Abstract
Despite the withdrawal of CB1 antagonists, such as rimonabant, from the market and from active clinical development due to concerns about their side effect profiles, research suggests that the endocannabinoid system may play an important role in modulating nicotine’s effects. We report the combined results, using a pooled analysis, of three previously unpublished trials assessing rimonabant as a smoking cessation pharmacotherapy conducted between 2002–2004. Smokers (n=2097) motivated to quit were enrolled in three randomized, double-blind, placebo-controlled trials, STRATUS EU, US, and META, which consisted of a 10-week treatment period with either rimonabant 20 mg (n=789), 5 mg (n=518; used in only 2 of the 3 studies), or placebo (n=790), in conjunction with brief counseling. The impact of drug on prolonged abstinence and adverse events was examined at 8 weeks (end-of-treatment; EOT) and at 48 weeks (available for STRATUS EU and US) after the targeted quit date (TQD). Rimonabant 20 mg resulted in significantly higher abstinence at EOT and at 48 weeks post-TQD compared with placebo, while rimonabant 5 mg and placebo did not differ. Serious AEs did not differ by drug group. The 20 mg rimonabant dose, compared to placebo, produced increased nausea, diarrhea, anxiety symptoms, hyporexia, and vomiting, and decreased headache, constipation, and cough. These results support rimonabant 20 mg as a modestly effective aid for smoking cessation. Although work on CB1 antagonists such as rimonabant has mostly been stopped due to unacceptable adverse events, these results may inform and spur the development of other endocannabinoids for smoking cessation.
Keywords: cannabinoid receptors, pharmacotherapy, rimonabant, smoking cessation, weight gain
INTRODUCTION
While much of the focus of previous research on nicotine addiction has been related to its direct effects on reward processes and mesolimbic dopamine (DA) neurotransmission (Pidoplichko et al., 1997; Pontieri et al., 1996), a growing body of literature suggests that the endocannabinoid system may play an important role in nicotine’s modulation of these neuroregulatory pathways. Three CB1 receptor antagonists, rimonabant (also known as Acomplia and Zimulti), surinabant (Tonstad & Aubin, 2012), and taranabant (Morrison et al., 2010), were evaluated as smoking cessation pharmacotherapies. However, none were ever approved for smoking cessation, primarily because rimonabant, which was approved for weight control, was removed from the market due to concerns and regulatory action about its potential for adverse events (AEs). Most of the clinical trials that evaluated these drugs for smoking cessation have not been published in a peer-reviewed forum. The purpose of this manuscript was to evaluate whether rimonabant, the most thoroughly evaluated CB1 receptor antagonist, demonstrated any indication as a smoking cessation aid, and to document its risks and impact on weight change. We conducted a pooled analysis of three unpublished rimonabant smoking cessation clinical trials with similar study designs. Our intention was to highlight the potential role for and spur further research on endocannabinoids as a smoking cessation pharmacotherapy, despite the failure of these first generation of CB1 receptor antagonist medications.
Endocannabinoids differ from other neurotransmitters in that they are not are not released from vesicles, but rather are generated on an as-needed basis in response to receptor activation or to the increase in intracellular Ca2+ associated with neuronal depolarization (Xie et al., 2007). The two most widely studied endocannabinoids are anandamide and 2-arachidonylglycerol (2-AG) (Mechoulam, Fride & Di, 1998). Studies suggest that these molecules act as synaptic retrograde messengers, which when released from postsynaptic neurons (following depolarization), activate CB1 receptors on axon terminals and inhibit subsequent neurotransmitter release from that neuron. In this sense, the endocannabinoid system provides a means for postsynaptic neurons to regulate their own synaptic activity (Alger, 2002; Wilson & Nicoll, 2002). CB1 receptors are present on axons throughout the CNS but are highly concentrated in olfactory and cortical regions (neocortex and pyriform cortex), cerebellar cortex, brainstem, and hypothalamic nuclei, and limbic areas including the amygdala, hippocampus, basal ganglia, and thalamic areas (Herkenham et al., 1990). It is important to note that these cortico-limbic areas have been implicated in smoking-related cue reactivity (Chiamulera, 2005; Engelmann et al., 2012).
In addition to the short-term regulation of synaptic activity, endocannabinoids may be involved in long-lasting synaptic plasticity, such as long-term depression in areas of the brain such as the nucleus accumbens (NAc) and the dorsal striatum (Gerdeman & Lovinger, 2003; Gerdeman, Ronesi & Lovinger, 2002; Hoffman et al., 2003; Lupica, Riegel & Hoffman, 2004; Robbe et al., 2002). This may have implications for drug addiction, including smoking, given the prominent role of DA release in the NAc in association with drug self-administration. For example, preclinical studies have shown that chronic nicotine administration stimulates endocannabinoid release in the NAc (Gonzalez et al., 2002). Specifically, CB1 receptor binding on presynaptic glutamatergic neurons in the NAc has been found to enhance VTA DA release by attenuating the inhibitory influence of GABAergic neurons on DA neurons (Cohen, Kodas & Griebel, 2005).
Rimonabant is a selective CB1 antagonist/inverse agonist that was initially developed and approved as a means for weight reduction, but was eventually removed from market due to concerns about adverse events (AEs). Rimonabant has a mean half-life of 16–32 days and is metabolized by CYP3A4 and amidohydrolase (predominantly hepatic) pathways in vitro, and has a mild inhibitory effect on CYP2C8 (European Medicines Agency, 2009). Rimonabant was found to reduce food intake in animal models (Di Marzo & Matias, 2005; Rinaldi-Carmona et al., 2004) and was evaluated for human use in a series of trials called Rimonabant in Obesity (RIO), which found that one year of the 20 mg dose led to modest weight reduction compared to placebo (Despres, Golay & Sjostrom, 2005; Pi-Sunyer et al., 2006; van Gaal et al., 2005; van Gaal et al., 2008). Rimonabant was approved in the EU in 2006, and eventually in 37 countries, but not in the US (Saul, 2007), as an adjunct to diet and exercise for the treatment of obese patients (BMI ≥ 30 kg/m2), and of overweight patients (BMI > 27 kg/m2) with associated risk factor(s), such as type 2 diabetes or dyslipidemia. However, in October 2008, rimonabant was withdrawn from the European market, due to concerns associated with psychiatric side effects, specifically depressive symptoms (European Medicines Agency, 2008). A meta-analysis of psychiatric AEs across the obesity trials highlighted these concerns, concluding that the risk-benefit ratio for weight loss did not favor continued use of the medication (Christensen et al., 2007).
Rimonabant’s pharmacological properties also led it to be evaluated as a smoking cessation intervention. Rimonabant has been shown to interfere with the action of endocannabinoids on GABAergic and glutamatergic neurons that contribute to the release of DA in the NAc (Schlicker & Kathmann, 2001). Given the centrality of the role NAc DA release is thought to have in the development and maintenance of nicotine dependence (Volkow et al., 2007), rimonabant was investigated for its ability to reduce nicotine use. Studies with experimental animals have shown that rimonabant reduces nicotine self-administration as well as the nicotine-induced DA release in the shell of the NAc (Cohen et al., 2002). In addition, pretreatment with rimonabant reduced responding maintained by nicotine-associated cues, in the absence of nicotine, several weeks after nicotine withdrawal (Cohen et al., 2005).
