Abstract
Objective:
Mood disorders and neurocognitive impairments are debilitating conditions among patients with HIV/AIDS. How these comorbidities interact and their relationships to systemic factors remain uncertain. Herein, we investigated factors contributing to depressive symptomatology (DS) in a prospective cohort of patients with HIV/AIDS in active care that included neuropsychological assessment.
Methods:
Among patients with HIV/AIDS receiving combination antiretroviral therapy (cART) and ongoing clinical assessments including measures of sleep, health-related quality of life (HQoL), neuropsychological testing, and mood evaluation (Patient Health Questionnaire–9 [PHQ-9]) were performed. Univariate and multivariate analyses were applied to the data.
Results:
In 265 persons, 3 categories of DS were established: minimal (PHQ-9: 0-4; n = 146), mild (PHQ-9: 5-9; n = 62), and moderate to severe (PHQ-9: 10+; n = 57). Low education, unemployment, diabetes, reduced adherence to treatment, HIV-associated neurocognitive disorders (HAND), low health-related quality of life (HQoL), reduced sleep times, and domestic violence were associated with higher PHQ-9 scores. Motor impairment was also associated with more severe DS. In a multinomial logistic regression model, only poor HQoL and shorter sleep duration were predictive of moderate to severe depression. In this multivariate model, the diagnosis of HAND and neuropsychological performance (NPz) were not predictive of DS.
Conclusions:
Symptoms of depression are common (45%) in patients with HIV/AIDS and represent a substantial comorbidity associated with multiple risk factors. Our results suggest that past or present immunosuppression and HAND are not linked to DS. In contrast, sleep quality and HQoL are important variables to consider in screening for mood disturbances among patients with HIV/AIDS and distinguishing them from neurocognitive impairments.
Keywords: HIV-1, depression, sleep, neuropsychology, PHQ-9, comorbidity
Abstract
Objectif:
Les troubles de l’humeur et les déficiences neurocognitives sont des affections débilitantes chez les patients vivant avec le VIH/sida. Comment ces comorbidités interagissent demeure incertain, tout comme leurs relations aux facteurs systémiques. Nous avons investigué aux présentes les facteurs contribuant à la symptomatologie dépressive (SD) dans une cohorte prospective de patients vivant avec le VIH/sida dans des soins actifs qui incluaient une évaluation neuropsychologique.
Méthodes:
Chez les patients vivant avec le VIH/sida qui recevaient une combinaison de thérapie antirétrovirale (cTAR) et chez qui des évaluations cliniques continues ont été effectuées, y compris des mesures du sommeil, de la qualité de vie liée à la santé (QdVS), des tests neuropsychologiques et l’évaluation de l’humeur (PHQ-9). Des analyses univariées et multivariées ont été appliquées aux données.
Résultats:
Chez 265 personnes, trois catégories de SD ont été établies: minimale [PHQ-9: 0-4 (n = 146)], bénigne [PHQ-9: 5-9 (n = 62)] et de modérée à grave [PHQ-9:10+ (n = 57)]. Le faible niveau d’instruction, le chômage, le diabète, une observance réduite du traitement, le trouble neurocognitif associé au VIH (TNAV), une faible qualité de vie liée à la santé (QdVS), les périodes de sommeil réduites et la violence domestique étaient associés à des scores plus élevés au PHQ-9. La déficience motrice était également associée à une SD plus grave. Dans un modèle de régression logistique multinomiale, seules la QdVS et la durée du sommeil écourtée prédisaient la dépression de modérée à grave. Dans ce modèle multivarié, le diagnostic de TNAV et le rendement neuropsychologique (NPz) n’étaient pas prédicteurs de la SD.
Conclusions:
Les symptômes de dépression sont communs (45%) chez les patients vivant avec le VIH/sida et représentent une comorbidité substantielle associée à de multiples facteurs de risque. Nos résultats suggèrent que l’immunosuppression passée ou présente ainsi que le TNAV ne sont pas liés à la SD. Par contre, la qualité du sommeil et la QdVS sont d’importantes variables à distinguer des déficiences neurocognitives et à considérer pour dépister les perturbations de l’humeur chez les patients vivant avec le VIH/sida.
