Abstract
Small molecules have extensive untapped potential to benefit society, but access to this potential is too often restricted by limitations inherent to the customized approach currently used to synthesize this class of chemical matter. In contrast, the “building block approach”, i.e., generalized iterative assembly of interchangeable parts, has now proven to be a highly efficient and flexible way to construct things ranging all the way from skyscrapers to macromolecules to artificial intelligence algorithms. The structural redundancy found in many small molecules suggests that they possess a similar capacity for generalized building block-based construction. It is also encouraging that many customized iterative synthesis methods have been developed that improve access to specific classes of small molecules. There has also been substantial recent progress toward the iterative assembly of many different types of small molecules, including complex natural products, pharmaceuticals, biological probes, and materials, using common building blocks and coupling chemistry. Collectively, these advances suggest that a generalized building block approach for small molecule synthesis may be within reach.
Introduction
Small molecules can serve as powerful tools for improving society, with wide-ranging applications in medicine, science, and technology. They make the world a more enjoyable place to see, touch, taste, and smell, by acting as popular colorants, lotions, flavorants, and perfumes. In fact, there is probably not a household on the planet that is not positively impacted by this class of chemical matter on a daily basis. Small molecules also possess substantial untapped potential to perform many frontier functions,1 including modulating protein-protein interactions,2 allosterically modifying protein function,3 acting as prostheses on the molecular scale,4,5 serving as next-generation biological probes,6–9 enabling miniaturized diagnostics,10 transducing energy,11–16 emitting light,17 initiating self-healing,18 acting as molecular magnets,19 enabling next generation computing,20 and superconducting.21–22 However, largely due to limitations in synthesis, much of this functional potential remains largely untapped. Eliminating this synthesis bottleneck thus represents both a major challenge and an extraordinary opportunity for the field of Chemistry.
Currently, most small molecules are synthesized using customized approaches. For each target, a unique set of starting materials and a specialized sequence of different chemical reactions are developed de novo and then extensively optimized. This approach is a useful strategy for the large scale production of a particular target, but also a laborious bottleneck for the discovery and optimization of new function, which depends on rapid access to many different chemical compounds. Although it is now widely considered possible to synthesize any physically accessible small molecule using this customized approach, both the design and execution phases of this process are time intensive, challenging to automate, and inherently restricted to specialists.
In many other disciplines that share the challenge of assembling complex structures to access new functions, a widespread innovation is the development of a more generalized strategy involving the iterative assembly of interchangeable building blocks. Early examples can be found in Samuel Bentham’s pioneering use of interchangeable parts to facilitate the rapid repair of wooden pulley blocks23 and Honore Blanc’s24 and Eli Whitney’s25 modularization of musket production. Henry Ford dramatically increased the efficiency of this approach with the development of the assembly line, which revolutionized human transportation by making automobiles a household commodity.26 The building block approach is now recognized in a remarkably wide range of different areas, including architecture,27 computers,28 space stations,29 robotics,30 college curricula,31 music,32 smart technology apps,28 and artificial intelligence algorithms.33 The advantages of building block-based construction on efficiency, flexibility, and scalability are well-documented and widely appreciated.34 Perhaps even more exciting is the capacity of this approach to inspire and enable innovation. This is evidenced in the explosion of applications for 3D printing35–38 and the joyful creativity unleashed in a child when handed their first bucket of Lego bricks.
During the latter half of the 20th century, iterative building block assembly was extended to the molecular scale, yielding automated synthesizers that now provide on-demand access to peptides39 and oligonucleotides40. The corresponding impact has been transformative. To highlight just a few examples, automatically synthesized oligonucleotide probes corresponding to every gene in the human genome printed on a glass slide helped usher in the era of genomics.41–42 Countless peptide- and oligonucleotide-based drug candidates were rapidly tested and optimized yielding entirely new classes of therapeutics.43–44 Total synthesis of genes, proteins, and even complete genomes became possible, launching the field of synthetic biology.45–47 Substantial recent progress in the iterative synthesis of oligosaccharides has also led to important impacts in vaccine development.48–49
Extending the building block method to small molecule synthesis brings a unique set of challenges. The remarkable structural complexity and diversity of small molecules will require a greater number of building blocks, more versatile assembly reactions, and new strategies for substrate-general purification conditions. Encouragingly, many different iterative building block-based synthesis approaches have already increased access to particular molecules and regions of chemical space. Heading towards an even more general platform, iteration of metal-mediated cross-coupling reactions has provided unprecedented access to a diverse range of small molecules and even demonstrated the possibility of automated synthesis. Many challenges remain, but as progress in this direction enables access to previously untapped small molecule functions, the impetus for finding solutions will continue to grow.
Many small molecules are inherently modular
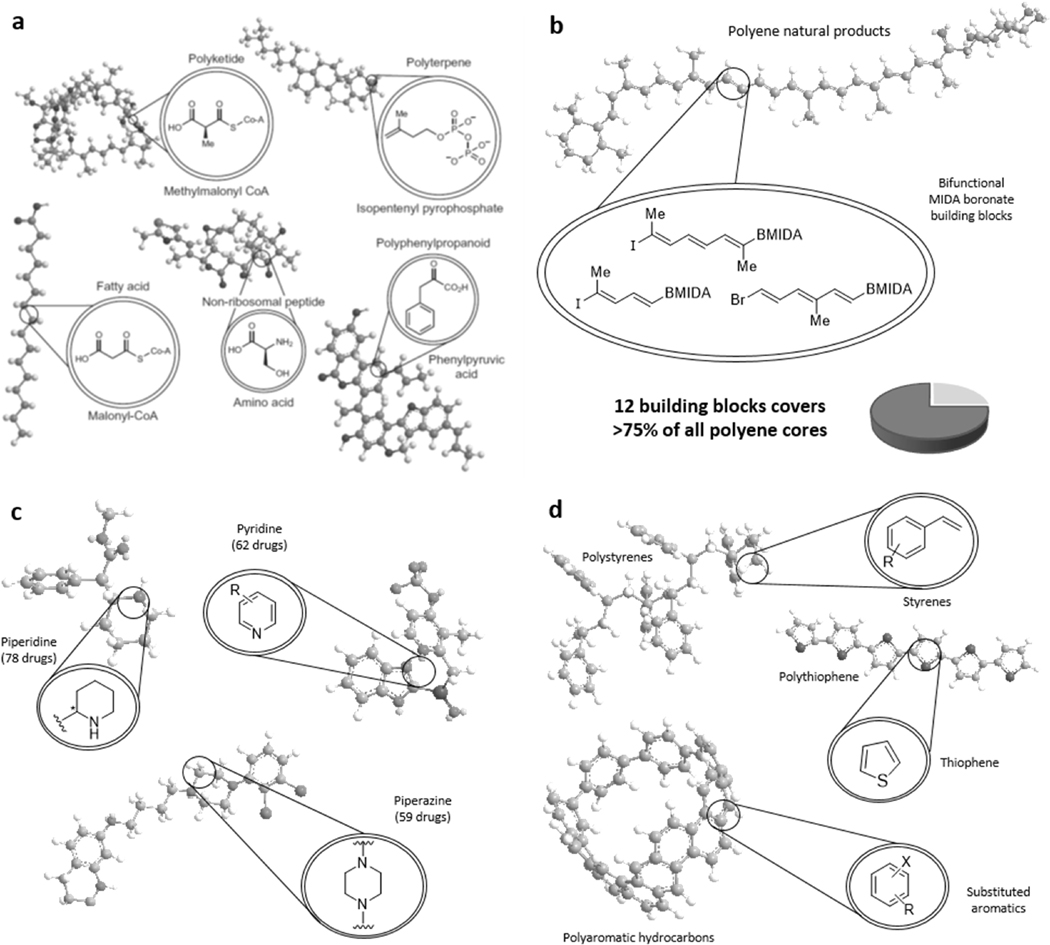
Small molecules are highly structurally diverse, which makes the development of a generalized building block approach for this class of chemical matter especially challenging. However, many types of small molecules are inherently modular, suggesting that such structures and their accompanying functions should be accessible using a generalized modular synthesis approach. Most natural products, which represent the source of inspiration for more than half of all human therapeutics,50,51 are derived from just a few major biosynthetic pathways that each involve the iterative assembly of a small number of discrete molecular building blocks (Figure 1a). For example, polyketides are biosynthesized from malonyl CoA and methyl malonyl CoA, polyterpenes from isopentenyl pyrophosphate and dimethylallylpyrophosphate, fatty acids primarily from malonyl CoA, non-ribosomal peptides from amino acids, and polyphenylpropanoids from phenylpyruvic acid.
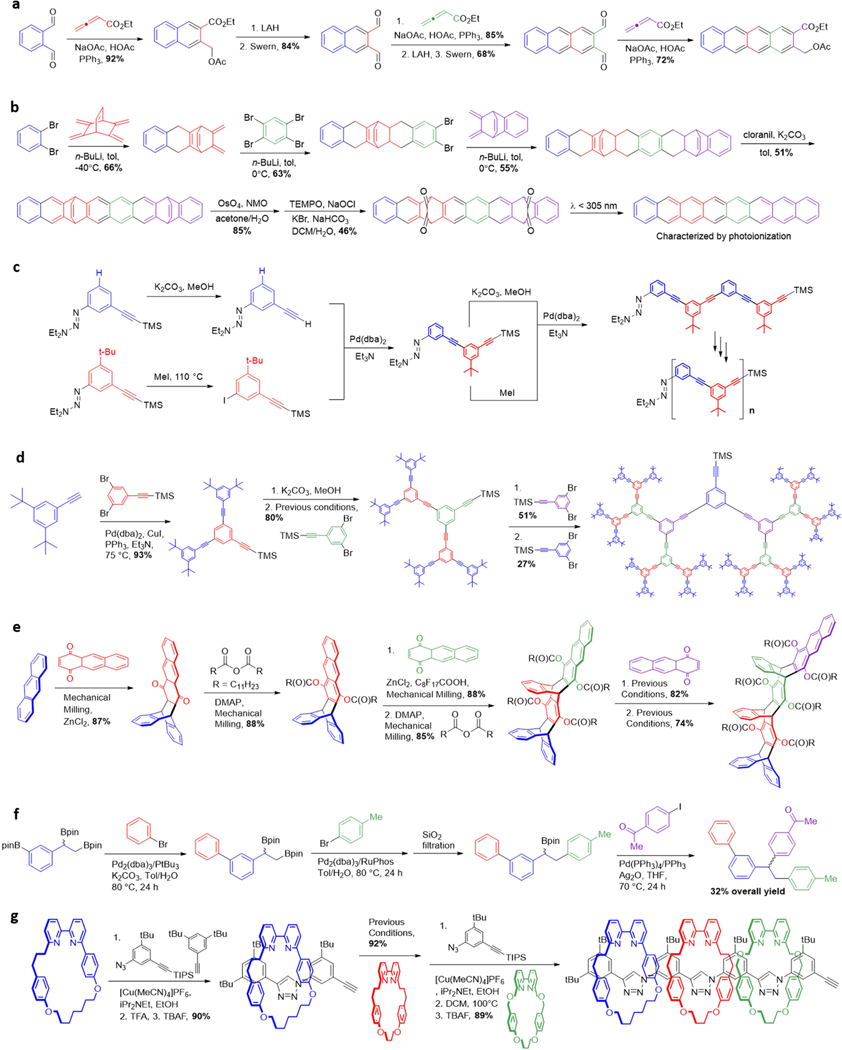
Figure 1. Modularity in small molecules.
a Biosynthesis of natural products from building blocks. b Coverage of > 75% of polyene natural product chemical space using 12 bifunctional halo MIDA boronate building blocks. c Common heteroaryl motifs found in pharmaceuticals. d Modularity in organic materials.
Even highly complex natural product molecular architectures can usually be traced back to these same modular pathways. Although many natural product biosyntheses also involve rearrangements, oxidations, and cyclizations, there is still evidence that this modularity shows up in the final products. A recent analysis revealed that more than 75% of all the polyene motifs found in natural products can be prepared with just 12 building blocks and one coupling reaction (Figure 1b).52 Increasing evidence further suggests that natural product chemical space is bounded,53 enabling consideration of generalized approaches for studying the complete natural productome.54 An expanded effort is now underway to find the minimal number of building blocks required to access most natural product chemical space.55
Even many non-natural small molecules, which lack such biosynthetic constraints, still contain a remarkable degree of structural redundancy. For example, a 2014 analysis of 1086 FDA approved small molecule drugs showed many recurring heterocyclic building blocks (Figure 1c), including piperidine (72 drugs), pyridine (62 drugs), piperazine (59 drugs), cephem (41 drugs), pyrrolidine (37 drugs), and thiazole (30 drugs).56 Sixteen additional heterocycles appear in at least 10 drugs. This modularity suggests that much of the chemical space relevant to synthesizing pharmaceuticals should also be accessible from a defined set of building blocks. Materials components also display a high degree of modularity. Despite performing a wide range of different functions, they are often composed of common repeating substructural motifs, such as oligoarenes, oligothiophenes, and polystyrenes (Figure 1d). Collectively, this inherent modularity suggests that a wide range of different molecular functions should be accessible by simply assembling building blocks that come from a finite set of common substructural motifs.
Many customized iterative synthesis methods have already been developed
Iterative synthesis of small molecules from building blocks has already streamlined access to a diverse range of molecular structures. In each case, building blocks are consecutively added to a growing molecule by repeating a series of chemical transformations. The two main distinguishing characteristics for different iterative synthesis methods are the type of bond used to connect building blocks and the reactions used to form that bond; both of these characteristics can be optimized for a given target or area of chemical space. Representative methods of iterative synthesis are summarized in Figure 2 (natural products) and Figure 3 (unnatural molecules), with building blocks highlighted in different colors.