Prior to voluntarily suspending all clinical trials involving the drug in November 2008 (Sanofi-aventis, 2008), Sanofi-Aventis evaluated the effectiveness of rimonabant for smoking cessation against placebo in a series of four trials called the Studies with Rimonabant and Tobacco Use (STRATUS), conducted in the US and EU. Following its withdrawal from the market, rimonabant was not submitted for regulatory approval as a smoking cessation therapy and none of the results of the initial STRATUS trials have been published. Two trials, one in Europe (STRATUS EU; study code EFC4474; ClinicalTrials.gov identifier: NCT00464165) and one in the US (STRATUS US; EFC4964; ClinicalTrials.gov identifier: NCT00358228) were identical in design and involved a 10-week treatment course with rimonabant (5- and 20-mg doses) with a 40 week post-treatment follow-up. A third trial conducted in the US (STRATUS META; EFC5794; ClinicalTrials.gov identifier: NCT00464256) involved only a 10-week treatment course, with no 5-mg dose or extended follow-up. The fourth, STRATUS World Wide (WW; EFC4796; ClinicalTrials.gov identifier: NCT00459173), evaluated the maintenance of smoking abstinence by exposing participants to 52 weeks of rimonabant, and re-randomized participants who were abstinent at 10 weeks. A fifth sponsored study, CIRRUS (EFC4798; ClinicalTrials.gov identifier: NCT00458718), examined rimonabant combined with the nicotine patch (Rigotti et al., 2009).
In this paper, we conducted pooled analysis of three of these Sanofi-Aventis-sponsored randomized double-blind clinical trials (STRATUS EU, US, and META) of rimonabant as a smoking cessation pharmacotherapy (because of differences in design, STRATUS WW and CIRRUS were not included in our analyses). Our primary objectives for these analyses involved evaluation of drug-associated prolonged abstinence, using a 2 week grace period following the targeted quit date (TQD), at end of treatment (EOT) and at 48 weeks post-TQD. We also report FDA 4-week continuous smoking abstinence at EOT because it was the primary outcome in the original protocols. Our secondary objectives involved examining the impact of drug on weight change and AEs, with particular emphasis on neuropsychiatric side effects. This manuscript differs from a meta-analysis conducted by the Cochrane Collaboration (Cahill & Ussher, 2011) because (1) we had access to the raw STRATUS data, rather than to unpublished reports, (2) we had access to and were able to include STRATUS META in our combined analyses, which extended our ability to compare the 20-mg dose against the placebo dose, and (3) we excluded STRATUS WW from our analyses because of its major differences in design. Our intentions are to present these previously unpublished findings in a peer-reviewed format, and to evaluate whether endocannabinoid antagonists may have a future role as a smoking cessation pharmacotherapy.
MATERIAL AND METHODS
Study Design
The studies were designed by the sponsor in consultation with several of the authors. STRATUS EU was performed in 32 centers in Europe (Belgium, Denmark, France, Spain, Sweden, Switzerland, and the United Kingdom). STRATUS US and META were conducted in the USA at 11 and 10 sites, respectively. STRATUS EU and US were identical in design and involved a 10-week treatment course with rimonabant (5 and 20 mg doses) with a 40 week post-treatment follow-up. STRATUS META involved only a 10-week treatment course, with no 5-mg dose or extended follow-up. All sites received approval to conduct the study from their institutional review boards (IRBs) or from Western IRB (Puyallup, WA, USA) if a local IRB was not used, and all study procedures were conducted in accordance with the Declaration of Helsinki. The study was monitored by an independent Data Safety and Monitoring Board established by the sponsor.
Study Selection
We conducted pooled analysis of three randomized, double-blind, placebo-controlled clinical trials of rimonabant as a smoking cessation pharmacotherapy, including STRATUS EU, US, and META. We excluded two other trials, STRATUS WW and CIRRUS, because of major differences in design. STRATUS WW differed from STRATUS EU, US, and META in that it exposed participants to 52 weeks of rimonabant and re-randomized participants who were abstinent at 10 weeks. CIRRUS examined rimonabant combined with the nicotine patch or placebo patch but had no true placebo condition (Rigotti et al., 2009).
Participants
Participants met inclusion criteria if they were 18 years of age or older, smoked >9 cigarettes per day (CPD), had a level of motivation to quit of 6 on a scale ranging from 1 to 10, completed a physical exam, had blood chemistries and 12-lead ECG within normal limits, were the only participant in a household, and provided informed consent. Participants were excluded if they were pregnant, were breast feeding, were of childbearing potential and not using a medically acceptable method of birth control, had taken an investigational drug within the month prior to screening, were currently being treated for a seizure disorder, had recent myocardial infarction, unstable angina, stroke or other major cardiovascular event within the 6 months prior to screening, had current clinically significant medical illness, met criteria for a current psychiatric disorder, had a history of cancer within the 5 years prior to screening, required previous systemic pharmacological treatment for drug related allergies, tested positive for marijuana metabolites the urine drug screen (UDS), had used smoking cessation, anti-depressant, amphetamine, non-selective antihistamine, or systemic long-acting corticosteroid pharmacotherapies, or used medication or herbal preparations for treatment of obesity or weight reduction, for more than 7 consecutive days within the month before the screening visit, or had participated in smoking cessation counseling on more than 3 days within the month preceding screening.
Procedures
Following a 2-week screening period, participants were randomized to placebo, 5 mg (STRATUS EU and US), or 20 mg of rimonabant. Randomization was assigned via a centralized computer system and balanced by site to maintain equal treatment assignment. Participants were seen weekly, in-clinic, for 12 weeks (all studies) including a 2 week pre-treatment screening, followed by randomization and 10-weeks of drug treatment. The target quit date (TQD) was set at the end of the second week of treatment in all studies (Day 15). In STRATUS EU and US, follow-up visits were conducted at 11, 14, 22, 30, 39, and 48 weeks after TQD. Visits at weeks 11, 22, and 39 were conducted via telephone.
Adverse event monitoring was carried out at each visit during treatment and for the first six weeks after treatment ended. Serious adverse events (SAEs) were recorded for the entire study period. Abstinence was assessed via self-report of any smoking since the last visit and was biochemically confirmed by expired CO ≤ 10 ppm and plasma cotinine concentration ≤ 8 μg/L. The Hospital Anxiety and Depression Scale (HADS) (Zigmond & Snaith, 1983) was administered at baseline and at 4 and 8 weeks (end of treatment) post-TQD. A measure of nicotine dependence, the Fagerström Test for Nicotine Dependence (FTND) (Heatherton et al., 1991), was carried out at the baseline visit. Plasma cotinine was collected at baseline and 6, 8, 14, 30, and 48 weeks post-TQD. All assessments noted above beyond the 8-week post-TQD time point were collected only in the STRATUS EU and US studies.
In all studies participants received brief (approximately 10 minute) behavioral counseling at each of the weekly and follow-up visits from a trained study assistant using intervention materials developed by the Mayo Clinic Nicotine Research Program (2000). This counseling was consistent with current smoking cessation guideline-based treatment (US Department of Health and Human Services, 2008) and other studies using counseling and a behavioral counseling intervention in smoking cessation trials (Cox, Tiffany & Christen, 2001; Hurt et al., 1997).