Depression represents a debilitating comorbidity among patients with HIV-1 infection.1,2 The risk of depression in HIV/AIDS patients is 2-fold higher than that observed in the general population,3,4 affecting as many as 20% to 40% of HIV-infected individuals.5,6 Moreover, depression is associated with reduced adherence to follow-up and antiretroviral therapy,7–9 increased risk of substance abuse and high-risk behaviours,10,11 and perhaps worsening cognition,12,13 all of which are associated with serious medical consequences. As the overall mortality declines in patients with HIV/AIDS receiving combination antiretroviral therapy (cART),14–16 the mean age of patients with HIV/AIDS has risen, as it has become a (manageable) chronic disease.17 Despite improvements in cART regimens over the past 20 years, the frequency of neurocognitive impairment remains high (20% to 40%)18–23 with HIV-associated neurocognitive disorders (HAND) affecting at least 25% of HIV/AIDS patients in active care.24 Patients with HAND exhibit a wide spectrum of signs and symptoms, including affective disorders such as apathy, mania, and other motor and executive impairments.25,26
Depression has previously been implicated as a risk factor for neurocognitive impairment in the general population27–30 and can be challenging to distinguish from apathy and related symptoms.31 Indeed, some symptoms of depression overlap with those observed with neurocognitive impairment,32,33 making the precise relationships and mechanisms underlying this link unclear. Thus, the relationship between depression and HAND occurrence remains uncertain.34–36 These circumstances prompted our working hypothesis: depression and neurocognitive impairment are both common in HIV/AIDS might have independent risk factors. The overarching aim of this study was to assess the prevalence and severity of depressive symptoms in a representative cohort of HIV/AIDS patients in Canada. This cohort was characterised by long-term clinical follow-up and access to universal health care, as well as composed of multiple ethnic and racial backgrounds with comprehensive neuropsychological testing coupled with rigorous clinical assessments. These circumstances permitted investigation of the risk factors for depression, including neuropsychological performance, HAND diagnosis, and multiple demographic and clinical variables.
Methods and Materials
Patient Cohort
HIV-1 seropositive patients were enrolled prospectively in a study with neurocognitive status being the primary outcome measure at the Southern Alberta (HIV) Clinic (SAC) in Calgary, Alberta. From a total of 322 individuals, 57 patients were excluded because cognitive impairment was deemed secondary to causes other than HIV infection, including substance abuse, traumatic brain injury, and developmental delay. The study was approved by the University of Calgary Ethics Committee (REB-130615), and written informed consent was obtained from all patients.
Setting
The SAC is a multidisciplinary clinic, delivering care for all patients with HIV/AIDS in southern Alberta since 1989 and offers regular clinical follow-up visits, laboratory investigations, and free and modern antiretroviral therapies, all without costs to patients. It is staffed by a clinical team including physicians, nurses, social workers, dieticians, and pharmacists. The SAC also maintains an in-house computerised database of all HIV-infected patients containing relevant patient, diagnostic, and treatment data.
Patient Clinical and Demographic Variables
The variables extracted from the clinical database included sex, age, ethnicity, birth continent, years of education, current employment status, sexual orientation, duration of HIV-1 infection, current and nadir CD4+ T-cell counts, current/peak plasma viral load, present cART treatment status, cART side effects (including cART toxicity), central nervous system (CNS) penetration-effectiveness (CPE) rank of current cART, and comorbidities (hepatitis C virus seropositivity; past and present substance use/abuse of alcohol, marijuana, cocaine, heroin, and other illicit substances; medical conditions: stroke, diabetes, hypothyroidism, head injury, seizures; HIV-related polyneuropathy; dyslipidemia; lipodystrophy; diabetes; and cardiac conditions [ischemic or coronary heart disease, hypertension, myocardial infarction, deep vein thrombosis]). On the test day, patients were asked about their current health-related quality of life (HQoL: “How has your health been in the past 5 days?” 1 = poor, 2 = fair; 3 = good; 4 = very good; 5 = excellent), the number of days with cART nonadherence in the past 5 days, concerns about sleep quality, and number of hours of sleep per night.
Mood assessment
The presence and severity of depressive symptoms was assessed by the Patient Health Questionnaire–9 (PHQ-9) assay and stratified in an established manner.37 For statistical analyses, 3 groups were analysed encompassing PHQ-9 scores in the range of 0 to 4 (minimal depression), 5 to 9 (mild depression), and 10 to 27 (moderate to severe depression). Chart reviews were conducted to validate the diagnosis of depression.
Neuropsychological Testing
Neuropsychological testing was conducted at the SAC (45-90 minutes) by an experienced psychometrist, comprising tests of attention/processing speed, memory, motor, language, and executive functions. Attention/processing speed was assessed with the Symbol Digit Modalities Test (SDMT; correct responses)38 and the Trail Making Test Number Sequencing subtest from the Delis-Kaplan Executive Function System (D-KEFS).39 Motor functions were assessed with the grooved pegboard (dominant and nondominant hand).40 Memory was assessed with the Hopkins Verbal Learning Test–Revised (HVLT-R; immediate and 25-minute delayed recall).41 Language functions were tested with the D-KEFS (letter fluency and category fluency, correct responses).39 Executive functions (EFs) were tested with the 64-card version of the Wisconsin Card Sorting Test (WCST-64; perseverative and nonperseverative errors)42 and Trail Making Test Number-Letter Switching subtest from the D-KEFS.39 Premorbid intelligence was estimated with the Word Reading subtest from the Wide-Range-Achievement Test, version 4 (WRAT-4).43 All test scores were interpreted relative to established normative results from healthy age-, sex-, and education-matched controls. Normative data were derived from the test manuals for the SDMT and WCST-64 as well as other sources, including D-KEFS Phonemic Fluency44 and D-KEFS Trails.45 Additional sex adjustments were applied to the HVLT-R, D-KEFS category fluency, and grooved pegboard scores.46
Neurocognitive impairment was defined as performance of at least 1.0 standard deviation (z < –1.0) below the normative means in at least 2 of the tested domains. The diagnosis and staging of HAND were established by the presence of impairment in 2 or more neurocognitive domains,17 followed by re-review of the patient by the SAC clinical team to ensure a confounding comorbidity did not contribute to neurocognitive impairment.