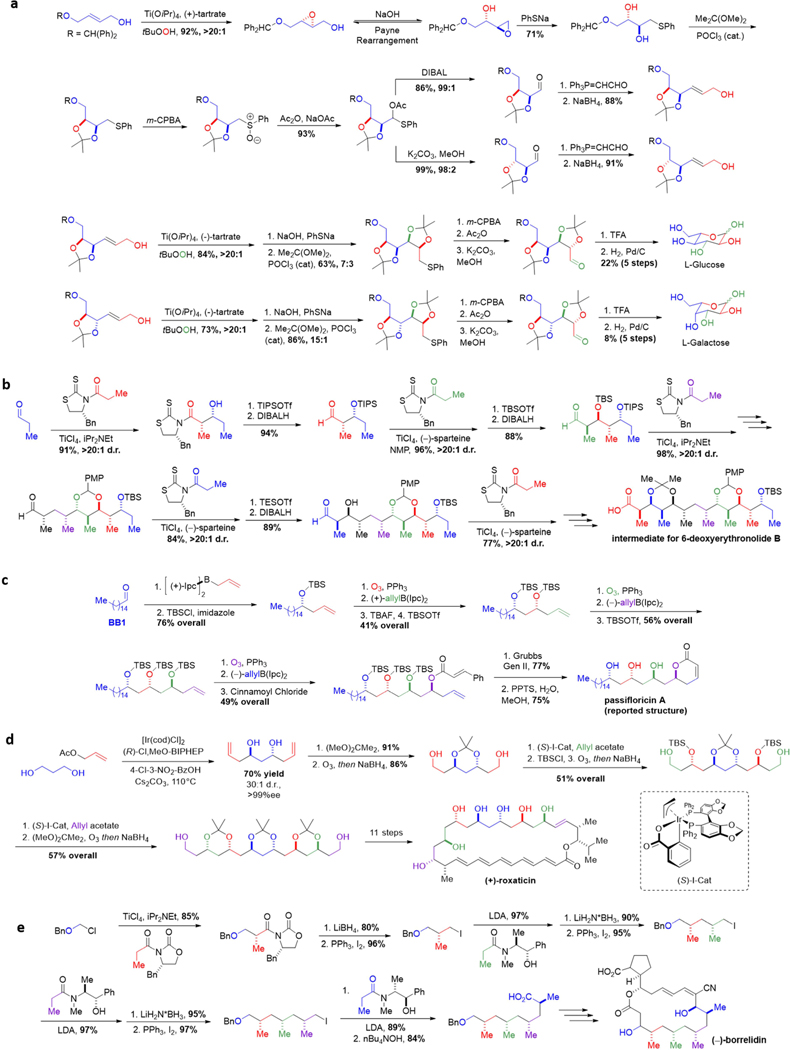
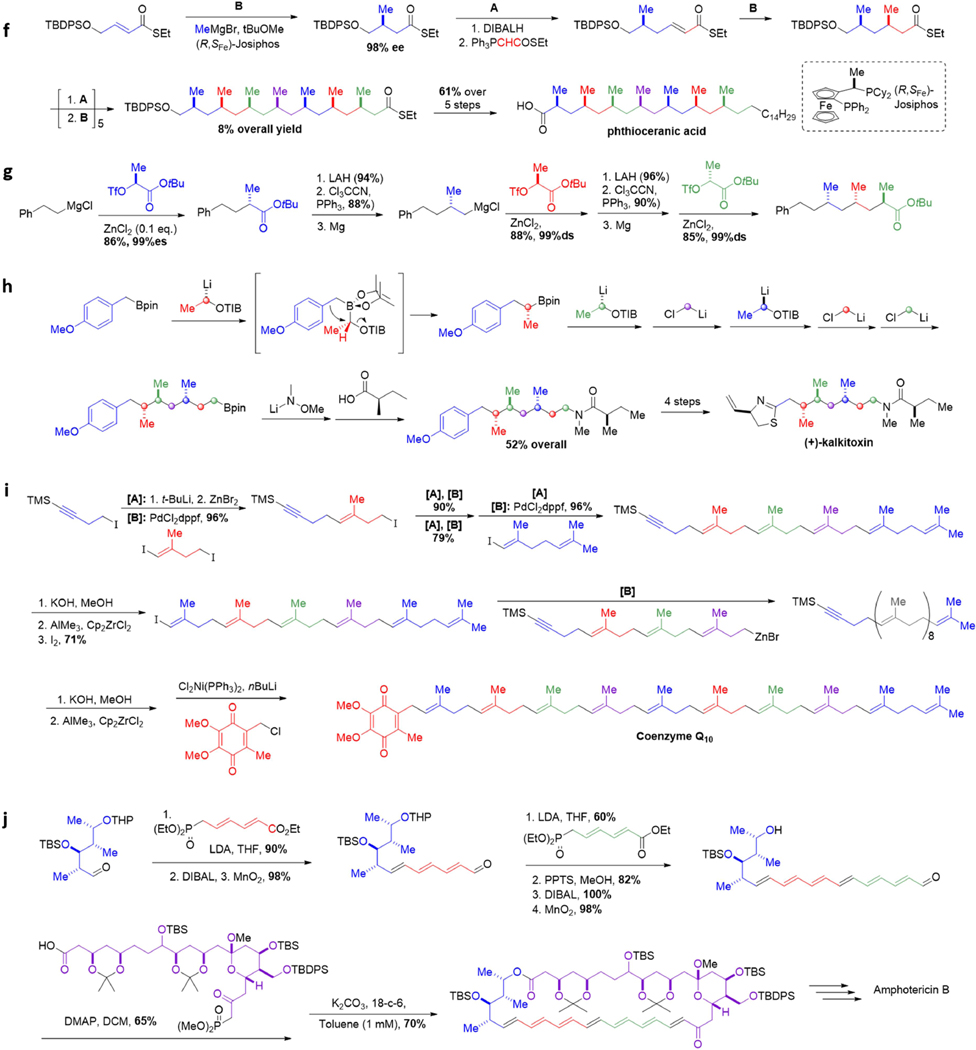
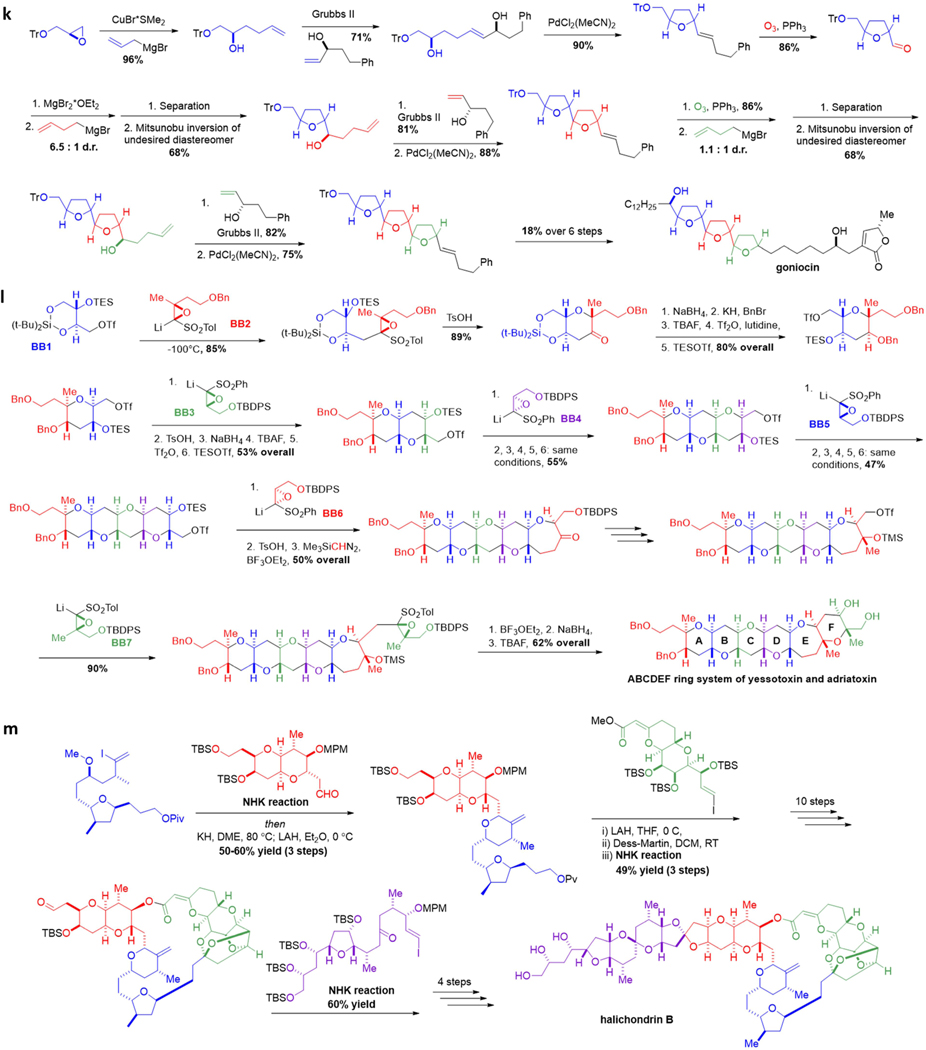
Figure 2. Customized iterative synthesis of naturally occurring small molecules.
a Aldohexose synthesis via iterative Sharpless epoxidation. b Formal synthesis of 6-deoxyerythronolide B via iterative aldol reactions. c Passifloricin A synthesis via iterative allylboration. d |(+)-Roxaticin via iterative C-C bond forming transfer hydrogenation. e (−)-Borrelidin via iterative Myers’ alkylation. f Phthioceranic acid via iterative conjugate addition. g Iterative synthesis of polydeoxypropionates via stereospecific displacement of tosylates. h (+)-Kalkitoxin via iterative homologation of boronic esters. i Coenzyme Q10 via iterative palladium-catalyzed couplings of alkylzinc reagents. j Amphotericin B via iterative Horner-Wadsworth-Emmons olefinations. k Synthesis of goniocin via iterative THF ring formation. l ABCDEF ring system of yessotoxin and adriatoxin via iterative oxiranyl anion strategy. m Halichondrin B via iterative NHK reactions.
Figure 3. Customized iterative synthesis of non-natural small molecules.
a Iterative arene homologation. b Iterative synthesis of octacene. c Iterative convergent synthesis of phenylacetylene oligomers. d Iterative convergent synthesis of hydrocarbon dendrimers. e Iterative synthesis of extended iptycenes. f Iterative arylation of multiply borylated compounds. g Iterative synthesis of rotaxanes.
These examples reflect some of the most efficient routes for accessing particular regions of chemical space, and in some cases they have led to impactful discoveries. Still, the assembly chemistries can impose some practical limitations, such as imperfect stereoselectivity in bond formations or harsh reaction conditions. The synthesis of more complex targets can require many transformations to complete one iteration cycle, lowering the overall yield and efficiency of the process. These methods also differ by how much structural variation is allowed in the building blocks, which is ultimately what determines the scope of molecules that can be made with an iterative method. Although these customized platforms each use different kinds of building blocks and assembly reactions and thus are limited with respect to their generality, they have substantially increased synthetic access to specific types of small molecule structures.
Seminal work by Masume and Sharpless established that an iterative four step cycle could be applied to access all eight L-hexoses (Figure 2a for illustrative examples). The cycle begins with asymmetric epoxidation of an allylic alcohol. In basic medium, the product rearranges to the more thermodynamically favorable terminal epoxide (Payne rearrangement), allowing for nucleophilic attack by sodium thiophenolate. A sequence of acetal protection of the nascent 1,2-diol followed by oxidation to the sulfoxide enables a Pummerer rearrangement to a gem-acetoxysulfide. Subsequent hydrolysis through reductive (DIBAL) or basic (K2CO3/MeOH) conditions allows selective retention or inversion of the C2 stereogenic center in formation of the corresponding aldehydes. Finally, Wittig olefination and aldehyde reduction produces allylic alcohols for further iteration. Changing the conditions of the two stereocontrolling steps, Sharpless asymmetric epoxidation and gem-acetoxysulfide reduction, allows for access to all eight L-hexoses. The versatility of this strategy has inspired the development of further methods for the de novo enantioselective synthesis of sugars,57 and many iterative methods have likewise been developed to access various different classes of small molecules.
The modular nature of polypropionates has inspired several customized iterative synthesis methods to access even highly stereochemically complex products. In early examples by Evans and Paterson, substrate controlled diastereoselective aldol reactions followed by stereodivergent ketone reductions, provided efficient access to a library of stereotetrad motifs.58–60 Auxiliary cleavage and regeneration of aldehyde intermediates allows both of these processes to be iterated. More recently, Crimmins developed a variant of the Evans oxazolidinone methodology61 that employs N-acylthiazolidinethiones as chiral auxiliaries to facilitate a more readily iterated aldol approach (Figure 2b).62–63 After a diastereoselective aldol reaction, cleavage of the chiral auxiliary directly generates an aldehyde, priming the substrate for further homologation. Notably, the same chiral building block can grant access to either stereoisomer of the syn aldol product depending on if the additive is TiC4or (−)-sparteine. In his synthesis of 6-deoxyerythronolide B, Crimmins iterated this sequence five times, setting 10 of the 11 stereocenters using a single type of reaction.64
A related polyketide motif, the 1,3-polyol unit, can also be prepared using several different types of iterative chemistry. Brown developed an iterative allylboration reaction using chiral boranes to carry out highly enantioselective reactions with aldehydes.65–67 The olefin motif of the resulting homoallylic alcohols can be oxidatively cleaved to reveal a new aldehyde for further allylation. This approach was employed to generate the 1,3-polyol fragment in the synthesis of passifloricin A (Figure 2c).68
Recently, Krische and co-workers pioneered a highly efficient C-C bond forming transfer hydrogenation strategy involving the in situ generation of an aldehyde and an organometallic nucleophile.69 Application of this iterative strategy in a two-directional manner expedited construction of the key polyol portion of (+)-roxaticin (Figure 2d).70 This methodology has proven to be highly versatile and readily scalable, enabling practical gram scale access to stereochemically complex building blocks for a wide range of highly complex polyketide natural products.71–73 This efficiency has further enabled practical synthesis and testing of bryostatin derivatives, shedding new light on the relationship between potency and biological activity.74
Polydeoxypropionates are an important subclass of the polyketide family, but the absence of functional handles has rendered their stereoselective synthesis challenging. Myers has developed a robust iterative alkylation protocol using stoichiometric ephedrine-based auxiliaries that provides access to all possible stereochemical variants,75–76 and Theodorakis has applied this methodology in the total synthesis of (−)-borrelidin (figure 2e).77–78
Minnaard and Feringa have recently developed an alternative iterative three step protocol to access syn deoxypropionate motifs based on catalytic asymmetric conjugate additions (Figure 2f). Starting from an α,β-unsaturated thioester, a highly enantioselective 1,4-addition of MeMgBr in the presence of a chiral copper catalyst sets the first methyl-bearing stereogenic center. A reduction and Wittig olefination sequence generates a new α,β-unsaturated thioester for further 1,4-addition reactions. Repetition of these three steps allows seven methyl stereocenters to be installed with excellent levels of stereoselectivity, enabling the first total synthesis of phthioceranic acid79 as well as the first total synthesis of sulfolipid-1.80
Breit has also developed an iterative zinc-catalyzed sp3-sp3 coupling method for the synthesis of deoxypropionates (Figure 2g).81 Treatment of an alkyl Grignard with ZnCl2 generates a triorganozincate species (R3ZnMgCl), which displaces a secondary triflate to generate a new carbon-carbon bond with inversion of configuration. Reduction of the ester followed by conversion to a primary alkyl chloride enables further iteration. The lack of reactivity with alkyl lithium species suggests that magnesium coordination to the triflate may play a role in Lewis acid activation of the electrophile. Using this iterative method, Breit was able to access a library of different diastereomers of trideoxypropionates, all in greater than 99% diastereomeric excess. In addition to the aforementioned work by Myers, Minnaard, and Breit, the modular nature of polydeoxypropionates has also inspired the development of several other iterative synthesis strategies.82–83
Aggarwal’s versatile approach for iterative synthesis of various stereochemically complex Csp3-rich motifs is demonstrated with his route to (+)-kalkitoxin (Figure 2h).84 This approach leverages the stereospecificity of the 1,2-metallate rearrangement of boronate complexes85 to install both stereochemistry and functionality via iterative chain extension of boronic esters. In a one pot procedure, a boronic ester was subjected to a series of six homologations, installing three methylene spacer units as well as three methyl-bearing stereocenters derived from the requisite enantiomerically-pure lithiated benzoates.86 Amination followed by amide formation furnished the core of (+)-kalkitoxin in an overall 52% yield. This same approach has been used to synthesize baulamycin A,87 tatanan A,88 fluorohexestrol,89 and C30 botryococcene90 and many other targets.91 More broadly, the versatility of this homologation method, which tolerates a diversity of structural variation in its building blocks, opens the door for divergent synthesis. This can be seen in Aggarwal’s assembly-line production method of hydrocarbons with tailored shapes.86
Polyisoprenoids are attractive targets for iterative synthesis due to the numerous 1,5-related trisubstituted olefins spanning their structures. Negishi has developed an iterative and convergent synthesis for these motifs and applied it to the synthesis of coenzyme Q10 (Figure 2i). A one pot iterative cycle begins with formation of a primary alkylzinc iodide followed by a chemoselective cross-coupling with a diiodo building block. Two more iterations and a further coupling with a diene containing building block installs five of the trisubstituted olefins of coenzyme Q10. To enable convergent synthesis, the TMS-protected alkyne also serves as an attachment. TMS deprotection subsequent carbometalation-iodonation generates a new vinyl iodide which undergoes a strategic and convergent cross-coupling with an earlier homologue. A final round of deprotection, hydrozirconation-iodination, and cross-coupling gave coenzyme Q10 in only 11 steps.