Data Analysis
The primary efficacy outcomes were prolonged and FDA abstinence at EOT (all studies) and at 48 weeks post-TQD (STRATUS EU & US), using the intention-to-treat (i.e., missing=relapsed) definition. Prolonged abstinence was defined using the Society for Research on Nicotine and Tobacco guidelines, which includes a 2-week grace period from the TQD and defines relapse either as “7 consecutive days of smoking” or as “smoking on at least 1 day on each of 2 consecutive weeks” (Hughes et al., 2003). FDA abstinence was defined as no smoking at all (i.e., continuous abstinence) during the last 4 weeks of treatment (US Food and Drug Administration, 1995). We evaluated the effects of treatment on abstinence, separately at each endpoint, using a logistic regression model (SAS PROC LOGISTIC; v9.4; SAS Institute Inc., Cary, NC) that included abstinence as a binary dependent variable, drug as a between-subject effect, and study as a covariate for pooled analyses. Odds ratios and confidence intervals (95% CI) are reported for the pooled analyses. In addition to the pooled results, results from the individual studies are presented for comparison.
In terms of secondary analyses, we analyzed pooled AEs at EOT (all studies) and at 48 weeks (STRATUS EU & US), by drug group, using χ2 tests (or Fisher’s exact test, if AE frequencies were < 5). We also examined drug differences in AEs among those who discontinued treatment, both for those who discontinued because of AEs and for those who discontinued for any reason. Serious Adverse Events (SAEs) were also examined. Weight, at EOT and 48 weeks, and HADS, at EOT, were calculated as change scores from the pre-medication baseline, and were analyzed using general linear models (SAS PROC GLM), with treatment, abstinence, and study (for pooled analyses) as predictors. Separate weight analyses were also conducted only on those who abstained from smoking at each time point. Comparisons involving significant multilevel effects were further explored using a least squares mean procedure to contrast pairwise differences among selected means participating in the effect. Student t-tests (continuous variables) and χ2 tests (discrete variables) were used to examine differences on baseline demographic characteristics.
RESULTS
Participant characteristics
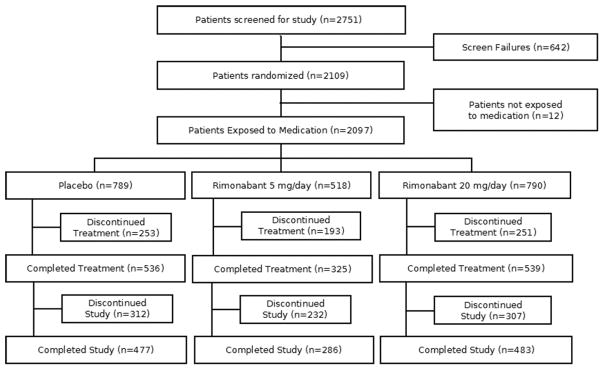
The study flow diagram, pooling all three studies, is presented in Figure 1. Across the three studies, 2751 participants were screened, 76.7% (2109) were randomized, and 76.2% (2097) were exposed to medication. Only the 2097 participants who were randomized and exposed to study medication are considered in the analyses for the intention-to-treat population are reported here. The baseline characteristics of the 2097 participants exposed to medication are summarized in Table 1. No differences were noted across the study samples in terms of gender, race, or CPD. However, the US samples (STRATUS US and META) were slightly older and showed higher BMIs, weight, expired CO, and HADS scores than their European counterparts (STRATUS EU).
Figure 1.
Consort table depicting the pooled sample sizes.
Table 1.
Participant Characteristics at baseline.
| Study | STRATUS EU (EFC4474; n=783) | STRATUS US (EFC4964; n=784) | META (EFC5794; n=530) | Total (n=2097) | ||||
|---|---|---|---|---|---|---|---|---|
| Frequency (n) | ||||||||
| Sex (Female) | 53.9 | (422) | 49.2 | (386) | 54.7 | (290) | 52.4 | (1098) |
| Race (Caucasian) | 97.7 | (765) | 87.4 | (685) | 89.4 | (474) | 91.7 | (1924) |
| Mean (SD) | ||||||||
| Age (years) | 42.43 | (10.51) | 42.28 | (11.35) | 44.61 | (11.78)a | 42.93 | (11.20) |
| BMI | 24.99 | (4.48) | 27.79 | (5.76)* | 27.73 | (5.41)* | 26.73 | (5.39) |
| Weight (kg) | 72.58 | (15.67) | 81.36 | (19.30)* | 80.70 | (18.29)* | 77.91 | (18.23) |
| Cigarettes (CPD) | 23.56 | (9.34) | 23.27 | (10.15) | 22.51 | (10.06) | 23.18 | (9.83) |
| Expired CO (ppm) | 23.38 | (12.86) | 21.68 | (11.22)* | 21.75 | (11.24)* | 22.33 | (11.88) |
| FTND | 5.55 | (2.15) | 5.43 | (2.17) | 5.47 | (2.07) | 5.49 | (2.14) |
| HADS Depression | 2.06 | (2.12) | 2.33 | (2.25)* | - | - | 2.20 | (2.19) |
| HADS Anxiety | 4.61 | (2.97) | 4.90 | (2.86)* | - | - | 4.76 | (2.92) |
Note:
Significant compared to STRATUS EU at the p < 0.05 level.
Significant compared to STRATUS EU and STRATUS US, both p’s < .05. CPD = cigarettes per day.
BMI = body mass index; CPD = cigarettes per day; FTND = Fagerström Test for Nicotine Dependence; HADS = The Hospital Anxiety and Depression Scale.
Abstinence outcomes
FDA 4-week continuous smoking abstinence at EOT
Table 2 shows the percentage of participants who met criteria for FDA-defined continuous abstinence at EOT (weeks 4–8 following the TQD) for each study and for the pooled results. The pooled analysis of the EU, US, and META studies indicated that the 20 mg rimonabant dose produced significantly greater abstinence rates than placebo (OR = 1.66, 95% CI: 1.23, 2.23). Smoking abstinence rates for participants who received 5 mg of rimonabant did not differ significantly from placebo at either time point for any of the individual or pooled analyses.
Table 2.