All patients with suspected neurocognitive impairment or declared neurocognitive symptomology underwent cranial magnetic resonance imaging (MRI) and/or cerebrospinal fluid (CSF) analyses. Patients with neurocognitive impairments from causes other than HAND (e.g., head trauma resulting in loss of consciousness exceeding 5 minutes), prior opportunistic CNS infections, and other neurological or psychiatric disorders (e.g., stroke, schizophrenia) were excluded from the analyses.
Statistical Analyses
Statistical analyses were performed with SPSS (version 23; SPSS, Inc., an IBM Company, Chicago, IL). First, demographic, clinical, and neuropsychological comparisons between the 3 PHQ-9 groups were conducted by analyses of variance (ANOVAs; for numerical data), χ2 tests (for categorical data), or Kruskal-Wallis tests (for highly skewed data). Post hoc t tests/ U tests were Bonferroni corrected between groups. Spearman correlations or Mann-Whitney U tests were also conducted between raw PHQ-9 scores and variables showing between-group differences in the PHQ-9. Multinomial and binary logistic regressions then tested all significant demographic, neuropsychological, and clinical variables together, predicting severity of depression symptomatology in the PHQ-9.
Results
Demographic and clinical differences between patients in the 3 PHQ-9 groups were investigated (Table 1). While the mean ages did not differ across groups, education, employment, HQoL, HAND diagnosis, reported sleep hours and sleep concerns, diabetes, and domestic violence differed significantly depending on the depressive symptomatology (DS) categorisation (Table 1). In addition, there was a trend toward a higher proportion of cART nonadherence in the moderate to severe group (P = 0.06). To confirm these group differences, we then examined the relationships between the raw PHQ-9 scores in the entire cohort and variables that had differentiated PHQ-9 groups, using Spearman rank correlations for continuous variables and U tests for categorical variables. DS scores were higher in individuals with lower education levels (U = 6652), unemployment (U = 5142), diabetes (U = 1848), domestic violence (U = 5731), and sleep concerns (U = 4651) (P < 0.01), as well as in individuals with lower HQoL (ρ[265] = −0.418), higher number of days with cART nonadherence (ρ[245] = 0.150), and lower reported sleep hours/night (ρ[262] = –0.342) (Ps < 0.05). Of note, sex, ethnicity, and clinical factors, including CD4+ T-cell nadir/current counts, were not significantly associated with PHQ-9 scores.
Table 1.
Demographic and Clinical Features of Patients (N = 265).
| PHQ-9: 0-4 (n = 146) | PHQ-9: 5-9 (n = 62) | PHQ-9: 10+ (n = 57) | P Valuea | |
|---|---|---|---|---|
| Sex (male), n (%) | 133 (91.1) | 54 (87.1) | 48 (84.2) | NS |
| Age, mean (SD), y | 47.0 (11.4) | 47.2 (11.2) | 48.3 (8.7) | NS |
| Ethnicity (Caucasian), n (%) | 33 (22.6) | 12 (19.4) | 16 (28.1) | NS |
| Birth continent (North America), n (%) | 113 (77.4) | 51 (82.3) | 42 (73.7) | NS |
| Education (postsecondary), n (%) | 99 (67.8)b | 37 (59.7)b,c | 24 (42.1)c | .003 |
| Employment (currently employed), n (%) | 115 (78.8)b | 47 (75.8)b | 25 (43.9)c | <.001 |
| Sexual orientation (heterosexual), n (%) | 42 (28.8) | 22 (35.5) | 21 (36.8) | NS |
| HQoL (1 = poor, 5 = excellent), mean (SD) | 4.00 (0.8)b | 3.5 (0.9)c | 3.0 (1.0)d | <.001 |
| Duration of HIV, mean (SD), y | 11.1 (8.0) | 12.9 (8.9) | 12.0 (8.5) | NS |
| Current viral load copies/mL (log), mean (SD) | 1.8 (0.6) | 1.9 (0.8) | 1.8 (0.6) | NS |
| Peak viral load copies/mL (log), mean (SD) | 4.7 (1.1) | 4.7 (1.1) | 4.7 (1.0) | NS |