Long polyene chains are another modular structure found in natural products. However, these motifs are often sensitive to many common reagents (e.g. protic and Lewis acids) and conditions (e.g. light and oxygen), rendering their synthesis challenging. In a landmark synthesis of the complex polyene macrolide amphotericin B, Nicolaou employed an iterative Horner-Wadsworth-Emmons strategy to complete the all-trans-polyene motif. Starting from an aldehyde, the first triene unit was installed using a diene-containing phosphonate (Figure 2j).92 Subsequent conversion of the terminal ethyl ester into an aldehyde set the stage for a second homologation using the phosphonate building block. Deprotection and redox modification furnished a hexaenal, which was esterified with a highly functionalized carboxylic acid containing a phosphonate to generate the open chain molecule. An intramolecular HWE reaction initiated by K2CO3/18-crown-6 formed the desired cyclic heptaene, completing the carbocyclic core of amphotericin B.93
Developing iterative routes to complex, polycyclic molecules represents a substantial challenge, but provided that common repeating units and assembly methods can be established, iterative synthesis can be both practical and expeditious. For example, Uenishi and co-workers developed an iterative protocol for the stereoselective synthesis of linked tetrahydrofuran (THF) rings (Figure 2k).94 The iterative sequence commences with formation of a homoallylic alcohol through Grignard addition to an aldehyde or epoxide, followed by cross-metathesis with a stereodefined allylic alcohol. Pd(II) mediated ring closure forms the THF ring, and finally, ozonolysis of the resultant olefin forms an aldehyde to allow further iteration. Synthesis of either trans-threo-trans and trans-threo-cis THF rings is simply a case of exchanging the allylic alcohol building blocks during the metathesis stage of the iterative cycle.
Mori and co-workers leveraged the inherent modularity within structurally complex polycyclic ether natural products to enabling their iterative construction (Figure 2l).95 In this approach, diastereomerically-pure oxiranyl anions were utilized to displace primary tosylates, and the resulting epoxy sulfone products were then subjected to an acid-catalyzed, 6-endo cyclization. A five-step sequence then generated a new triflate for further iteration. Six repetitions of this protocol led to efficient construction of the ABCDEF-ring fragments of yessotoxin and adriatoxin, with the stereochemistry of the cyclic ethers dialed in through selection of the appropriate oxirane building blocks. This iterative oxiranyl anion strategy has further been employed for the synthesis of hemibrevetoxin B,96 gambierol,97 and even gymnocin-A with 14 contiguous fused rings.98 Other iterative synthesis strategies have also been developed for polycyclic eithers, including iterative ring closing metathesis/hydroboration, iterative reductive cyclizations, and iterative oxonium ylide formation/[2,3]-shift processes.99
Kishi’s highly impactful synthesis of halichondrin B utilized iterative Nozaki-Hiyama-Kishi (NHK) reactions interspaced by tailoring steps to build cyclic ether moieties in a recursive fashion (Figure 2m). This strategy allowed highly complex building blocks to be efficiently assembled in an iterative manner.88 Other family members of the halichondrin family could also be accessed by incorporating variant building blocks into a similar synthetic sequence.100 This efficient and modular synthesis enabled Kishi and coworkers to discover that half of halichondrin B, the portion that contains the macrocycle, is nearly equipotent as an anticancer agent to the exceptionally potent natural product. A close variant of this iterative synthesis was successfully scaled up by chemists at Eisai to produce enough of this portion of the molecule, dubbed Eribulin, to enable its clinical utlization. Significant benefits were observed for patients with metastatic breast cancer and liposarcoma, leading to FDA approval of Eribulin for treatment of these cancers in 2010 and 2016, respectively.101–102
Beyond natural products, iterative assembly methods have also enabled the synthesis of many other types of inherently oligomeric materials (Figure 3). For instance, polyaromatic hydrocarbons have numerous applications in solar cells and light emitting diodes but can often prove challenging to prepare when site specific functionalization is required. Kwon and co-workers have developed an efficient strategy for the iterative synthesis of polyaromatic hydrocarbons through the union of a 1,2-dialdehyde and ethyl allenoate (Figure 3a).103 Oxidative modification of the annulated product furnishes a 2,3-dialdehyde that is primed for another annulation reaction. This iterative cycle rapidly generates 2,3-substituted anthracene and tetracene structures.
The synthesis of larger acenes is complicated by their higher reactivity, but these structures are highly sought after for their applications in organic electronic materials. For example, pentacene is the best available organic p-type semiconductor, but larger members could be even more useful.104 Excellent syntheses of octacene and nonacene derivatives have been carried out by Echavarren,105–106 and Bettinger and coworkers have developed a building block method using iterative Diels Alder reactions (Figure 3b).107 Combination of 5,6,7,8-tetramethylenebicyclo[2.2.2]oct-2-ene and an aryne dienophile generated by treatment of 1,2-dibromobenzene with n-BuLi led to formation of a cycloadduct with a terminating diene moiety. This was treated with an aryne generated from 1,2,4,5-tetrabromobenzene, resulting in a product terminating in another dibromide moiety. One more iteration of aryne formation and cycloaddition gave a stable precursor to octacene. After a sequence of aromatization and oxidation reactions, exposure to low wavelength UV-light generated octacene for characterization and some initial studies.
While most iterative synthesis methods are based on the linear assembly of building blocks, Moore and co-workers have developed a convergent iterative synthesis of phenylacetylene oligomers using Sonogashira coupling.108–109 Moore’s work highlights a critical advance enabling the application of iterative synthesis in a convergent fashion, the ability to orthogonally protect and deprotect each of the two different functional groups required for building block assembly. Here, a bifunctional building block can be selectively activated in two different ways; a dialkyltriazene can be converted to an iodide, or a TMS protecting group can be removed to reveal a reactive terminal alkyne (Figure 3c). These two differently activated building blocks can then be assembled via Sonagashira coupling to form an advanced intermediate containing a dialkyltriazene and a protected alkyne at opposite termini. Repeating this process of using advanced intermediates as building blocks allows for exponential molecule growth. In such a manner, it is possible to generate repeating tetramers, octamers, and longer oligomers with control of the sequence of building blocks. Additionally, building blocks with other functional groups can also be incorporated to prepare diverse oligomeric products.110
Moore has also developed a convergent approach to iterative synthesis for the assembly of large dendrimers (Figure 3d).111–113 Compared to his strategy for making oligomers (Figure 3c), this dendrimer method is different in two fundamental respects. Here, the building blocks can only be activated in one way, by unmasking a TMS group to reveal a reactive terminal alkyne for Sonogashira coupling. However, exponential molecule growth can still be achieved via a double Sonogashira coupling onto a trifunctional monomer containing two bromines. Four iterations of TMS deprotection and double Sonogashira coupling allowed for rapid construction of a monodedron containing 31 building blocks.111 This double Sonogashira approach is capable of quickly making large molecules, but it also lacks versatility needed to make unsymmetrical targets. Such a limitation is not problematic for dendrimer synthesis, but it does illustrate that convergent iterative synthesis can be most versatile when it has two separate masking and deprotection strategies for orthogonal functional groups (Figure 3c).
Iterative synthesis methods have also been developed for molecules of defined threedimensional architectures. Iptycenes are of interest in materials science and supramolecular chemistry due to their structural rigidity and three-dimensionality. Swager and co-workers devised an iterative solid-state synthesis of extended iptycenes in which a Diels-Alder reaction between anthracene and 1,4-anthraquinone was followed by rearomatization to form a new anthracene unit for further iteration (Figure 3e). This cycle was then repeated to form longer iptycenes in a modular fashion.114
Many methods have recently been developed which allow near-identical boronic esters to be chemoselectively functionalized, and Crudden has harnessed this selectivity to facilitate a fundamentally different approach to iterative synthesis (Figure 3f). Instead of generating new positions for appending building blocks during each iteration cycle, Crudden has preinstalled all of the attachment points in advance. In a one-pot procedure, a Csp2-Csp2 coupling of an aryl boronic ester was followed by group selective cross coupling of a Csp3 primary boronic ester. Simple filtration through silica gel enabled a Csp3 cross coupling of the remaining secondary boronic acid in the presence of Ag2O, creating a defined polyarylated structure.115
Iterative synthesis has also been applied to create mechanically-interlocked molecules. In Goldup’s iterative synthesis of oligo[n]rotaxanes, copper-templating via a bipyridyl-containing macrocycle is harnessed to control a three component click reaction (Figure 3g). The product rotaxane has a terminus containing a TIPS-protected alkyne. Cleavage of the silyl group and iteration of this reaction allows for the incorporation of different macrocyclic moieties.116
Each of these examples represent important advances toward efficient and flexible synthetic access to specific types of small molecule motifs. In most cases, the iterative syntheses proceed in a linear way, but there are also multiple strategies for convergent iterative methods (Figure 3c-d) that allow for even more efficient molecule growth. There is tradeoff; the more convergent an iterative synthesis method, the more limited its scope of targets. In contrast, divergent approaches to a iterative synthesis have the greatest potential to accelerate discovery of new function. It is evident from several divergent iterative synthesis methods59, 76, 81, 86, 117 that the greater the potential for structural variation in the building blocks, the greater the scope of possible products. Given the arguments that most small molecules could be made from a defined set of molecular building blocks, is it possible to develop a suitably general iterative synthesis method?
Advances towards a general platform for iterative small molecule synthesis
A general iterative platform for small molecule synthesis requires a common type of assembly chemistry that can form a wide range of different types of C-C and C-X bonds whilst being very tolerant of many functional groups. Although many methods fall short of the high bar required to enable generalized synthesis, metal-mediated cross-coupling represents an exceptionally attractive candidate. The Suzuki-Miyaura and Buchwald-Hartwig couplings in particular can employ non-toxic and shelf-stable building blocks, are highly efficient and stereospecific, and proceed under mild reaction conditions with high levels of functional group tolerance. Moreover, the scope of both C-C and C-X bonds that can be formed using such methodology is already very broad and continues to expand, and the versatility of these types of couplings has placed them among the most widely employed reactions in both academic and industrial synthesis groups. Most recently, this scope has been extended to include a wide range of Csp3 and Xsp3 coupling partners,118–120 including even stereospecific Csp3 cross-couplings of stereochemically defined chiral non-racemic building blocks, originally pioneered by Crudden.121–131 These new methods, combined with anticipated major additional advances in the area of sp3 cross-coupling over the next few decades, suggest that iterative cross-coupling could represent a generalizable approach for building block-based small molecule synthesis.
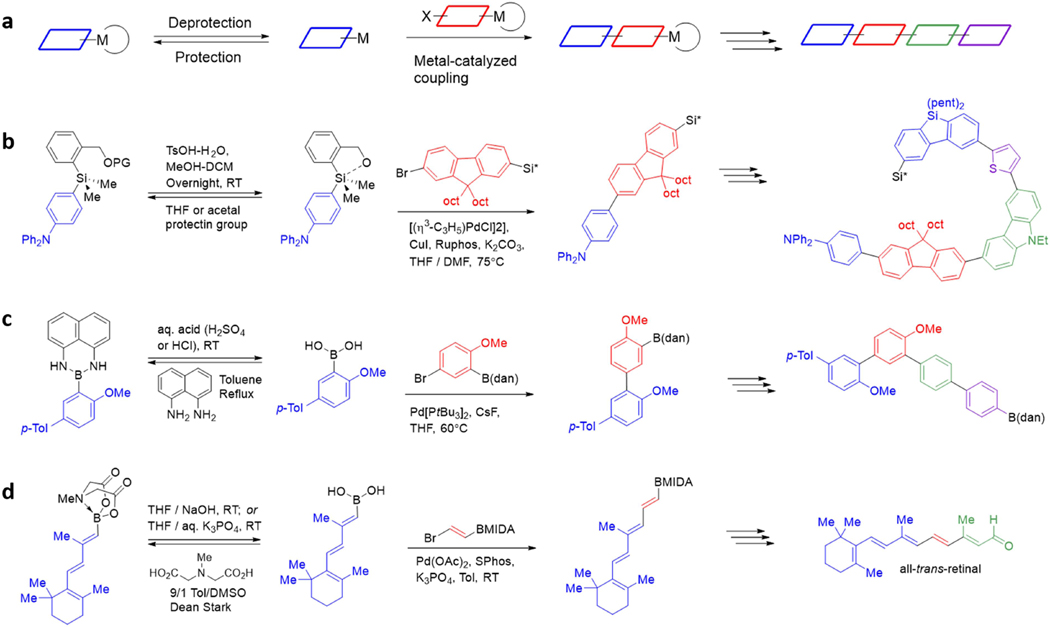
In such a platform, the complex problem of molecular construction can be simplified into just making and coupling building blocks. In theory, all the required functional groups, oxidation states, and stereochemistry can be pre-installed into such building blocks and then faithfully translated into the growing target structure using only mild and stereospecific cross-coupling reactions. Achieving this goal requires compatible bifunctional building blocks that can be iteratively assembled in a precisely controlled manner. This, in turn required the development of methodology for reversibly attenuating the reactivity of such building blocks toward metal-mediated coupling (Figure 4a).108–109
Figure 4. Approaches to Iterating Metal-Mediated Coupling Reactions.
a Reversible attenuation of organometallic reactivity enables iterative coupling. b Iterative Hiyama couplings by reversible silane activation. c Iterative Suzuki couplings by reversible protection with 1,8-diaminonaphthalene (dan). d Iterative Suzuki couplings by reversible protection with N-methyliminodiacetic acid (MIDA).