Smoking abstinence rates and odds ratios relative to placebo.a
| Time Point | Percentage Abstinent By Group | Logistic Regression Results | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Placebo | 5 mg | 20 mg | 5 mg versus placebo | 20 mg versus placebo | |||||
|
|
|||||||||
| (N=789) | (N=518) | (N=790) | |||||||
|
|
|
||||||||
| EOT (FDA Continuous) | P | OR | 95% CI | P | OR | 95% CI | |||
| STRATUS EU | 19.6% (51) | 25.0% (64) | 25.5% (68) | 0.14 | 1.37 | (0.90, 2.08) | 0.11 | 1.40 | (0.93, 2.18) |
| STRATUS US | 16.1% (42) | 16.0% (42) | 27.6% (72) | 0.98 | 1.00 | (0.62, 1.59) | 0.002 | 1.99 | (1.29, 3.05) |
| STRATUS META | 9.3% (25) | - | 13.7% (36) | - | - | - | 0.11 | 1.55 | (0.90, 2.67) |
| POOLED EU/US | 17.8% (93) | 20.5% (106) | 26.5% (140) | 0.28 | 1.19 | (0.87, 1.62) | .0008 | 1.66 | (1.23, 2.23) |
| POOLED EU/US/META | 15.0% (118) | - | 22.3% (176) | - | - | - | .0002 | 1.63 | (1.26, 2.11) |
| EOT (SRNT Prolonged) | |||||||||
| STRATUS EU | 19.2% (50) | 24.6% (63) | 25.8% (69) | 0.14 | 1.37 | (0.90, 2.09) | 0.07 | 1.46 | (0.97, 2.21) |
| STRATUS US | 16.1% (42) | 14.9% (39) | 25.3% (66) | 0.70 | 0.91 | (0.57, 1.47) | 0.01 | 1.77 | (1.14, 2.72) |
| STRATUS META | 9.0% (24) | - | 13.7% (36) | - | - | - | 0.09 | 1.62 | (0.93, 2.81) |
| POOLED EU/US | 17.7% (92) | 19.7% (102) | 25.6% (135) | 0.40 | 1.14 | (0.84, 1.56) | .002 | 1.60 | (1.19, 2.16) |
| POOLED EU/US/META | 14.7% (116) | - | 21.6% (171) | - | - | - | .0004 | 1.61 | (1.24, 2.08) |
| 48 Weeks (SRNT Prolonged) | |||||||||
| STRATUS EU | 12.3% (32) | 13.3% (34) | 14.2% (38) | 0.74 | 1.09 | (0.65, 1.83) | 0.52 | 1.18 | (0.71, 1.96) |
| STRATUS US | 8.4% (22) | 8.4% (22) | 15.3% (40) | 0.99 | 1.00 | (0.54, 1.85) | 0.02 | 1.97 | (1.13, 3.42) |
| POOLED EU/US | 10.4% (54) | 10.8% (56) | 14.8% (78) | 0.81 | 1.05 | (0.71, 1.56) | 0.04 | 1.50 | (1.03, 2.17) |
Note:
Self-reported smoking abstinence was biochemically confirmed by expired CO ≤ 10 ppm and plasma cotinine concentration ≤8 μg/L. EOT = end of treatment; TQD = targeted quit date. FDA continuous abstinence defined as no smoking at all during the last 4 weeks of treatment. SRNT abstinence included a 2-week grace period from the TQD, with relapse defined as either 7 consecutive days of smoking or smoking on at least 1 day on each of 2 consecutive weeks.
CI = confidence interval; EOT = end of treatment; OR = odds ratio; TQD = targeted quit date.
SRNT Prolonged abstinence at EOT (2-week grace period)
Table 2 shows the percentage of participants who met criteria for prolonged abstinence at EOT (from two weeks grace period to 8 weeks following TQD) for each study and for the pooled results. The pooled analysis of the EU, US, and META studies indicated that the 20 mg rimonabant dose produced significantly greater abstinence rates than placebo (OR = 1.61, 95% CI: 1.24, 2.09). Smoking abstinence rates for participants who received 5 mg of rimonabant did not differ significantly from placebo at either time point for any of the individual or pooled analyses.
SRNT Prolonged abstinence at 48 weeks post-TQD (2-week grace period)
At 48 weeks (from two weeks grace to 48 weeks following TQD), the pooled analysis of the STRATUS EU and US studies indicated that the 20 mg rimonabant dose produced significantly greater abstinence rates than placebo (OR = 1.50, 95% CI: 1.03, 2.17; see Table 2). Smoking abstinence rates for participants who received 5 mg of rimonabant did not differ significantly from placebo at either time point for any of the individual or pooled analyses.
Weight
Weight change was calculated as change scores from the pre-medication baseline, for both EOT and 48 weeks post-TQD, and are depicted in Table 3 for the individual and pooled analyses. From the start of medication to EOT, the pooled analysis of the EU, US, and META studies indicated that the 20 mg rimonabant dose resulted in less weight gain than placebo. However, by 48 weeks post-TQD, no differences were found between the three doses for the pooled EU & US analysis. Similar results were found when restricting the analyses to those who were abstinent from smoking at both time points.
Table 3.
Weight change (kg) from pre-medication baseline, for both the total sample and for abstainers only, at EOT and 48-weeks post-TQD. All comparisons are relative to placebo. Values are presented as least square means (standard error).
| Study | Placebo | 5 mg | 20 mg | |||
|---|---|---|---|---|---|---|
| EOT (8 weeks post-TQD) | ||||||
| STRATUS EU | 1.70 | (0.17) | 0.86 | (0.17)** | −0.06 | (0.16)*** |
| Abstainers only | 2.61 | (0.33) | 1.72 | (0.30)* | 0.42 | (0.28)** |
| STRATUS US | 2.00 | (0.18) | 1.45 | (0.18)* | 0.35 | (0.17)*** |
| Abstainers only | 3.51 | (0.38) | 2.32 | (0.39)* | 0.90 | (0.30)*** |
| STRATUS META | 1.82 | (0.21) | - | - | −0.10 | (0.19)*** |
| Abstainers only | 2.64 | (0.47) | - | - | 1.62 | (0.36) |
| POOLED EU/US | 1.84 | (0.12) | 1.14 | (0.12)** | 0.14 | (0.12)*** |
| Abstainers only | 3.04 | (0.25) | 2.03 | (0.24)** | 0.66 | (0.21)*** |
| POOLED EU/US/META | 1.76 | (0.11) | - | - | −0.01 | (0.10)*** |
| Abstainers only | 3.03 | (0.23) | - | - | 0.92 | (0.19)*** |
| 48 Weeks (Post-TQD) | ||||||
| STRATUS EU | 3.27 | (0.37) | 3.33 | (0.36) | 3.24 | (0.36) |
| Abstainers only | 4.94 | (0.74) | 5.56 | (0.72) | 4.72 | (0.68) |
| STRATUS US | 3.17 | (0.45) | 4.43 | (0.44)* | 3.92 | (0.41) |
| Abstainers only | 7.35 | (1.31) | 7.13 | (1.31) | 5.09 | (0.97) |
| POOLED EU/US | 3.16 | (0.29) | 3.85 | (0.28) | 3.58 | (0.27) |
| Abstainers only | 6.04 | (0.70) | 6.31 | (0.69) | 4.89 | (0.58) |
Note:
p < .05,
p < .001,
p < .0001.
EOT = end of treatment; TQD = targeted quit date.
Safety
Adverse Events
From the start of the medication phase to 6 weeks after medication completion, the frequency of several AEs significantly differed between the 20 mg rimonabant dose and placebo (see Table 4). In our pooled analysis of the EU, US, and META studies, the 20 mg rimonabant dose, led to increased reports of nausea, diarrhea, anxiety, hyporexia, and vomiting, but decreased reports of headache, constipation, and cough, when compared to placebo. There were no differences in AE frequencies between the 5 mg rimonabant dose and placebo, besides increased upper respiratory disturbance and decreased cough.
Table 4.