| Current CD4+ T cell (count/mm3), mean (SD) | 568.2 (217.19) | 609.3 (291.9) | 577.9 (283.2) | NS |
| Nadir CD4+ T cell (count/mm3), mean (SD) | 218.5 (156.7) | 207.7 (175.9) | 209.8 (184.0) | NS |
| HAND status, n (%) | 36 (24.7)b,c | 9 (14.5)b | 20 (35.1)c | .034 |
| On cART at test date, n (%) | 135 (92.5) | 59 (95.2) | 54 (94.7) | NS |
| cART side effects, n (%) | 27 (18.5) | 10 (16.1) | 12 (21.1) | NS |
| cART nonadherence,e n (%) | 6 (4.5) | 5 (8.6) | 8 (14.8) | NS |
| CPE rank | 7 (0-12) | 7 (0-11) | 7 (0-12) | NS |
| Diabetes, n (%) | 6 (4.1)b | 8 (12.9)b,c | 9 (15.8)c | .012 |
| Sleep hours, hf | 7 (3.5-10)b | 6.5 (3.5-11.5)b | 6 (2.5-9)c | <.001 |
| Sleep concerns (yes), n (%) | 45 (30.8)b | 43 (69.4)c | 47 (82.5)c | <.001 |
| Cardiovascular conditions, n (%) | 17 (11.6) | 9 (14.5) | 11 (19.3) | NS |
| Domestic violence, n (%) | 33 (22.6)b | 14 (22.6)b,c | 24 (42.1)c | .013 |
| Alcohol (binge), n (%) | 3 (2.1) | 3 (4.8) | 3 (5.3) | NS |
| Marijuana, n (%) | 34 (23.3) | 17 (27.4) | 16 (25.3) | NS |
| Crack/cocaine use, n (%) | 6 (4.1) | 4 (6.5) | 6 (10.5) | NS |
| Other illicit drugs, n (%) | 4 (2.7) | 2 (3.2) | 1 (1.8) | NS |
Abbreviations: cART, combination antiretroviral therapy; CPE, antiretroviral central nervous system penetration-effectiveness score; DS, depressive symptomatology; HAND, HIV-associated neurocognitive disorders; NS, not significant; PHQ-9, Patient Health Questionnaire–9; QoL, quality of life.
aOne-way analysis of variance (numerical values, reported as means), χ2 tests (nominal variables, reported as percentages), and Kruskal-Wallis tests (nonparametric variables, reported as median and ranges) were used to test group differences. All tests were Bonferroni corrected for multiple comparisons (unadjusted P value multiplied by total number of comparisons, significance remains at P < 0.05).
b,c,dMatching-letter superscripts denote groups that do not differ significantly from each other at the P < 0.05 level.
eBased on 245 patients receiving cART on test date.
fSelf-reported hours of sleep/night available for n = 262.
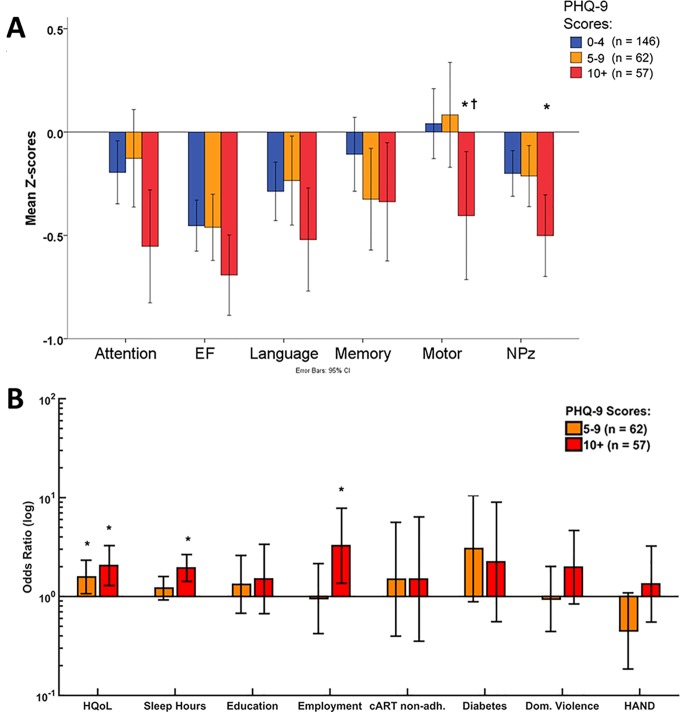
Depression has been linked to neuropsychological performance in multiple disorders,47,48 and we found the highest proportion of individuals with HAND diagnosis in the moderate to severe PHQ-9 group (Table 1). The groups differed in composite neuropsychological performance (NPz) (F(2, 262) = 3.83, P = 0.023), as well as in attention (F(2, 262) = 3.37, P = 0.036) and motor functions (F(2, 262) = 4.48, P = 0.021). Post hoc tests showed that the moderate to severe depressed group had lower motor function scores than the minimally and mildly depressed groups, as well as significantly lowered NPz compared to the minimal DS group only (Figure 1A). Post hoc tests showed no significant differences in attention scores between the groups. However, there were no significant correlations between PHQ-9 scores and neuropsychological scores. Thus, although HAND status and neurocognitive impairment were significantly higher in the moderate-to-severe DS group, neuropsychological performance had limited associations with DS across the entire cohort. In a subset of 65 patients within the cohort who had a formal psychiatric consultation, 24 were found to have major depressive disorder, although anxiety disorders were also present in 11 of the remaining 41 patients; these findings were verified by examining individual patients’ cumulative problem lists. These findings indicated that DS was common in this cohort, and multiple factors were associated with its presence.