Several approaches to achieve such reactivity attenuation have leveraged the sensitivity of Lewis acidic organometallic coupling partners in different ways. Hiyama has devised a strategy for reversibly attenuating the reactivity of arylsilanes using specially designed organo[(2-hydroxymethyl)phenyl]dimethylsilanes (Figure 4b). Under normal cross-coupling conditions, these arylsilanes are “switched off” and unreactive towards transmetalation, but upon deprotection of a strategically-positioned neighboring alcohol, the resulting intramolecular O-Si coordination activates the silane and promotes cross-coupling. Using this method of building block assembly in an iterative fashion, Hiyama completed the synthesis of highly conjugated linear oligoarenylsilanes.132
Suginome reported an alternative method for switching off organometallic coupling partners (Figure 4c). Complexing boronic acids with the 1,8-diaminonaphthalene (DAN) group decreases the Lewis acidity of the p orbital of the sp2-hybridized boron atom via electron donation from neighboring lone pairs on planar nitrogen atoms. The resulting BDAN compounds are stable to anhydrous as well as aqueous biphasic cross-coupling conditions, but exposure to strong aqueous HCl or H2SO4 removes the DAN group and releases the corresponding boronic acid. This iterative building block-based method has been applied in the synthesis of oligoarenes133 and oligo(phenylenevinylene)s.134
Parallel work by our group identified that complexation with the trivalent ligand N-methyliminodiacetic acid (MIDA) can alternatively attenuate boronic acid reactivity by rehybridizing the boron atom from sp2 to sp3 (Figure 4d).135 MIDA boronates136 represent a very promising platform for generalized building block-based molecule synthesis. It is of course possible that even better strategies are yet to be discovered for reversibly attenuating the reactivity of organometallic building blocks. Such enabling advances would be a very exciting opportunity for generalized synthesis of small molecules and the discovery of new molecular functions. However, due the collective efforts of many different research groups, the MIDA boronate platform currently represents the most well-developed strategy for iteration of metal-mediated couplings.
MIDA boronates have many advantageous physical and chemical features. They are readily purified by silica gel chromatography and/or recrystalization and typically are indefinitely stable on the benchtop as free-flowing crystalline solids.135 Moreover, many alkyl-, vinyl-, and arylboronic acids can be directly converted to their MIDA boronate counterparts in quantitative yield under mild conditions. To drive the MIDA complexation to completion, water can be removed simply via toluene azeotrope with a Dean-Stark trap136 or by addition of a drying agent like magnesium sulfate. MIDA boronates are inert to anhydrous cross-coupling reactions, but they can be quickly deprotected under mild aqueous basic conditions. Deprotection can proceed through two different mechanisms: (1) under strong aqueous base, rate-limiting attack of hydroxide on the carbonyl carbon, or (2) under weakly basic or neutral aqueous conditions, slower rate-limiting attack of water on the boron-nitrogen bond.137 This mechanistic divergence is consistent with extensive reaction kinetics, kinetic isotope effects, 18O labelling, and computational data. In-situ slow release of MIDA boronates has been advantageous for many reactions including couplings of unstable heteroarylboronates,138,139 polymerization reactions,140–141 asymmetric methodologies,142 the synthesis of organic photovoltaics,143 and a one-pot homologation of boronic acids.144
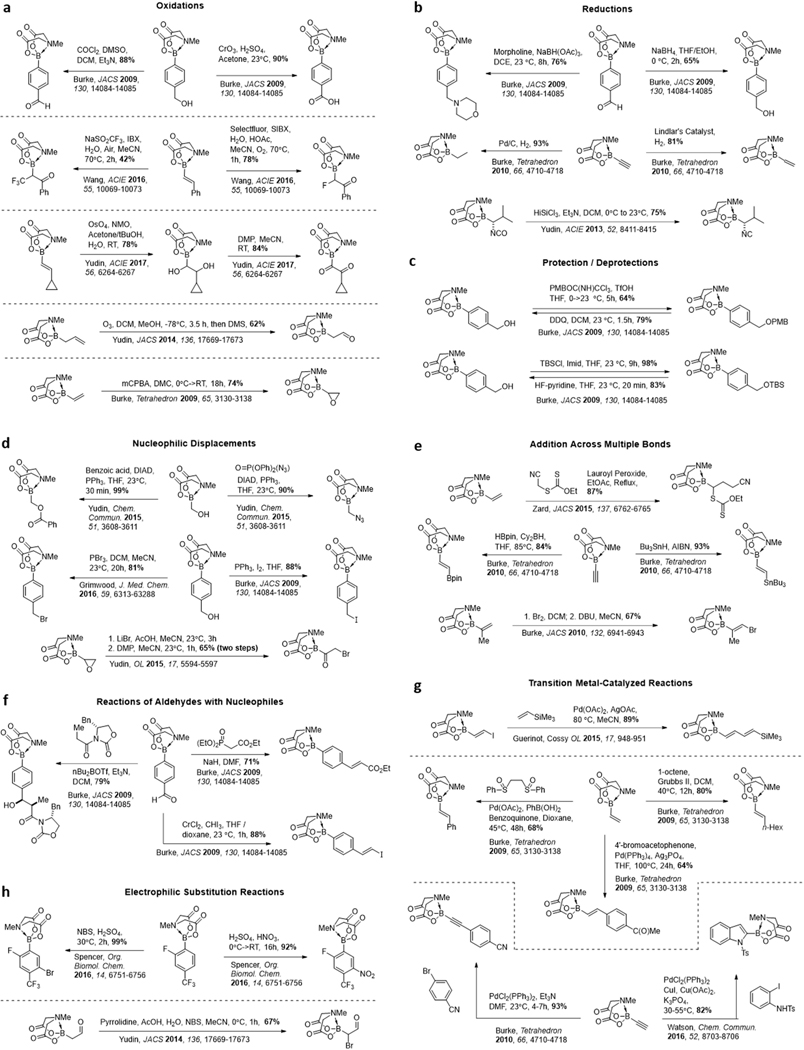
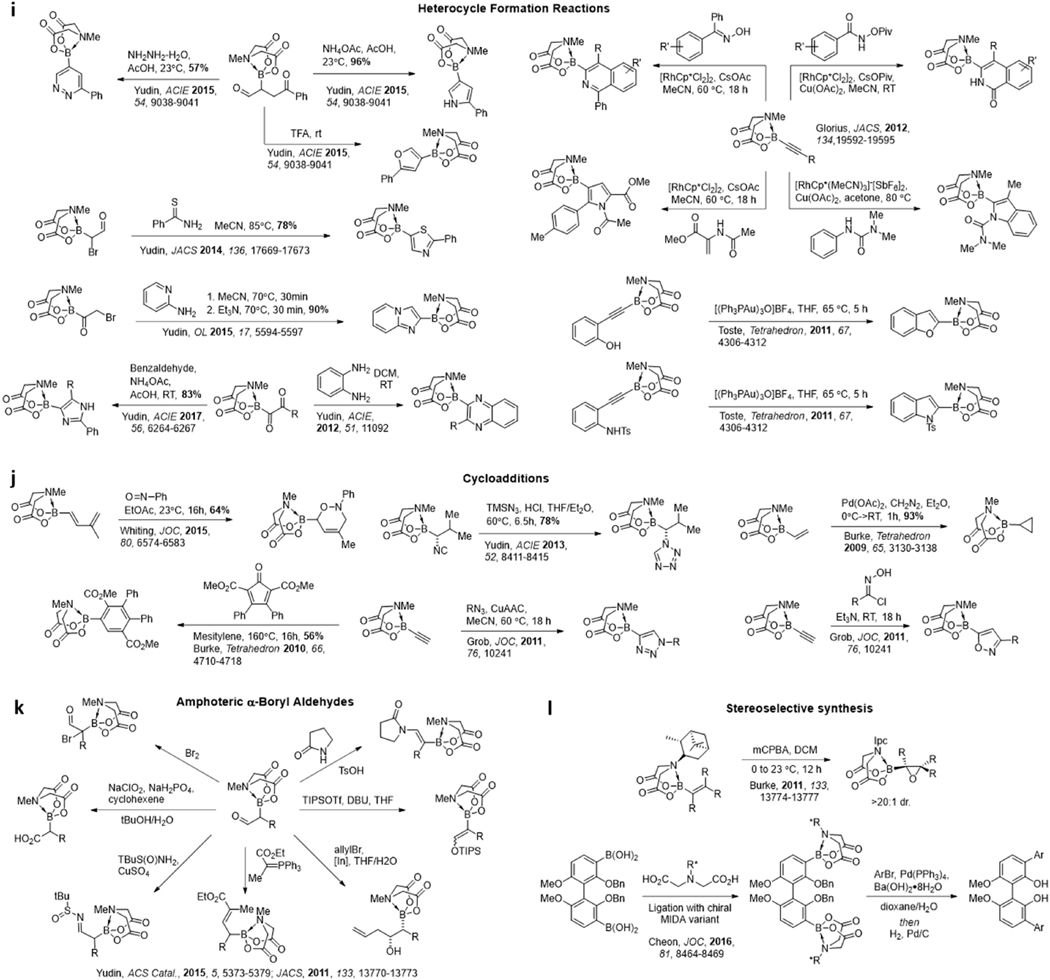
The durability of MIDA boronates to a wide range of different reagents and reaction conditions further facilitates the synthesis of otherwise challenging to access boronate building blocks from simple boron-containing starting materials (Figure 5). For example, many standard oxidations (Figure 5a), reductions (Figure 5b), and protecting group manipulations (Figure 5c) are well-tolerated.145–151 Numerous other common synthetic transformations leave MIDA boronates intact, including aldol reactions,145 carbonyl olefination reactions,145 Mitsunobu reactions,145 electrophilic substitution reactions,149, 152 hydroborations and –stannylations,151 Diels Alder cycloadditions (Figure 5j),151, 153 and cyclopropanations.147 A variety of transition metal-catalyzed reactions are also well-tolerated (Figure 5g), including Heck reactions,147, 154 Grubbs metathesis,147 Sonagashira couplings,151 and Suzuki cross-couplings.135
Figure 5. Reaction conditions that tolerate. MIDA boronates.
a Oxidations. b Reductions. c Protections and Deprotections. d Nucleophilic Displacements. e Addition across multiple bonds. f Reactions of aldehydes with nucleophiles. g Transition metal-catalyzed reactions. h Electrophilic substitution reactions. i Heterocycle formation reactions. j Cycloadditions. k Reactions of α-boryl aldehydes. l Stereoselective synthesis with chiral MIDA variants
MIDA boronates are not without limitations in terms of scope and application. A large number of Suzuki-Miyaura cross coupling reactions require the use of aqueous base which cause hydrolsis of othe MIDA boronate, and Buchwald-Hartwig aminations can involve the use of strong base which is incompatible with the acidic protons on the backbone of the MIDA ligand. The former point is a significant limitation as the majority of recent important advances in Csp3 coupling involve aquoues basic reaction conditions thus preventing their use in MIDA-boronate based ICC.
A few of the many recent advances in this area are highlighted here. By harnessesing the ability of MIDA to rehybridize boron from sp2 to sp3, Zard has used vinyl MIDA boronates as acceptors for xanthate-derived radicals.155 The resulting α-boryl radicals are sufficiently destabilized to ensure that the reaction is irreversible. Making use of similar vinyl MIDA boronate substrates, Wang has developed a new oxidative difunctionalization reaction.146 After treating with SIBX along with either a fluorinating or trifluoromethylating reagent, the resulting α-boryl ketones can be converted to highly functionalized furans bearing MIDA boronates. A variety of other heterocycle-forming reactions have also been developed using MIDA boronate-containing substrates. Making use of kinetically amphoteric molecules containing MIDA boronates and electrophilic ketones/aldehydes, Yudin has developed syntheses of borylated pyridazines/pyrroles,156 imidazoles,148 thiazoles,149 imidazo[1,2-a]pyridines,157 and many other motifs.158 Watson has also developed a synthesis of 2-borylated indoles/benzofurans involving a palladium catalyzed cascade reaction with ethynyl BMIDA and 2-iodoanilines.159 Similarly, borylated indolenes and benzofurans have been synthesized through gold-catalyzed intramolecular cyclizations onto alkynes.160 These reactions are expanding the collection of MIDA boronate building blocks available for small molecule construction, and hundreds of MIDA boronates are already commercially available.