Analysis of the pooled STRATUS EU, US, and META adverse events (AEs) that occurred during the 10-week medication phase and for 6 weeks after medication ended for the studies. The listed AEs impacted at least 2% of the total sample.
| Adverse Event (AE) | Placebo (N=789) | 5 mga (N=518) | 20 mg (N=790) |
|---|---|---|---|
| Any AEs reported (%) | 60.96% (481) | 61.00 (316) | 69.62 (550)*** |
| Upper Respiratory Disturbance | 21.67% (171) | 27.03% (140)* | 22.91% (181) |
| Headache | 15.72% (124) | 19.11% (99) | 11.39% (90)* |
| Sleep Disturbance | 12.29% (97) | 13.51% (70) | 15.19% (120) |
| Nausea | 6.84% (54) | 7.92% (41) | 18.48% (146)*** |
| Dyspepsia | 10.14% (80) | 9.85% (51) | 9.75% (77) |
| Diarrhea | 6.46% (51) | 7.34% (38) | 11.39% (90)*** |
| Depressive Symptoms | 7.35% (58) | 6.56% (34) | 9.24% (73) |
| Anxiety Symptoms | 5.70% (45) | 7.34% (38) | 9.75% (77)** |
| Dizziness | 6.59% (52) | 6.37% (33) | 9.11% (72) |
| Fatigue | 5.45% (43) | 4.83% (25) | 6.08% (48) |
| Influenza | 4.69% (37) | 6.18% (32) | 5.19% (41) |
| Irritability | 5.45% (43) | 4.63% (24) | 5.06% (40) |
| Hyporexia | 2.15% (17) | 3.28% (17) | 6.20% (49)*** |
| Back Pain | 3.68% (29) | 3.67% (19) | 4.30% (34) |
| Vomiting | 2.03% (16) | 2.12% (11) | 5.32% (42)*** |
| Dry Mouth | 2.28% (18) | 2.51% (13) | 3.42% (27) |
| Attentional Disturbance | 2.41% (19) | 1.54% (8) | 3.42% (27) |
| Constipation | 3.68% (29) | 1.93% (10) | 1.52% (12)** |
| Cough | 3.42% (27) | 1.54% (8)* | 1.77% (14)* |
| Arthralgia | 2.79% (22) | 2.12% (11) | 1.39% (11) |
Note:
p < .05,
p < .01,
p < .001.
The AEs report for the 5 mg sample are for the EU & US samples only.
EOT = end of treatment; AEs = adverse events.
Among those who discontinued drug treatment by EOT (8 weeks post-TQD), pooled analysis indicated that participants in the 20 mg rimonabant group (29.9%; n=75) were more likely to cite AEs as the reason for drug discontinuation than those on placebo (16.2%; n=41), p < .001. Among those citing AEs as a reason for discontinuation, significant differences (p<.05) were noted between placebo and the 20 mg groups for headache (34.2% vs. 13.3%), nausea (12.2% vs. 32.0%), and anxiety symptoms (2.4% vs. 29.3%), respectively. No differences were noted for the 5 mg condition in any of the comparisons of those who discontinued drug.
We also examined AE’s among those who discontinued drug treatment for any reason, because patients may not always specify that an AE was the reason for their discontinuation (or were lost to follow-up). As noted in Figure 1, there were 253 (32.1%), 193 (37.3%), and 251 (31.8%) patients who discontinued drug for any reason in the placebo, 5 mg, and 20 mg conditions, respectively. Significant differences (p < .05) between placebo and the 20 mg condition were noted among those discontinuing drug for any reason in the frequencies of headache (17.4% vs. 10.0%), sleep disturbance (8.7% vs. 18.3%), nausea (5.9% vs. 19.9%), anxiety symptoms (2.4% vs. 14.7%), hyporexia (1.6% vs. 6.8%), and vomiting (2.0% vs. 5.6%), respectively. Those in the 5 mg group who discontinued drug were more likely to report anxiety symptoms (6.2%) compared to placebo (2.4%).
Serious Adverse Events
In terms of SAEs, a total of 48 were reported for the pooled EU, US, & META samples, with no deaths reported in any study. The most frequent SAEs were hospitalizations due to injuries (7; 5 in placebo; 2 in 20 mg), unspecified infections (5 at 20 mg), coronary artery disorders (3 in placebo), and gastrointestinal motility conditions (3; 1 in placebo, 2 in 20 mg). There was one suicide attempt in the EU study by a participant in the placebo condition. In the STRATUS US sample, there were 4 pregnancies (1 in placebo, 3 in 5 mg), with one in the 5 mg group resulting in a spontaneous abortion.
HADS
The HADS, a measure of depression and anxiety, was examined as a change score from baseline to EOT (8 weeks post-TQD) for the combined STRATUS EU & US sample. Compared to placebo, neither the 20 mg nor the 5 mg rimonabant dose produced significant differences in 8 weeks post-TQD change scores for either the HADS Depression or Anxiety scales (see Table 5). There were similar findings when restricting the analyses to those who were abstinent from smoking.
Table 5.
HADS Depression and Anxiety change from pre-medication baseline, for both the total sample and for abstainers only, at EOT (8-weeks post-TQD) for STRATUS EU & US (STRATUS META did not administer the HADS). None of the comparisons were significant compared to placebo. Values are presented as least square means (standard error).
| Study | Placebo | 5 mg | 20 mg | |||
|---|---|---|---|---|---|---|
| HADS Anxiety | ||||||
| STRATUS EU | −0.82 | (0.23) | −0.59 | (0.23) | −0.24 | (0.22) |
| Abstainers only | −1.15 | (0.41) | −0.92 | (0.36) | −0.70 | (0.35) |
| STRATUS US | −0.56 | (0.21) | −0.89 | (0.21) | −0.52 | (0.20) |
| Abstainers only | −1.07 | (0.44) | −1.29 | (0.45) | −0.86 | (0.35) |
| POOLED EU/US | −0.68 | (0.15) | −0.75 | (0.15) | −0.38 | (0.15) |
| Abstainers only | −1.11 | (0.30) | −1.06 | (0.28) | −0.80 | (0.25) |
| HADS Depression | ||||||
| STRATUS EU | −0.31 | (0.20) | −0.18 | (0.20) | 0.19 | (0.19) |
| Abstainers only | −0.42 | (0.36) | −0.05 | (0.32) | −0.08 | (0.31) |
| STRATUS US | −0.45 | (0.18) | −0.89 | (0.19) | −0.32 | (0.17) |
| Abstainers only | −1.15 | (0.37) | −0.82 | (0.37) | −0.59 | (0.29) |
| POOLED EU/US | −0.35 | (0.13) | −0.53 | (0.13) | −0.06 | (0.13) |
|
| ||||||
| Abstainers only | −0.76 | (0.26) | −0.35 | (0.24) | −0.34) | (0.22) |
Note: HADS = The Hospital Anxiety and Depression Scale. EOT = end of treatment; TQD = targeted quit date.
DISCUSSION
While individual study results varied, pooled analyses of the Phase III STRATUS program showed that 20 mg of rimonabant is a moderately effective aid to smoking cessation, but with increased incidence of several AEs. Abstinence was more likely at EOT (OR = 1.6, 95% CI: 1.26, 2.12) and at 48 weeks (OR = 1.50, 95% CI: 1.03, 2.17) for the rimonabant 20 mg dose compared to placebo. In terms of AEs, the 20 mg rimonabant dose, compared to placebo, produced significantly increased rates of nausea, diarrhea, anxiety symptoms, hyporexia, and vomiting, as well as decreased headache, constipation, and cough. There were no differences in abstinence rates or in AE frequencies between the 5 mg rimonabant dose and placebo (except for increased upper respiratory disturbance and decreased cough). Although rimonabant was revoked from the EU market, and never approved for the US market, due to AE concerns, we believe that these data support a research role for endocannabinoids as a potential pharmacotherapy for smoking cessation.