Figure 1.
Neuropsychological testing and multivariate analysis of depression predictors. (A) Neurocognitive performance (mean z score) for individual domains (attention, executive function, language, memory, motor function) and total composite z score among patient groups categorized by level of depression (minimal: 0-4; mild: 5-9; moderate/severe: 10+). *P <0.05 (minimal vs. moderate to severe) † P < 0.05 (mild vs. moderate to severe). (B) Relative to the nondepressed (minimal) group (PHQ-9: 0-4 points), quality of life and hours of sleep were independent predictors of moderate to severe depression (PHQ-9: 10+ points) indicated by increased odds ratios, while employment, HAND, and cART adherence were not predictive of depression. *P < 0.05 (minimal vs. mild or moderate/severe). cART, combination antiretroviral therapy; EF, executive function; HAND, HIV-associated neurocognitive disorders; HQoL, health-related quality of life; NPz, neuropsychological performance; PHQ-9, Patient Health Questionnaire–9.
As multiple variables were associated with depression in the univariate analyses (Table 1), we sought to identify the most important and independent predictors of DS. A multinomial logistic regression was conducted to identify predictors for mild (PHQ-9: 5-9) or moderate to severe (PHQ-9: 10+) depression compared to the minimally depressed group as a reference group (PHQ-9: 0-4). Predictors were limited to those that were significantly correlated to the raw PHQ-9 score (HQoL, education level, employment status, sleep hours, diabetes, domestic violence, cART nonadherence), as well as HAND status. Inclusion of HAND status was motivated by the significantly high proportion of HAND patients in the most depressed group (Table 1).
The full model was statistically significant, indicating that the chosen predictors as a set reliably distinguished between PHQ-9 groups (χ2(16) = 84.86, P < 0.001; Nagelkerke R 2 = 0.34), with significant likelihood ratio tests for individual predictors: sleep (hours) (χ2(2) = 20.32, P < 0.01), HQoL (χ2(2) = 11.85, P < 0.01), and employment status (χ2(2) = 8.22, P < 0.05). The odds ratios (ORs) and 95% confidence intervals for each predictor in both groups are displayed relative to the reference group with no depressive symptoms (Suppl. Table S1). All predictors were scaled such that positive ORs are unfavourable (i.e., sleep [hours], education, HQoL, and work status were inverted). Low HQoL was the only significant factor increasing the risk for mild DS, by a factor (OR) of 1.57 (P = 0.022, Figure 1B). Risk factors for moderate to severe DS also included lower HQoL (OR = 2.05), less sleep (hours) (OR = 1.94), and employment status (OR = 3.26) (P < 0.01; Figure 1B). A binary logistic regression was also performed to compare the minimally DS group (PHQ-9: 0-4) to all other patients (PHQ-9 >4) (χ2(8) = 51.37, P < 0.001; Nagelkerke R 2 = 0.34). Only low HQoL (OR = 1.89, P < 0.01) and less sleep (hours) (OR = 1.36, P = 0.045) were predictive of any depressive symptom (Suppl. Table S2). Thus, the presence and severity of DS in this cohort were not linked to systemic immunity or neurocognitive impairment but instead were associated with HQoL, sleep, and employment status.
Discussion
The current findings indicate that symptoms of depression are frequent (45%) among patients with HIV/AIDS in active care, although the severity of DS ranged widely. These results also suggest that DS is associated with specific clinical and demographic factors but not with sustained immunosuppression or the development of HAND or neurocognitive impairments. Previous studies have reported associations between depression and neurocognitive impairments, including HAND during HIV/AIDS.22,49–51 Although univariate relationships between depression and cognition were observed in the present studies, these did not persist in the multivariate analyses. Instead, severity of DS in this cohort was linked to other factors, including HQoL, sleep disturbances, and unemployment. Systemic immunity was also unrelated to depressive symptoms. These observations underscore the high prevalence of mood dysfunction among treated HIV/AIDS patients receiving modern antiretroviral therapy52 and the complex comorbidities contributing to symptoms of depression.53
The lack of association between DS and HAND (or immunosuppression) in the present study emphasises the importance of clearly differentiating depression-like syndromes and HAND because the treatment options differ depending on the disorder. Nonetheless, patients’ predictions of their neurocognitive status are usually good, highlighting the importance of asking patients and caregivers about neurocognitive or depressive symptoms.54
Depression has been associated with neurocognitive impairments through different mechanisms, including sustained exposure of the brain to glucocorticoids,55 particularly in the hippocampus, and perhaps inflammatory molecules such as cytokines (e.g., interleukin-1β or tumour necrosis factor–α) (reviewed in Reus et al.56). Both of these mechanistic pathways are recognised features of HIV/AIDS pathogenesis,15 but the presence of the viral (HIV-1) genome and/or proteins in the brain might tip the balance toward the development of HAND depending on the severity and duration of glucocorticoid or cytokine exposure. In fact, HAND has been associated with symptoms and signs of apathy and mania,57,58 which is related to the expression of the HIV-1 Nef and Vpr proteins in the brain and perturbations in biogenic amine levels.59,60 These experimental findings highlight the multifaceted interactions between host and virus producing either mood dysfunction or neurocognitive impairments.