The protection of boron with the MIDA ligand has also enabled previously inaccessible borylated molecules to be created such as α-boryl adehydes (Figure 5k), which without MIDA complexation would rearrange to the O-boryl enolates. Yudin has pioneered the application of this unique class of molecules by exploiting the amphoteric nature of the boron centre and the aldehyde.161 This has served as a platform for the synthesis of a variety of heterocycles and functionalized boronic esters and even the formation of complex tertiary organoboronates through Tsuji-Trost allylations.162
Beyond the synthesis of simple achiral and racemic building blocks, chiral non-racemic MIDA ligand varients (Figure 5l) can direct diastereoselective epoxidation of vinyl boronates, to generate stereoisomerically pure oxyranyl boronates.163 Futhermore, installation of enantiopure chiral MIDA ligands onto racemic boronic acids generates diastereomers that can be separated via column chromatography to ultimately yield enantiomerically pure boronic acids.164
Iterative cross-coupling with MIDA boronates
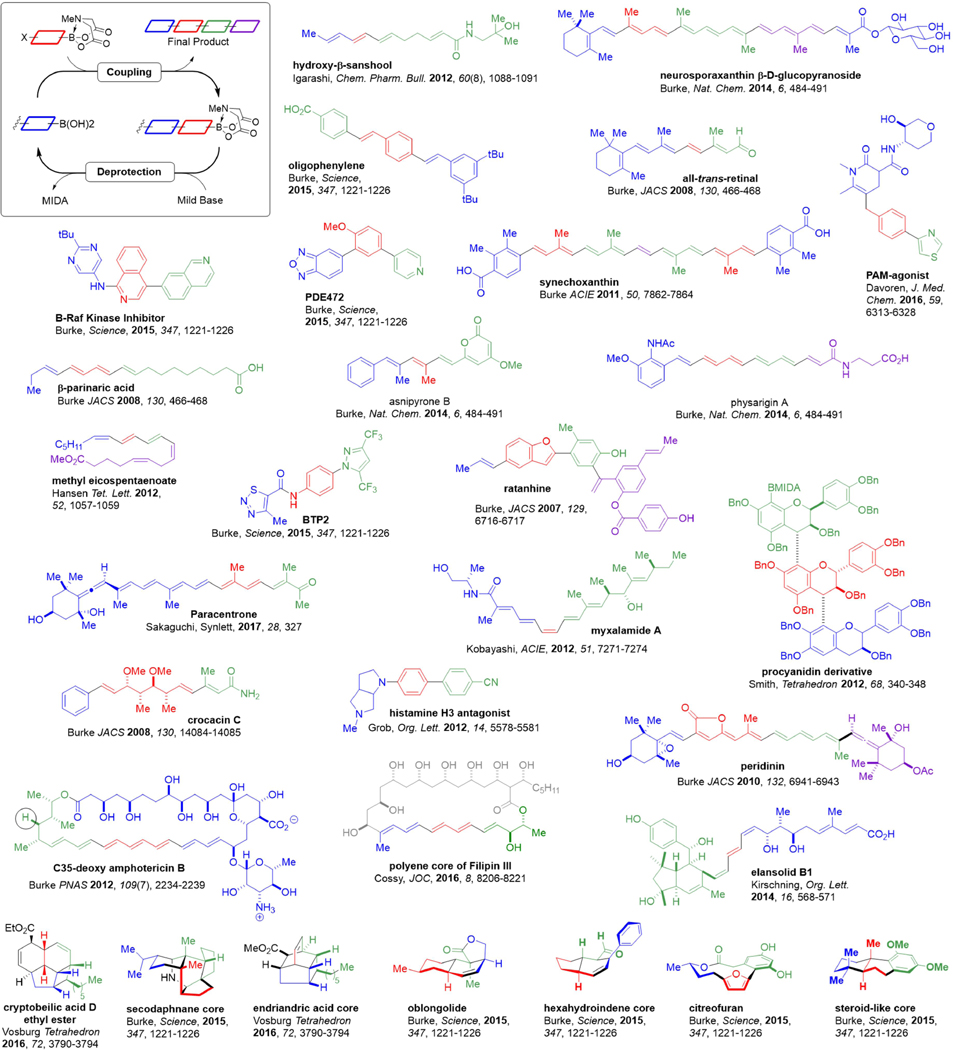
Leveraging these many advantageous features, iterative cross-coupling (ICC) of MIDA boronates has now been applied by many research groups to synthesize a wide range of structurally-diverse small molecules (Figure 6).135, 145, 163, 165–178 This growing list includes natural products from every major biosynthetic pathway, pharmaceuticals, biological probes, and materials components. Moreover, these completed syntheses include linear molecules, for which the capacity for iterative building block-based assembly is more apparent, as well as an increasing number of highly complex macro- and polycyclic frameworks for which inherent modularity is less obvious. Moreover, many of these syntheses took advantage of common off-the-shelf MIDA boronate building blocks many of which are now commercially available. Collectively, this rapidly growing collection of successful applications of ICC to make a wide range of complex small molecules suggests the opportunity to drive functional discovery with this type of chemical matter will be increasingly accessible.
Figure 6.
Small molecules made via iterative cross-coupling with MIDA boronate building blocks.
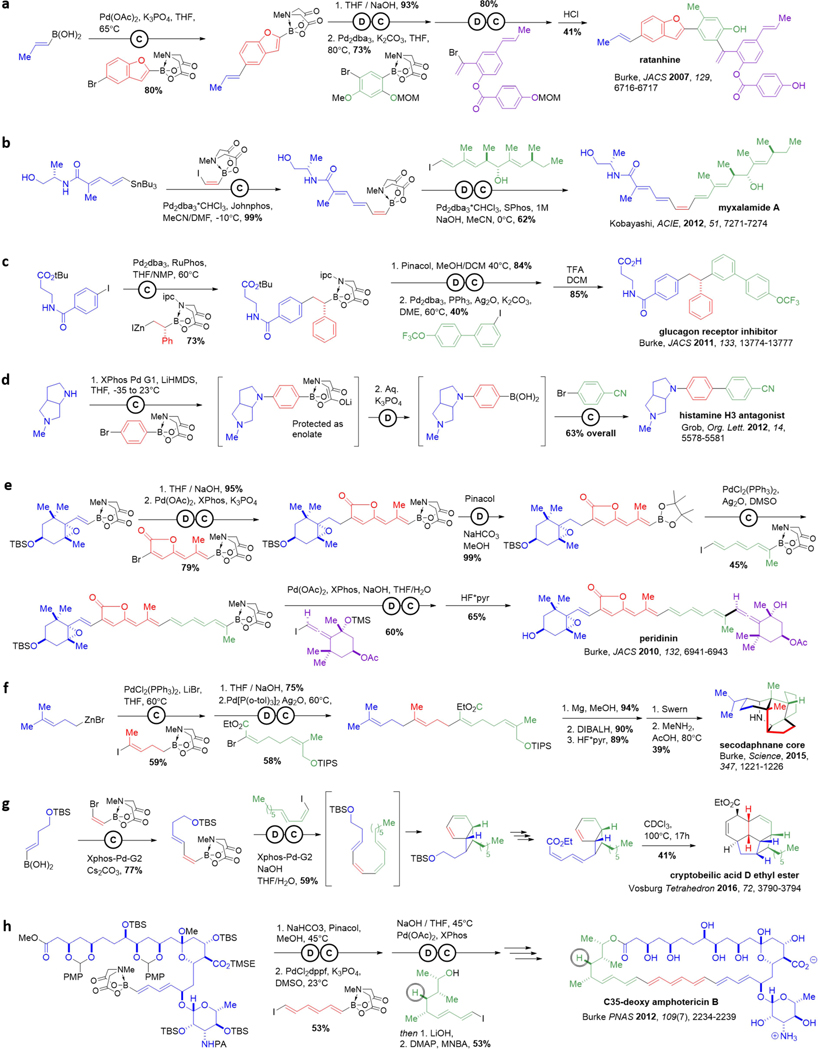
A few illustrative examples are highlighted in detail in Figure 7. Ratanhine was the first natural product to be synthesized by ICC (Figure 7a).135 Notably, the mild nature of the deprotection and coupling reactions preserved even hydrolytically-sensitive functional groups such as esters. The stability of MIDA also assisted in several additional ways. The benzofuran building block, which in its boronic acid form is prone to decomposition, proved to be bench stable under air. Even couplings that required elevated temperatures of 80°C tolerated the MIDA boronate and proceeded in high yield.
Figure 7. Illustrative examples of iterative cross-coupling.
a Ratanhine. b Mixalamide A. c Glucagon receptor inhibitor. d Histamine H3 antagonist. e Peridinin. f Secodaphnane core via biomimetic linear-cyclized strategy. g Cryptobeilic acid D methyl ester. h C35-deoxy amphotericin B.
Kobayashi developed an efficient ICC-based synthesis to the natural product myxalamide A (Figure 6b).171 A key challenge in this synthesis was construction of the central trans-trans-cis-trans tetraene, as such motifs are difficult to install in a stereocontrolled fashion. The capacity of olefinic stereochemical elements pre-installed in shelf-stable bifunctional MIDA boronate building blocks to be faithfully translated into growing targets by using mild and stereospecific cross-coupling methods simplifies this type of complex problem.165–166, 179 Specifically in this case, a cis-bifunctional vinyl MIDA boronate building block was iteratively cross-coupled to unite the two key fragments of the polyene core of myxalamide A in a stereospecific fashion. Moreover, no protecting groups were required, as the cross-coupling reactions proceeded in the presence of two free alcohols.
This same concept of leveraging stereospecific cross-couplings to transfer pre-installed stereochemistry from pre-fabricated building blocks into products has been extended to stereogenic Csp3 centers as well. For the glucagon receptor inhibitor (Figure 7c), the presence of several Csp3 carbons offered strategic disconnection points. The first two building blocks were assembled by a Csp3 coupling between an aryl iodide and a primary alkylzinc reagent.163 In the second coupling reaction, an enantiomerically-enriched benzylic boronate was then subjected to Crudden’s stereoretentive Csp3 coupling to yield the targeted chiral pharmaceutical candidate in a very simple manner.121
Another powerful reaction in the synthesis of pharmaceutical compounds is C-N bond formation, however strong bases are sometimes required for less reactive amines, and these conditions can be incompatible with MIDA boronates. Hamann and coworkers have pioneered a strategy that selectively protects MIDA boronates in situ through enolization with LiHMDS at low temperatures.180 This more robust Li-MIDA enolate enabled the authors to perform an efficient one-pot ICC synthesis of the histamine H3 agonist through iterative C-N then C-C coupling reactions (Figure 7d). This Li-MIDA enolate strategy may have applications beyond C-N coupling where strong base causes loss/cleavage of the MIDA protecting group.
Even some highly complex small molecules have been prepared via ICC. For example, the natural product peridinin is a norcarotenoid containing several complex functional groups and stereochemical elements, including a butenolide, an epoxide, and an allene motif. Because of the mild and stereospecific nature of ICC, all of these functional groups and stereochemical elements were preinstalled in the four complex building blocks and faithfully translated into the final product, yielding the first fully stereocontrolled synthesis of peridinin.167 Each MIDA boronate intermediate, including even the final heptaenyl MIDA boronate, proved stable to chromatography and storage. Although the boronic acid in the final coupling reaction proved unstable to isolation, an in situ deprotection of the MIDA boronate allowed for the coupling to proceed both in high yield and with complete stereoretention.
Polycyclic molecules present an especially formidable challenge for building block-based construction. However, the biosynthesis of complex, Csp3-rich polycyclic natural products often involves an actionable two-part strategy: assembly of building blocks into a linear precursor and then a cyclization reaction to transform this linear molecule into a complex (poly)cyclic skeleton.181 In theory, ICC could be employed in a similar linear-to-cyclized approach. This was confirmed in the synthesis of the pentacyclic core of secodaphnane natural products (Figure 7f).178 Two cycles of Csp3 couplings were initially used to construct a linear precursor, which was then subjected to a bioinspired cyclization cascade involving amine condensation and intramolecular Diels-Alder and Prins cyclizations.182
A linear-to-cyclized strategy was also employed by Vosberg to make other highly complex polycyclic natural product frameworks, including the ethyl ester of the tetracyclic natural product cryptobeilic acid D (Figure 7g). Vosburg and co-workers used all commercially-available MIDA boronate building blocks to stereospecifically construct the complex trans-cis-cis-trans-tetraene linear precursor. This tetraene was primed for an 8π/6π electrocylization cascade to generate a fused 4-6 ring system, which led to the completion of cryptobeilic acid D after some additional final steps (Figure 7g).176
This linear-to-cyclized strategy has also enabled biological studies of clinically-relevant natural products. Having served for over 50 years as the last line of defense against invasive and drug-resistant fungal infections, the polyene macrolide amphotericin B (AmB) still suffers from dose-limiting side effects. With the goal of gaining a better understanding of AmB’s mechanism of toxicity, an AmB derivative lacking a single hydroxyl group was synthesized via ICC followed by macrocyclization (Figure 7h). This new compound, C35deOAmB, lacked the ability to form ion channels yet retained its toxicity to fungal pathogens, overturning decades of prior thinking about the primary mechanism of action of this clinically vital but unfortunately highly toxic natural product.183 This discovery helped build a strong foundation for ongoing efforts to rationally optimize the therapeutic index of AmB.184–185 It also enabled the ion channel-forming capacity of this natural product to be rationally separated from its cell killing effects. This, in turn, facilitated the development of small molecules that replace missing protein ion channels and thereby restore physiology, akin to acting as prostheses on the molecular scale.4–5 MIDA boronates have been employed independent of iteration on a wide range of other small molecule synthesis applications as well.140–141, 143, 150, 180, 186–193
Automation of small molecule synthesis
The automation of small molecule synthesis has the potential to dramatically improve the efficiency with which new molecular functions can be discovered and optimized. It also represents an actionable path for bringing the power of making small molecules to non-specialists. One strategy for automating small molecule synthesis involves translating current customized synthesis approaches to an automated format.194–197 While this approach has the advantage of utilizing known manual solutions, it necessitates the automation of many different kinds of chemical reactions, each of which requires different types of reagents, conditions, optimization protocols, reaction vessels, and/or purification protocols.