While the likelihood of quitting smoking for those using rimonabant 20 mg was significantly better than placebo at 48 weeks post-TQD (OR = 1.50, 95% CI: 1.03, 2.17), it is somewhat lower than that of other approved pharmacotherapies. For example, meta-analyses (Cahill et al., 2013) of varenicline (OR = 2.88, 95% credible interval [credI]: 2.4, 3.47), NRT (OR = 1.84, 95% credI: 1.71, 1.99), and bupropion (OR = 1.82, 95% credI: 1.60, 2.06), compared to placebo, indicate higher likelihoods of remaining abstinent at 6 months or more with these first-line medications than what we found for rimonabant 20 mg at 48 weeks post-TQD (OR = 1.50). In comparison with second-line medications, as classified by the US Public Health Service’s Clinical Practice Guidelines (US Department of Health and Human Services, 2008), the relative risk performance of rimonabant 20 mg versus placebo at 48 weeks post-TQD in our pooled analyses indicates that it is somewhat less likely to lead to abstinence (RR = 1.45, 95% CI: 1.02, 1.97) than clonidine (RR = 1.63, 95% CI: 1.22, 2.18) or nortriptyline (RR = 2.03, 95% CI: 1.48, 2.78) (Cahill et al., 2013). However, it should be noted that the efficacy of rimonabant has not, to our knowledge, been formally compared to other smoking cessation pharmacotherapies, using network meta-analysis or other statistical procedures.
Rimonabant minimized weight gain among quitting smokers, but only while on medication. At EOT, rimonabant 20 mg resulted in less weight gain than placebo, and, consistent with its effect on weight, this dose lead to more AE reports of hyporexia compared to placebo. This effect on weight is consistent with those of the pooled analysis of the RIO trials (van Gaal et al., 2008), which found that rimonabant 20 mg led to a decrease in weight by 4 weeks on medication, compared to placebo. However, once off medication at 48 weeks post-TQD, there were no weight differences by drug group in our pooled analyses. This is consistent with findings from the RIO-North America trial (Pi-Sunyer et al., 2006), which found no weight differences by drug at 2 years among those who were switched from rimonabant to placebo after the first year. Given that most smokers typically gain weight after quitting, our findings support the idea that endocannabinoid antagonists, such as rimonabant, can minimize the weight gain associated with smoking cessation.
Rimonabant 20 mg led to greater reports of AEs, and treatment discontinuation due to AEs, than the 5-mg and placebo doses. The 20-mg rimonabant dose produced increased nausea, diarrhea, anxiety, hyporexia, and vomiting, as well as decreased headache, compared to placebo. A similar pattern was found by the FDA for GI events across all rimonabant trials (US Food and Drug Administration, 2007a) and in the published pooled analyses of the RIO trials (van Gaal et al., 2008). Among those who discontinued treatment, our findings indicated that those in the 20-mg rimonabant group (11.0%) were more likely to cite AEs as the reason for study discontinuation by EOT than those on placebo (5.6%). These rates are somewhat lower than those reported in the pooled analysis of the RIO weight reduction trials (van Gaal et al., 2008), which found that 13.8% of patients on rimonabant 20 mg discontinued treatment due to AEs, compared to 7.2% on placebo, though the RIO frequencies were measured at one year and would be expected to be higher. Thus, our findings support previous published and unpublished findings that rimonabant 20 mg results in more AEs than placebo, particularly GI disturbances.
The primary reason for the removal of rimonabant from the EU market, and of non-approval for the US market, was concern for the potential for neuropsychiatric AEs. Our findings support findings from the pooled RIO analysis that suggest that rimonabant use leads to increased anxiety symptoms (van Gaal et al., 2008), as our pooled analysis indicated that those on rimonabant 20 mg were significantly more likely to report anxiety AEs than those on placebo (9.7% vs. 5.7%). Additionally, participants who discontinued the STRATUS studies included here due to AEs were more likely to have reported anxiety symptoms in the 20-mg (28.3%) compared to the placebo (2.4%) group. However, consistent with meta-analysis of the RIO trials (Christensen et al., 2007), we found no differences on the HADS Anxiety scale by drug dose. Given the sensitivity and specificity of the HADS Anxiety scale has demonstrated with identifying anxiety disorders (Bjelland et al., 2002), it is unclear why there is a discrepancy between the AE symptom reports and HADS scores across the rimonabant trials.
Our pooled analyses found no statistically significant evidence that 10 weeks of rimonabant led to increased rates of depressive symptoms. Neither the 20-mg nor the 5-mg rimonabant doses led to a significant increase in depressive symptom AEs compared to placebo. Likewise, depressive symptoms did not differ by drug group among those who discontinued treatment. Both of these findings are inconsistent with the pooled findings of the RIO studies (van Gaal et al., 2008), which found that rimonabant 20 mg, compared to placebo, led to increased depressive symptoms (including depressive disorders) overall (8.0% vs. 4.7%), and to treatment discontinuation due to depressive symptoms (2.9% vs. 1.4%). However, it should be noted that the RIO trials exposed participants to one year of rimonabant, compared to 10 weeks in the STRATUS studies that were the subject of our analyses. Our finding of no differences on the HADS Depression scale by drug dose is consistent with meta-analysis of the RIO trials (Christensen et al., 2007). Thus, there was no evidence from our analyses that rimonabant leads to greater depressive symptoms among smokers taking 20 mg of rimonabant for 10 weeks, compared to placebo, although the RIO trials suggest that taking rimonabant for one year increases the likelihood of these symptoms.
Using the original dataset provided to us by Sanofi-Aventis, we found only one report of suicidality, a completed suicide in the STRATUS-EU trial in the placebo group. However, in June 2007 (US Food and Drug Administration, 2007a, 2007b), at the request of the FDA, a blinded retrospective analysis was performed by Dr. Kelley Posner that examined 1201 patient narratives for possible suicidality across 13 rimonabant clinical trials using the Columbia Classification Algorithm of Suicide Assessment (C-CASA) (Posner et al., 2007). A total of 91 cases were identified as either possibly or definitely suicidal, 74 of which occurred in studies during initial randomization to placebo (N=20), 5 mg (N=8), or 20 mg (N=46) rimonabant. Rimonabant 20 mg was found to have an increased likelihood of suicidality compared to placebo, OR = 1.9 (95% CI: 1.1, 3.1). Section 1 (Table 15) of the FDA report (US Food and Drug Administration, 2007b) indicates that there were 8 total cases of suicidality among the 3 studies (EFC4474, EFC4964, and EFC5794) included in our pooled analyses. The suicidality-related AEs in the smoking cessation trials analyzed here were too few (0–2 AEs per drug group per study) to make conclusions about the influence of rimonabant and suicidality among smokers receiving 10 weeks of rimonabant pharmacotherapy. However, one study not included in our analyses, STRATUS-WW (EFC4796), which provided rimonabant for a year, did find more suicidality-related AEs for the rimonabant 20 mg group (n=12) than the 5 mg (n=0) or placebo group (n=0) (US Food and Drug Administration, 2007b). Additionally, the pooled analysis of obesity (RIO) trials found more instances of suicidal ideation (n=17) among the rimonabant 20 mg group compared to the 5 mg (n=8) and placebo (n=8) groups (van Gaal et al., 2008). Taken together, these data suggest that there is a small increase in suicidality associated with 20 mg of rimonabant vs. placebo, but only among the studies where it was taken for a year.