Several challenges are evident in the present study. The small group sizes as well as the limited neuropsychological battery implemented herein limit the conclusions that can be drawn from these results, although the present cohort represents one of the largest prospective cohorts of HAND patients to be examined from a mood perspective. These results await larger cohorts of repeated neuropsychological and mood testing together with psychiatric validation of the mood assessment. Moreover, the present findings are complicated by the broad diversity of variables (e.g., education, medical conditions) contributing to symptoms of depression (Table 1). Other issues that may affect the prevalence (and incidence) of depression and its relationship to neurocognitive impairment include the frequencies of antiretroviral drug resistance as well as viral burden in reservoir sites such as brain. Future studies will need to dissect these issues, bearing in mind other emerging issues facing patients with HIV/AIDS, including ageing, potential cART neurotoxicity, and other comorbidities (e.g., hepatitis C virus infections).
In summary, these observations underline the importance of evaluating HIV/AIDS patients for depression, using an easily deliverable scale but also maintaining a high level of suspicion for depression in patients with sleep disturbances, poor health-related quality of life, and unemployment. Identification of depression using effective tools warrants treatment with antidepressants61 and/or behavioural interventions unlike neurocognitive disorders, for which therapeutic interventions are limited. The development of biomarkers in conjunction with rigorous bedside mood and neuropsychological testing protocols is imperative for addressing the impact and response to therapy for depression in HIV/AIDS.
Supplementary Material
Supplemental Material, Logistic_regression_tables_28viii17 for Associations between Depressive Symptomatology and Neurocognitive Impairment in HIV/AIDS by Sarah Tymchuk, Daniela Gomez, Noshin Koenig, M. John Gill, Esther Fujiwara, and Christopher Power in The Canadian Journal of Psychiatry
Acknowledgements
We thank the patients and staff at the Southern Alberta Clinic for their willingness to participate in the study and Brenda Beckthold for technical assistance.
Authors’ Note: Sarah Tymchuk and Daniela Gomez contributed equally to the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: These studies were supported by a Canadian Institutes of Health Research, Emerging Team Grant (E.F., M.J.G., and C.P.).
Supplementary Material: Supplementary material for this article is available online.
References
- 1. Dube B, Benton T, Cruess DG, et al. Neuropsychiatric manifestations of HIV infection and AIDS. J Psychiatry Neurosci. 2005;30(4):237–246. [PMC free article] [PubMed] [Google Scholar]
- 2. Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep. 2008;5(4):163–171. [DOI] [PubMed] [Google Scholar]
- 3. Maj M, Janssen R, Starace F, et al. WHO Neuropsychiatric AIDS study, cross-sectional phase I. Study design and psychiatric findings. Arch Gen Psychiatry. 1994;51(1):39–49. [DOI] [PubMed] [Google Scholar]
- 4. Patterson K, Young C, Woods SP, et al. Screening for major depression in persons with HIV infection: the concurrent predictive validity of the Profile of Mood States Depression-Dejection Scale. Int J Methods Psychiatr Res. 2006;15(2):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58(8):721–728. [DOI] [PubMed] [Google Scholar]
- 6. Leserman J, Ironson G, O’Cleirigh C, et al. Stressful life events and adherence in HIV. AIDS Patient Care STDS. 2008;22(5):403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beer L, Skarbinski J. Adherence to antiretroviral therapy among HIV-infected adults in the United States. AIDS Educ Prev. 2014;26(6):521–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonzalez JS, Batchelder AW, Psaros C, et al. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Do NT, Phiri K, Bussmann H, et al. Psychosocial factors affecting medication adherence among HIV-1 infected adults receiving combination antiretroviral therapy (cART) in Botswana. AIDS Res Hum Retroviruses. 2010;26(6):685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wagner GJ, Goggin K, Remien RH, et al. A closer look at depression and its relationship to HIV antiretroviral adherence. Ann Behav Med. 2011;42(3):352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Y, Gong H, Yang G, et al. Perceived stigma, mental health and unsafe sexual behaviors of people living with HIV/AIDS. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014;39(7):658–663. [DOI] [PubMed] [Google Scholar]