A major advantage of iterative cross-coupling is that, once the necessary building blocks are in hand, it only requires the same two reactions, deprotection and coupling, to complete the molecular assembly process. This presents the strategic opportunity for collective efforts to be deployed to extensively optimize these two reactions and ultimately reach highly generalized conditions, as was previously achieved for iterative peptide198 and oligonucleotide synthesis,199 and is now being increasingly realized for oligosaccharides.200
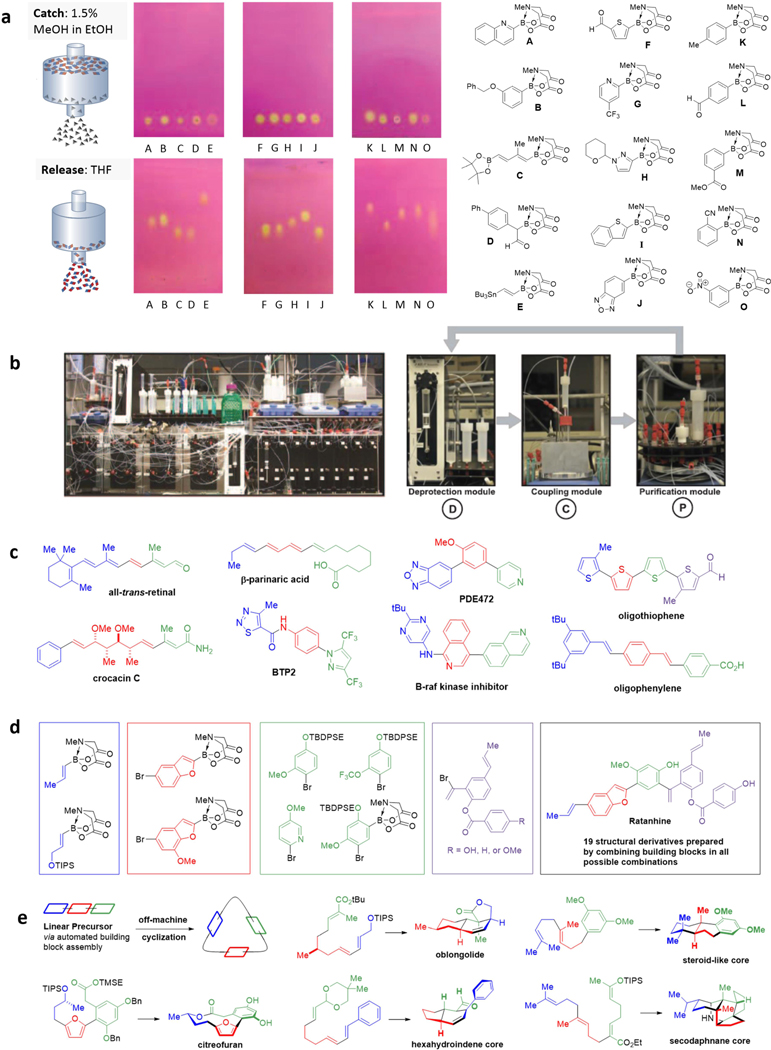
It is also notable that every intermediate in a MIDA boronate-based ICC sequence contains the same common MIDA boronate functional group. It was recognized that this could potentially serve as a handle for generalized purification. In this vein, MIDA boronates were discovered to possess an unusual binary affinity for silica gel with certain pairs of eluents (Figure 8a).178 Regardless of size, functional group content, and polarity, MIDA boronates show minimal mobility when eluting with MeOH:Et2O, but simply switching the solvent to THF causes rapid elution. This was the key to developing a generalized “catch-and-release” purification platform (Figure 8a). A crude reaction is first loaded onto a silica gel plug and washed with 1.5% MeOH in Et2O to remove any excess reagents, catalysts or other impurities. Then by simply switching the solvent to THF, the purified MIDA boronate is rapidly released. This purification was readily transformed into a general automated purification module.
Figure 8. Automated Synthesis Machine.
a Binary affinities of MIDA boronates for silica gel enable catch-and-release purification. b Design of a machine for automated iterative cross-coupling. c Linear small molecules prepared by using automated iterative cross-coupling. d Automated synthesis of ratanhine and 19 derivatives. e Linear-to-cyclized strategy for automated synthesis of complex natural products.
This catch-and-release purification module was then combined with a deprotection module and a cross-coupling module to create a machine capable of carrying out each step of ICC in a fully automated fashion (Figure 8b).178 This synthesis machine proved capable of making natural products from every major biosynthetic pathway (crocacin C, β-parinaric acid, all-trans-retinal, and ratanhine) and many natural product derivatives (Figure 8c). In additional, materials components (oligophenylene and oligothiophene), pharmaceuticals (PDE472, B-Raf kinase inhibitor), and biological probes (BTP2) were also made in the same fully automated fashion. In each case, all the functional groups, oxidation states, and stereochemical information were pre-installed into the corresponding building blocks and then faithfully translated to the products via automated stereospecific couplings.
Another advantage of building block-based synthesis is the ability to rapidly prepare many different structural derivatives of a targeted compound. For the natural product ratanhine, the automated synthesizer was capable of combining four sets of variant building blocks in all possible combinations to make 20 unique structural derivatives of the parent natural product (Figure 8d). All 20 of these syntheses were completed without any customized optimization of the conditions for deprotection or coupling.
It is not immediately obvious that the same building block-based approach can be used to create complex polycyclic scaffolds consisting of a network of Csp3-linkages. However, by integrating the aforementioned biomimetic “linear-to-cyclized” strategy, even Csp3-rich cyclic and polycyclic natural products can be accessed via automated synthesis (Figure 8e). For the macrocyclic natural product citreofuran, the fully automated assembly of building blocks into a linear precursor set the stage for an atropdiastereoselective Mitsunobu cyclization. In the case of oblongolide, the stereochemical information encoded in the building blocks was faithfully translated through the automated assembly of the linear precursor and then used to direct the subsequent diastereoselective polycyclization. A steroid-like core was made through an automatically-assembled linear precursor followed by a catalyst-promoted enantio- and diastereoselective cation-π cyclization. The same first two building blocks were also used to achieve the automated synthesis of the linear precursor for the pentacyclic natural product secodaphnane core. This strategy of combining automated assembly of prefabricated molecular building blocks with biomimetic cyclization reactions has substantial potential to remove the synthetic bottleneck to accessing many other complex, biologically-relevant natural products. Thus, while many important challenges remain, the scope of the now fully automated MIDA boronate-based ICC is already substantial and rapidly expanding.
Summary and prospectus
Over the past several centuries, the strategy of iterative assembly of building blocks has repeatedly accelerated the discovery of new functions. The recent development of automated synthesis platforms for oligopeptides and oligonucleotides removed the synthetic barrier and led to countless discoveries and applications. Small molecules are very different from biopolymers. Given that their structures are far more diverse and complex, the development of a highly general building block synthesis of small molecules will require solving a unique set of challenges. Principally, this endeavor will require very efficient and versatile ways of both making and coupling a wide range of building blocks corresponding to some of the most common substructural motifs found in small molecules. For specific classes of small molecules, customized iterative strategies have already demonstrated some of this potential and led to important discoveries. However, a general platform for small molecule synthesis would be far more impactful. As an important step in that direction, iterative cross-coupling with MIDA boronates has already enabled the synthesis of many different kinds of small molecules in a fully automated fashion.
Continued progress towards this goal will depend on the development of new chemical methodologies. The last several decades have seen tremendous progress in the expansion of cross-coupling chemistry, including even stereocontrolled Csp3 coupling methods. Another important avenue of investigation will be integration of customized iterative approaches into a generalized synthesis platform, as they represent especially efficient routes to specific areas of chemical space. These advances will enable generalized synthesis strategies to become capable of making a wider range of different small molecules, and resulting discoveries of new molecular functions will inspire further efforts to improve the versatility of generalized synthesis. It is worth noting that customized synthesis still retains many practical advantages in the scale-up of routes to particular target compounds, where a premium is placed on step-count, atom economy, and overall efficiency. It is thus exciting to imagine how removing the bottleneck to discovering new molecular functions will in turn create more opportunities for chemists to find the best ways of making those functions available to society.
References
- 1.Wender PA, Miller BL. Synthesis at the molecular frontier. Nature. 2009;460(7252):197–201. doi: 10.1038/460197a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villar EA, Beglov D, Chennamadhavuni S, Porco JAJr, Kozakov D, Vajda S, Whitty A. How proteins bind macrocycles. Nature chemical biology. 2014;10(9):723–31. doi: 10.1038/nchembio.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du J, Lu W, Wu S, Cheng Y, Gouaux E. Glycine receptor mechanism elucidated by electron cryo-microscopy. Nature. 2015;526(7572):224–9. doi: 10.1038/nature14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cioffi AG, Hou J, Grillo AS, Diaz KA, Burke MD. Restored Physiology in Protein-Deficient Yeast by a Small Molecule Channel. Journal of the American Chemical Society. 2015;137(32):10096–9. doi: 10.1021/jacs.5b05765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grillo AS, SantaMaria AM, Kafina MD, Cioffi AG, Huston NC, Han M, Seo YA, Yien YY, Nardone C, Menon AV, Fan J, Svoboda DC, Anderson JB, Hong JD, Nicolau BG, Subedi K, Gewirth AA, Wessling-Resnick M, Kim J, Paw BH, Burke MD. Restored iron transport by a small molecule promotes absorption and hemoglobinization in animals. Science. 2017;356(6338):608–616. doi: 10.1126/science.aah3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kocaoglu O, Carlson EE. Progress and prospects for small-molecule probes of bacterial imaging. Nature chemical biology. 2016;12(7):472–8. doi: 10.1038/nchembio.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan J, Dodani SC, Chang CJ. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nature chemistry. 2012;4(12):973–84. doi: 10.1038/nchem.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber J, Beard PC, Bohndiek SE. Contrast agents for molecular photoacoustic imaging. Nature methods. 2016;13(8):639–50. doi: 10.1038/nmeth.3929. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Zhang P, Smaga LP, Hoffman RA, Chan J. Photoacoustic Probes for Ratiometric Imaging of Copper(II) Journal of the American Chemical Society. 2015;137(50):15628–31. doi: 10.1021/jacs.5b10504. [DOI] [PubMed] [Google Scholar]
- 10.Schumacher S, Sartorius D, Ehrentreich-Forster E, Bier FF. Miniaturization for Point-of-Care Analysis: Platform Technology for Almost Every Biomedical Assay. Ejifcc. 2012;23(3):70–5. [PMC free article] [PubMed] [Google Scholar]
- 11.Romero NA, Nicewicz DA. Organic Photoredox Catalysis. Chemical reviews. 2016;116(17):10075–166. doi: 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- 12.Kiser PD, Golczak M, Palczewski K. Chemistry of the retinoid (visual) cycle. Chemical reviews. 2014;114(1):194–232. doi: 10.1021/cr400107q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mart RJ, Allemann RK. Azobenzene photocontrol of peptides and proteins. Chemical Communications. 2016;52(83):12262–12277. doi: 10.1039/c6cc04004g. [DOI] [PubMed] [Google Scholar]
- 14.Koumura N, Zijlstra RW, van Delden RA, Harada N, Feringa BL. Light-driven monodirectional molecular rotor. Nature. 1999;401(6749):152–5. doi: 10.1038/43646. [DOI] [PubMed] [Google Scholar]
- 15.Hickenboth CR, Moore JS, White SR, Sottos NR, Baudry J, Wilson SR. Biasing reaction pathways with mechanical force. Nature. 2007;446(7134):423–7. doi: 10.1038/nature05681. [DOI] [PubMed] [Google Scholar]
- 16.Davis DA, Hamilton A, Yang J, Cremar LD, Van Gough D, Potisek SL, Ong MT, Braun PV, Martinez TJ, White SR, Moore JS, Sottos NR. Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature. 2009;459(7243):68–72. doi: 10.1038/nature07970. [DOI] [PubMed] [Google Scholar]
- 17.Aizawa N, Pu YJ, Watanabe M, Chiba T, Ideta K, Toyota N, Igarashi M, Suzuri Y, Sasabe H, Kido J. Solution-processed multilayer small-molecule light-emitting devices with high-efficiency white-light emission. Nat Commun. 2014;5:5756. doi: 10.1038/ncomms6756. [DOI] [PubMed] [Google Scholar]
- 18.Wilson GO, Caruso MM, Reimer NT, White SR, Sottos NR, Moore JS. Evaluation of Ruthenium Catalysts for Ring-Opening Metathesis Polymerization-Based Self-Healing Applications. Chemistry of Materials. 2007;20(10):3288–3297. [Google Scholar]
- 19.Thiele S, Balestro F, Ballou R, Klyatskaya S, Ruben M, Wernsdorfer W. Electrically driven nuclear spin resonance in single-molecule magnets. Science. 2014;344(6188):1135–8. doi: 10.1126/science.1249802. [DOI] [PubMed] [Google Scholar]
- 20.Heinrich BW, Braun L, Pascual JI, Franke KJ. Protection of excited spin states by a superconducting energy gap. Nature Physics. 2013;9:765–768. [Google Scholar]
- 21.Valade L, Caro Dd, Faulmann C, Jacob K. TTF[Ni(dmit)2]2: From single-crystals to thin layers, nanowires, and nanoparticles. Coordination Chemistry Reviews. 2016;308:433–444. [Google Scholar]
- 22.Cui H, Kobayashi H, Ishibashi S, Sasa M, Iwase F, Kato R, Kobayashi A. A single-component molecular superconductor. Journal of the American Chemical Society. 2014;136(21):7619–22. doi: 10.1021/ja503690m. [DOI] [PubMed] [Google Scholar]
- 23.Cooper CC. The Portsmouth System of Manufacture. Technology and Culture. 1984;25(2):185–225. [Google Scholar]
- 24.Alder K. Innovation and Amnesia: Engineering Rationality and the Fate of Interchangeable Parts Manufacturing in France. Technology and Culture. 1997;38(2):273–311. [Google Scholar]
- 25.Rattenbury RC. A Legacy in Arms: American Firearm Manufacture, Design, and Artistry, 1800-1900. University of Oklahoma Press; Norman, Oklahoma: 2014. [Google Scholar]
- 26.Hounshell D. From the American System to Mass Production, 1800-1932: The Development of Manufacturing Technology in the United States. Johns Hopkins University Press; Baltimore, Maryland: 1985. [Google Scholar]
- 27.Ali MM, Moon KS. Structural Developments in Tall Buildings: Current Trends and Future Prospects. Architectural Science Review. 2007;50(3):205–223. [Google Scholar]
- 28.Telamarthi K, Aman MS, Abdelgawad A. An Application-Driven Modular IoT Architecture. Wireless Communications and Mobile Computing. 2017;2017:16. [Google Scholar]
- 29.Kitmacher GH. In: Design of the space station habitable modules. Congress, T. W. S., editor. Houston, Texas: 2002. (IAC-02-IAA.8.2.04, IAA.8.2). [Google Scholar]
- 30.Meng Y, Johnson K, Simms B, Conforth M. A Generic Architecture of Modular Embedded System for Miniature Mobile Robots. International Conference on Intelligent Robots and Systems. 2008 [Google Scholar]
- 31.Garud R, Kumaraswamy A, Langlois R. Managing in the Modular Age: Architectures, Networks, and Organizations. Blackwell; 2002. [Google Scholar]
- 32.Peretz I, Coltheart M. Modularity of music processing. Nature neuroscience. 2003;6(7):688–91. doi: 10.1038/nn1083. [DOI] [PubMed] [Google Scholar]
- 33.D’ORAZIO F. Introducing Modules: Artificial Intelligence On Demand on Pulsar. https://www.pulsarplatform.com/blog/2016/introducing-modules-artificial-intelligence-on-demand-on-pulsar/ (accessed September 9, 2017).