While work on CB1 antagonists has largely halted, there is evidence warranting examination of other drugs in this class that might be effective and safer. Following revocation of rimonabant’s approval on the EU market, trials evaluating other CB1 antagonists were stopped, including ibipinabant (Solvay/Bristol-Myers Squibb), otenabant (Pfizer), surinabant (Sanofi-Aventis), and taranabant (Merck) (Jones, 2008). However, work on other endocannabinoid-related drugs have continued. For example, cannabidiol, a weak CB1 inverse agonist with demonstrated neuroprotective properties, including anxiolysis (Bergamaschi et al., 2011), has been found to reduce smoking (Morgan et al., 2013) and consumption of other addictive drugs (Prud’homme, Cata & Jutras-Aswad, 2015). Other substances that impact the CB1 receptor, including URB597 (Forget et al., 2016), a fatty-acid-amide-hydrolase (FAAH) inhibitor, along with VDM11 (Gamaleddin et al., 2011) and AM404 (Gamaleddin et al., 2013), both anandamide reuptake inhibitors, have been found to reduce cue-induced reinstatement in rats. With the exception of cannabidiol, these drugs have not been tested beyond pre-clinical trials, and their potential for AEs among humans are largely unknown. Thus, additional work with other cannabinoid-related drugs suggests that CB1 antagonists might have a role in smoking behavior.
Despite the increased incidence of psychiatric and GI-related AEs, and its moderate impact on cessation, these rimonabant trials demonstrate that the endocannabinoid system is a potential target as a smoking cessation pharmacotherapy. A major impetus for making these data public is to stimulate the development of future medications targeting the cannabinoid system. However, the side effect profiles of these endocannabinoid antagonists necessitate that other endocannabinoid receptors and mechanisms be explored. For example, a partial agonist of the CB2 receptors, such as Δ9-tetrahydrocannabivarin (THCV), may avoid many of the side effects associated with CB1 antagonism (McPartland et al., 2015), yet potentially reduce drug self-administration (Xi et al., 2011). Additionally, cannabidiol, a cannabinoid that does not target either the CB1 or CB2 receptors, but instead acts on a variety of receptor types, has been found to impact drug-related behavior (Zlebnik & Cheer, 2016). Finally, it is worth mentioning that the beneficial effects of rimonabant on weight loss, triglyceride levels, high-density lipoproteins levels, and insulin resistance that have been demonstrated in the RIO clinical trials (Despres, Golay, & Sjostrom, 2005; Pi-Sunyer et al., 2006; van Gaal et al., 2005) may make this medication class a particularly attractive choice for smokers with multiple metabolic risk factors for chronic disease.
In conclusion, we found that 10 weeks of rimonabant 20 mg was moderately effective at promoting abstinence at smoking compared to placebo, and that the effect was still present months after stopping medication, at week 48. Compared to placebo, rimonabant 20 mg minimized weight loss, but only while the drug was being taken. In terms of safety, the rimonabant 20 mg led to a significantly greater likelihood of side effects compared to placebo, including increased GI disturbances and anxiety symptoms. However, unlike previous reports, we did not find conclusive evidence that rimonabant 20 mg led to increased likelihood of depressive symptoms or suicidality compared to placebo. We believe that these data support a research role for endocannabinoids as a potential pharmacotherapy for smoking cessation.
Acknowledgments
This study was supported by Sanofi-Aventis, manufacturer of rimonabant. Sanofi-Aventis designed the study, funded data collection, and provided the dataset, but did not analyze or provide data interpretation for this manuscript or provide input regarding its publication.
The authors wish to thank the principal investigators and study staff of the sites that participated in the STRATUS EU, US, META trials, and Owen Hagino, Bernard Sebastien, and Marie Sebille of Sanofi-Aventis. Work on this manuscript was supported by MD Anderson’s Cancer Center Support Grant (CA016672), funded by the National Cancer Institute.
STRATUS EU site principal investigators: Pierre Bartsch, MD; Laurence Galanti, MD; Wilfried De Backer, MD; Paul De Vuyst, MD; Hedwig Boudrez, MD; Henri-Jean Aubin, MD; Anne Borgne, MD; Anne-Marie Clauzel, MD; Gilbert Lagrue, MD; Anne-Laurence Le Faou, MD; Patrick Maillon, MD; Jean Perriot, MD; Gérard Peiffer, MD; Raymond Philippe Sarfati, MD; Véronique Peim Boujenah, MD; José Luís Izquierdo, MD; Miguel Barrueco, MD; Pilar De Lucas, MD; José Luís Álvarez-Sala, MD; Hans Gilljam, MD; Matz Larsson, MD; Johan Herlitz, MD; Peter Lange, MD; Jacques Cornuz, MD; Michael Tamm, MD; Erich Russi, MD; Jean-Paul Humair, MD; Jacqueline James, MD; Gareth Dovey, MD; Caroline Naik, MD; John Robinson, MD; Mansur Salman, MD; Guy Newcombe, MD; Francis Arnold, MD; Hawys Thomas, MD; Kevin Lewis, MD; Carlos Jiménez Ruiz, MD.
STRATUS US site principal investigators: Lowell C. Dale, MD; Robert M. Anthenelli, MD; Paul M. Cinciripini, PhD; Raymond Niaura, PhD; Elbert D. Glover, PhD; Darrell R. Schroeder, MS; Stephen Rennard, MD; Mitchell A. Nides, PhD; Charles O’Brien, MD; Tony George, MD.
STRATUS META site principal investigators: Paul M. Cinciripini, PhD; Lowell C. Dale, MD; James M. Ferguson, MD; Maggie DeBon, MD; Jon F. Heiser, MD; Jon F. Heiser, MD; Peter D. Londborg, MD; Charles H. Merideth, MD; Geri E. Poss, MD; Stephen I. Rennard, MD; Mark Roffman, MD.
Data and Safety Monitoring Board: Alain Leizorovicz MD (Chairman), Faculté RTH Laënnec – EA643, Université Claude Bernard, Lyon, France ; Michael Weintraub MD, University of Rochester School of Medicine, New York, USA; Jean-Louis Imbs MD, Hôpital Civil – Centre Régional de Pharmacovigilance 1, Strasbourg, France; Elliot Danforth MD, University of Vermont, Burlington, VT, USA; David P.L. Sachs MD, Palo Alto Center for Pulmonary Disease, Palo Alto, CA, USA.
