubMed] [Google Scholar]
- 2.Etienne-Manneville S andHall A.. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell 2001; 106: 489–498. [DOI] [PubMed] [Google Scholar]
- 3.Han B Zhao JY Wang WT Li ZW He AP andSong XY.. Cdc42 promotes Schwann cell proliferation and migration through Wnt/β-catenin and p38 MAPK signaling pathway after sciatic nerve injury. Neurochem Res 2017; 42: 1317–1324. [DOI] [PubMed] [Google Scholar]
- 4.Vadodaria KC Brakebusch C Suter U andJessberger S.. Stage-specific functions of the small Rho GTPases Cdc42 and Rac1 for adult hippocampal neurogenesis. J Neurosci 2013; 33: 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SS Kim MO Yun SP Ryu JM Park JH Seo BN Jeon JH andHan HJ.. C(16)-Ceramide-induced F-actin regulation stimulates mouse embryonic stem cell migration: involvement of N-WASP/Cdc42/Arp2/3 complex and cofilin-1/α-actinin. Biochim Biophys Acta 2013; 1831: 350–360. [DOI] [PubMed] [Google Scholar]
- 6.Katayama K Imai F Campbell K Lang RA Zheng Y andYoshida Y.. RhoA and Cdc42 are required in pre-migratory progenitors of the medial ganglionic eminence ventricular zone for proper cortical interneuron migration. Development 2013; 140: 3139–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y Ransom JF Li A Vedantham V Drehle MV Muth AN Tsuchihashi T Mcmanus MT Schwartz RJ andSrivastava D.. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell 2007; 129: 303–317. [DOI] [PubMed] [Google Scholar]
- 8.Jiang L Liu X Chen Z Jin Y Heidbreder CE Kolokythas A Wang A Dai Y andZhou X.. MicroRNA-7 targets IGF1R (insulin-like growth factor 1 receptor) in tongue squamous cell carcinoma cells. Biochem J 2010; 432: 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanek NA andYoung WS.. Investigating the in vivo expression patterns of miR-7 microRNA family members in the adult mouse brain. Microrna 2012; 1: 11–18. [DOI] [PubMed] [Google Scholar]
- 10.Cui Y Xiao Chen Z Wei T Chen J Liu L Chen L Wang B Li XX andDai J.. The miR-7 identified from collagen biomaterial-based three-dimensional cultured cells regulates neural stem cell differentiation. Stem Cells Dev 2014; 23: 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevanato L andSinden JD.. The effects of microRNAs on human neural stem cell differentiation in two- and three-dimensional cultures. Stem Cell Res Ther 2014; 5: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng F Ming Chen L Yan QZ Ding JH andGang H.. MicroRNA-7 enhances subventricular zone neurogenesis by inhibiting NLRP3/Caspase-1 axis in adult neural stem cells. Mol Neurobiol 2015; 53: 7057–7069. [DOI] [PubMed] [Google Scholar]
- 13.Sowa Y Kishida T Imura T Numajiri T Nishino K Tabata Y andMazda O.. Adipose-derived stem cells promote peripheral nerve regeneration in vivo without differentiation into Schwann-like lineage. Plast Reconstr Surg 2016; 137: 318e–330e. [DOI] [PubMed] [Google Scholar]
- 14.Addington CP, Dharmawaj S, Heffernan JM, Sirianni RW and Stabenfeldt SE Hyaluronic acid-laminin hydrogels increase neural stem cell transplant retention and migratory response to SDF-1α. Matrix Biol 2017; 60–61: 206–216. [DOI] [PMC free article] [PubMed]
- 15.Clavijoalvarez JA Nguyen VT Santiago LY Doctor JS Lee WP andMarra KG.. Comparison of biodegradable conduits within aged rat sciatic nerve defects. Plast Reconstr Surg 2008; 121: 344–345.18176255 [Google Scholar]
- 16.Sowa Y Kishida T Tomita K Yamamoto K Numajiri T andMazda O.. Direct conversion of human fibroblasts into Schwann cells that facilitate regeneration of injured peripheral nerve in vivo. Stem Cells Transl Med 2017; 6: 1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inserra MM Bloch DA andTerris DJ.. Functional indices for sciatic, peroneal, and posterior tibial nerve lesions in the mouse. Microsurgery 1998; 18: 119–124. [DOI] [PubMed] [Google Scholar]
- 18.Imitola J andKhoury SJ.. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA 2004; 101: 18117–18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berrocal YA Almeida VW Gupta R andLevi AD.. Transplantation of Schwann cells in a collagen tube for the repair of large, segmental peripheral nerve defects in rats. J Neurosurg 2013; 119: 720–732. [DOI] [PubMed] [Google Scholar]
- 20.Guerout N Paviot Bonmardion A Honoré N Obongo A Duclos RC andMarie JP.. Transplantation of olfactory ensheathing cells to evaluate functional recovery after peripheral nerve injury. J Vis Exp 2014; 84: e50590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruven C Wen L Li H Wong WM andWu W.. Transplantation of embryonic spinal cord derived cells helps to prevent muscle atrophy after peripheral nerve injury. Int J Mol Sci 2017; 18: 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amoh Y Aki R Hamada Y Niiyama S Eshima K Kawahara K Sato Y Tani Y Hoffman RM andKatsuoka K.. Nestin-positive hair follicle pluripotent stem cells can promote regeneration of impinged peripheral nerve injury. J Dermatol 2012; 39: 33–38. [DOI] [PubMed] [Google Scholar]
- 23.Abraham AB Bronstein R Chen EI Koller A Ronfani L Maleticsavatic M andTsirka SE.. Members of the high mobility group B protein family are dynamically expressed in embryonic neural stem cells. Proteome Sci 2013; 11: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary Figure for MiR-7 inhibited peripheral nerve injury repair by affecting neural stem cells migration and proliferation through cdc42 by Nan Zhou, Shuang Hao, Zongqiang Huang, Weiwei Wang, Penghui Yan, Wei Zhou, Qihang Zhu and Xiaokang Liu in Molecular Pain