- 34.Baldwin CY, Clark KB. Managing in an age of modularity. Harvard business review. 1997;75(5):84–93. [PubMed] [Google Scholar]
- 35.Schubert C, van Langeveld MC, Donoso LA. Innovations in 3D printing: a 3D overview from optics to organs. The British journal of ophthalmology. 2014;98(2):159–61. doi: 10.1136/bjophthalmol-2013-304446. [DOI] [PubMed] [Google Scholar]
- 36.MacDonald E, Salas R, Espalin D, Perez M, Aguilera E, Muse D, Wicker RB. 3D Printing for the Rapid Prototyping of Structural Electronics. IEEE Access. 2014;2:234–242. [Google Scholar]
- 37.Ventola CL. Medical Applications for 3D Printing: Current and Projected Uses. P & T : a peer-reviewed journal for formulary management. 2014;39(10):704–11. [PMC free article] [PubMed] [Google Scholar]
- 38.Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Analytical chemistry. 2014;86(7):3240–53. doi: 10.1021/ac403397r. [DOI] [PubMed] [Google Scholar]
- 39.Merrified RB. Automated Synthesis of Peptides. Science. 1965;150(3693):178–185. doi: 10.1126/science.150.3693.178. [DOI] [PubMed] [Google Scholar]
- 40.Caruthers MH. Gene synthesis machines: DNA chemistry and its uses. Science. 1985;230(4723):281–5. doi: 10.1126/science.3863253. [DOI] [PubMed] [Google Scholar]
- 41.Trevino V, Falciani F, Barrera-Saldana HA. DNA microarrays: a powerful genomic tool for biomedical and clinical research. Molecular medicine (Cambridge, Mass) 2007;13(9-10):527–41. doi: 10.2119/2006-00107.Trevino. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lenoir T, Giannella E. The emergence and diffusion of DNA microarray technology. Journal of biomedical discovery and collaboration. 2006;1:11. doi: 10.1186/1747-5333-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug discovery today. 2015;20(1):122–8. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Khvorova A, Watts JK. The chemical evolution of oligonucleotide therapies of clinical utility. Nature biotechnology. 2017;35(3):238–248. doi: 10.1038/nbt.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, Algire MA, Benders GA, Montague MG, Ma L, Moodie MM, Merryman C, Vashee S, Krishnakumar R, Assad-Garcia N, Andrews-Pfannkoch C, Denisova EA, Young L, Qi ZQ, Segall-Shapiro TH, Calvey CH, Parmar PP, Hutchison CA, 3rd, Smith HO, Venter JC. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010;329(5987):52–6. doi: 10.1126/science.1190719. [DOI] [PubMed] [Google Scholar]
- 46.Khorana HG. Total synthesis of a gene. Science. 1979;203(4381):614–25. doi: 10.1126/science.366749. [DOI] [PubMed] [Google Scholar]
- 47.Kent SB. Total chemical synthesis of proteins. Chemical Society reviews. 2009;38(2):338–51. doi: 10.1039/b700141j. [DOI] [PubMed] [Google Scholar]
- 48.Seeberger PH, Werz DB. Automated synthesis of oligosaccharides as a basis for drug discovery. Nature reviews. Drug discovery. 2005;4(9):751–63. doi: 10.1038/nrd1823. [DOI] [PubMed] [Google Scholar]
- 49.Plante OJ, Palmacci ER, Seeberger PH. Automated solid-phase synthesis of oligosaccharides. Science. 2001;291(5508):1523–7. doi: 10.1126/science.1057324. [DOI] [PubMed] [Google Scholar]
- 50.Newman DJ, Cragg GM. Natural Products as Sources of New Drugs from 1981 to 2014. Journal of natural products. 2016;79(3):629–61. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 51.Rodrigues T, Reker D, Schneider P, Schneider G. Counting on natural products for drug design. Nature chemistry. 2016;8(6):531–41. doi: 10.1038/nchem.2479. [DOI] [PubMed] [Google Scholar]
- 52.Woerly EM, Roy J, Burke MD. Synthesis of most polyene natural product motifs using just 12 building blocks and one coupling reaction. Nature chemistry. 2014;6(6):484–91. doi: 10.1038/nchem.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pye CR, Bertin MJ, Lokey RS, Gerwick WH, Linington RG. Retrospective analysis of natural products provides insights for future discovery trends. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(22):5601–5606. doi: 10.1073/pnas.1614680114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palazzolo AME, Simons CLW, Burke MD. The natural productome. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(22):5564–5566. doi: 10.1073/pnas.1706266114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Service RF. Billion-dollar project would synthesize hundreds of thousands of molecules in search of new medicines. http://www.sciencemag.org/news/2017/04/billion-dollar-proiect-would-synthesize-hundreds-thousands-molecules-search-new (accessed August 8, 2017).
- 56.Vitaku E, Smith DT, Njardarson JT. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. Journal of medicinal chemistry. 2014;57(24):10257–74. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 57.Frihed TG, Bols M, Pedersen CM. Synthesis of L-Hexoses. Chemical reviews. 2015;115(9):3615–76. doi: 10.1021/acs.chemrev.5b00104. [DOI] [PubMed] [Google Scholar]
- 58.Paterson I, Scott JP. Polyketide Library Synthesis: Iterative Assembly of Extended Polypropionates Using (R)- and (S)-I-(Benzyloxy)-2-methylpentan-3-one. Tetrahedron Letters. 1997;38(42):4. [Google Scholar]
- 59.Paterson II, Donghi M, Gerlach K. A Combinatorial Approach to Polyketide-Type Libraries by Iterative Asymmetric Aldol Reactions Performed on Solid Support. Angewandte Chemistry International Edition. 2000;39(18):3315–3319. doi: 10.1002/1521-3773(20000915)39:18<3315::aid-anie3315>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 60.Evans DA, Clark JS, Metternich R, Novack VJ, Sheppard GS. Diastereoselective aldol reactions using .beta.-keto imide derived enolates. A versatile approach to the assemblage of polypropionate systems. Journal of the American Chemical Society. 1990;112(2):866–868. [Google Scholar]
- 61.Evans DA, Nelson JV, Vogel E, Taber TR. Stereoselective aldol condensations via boron enolates. Journal of the American Chemical Society. 1981;103(11):3099–3111. [Google Scholar]
- 62.Crimmins MT, Chaudhary K. Titanium enolates of thiazolidinethione chiral auxiliaries: versatile tools for asymmetric aldol additions. Organic letters. 2000;2(6):775–7. doi: 10.1021/ol9913901. [DOI] [PubMed] [Google Scholar]
- 63.Crimmins MT, King BW, Tabet EA, Chaudhary K. Asymmetric aldol additions: use of titanium tetrachloride and (−)-sparteine for the soft enolization of N-acyl oxazolidinones, oxazolidinethiones, and thiazolidinethiones. The Journal of organic chemistry. 2001;66(3):894–902. doi: 10.1021/jo001387r. [DOI] [PubMed] [Google Scholar]
- 64.Crimmins MT, Slade DJ. Formal synthesis of 6-deoxyerythronolide B. Organic letters. 2006;8(10):2191–4. doi: 10.1021/ol0607241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown HC, Bhat KS, Randad RS. beta.-Allyldiisopinocampheylborane: a remarkable reagent for the diastereoselective allylboration of .alpha.-substituted chiral aldehydes. Journal of Organic Chemistry. 1987;52(2):319–320. [Google Scholar]
- 66.Brown HC, Bhat KS. Enantiomeric Z- and E-crotyldiisopinocampheylboranes. Synthesis in high optical purity of all four possible stereoisomers of .beta.-methylhomoallyl alcohols. Journal of the American Chemical Society. 1986;108(2):293–294. doi: 10.1021/ja00279a042. [DOI] [PubMed] [Google Scholar]
- 67.Brown HC, Bhat KS. Chiral synthesis via organoboranes. 7. Diastereoselective and enantioselective synthesis of erythro- and threo-.beta.-methylhomoallyl alcohols via enantiomeric (Z)- and (E)-crotylboranes. Journal of the American Chemical Society. 1986;108(19):5919–23. doi: 10.1021/ja00279a042. [DOI] [PubMed] [Google Scholar]
- 68.Garcia-Fortanet J, Murga J, Carda M, Marco JA. On the structure of passifloricin A: asymmetric synthesis of the delta-lactones of (2Z,5S,7R,9S,11S)- and (2Z,5R,7R,9S,11S)tetrahydroxyhexacos-2-enoic acid. Organic letters. 2003;5(9):1447–9. doi: 10.1021/ol034182o. [DOI] [PubMed] [Google Scholar]
- 69.Dechert-Schmitt AM, Schmitt DC, Gao X, Itoh T, Krische MJ. Polyketide construction via hydrohydroxyalkylation and related alcohol C-H functionalizations: reinventing the chemistry of carbonyl addition. Natural product reports. 2014;31(4):504–13. doi: 10.1039/c3np70076c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han SB, Hassan A, Kim IS, Krische MJ. Total synthesis of (+)-roxaticin via C-C bond forming transfer hydrogenation: a departure from stoichiometric chiral reagents, auxiliaries, and premetalated nucleophiles in polyketide construction. Journal of the American Chemical Society. 2010;132(44):15559–61. doi: 10.1021/ja1082798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shin I, Hong S, Krische MJ. Total Synthesis of Swinholide A: An Exposition in Hydrogen-Mediated C-C Bond Formation. Journal of the American Chemical Society. 2016;138(43):14246–14249. doi: 10.1021/jacs.6b10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao X, Woo SK, Krische MJ. Total synthesis of 6-deoxyerythronolide B via C-C bond-forming transfer hydrogenation. Journal of the American Chemical Society. 2013;135(11):4223–6. doi: 10.1021/ja4008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feng J, Kasun ZA, Krische MJ. Enantioselective Alcohol C-H Functionalization for Polyketide Construction: Unlocking Redox-Economy and Site-Selectivity for Ideal Chemical Synthesis. Journal of the American Chemical Society. 2016;138(17):5467–78. doi: 10.1021/jacs.6b02019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ketcham JM, Volchkov I, Chen TY, Blumberg PM, Kedei N, Lewin NE, Krische MJ. Evaluation of Chromane-Based Bryostatin Analogues Prepared via Hydrogen-Mediated C-C Bond Formation: Potency Does Not Confer Bryostatin-like Biology. Journal of the American Chemical Society. 2016;138(40):13415–13423. doi: 10.1021/jacs.6b08695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Myers AG, Yang BH, Chen H, Gleason JL. Use of Pseudoephedrine as a Practical Chiral Auxiliary for Asymmetric Synthesis. Journal of the American Chemical Society. 1994;116(20):9361–9362. [Google Scholar]
- 76.Myers AG, Yang BH, Chen H, Kopecky DJ. Asymmetric Synthesis of 1,3-Dialkyl-Substituted Carbon Chains of any Stereochemical Configuration by an Iterable Process. Synlett. 1997:457–459. [Google Scholar]
- 77.Vong BG, Abraham S, Xiang AX, Theodorakis EA. Synthetic studies on borrelidin: enantioselective synthesis of the C1-C12 fragment. Organic letters. 2003;5(10):1617–20. doi: 10.1021/ol034243i. [DOI] [PubMed] [Google Scholar]
- 78.Vong BG, Kim SH, Abraham S, Theodorakis EA. Stereoselective total synthesis of (−)-borrelidin. Angewandte Chemie (International ed in English) 2004;43(30):3947–51. doi: 10.1002/anie.200460203. [DOI] [PubMed] [Google Scholar]
- 79.ter Horst B, Feringa BL, Minnaard AJ. Catalytic asymmetric synthesis of phthioceranic acid, a heptamethyl-branched acid from Mycobacterium tuberculosis. Organic letters. 2007;9(16):3013–5. doi: 10.1021/ol071078o. [DOI] [PubMed] [Google Scholar]
- 80.Geerdink D, Minnaard AJ. Total synthesis of sulfolipid-1. Chemical communications (Cambridge, England) 2014;50(18):2286–8. doi: 10.1039/c3cc48087a. [DOI] [PubMed] [Google Scholar]
- 81.Brand GJ, Studte C, Breit B. Iterative synthesis of (oligo)deoxypropionates via zinc-catalyzed enantiospecific sp3-sp3 cross-coupling. Organic letters. 2009;11(20):4668–70. doi: 10.1021/ol901944b. [DOI] [PubMed] [Google Scholar]
- 82.ter Horst B, Feringa BL, Minnaard AJ. Iterative strategies for the synthesis of deoxypropionates. Chemical communications (Cambridge, England) 2010;46(15):2535–47. doi: 10.1039/b926265b. [DOI] [PubMed] [Google Scholar]
- 83.Schmid F, Baro A, Laschat S. Strategies for the Synthesis of Deoxypropionates. Synthesis. 2017;49:237–251. [Google Scholar]
- 84.Balieu S, Hallett GE, Burns M, Bootwicha T, Studley J, Aggarwal VK. Toward ideality: the synthesis of (+)-kalkitoxin and (+)-hydroxyphthioceranic acid by assembly-line synthesis. Journal of the American Chemical Society. 2015;137(13):4398–403. doi: 10.1021/ja512875g. [DOI] [PubMed] [Google Scholar]
- 85.Thomas SP, French RM, Jheengut V, Aggarwal VK. Homologation and alkylation of boronic esters and boranes by 1,2-metallate rearrangement of boronate complexes. Chemical record (New York, NY) 2009;9(1):24–39. doi: 10.1002/tcr.20168. [DOI] [PubMed] [Google Scholar]
- 86.Burns M, Essafi S, Bame JR, Bull SP, Webster MP, Balieu S, Dale JW, Butts CP, Harvey JN, Aggarwal VK. Assembly-line synthesis of organic molecules with tailored shapes. Nature. 2014;513(7517):183–8. doi: 10.1038/nature13711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu J, Lorenzo P, Zhong S, Ali M, Butts CP, Myers EL, Aggarwal VK. Synergy of synthesis, computation and NMR reveals correct baulamycin structures. Nature. 2017;547(7664):436–440. doi: 10.1038/nature23265. [DOI] [PubMed] [Google Scholar]
- 88.Noble A, Roesner S, Aggarwal VK. Short Enantioselective Total Synthesis of Tatanan A and 3-epi-Tatanan A Using Assembly-Line Synthesis. Angewandte Chemie (International ed in English) 2016;55(51):15920–15924. doi: 10.1002/anie.201609598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roesner S, Blair DJ, Aggarwal VK. Enantioselective installation of adjacent tertiary benzylic stereocentres using lithiation–borylation–protodeboronation methodology. Application to the synthesis of bifluranol and fluorohexestrol. Chemical Science. 2015;6:3718–3723. doi: 10.1039/c4sc03901g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pulis AP, Aggarwal VK. Synthesis of enantioenriched tertiary boronic esters from secondary allylic carbamates. Application to the synthesis of C30 botryococcene. Journal of the American Chemical Society. 2012;134(17):7570–4. doi: 10.1021/ja303022d. [DOI] [PubMed] [Google Scholar]