- 12. Gibbie T, Mijch A, Ellen S, et al. Depression and neurocognitive performance in individuals with HIV/AIDS: 2-year follow-up. HIV Med. 2006;7(2):112–121. [DOI] [PubMed] [Google Scholar]
- 13. Starace F, Bartoli L, Aloisi MS, et al. Cognitive and affective disorders associated to HIV infection in the HAART era: findings from the NeuroICONA study. Cognitive impairment and depression in HIV/AIDS. The NeuroICONA study. Acta Psychiatr Scand. 2002;106(1):20–26. [DOI] [PubMed] [Google Scholar]
- 14. Nakagawa F, May M, Phillips A. Life expectancy living with HIV: recent estimates and future implications. Curr Opin Infect Dis. 2013;26(1):17–25. [DOI] [PubMed] [Google Scholar]
- 15. Roehr B. UNAIDS celebrates success in halting spread of HIV and sets goals for 2030. BMJ. 2015;351:h3832. [DOI] [PubMed] [Google Scholar]
- 16. Puhan MA, Van Natta ML, Palella FJ, et al. ; Ocular Complications of AIDS Research Group. Excess mortality in patients with AIDS in the era of highly active antiretroviral therapy: temporal changes and risk factors. Clin Infect Dis. 2010;51(8):947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schouten J, Cinque P, Gisslen M, et al. HIV-1 infection and cognitive impairment in the cART era: a review. AIDS. 2011;25(5):561–575. [DOI] [PubMed] [Google Scholar]
- 19. Sirois JL III, Drennan JC. Dystrophic spinal deformity in neurofibromatosis. J Pediatr Orthop. 1990;10(4):522–526. [PubMed] [Google Scholar]
- 20. Winston A, Arenas-Pinto A, Stohr W, et al. Neurocognitive function in HIV infected patients on antiretroviral therapy. PLoS One. 2013;8(4):e61949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243–1250. [DOI] [PubMed] [Google Scholar]
- 22. Fellows RP, Byrd DA, Morgello S;, Manhattan HIVBB. Major depressive disorder, cognitive symptoms, and neuropsychological performance among ethnically diverse HIV+ men and women. J Int Neuropsychol Soc. 2013;19(2):216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ammassari A, Antinori A, Aloisi MS, et al. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics. 2004;45(5):394–402. [DOI] [PubMed] [Google Scholar]
- 24. Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13(11):976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rabkin JG, Ferrando SJ, van Gorp W, et al. Relationships among apathy, depression, and cognitive impairment in HIV/AIDS. J Neuropsychiatry Clin Neurosci. 2000;12(4):451–457. [DOI] [PubMed] [Google Scholar]
- 27. Lee RS, Hermens DF, Porter MA, et al. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J Affect Disord. 2012;140(2):113–124. [DOI] [PubMed] [Google Scholar]
- 28. Beblo T, Sinnamon G, Baune BT. Specifying the neuropsychology of affective disorders: clinical, demographic and neurobiological factors. Neuropsychol Rev. 2011;21(4):337–359. [DOI] [PubMed] [Google Scholar]
- 29. Ebmeier K, Rose E, Steele D. Cognitive impairment and fMRI in major depression. Neurotox Res. 2006;10(2):87–92. [DOI] [PubMed] [Google Scholar]
- 30. Rock PL, Roiser JP, Riedel WJ, et al. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029–2040. [DOI] [PubMed] [Google Scholar]
- 31. Tagariello P, Girardi P, Amore M. Depression and apathy in dementia: same syndrome or different constructs? A critical review. Arch Gerontol Geriatr. 2009;49(2):246–249. [DOI] [PubMed] [Google Scholar]
- 32. Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200–206. [DOI] [PubMed] [Google Scholar]
- 33. Hudon C, Belleville S, Gauthier S. The association between depressive and cognitive symptoms in amnestic mild cognitive impairment. Int Psychogeriatr. 2008;20(4):710–723. [DOI] [PubMed] [Google Scholar]
- 34. Cysique LA, Deutsch R, Atkinson JH, et al. Incident major depression does not affect neuropsychological functioning in HIV-infected men. J Int Neuropsychol Soc. 2007;13(1):1–11. [DOI] [PubMed] [Google Scholar]
- 35. Gold JA, Grill M, Peterson J, et al. Longitudinal characterization of depression and mood states beginning in primary HIV infection. AIDS Behav. 2014;18(6):1124–1132. [DOI] [PubMed] [Google Scholar]
- 36. Cysique LA, Dermody N, Carr A, et al. The role of depression chronicity and recurrence on neurocognitive dysfunctions in HIV-infected adults. J Neurovirol. 2016;22(1):56–65. [DOI] [PubMed] [Google Scholar]
- 37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith A. Symbol digit modalities test. Los Angeles (CA): Western Psychological Services; 1973. [Google Scholar]