Footnotes
AUTHOR CONTRIBUTIONS
PMC, HJA, LCD, RN, and RMA were responsible for the study concept and design. JDR performed the data analyses. PMC and MKH assisted with data analysis and interpretation of the findings. JDR drafted the manuscript. All authors provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
References
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Bergamaschi MM, Queiroz RHC, Crippa JAS, Zuardi AW. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011;6:237–249. doi: 10.2174/157488611798280924. [DOI] [PubMed] [Google Scholar]
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: An overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:CD009329–CD009329. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K, Ussher MH. Cannabinoid type 1 receptor antagonists for smoking cessation. Cochrane Database Syst Rev. 2011:CD005353. doi: 10.1002/14651858.CD005353.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamulera C. Cue reactivity in nicotine and tobacco dependence: A “multiple-action” model of nicotine as a primary reinforcement and as an enhancer of the effects of smoking-associated stimuli. Brain Res Rev. 2005;48:74–97. doi: 10.1016/j.brainresrev.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: A meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Cohen C, Kodas E, Griebel G. CB1 receptor antagonists for the treatment of nicotine addiction. Pharmacol Biochem Behav. 2005;81:387–395. doi: 10.1016/j.pbb.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmacol. 2005;30:145–155. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13:451–463. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8:585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: A meta-analysis of fMRI studies. Neuroimage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency. [Accessed 2016.04.13];The European Medicines Agency recommends suspension of the marketing authorisation of Acomplia. 2008 Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/11/WC500014774.pdf.
- European Medicines Agency. [Accessed 2016.03.08];Acomplia: EPAR - Product Information. 2009 Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000666/WC500021287.pdf.
- Forget B, Guranda M, Gamaleddin I, Goldberg SR, Le Foll B. Attenuation of cue-induced reinstatement of nicotine seeking by URB597 through cannabinoid CB1 receptor in rats. Psychopharmacology (Berl) 2016;233:1823–1828. doi: 10.1007/s00213-016-4232-y. [DOI] [PubMed] [Google Scholar]
- Gamaleddin I, Guranda M, Goldberg SR, Le Foll B. The selective anandamide transport inhibitor VDM11 attenuates reinstatement of nicotine seeking behaviour, but does not affect nicotine intake. Br J Pharmacol. 2011;164:1652–1660. doi: 10.1111/j.1476-5381.2011.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamaleddin I, Guranda M, Scherma M, Fratta W, Makriyannis A, Vadivel SK, Goldberg SR, Le Foll B. AM404 attenuates reinstatement of nicotine seeking induced by nicotine-associated cues and nicotine priming but does not affect nicotine- and food-taking. J Psychopharmacol. 2013;27:564–571. doi: 10.1177/0269881113477710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Lovinger DM. Emerging roles for endocannabinoids in long-term synaptic plasticity. Br J Pharmacol. 2003;140:781–789. doi: 10.1038/sj.bjp.0705466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. P Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Caulder T, Lupica CR. Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neurosci. 2003;23:4815–4820. doi: 10.1523/JNEUROSCI.23-12-04815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Jones D. End of the line for cannabinoid receptor 1 as an anti-obesity target? Nat Rev Drug Discov. 2008;7:961–962. doi: 10.1038/nrd2775. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF. Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol. 2004;143:227–234. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo Clinic Nicotine Research Program. Smoke-Free and Living It. Rochester, MN: Mayo Foundation for Medical Education and Research; 2000. [Google Scholar]
- McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172:737–753. doi: 10.1111/bph.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Di M. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- Morgan CJA, Das RK, Joye A, Curran HV, Kamboj SK. Cannabidiol reduces cigarette consumption in tobacco smokers: Preliminary findings. Addict Behav. 2013;38:2433–2436. doi: 10.1016/j.addbeh.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Morrison MF, Ceesay P, Gantz I, Kaufman KD, Lines CR. Randomized, controlled, double-blind trial of taranabant for smoking cessation. Psychopharmacology (Berl) 2010;209:245–253. doi: 10.1007/s00213-010-1790-2. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VL, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di CG. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): Classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud’homme M, Cata R, Jutras-Aswad D. Cannabidiol as an intervention for addictive behaviors: A systematic review of the evidence. Subst Abuse. 2015;9:33–38. doi: 10.4137/SART.S25081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti NA, Gonzales D, Dale LC, Lawrence D, Chang Y. A randomized controlled trial of adding the nicotine patch to rimonabant for smoking cessation: Efficacy, safety and weight gain. Addiction. 2009;104:266–276. doi: 10.1111/j.1360-0443.2008.02454.x. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Congy C, Martinez S, Oustric D, Perio A, Poncelet M, Maruani J, Arnone M, Finance O, Soubrie P, Le Fur G. SR147778 [5-(4-bromophenyl)-1-(2,4-dichlorophenyl)-4-ethyl-N-(1-piperidinyl)-1H-pyrazole-3-carboxamide], a new potent and selective antagonist of the CB1 cannabinoid receptor: Biochemical and pharmacological characterization. J Pharmacol Exp Ther. 2004;310:905–914. doi: 10.1124/jpet.104.067884. [DOI] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. P Natl Acad Sci USA. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanofi-aventis. [Accessed 2016.04.06];Sanofi-aventis to discontinue all clinical trials with rimonabant. 2008 Available at: http://en.sanofi.com/Images/14245_20081105_rimonabant_en.pdf.
- Saul S. [Accessed 2016.04.12];FDA panel rejects drug for obesity. 2007 Available at: http://www.nytimes.com/2007/06/14/business/14drugs.html.
- Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- Tonstad S, Aubin H-J. Efficacy of a dose range of surinabant, a cannabinoid receptor blocker, for smoking cessation: a randomized controlled clinical trial. J Psychopharmacol. 2012;26:1003–1009. doi: 10.1177/0269881111431623. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. [Accessed 2016.04.06];Treating Tobacco Use and Dependence: 2008 Update. 2008 Available at: http://bphc.hrsa.gov/buckets/treatingtobacco.pdf.
- US Food and Drug Administration. Transcript of the Joint Meeting of the Nonprescription Drugs Advisory Committee and the Drug Abuse Advisory Committee of the Food and Drug Administration. Rockville, MD: US Food and Drug Administration; 1995. [Google Scholar]
- US Food and Drug Administration. [Accessed 2016.04.13];Briefing Information for FDA Advisory Committee Meeting on ZIMULTI (Rimonabant), May 10, 2007. 2007a Available at: http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4306b1-01-sponsor-backgrounder.pdf.
- US Food and Drug Administration. FDA briefing document. [Accessed 2016.03.29];NDA 21-888 Zimulti (rimonabant) tablets, 20 mg Sanofi Aventis advisory committee - 13 June 2007. 2007b Available at: http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4306b1-fda-backgrounder.pdf.
- van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- van Gaal L, Pi-Sunyer X, Despres J-P, McCarthy C, Scheen A. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care. 2008;31(Suppl 2):S229–40. doi: 10.2337/dc08-s258. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: Results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Xi Z-X, Peng X-Q, Li X, Song R, Zhang H-Y, Liu Q-R, Yang H-J, Bi G-H, Li J, Gardner EL. Brain cannabinoid CB2 receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Furjanic MA, Ferrara JJ, McAndrew NR, Ardino EL, Ngondara A, Bernstein Y, Thomas KJ, Kim E, Walker JM, Nagar S, Ward SJ, Raffa RB. The endocannabinoid system and rimonabant: a new drug with a novel mechanism of action involving cannabinoid CB1 receptor antagonism--or inverse agonism--as potential obesity treatment and other therapeutic use. J Clin Pharm Ther. 2007;32:209–231. doi: 10.1111/j.1365-2710.2007.00817.x. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- Zlebnik NE, Cheer JF. Beyond the CB1 Receptor: Is Cannabidiol the Answer for Disorders of Motivation? Annu Rev Neurosci. 2016;39:1–17. doi: 10.1146/annurev-neuro-070815-014038. [DOI] [PMC free article] [PubMed] [Google Scholar]