- 39. Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio (TX): The Psychological Corporation; 2001. [Google Scholar]
- 40. Trites RL. Neuropsychological test manual. Ottawa (Canada): Royal Ottawa Hospital; 1977. [Google Scholar]
- 41. Brandt J, Benedict R. Hopkins Verbal Learning Test–Revised (HVLT-R). Lutz (FL): Psychological Assessment Resources; 2001. [Google Scholar]
- 42. Kongs SK, Thompson LL, Iverson GL, et al. Wisconsin Card Sorting Test–64 Card Version (WCST-64). Ann Arbor (MI): Ann Arbor Publishers; 2000. [Google Scholar]
- 43. Wilkinson GS, Robertson GJ. WRAT-4 Wide Range Achievement Test professional manual. Lutz (FL): Psychological Assessment Resources; 2006. [Google Scholar]
- 44. Mitrushina M, Boone K, Razani J, et al. Handbook of normative data for neuropsychological assessment. 2nd ed Oxford (UK: ): Oxford University Press; 2005. [Google Scholar]
- 45. Fine EM, Delis DC, Holdnack J. Normative adjustments to the D-KEFS trail making test: corrections for education and vocabulary level. Clin Neuropsychol. 2011;25(8):1331–1344. [DOI] [PubMed] [Google Scholar]
- 46. Schretlen D, Testa S, Pearlson G. Calibrated neuropsychological normative system professional manual. Lutz (FL): Psychological Assessment Resources; 2010. [Google Scholar]
- 47. Hammar A. Automatic and effortful information processing in unipolar major depression. Scand J Psychol. 2003;44(5):409–413. [DOI] [PubMed] [Google Scholar]
- 48. Hartlage S, Alloy LB, Vazquez C, et al. Automatic and effortful processing in depression. Psychol Bull. 1993;113(2):247–278. [DOI] [PubMed] [Google Scholar]
- 49. Pinheiro CA, Souza LD, Motta JV, et al. Depression and diagnosis of neurocognitive impairment in HIV-positive patients. Braz J Med Biol Res. 2016;49(10):e5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bonnet F, Amieva H, Marquant F, et al. Cognitive disorders in HIV-infected patients: are they HIV-related? AIDS. 2013;27(3):391–400. [DOI] [PubMed] [Google Scholar]
- 51. Troncoso FT, Conterno Lde O. Prevalence of neurocognitive disorders and depression in a Brazilian HIV population. Rev Soc Bras Med Trop. 2015;48(4):390–398. [DOI] [PubMed] [Google Scholar]
- 52. Jallow A, Ljunggren G, Wandell P, et al. HIV-infection and psychiatric illnesses—a double edged sword that threatens the vision of a contained epidemic: The Greater Stockholm HIV Cohort Study. J Infect. 2017;74(1):22–28. [DOI] [PubMed] [Google Scholar]
- 53. Morrison MF, Petitto JM, Ten Have T, et al. Depressive and anxiety disorders in women with HIV infection. Am J Psychiatry. 2002;159(5):789–796. [DOI] [PubMed] [Google Scholar]
- 54. Blackstone K, Moore DJ, Heaton RK, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc. 2012;18(1):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hayley S, Audet MC, Anisman H. Inflammation and the microbiome: implications for depressive disorders. Curr Opin Pharmacol. 2016;29:42–46. [DOI] [PubMed] [Google Scholar]
- 56. Reus GZ, Fries GR, Stertz L, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141–154. [DOI] [PubMed] [Google Scholar]
- 57. Kamat R, Doyle KL, Iudicello JE, et al. Neurobehavioral disturbances during acute and early HIV infection. Cogn Behav Neurol. 2016;29(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McIntosh RC, Rosselli M, Uddin LQ, et al. Neuropathological sequelae of human immunodeficiency virus and apathy: a review of neuropsychological and neuroimaging studies. Neurosci Biobehav Rev. 2015;55:147–164. [DOI] [PubMed] [Google Scholar]
- 59. Acharjee S, Branton WG, Vivithanaporn P, et al. HIV-1 Nef expression in microglia disrupts dopaminergic and immune functions with associated mania-like behaviors. Brain Behav Immun. 2014;40:74–84. [DOI] [PubMed] [Google Scholar]
- 60. Mamik MK, Hui E, Branton WG, et al. HIV-1 viral protein R activates NLRP3 inflammasome in microglia: implications for HIV-1 associated neuroinflammation. J Neuroimmune Pharmacol. 2017;12(2):233–248. [DOI] [PubMed] [Google Scholar]
- 61. Relf MV, Eisbach S, Okine KN, et al. Evidence-based clinical practice guidelines for managing depression in persons living with HIV. J Assoc Nurses AIDS Care. 2013;24(1 Suppl):S15–S28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Logistic_regression_tables_28viii17 for Associations between Depressive Symptomatology and Neurocognitive Impairment in HIV/AIDS by Sarah Tymchuk, Daniela Gomez, Noshin Koenig, M. John Gill, Esther Fujiwara, and Christopher Power in The Canadian Journal of Psychiatry