- 91.Leonori D, Aggarwal VK. Lithiation-borylation methodology and its application in synthesis. Accounts of chemical research. 2014;47(10):3174–83. doi: 10.1021/ar5002473. [DOI] [PubMed] [Google Scholar]
- 92.Nicolaou KC, Chakraborty TK, Daines RA, Simpkins NS. Retrosynthetic and Synthetic Chemistry on Amphotericin B. Synthesis of C(1)-C(20) and C(21)-C(38) Fragments and Construction of the 38-Membered Macrocycle. Journal of the Chemical Society, Chemical Communications. 1986:4. [Google Scholar]
- 93.Nicolaou KC, Daines RA, Chakraborty TK, Ogawa Y. Total Synthesis of Amphoteronolide B and Amphotericin B. 2. Total Synthesis of Amphoteronolide B. Journal of the American Chemical Society. 1988;110:12. [Google Scholar]
- 94.Suzuki A, Sasaki M, Nakagishi T, Ueda T, Hoshiya N, Uenishi J. Construction of Iterative Tetrahydrofuran Ring Units and Total Synthesis of (+)-Goniocin. Organic letters. 2016;18(9):2248–51. doi: 10.1021/acs.orglett.6b00877. [DOI] [PubMed] [Google Scholar]
- 95.Mori Y, Nogami K, Hayashi H, Noyori R. Sulfonyl-stabilized oxiranyllithium-based approach to polycyclic ethers. Convergent synthesis of the ABCDEF-ring system of yessotoxin and adriatoxin. The Journal of organic chemistry. 2003;68(23):9050–60. doi: 10.1021/jo035145d. [DOI] [PubMed] [Google Scholar]
- 96.Mori Y, Yaegashi K, Furukawa H. Formal Total Synthesis of Hemibrevetoxin B by an Oxiranyl Anion Strategy. The Journal of organic chemistry. 1998;63(18):6200–6209. doi: 10.1021/jo980320p. [DOI] [PubMed] [Google Scholar]
- 97.Furuta H, Hasegawa Y, Hase M, Mori Y. Total synthesis of gambierol by using oxiranyl anions. Chemistry (Weinheim an der Bergstrasse, Germany) 2010;16(25):7586–95. doi: 10.1002/chem.201000497. [DOI] [PubMed] [Google Scholar]
- 98.Sakai T, Matsushita S, Arakawa S, Mori K, Tanimoto M, Tokumasu A, Yoshida T, Mori Y. Total Synthesis of Gymnocin-A. Journal of the American Chemical Society. 2015;137(45):14513–6. doi: 10.1021/jacs.5b10082. [DOI] [PubMed] [Google Scholar]
- 99.Marmsater FP, West FG. New efficient iterative approaches to polycyclic ethers. Chemistry (Weinheim an der Bergstrasse, Germany) 2002;8(19):4346–53. doi: 10.1002/1521-3765(20021004)8:19<4346::AID-CHEM4346>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 100.Yamamoto A, Ueda A, Bremond P, Tiseni PS, Kishi Y. Total synthesis of halichondrin C. Journal of the American Chemical Society. 2012;134(2):893–6. doi: 10.1021/ja2108307. [DOI] [PubMed] [Google Scholar]
- 101.Ledford H. Complex synthesis yields breast-cancer therapy. Nature. 2010;468(7324):608–9. doi: 10.1038/468608a. [DOI] [PubMed] [Google Scholar]
- 102.Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Dieras V, Delozier T, Vladimirov V, Cardoso F, Koh H, Bougnoux P, Dutcus CE, Seegobin S, Mir D, Meneses N, Wanders J, Twelves C. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet (London, England) 2011;377(9769):914–23. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 103.Zhang K, Cai L, Jiang X, Garcia-Garibay MA, Kwon O. Phosphine-Mediated Iterative Arene Homologation Using Allenes. Journal of the American Chemical Society. 2015;137(35):11258–61. doi: 10.1021/jacs.5b07403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anthony JE. The larger acenes: versatile organic semiconductors. Angewandte Chemie (International ed in English) 2008;47(3):452–83. doi: 10.1002/anie.200604045. [DOI] [PubMed] [Google Scholar]
- 105.Zuzak R, Dorel R, Krawiec M, Such B, Kolmer M, Szymonski M, Echavarren AM, Godlewski S. Nonacene Generated by On-Surface Dehydrogenation. ACS nano. 2017;11(9):9321–9329. doi: 10.1021/acsnano.7b04728. [DOI] [PubMed] [Google Scholar]
- 106.Dorel R, Echavarren AM. Strategies for the Synthesis of Higher Acenes. European journal of organic chemistry. 2017;2017(1):14–24. doi: 10.1002/ejoc.201601129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tonshoff C, Bettinger HF. Photogeneration of octacene and nonacene. Angewandte Chemie (International ed in English) 2010;49(24):4125–8. doi: 10.1002/anie.200906355. [DOI] [PubMed] [Google Scholar]
- 108.Zhang J, Moore JS, Xu Z, Aguirre RA. Nanoarchitectures. 1. Controlled Synthesis of Phenylacetylene Sequences. Journal of the American Chemical Society. 1992;114(6):2. [Google Scholar]
- 109.Moore JS. Shape-Persistent Molecular Architectures of Nanoscale Dimension. Accounts of chemical research. 1997;30(10):12. [Google Scholar]
- 110.Zhang J, Pesak DJ, Ludwick JL, Moore JS. Geometrically-Controlled and Site-Specifically-Functionalized Phenylacetylene Macrocycles. Journal of the American Chemical Society. 1993;116(10):13. [Google Scholar]
- 111.Bharathi P, Patel U, Kawaguchi T, Pesak DJ, Moore JS. Improvements in the Synthesis of Phenylacetylene Monodendrons Including a Solid-Phase Convergent Method. Macromolecules. 1995;28(17):9. [Google Scholar]
- 112.Xu Z, Kahr M, Walker KL, Wilkins CL, Moore JS. Phenylacetylene Dendrimers by the Divergent, Convergent, and Double-Stage Convergent Methods. Journal of the American Chemical Society. 1994;116(11):14. [Google Scholar]
- 113.Xu Z, Moore JS. Rapid Construction of Large-size Phenylacetylene Dendrimers up to 12.5 Nanometers in Molecular Diameter. Angewandte Chemie (International ed in English) 1993;32(9):4. [Google Scholar]
- 114.Zhao Y, Rocha SV, Swager TM. Mechanochemical Synthesis of Extended Iptycenes. Journal of the American Chemical Society. 2016;138:13834–13837. doi: 10.1021/jacs.6b09011. [DOI] [PubMed] [Google Scholar]
- 115.Crudden CM, Ziebenhaus C, Rygus JP, Ghozati K, Unsworth PJ, Nambo M, Voth S, Hutchinson M, Laberge VS, Maekawa Y, Imao D. Iterative protecting group-free cross-coupling leading to chiral multiply arylated structures. Nat Commun. 2016;7:11065. doi: 10.1038/ncomms11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lewis JE, Winn J, Cera L, Goldup SM. Iterative Synthesis of Oligo[n]rotaxanes in Excellent Yield. Journal of the American Chemical Society. 2016;138(50):16329–16336. doi: 10.1021/jacs.6b08958. [DOI] [PubMed] [Google Scholar]
- 117.Bredenkamp A, Wegener M, Hummel S, Haring AP, Kirsch SF. Versatile process for the stereodiverse construction of 1,3-polyols: iterative chain elongation with chiral building blocks. Chemical communications (Cambridge, England) 2016;52(9):1875–8. doi: 10.1039/c5cc09328g. [DOI] [PubMed] [Google Scholar]
- 118.Johansson Seechurn CC, Kitching MO, Colacot TJ, Snieckus V. Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel Prize. Angewandte Chemie (International ed in English) 2012;51(21):5062–85. doi: 10.1002/anie.201107017. [DOI] [PubMed] [Google Scholar]
- 119.Hartwig JF. Organotransition Metal Chemistry: From Bonding to Catalysis. University Science; 2010. [Google Scholar]
- 120.Martin R, Buchwald SL. Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Accounts of chemical research. 2008;41(11):1461–73. doi: 10.1021/ar800036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Imao D, Glasspoole BW, Laberge VS, Crudden CM. Cross coupling reactions of chiral secondary organoboronic esters with retention of configuration. Journal of the American Chemical Society. 2009;131(14):5024–5. doi: 10.1021/ja8094075. [DOI] [PubMed] [Google Scholar]
- 122.Matthew SC, Glasspoole BW, Eisenberger P, Crudden CM. Synthesis of enantiomerically enriched triarylmethanes by enantiospecific Suzuki-Miyaura cross-coupling reactions. Journal of the American Chemical Society. 2014;136(16):5828–31. doi: 10.1021/ja412159g. [DOI] [PubMed] [Google Scholar]
- 123.Sandrock DL, Jean-Gerard L, Chen CY, Dreher SD, Molander GA. Stereospecific cross-coupling of secondary alkyl beta-trifluoroboratoamides. Journal of the American Chemical Society. 2010;132(48):17108–10. doi: 10.1021/ja108949w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Molander GA, Wisniewski SR. Stereospecific cross-coupling of secondary organotrifluoroborates: potassium 1-(benzyloxy)alkyltrifluoroborates. Journal of the American Chemical Society. 2012;134(40):16856–68. doi: 10.1021/ja307861n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ohmura T, Awano T, Suginome M. Stereospecific Suzuki-Miyaura coupling of chiral alpha-(acylamino)benzylboronic esters with inversion of configuration. Journal of the American Chemical Society. 2010;132(38):13191–3. doi: 10.1021/ja106632j. [DOI] [PubMed] [Google Scholar]
- 126.Awano T, Ohmura T, Suginome M. Inversion or retention? Effects of acidic additives on the stereochemical course in enantiospecific Suzuki-Miyaura coupling of alpha-(acetylamino)benzylboronic esters. Journal of the American Chemical Society. 2011;133(51):20738–41. doi: 10.1021/ja210025q. [DOI] [PubMed] [Google Scholar]
- 127.Lee JC, McDonald R, Hall DG. Enantioselective preparation and chemoselective cross-coupling of 1,1-diboron compounds. Nature chemistry. 2011;3(11):894–9. doi: 10.1038/nchem.1150. [DOI] [PubMed] [Google Scholar]
- 128.Li L, Wang CY, Huang R, Biscoe MR. Stereoretentive Pd-catalysed Stille cross-coupling reactions of secondary alkyl azastannatranes and aryl halides. Nature chemistry. 2013;5(7):607–12. doi: 10.1038/nchem.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li L, Zhao S, Joshi-Pangu A, Diane M, Biscoe MR. Stereospecific pd-catalyzed cross-coupling reactions of secondary alkylboron nucleophiles and aryl chlorides. Journal of the American Chemical Society. 2014;136(40):14027–30. doi: 10.1021/ja508815w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang CY, Ralph G, Derosa J, Biscoe MR. Stereospecific Palladium-Catalyzed Acylation of Enantioenriched Alkylcarbastannatranes: A General Alternative to Asymmetric Enolate Reactions. Angewandte Chemie (International ed in English) 2017;56(3):856–860. doi: 10.1002/anie.201609930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hoang GL, Takacs JM. Enantioselective gamma-borylation of unsaturated amides and stereoretentive Suzuki-Miyaura cross-coupling. Chem Sci. 2017;8(6):4511–4516. doi: 10.1039/c7sc01093a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nakao Y, Chen J, Tanaka M, Hiyama T. A silicon-based approach to oligoarenes by iterative cross-coupling reactions of halogenated organo[(2-hydroxymethyl)phenyl]dimethylsilanes. Journal of the American Chemical Society. 2007;129(38):11694–5. doi: 10.1021/ja074728s. [DOI] [PubMed] [Google Scholar]
- 133.Noguchi H, Hojo K, Suginome M. Boron-masking strategy for the selective synthesis of oligoarenes via iterative Suzuki-Miyaura coupling. Journal of the American Chemical Society. 2007;129(4):758–9. doi: 10.1021/ja067975p. [DOI] [PubMed] [Google Scholar]
- 134.Iwadate N, Suginome M. Synthesis of B-protected beta-styrylboronic acids via iridium-catalyzed hydroboration of alkynes with 1,8-naphthalenediaminatoborane leading to iterative synthesis of oligo(phenylenevinylene)s. Organic letters. 2009;11(9):1899–902. doi: 10.1021/ol9003096. [DOI] [PubMed] [Google Scholar]
- 135.Gillis EP, Burke MD. A simple and modular strategy for small molecule synthesis: iterative Suzuki-Miyaura coupling of B-protected haloboronic acid building blocks. Journal of the American Chemical Society. 2007;129(21):6716–7. doi: 10.1021/ja0716204. [DOI] [PubMed] [Google Scholar]
- 136.Mancilla T, Contreras R. New Bicyclic Organylboronic Esters Derived from Iminodiacetic Acids. Journal of Organometallic Chemistry. 1986;307:1–6. [Google Scholar]
- 137.Gonzalez JA, Ogba OM, Morehouse GF, Rosson N, Houk KN, Leach AG, Cheong PH, Burke MD, Lloyd-Jones GC. MIDA boronates are hydrolysed fast and slow by two different mechanisms. Nature chemistry. 2016;8(11):1067–1075. doi: 10.1038/nchem.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Knapp DM, Gillis EP, Burke MD. A general solution for unstable boronic acids: slow-release cross-coupling from air-stable MIDA boronates. Journal of the American Chemical Society. 2009;131(20):6961–3. doi: 10.1021/ja901416p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dick GR, Woerly EM, Burke MD. A general solution for the 2-pyridyl problem. Angewandte Chemie (International ed in English) 2012;51(11):2667–72. doi: 10.1002/anie.201108608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Carrillo JA, Ingleson MJ, Turner ML. Thienyl MIDA Boronate Esters as Highly Effective Monomers for Suzuki-Miyaura Polymerization Reactions. Macromolecules. 2015;48:8. [Google Scholar]
- 141.Carrillo JA, Turner ML, Ingleson MJ. A General Protocol for the Polycondensation of Thienyl N-Methyliminodiacetic Acid Boronate Esters To Form High Molecular Weight Copolymers. Journal of the American Chemical Society. 2016;138(40):13361–13368. doi: 10.1021/jacs.6b07666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brak K, Ellman JA. Asymmetric Rh(I)-catalyzed addition of MIDA boronates to N-tert-butanesulfinyl aldimines: development and comparison to trifluoroborates. The Journal of organic chemistry. 2010;75(9):3147–50. doi: 10.1021/jo100318s. [DOI] [PubMed] [Google Scholar]
- 143.Davy NC, Man G, Kerner RA, Fusella MA, Purdum GE, Sezen M, Rand BP, Kahn A, Loo Y-L. Contorted Hexabenzocoronenes with Extended Heterocyclic Moieties Improve Visible-Light Absorption and Performance in Organic Solar Cells. Chemistry of Materials. 2016;28:673–681. [Google Scholar]
- 144.Muir CW, Vantourout JC, Isidro-Llobet A, Macdonald SJ, Watson AJ. One-Pot Homologation of Boronic Acids: A Platform for Diversity-Oriented Synthesis. Organic letters. 2015;17(24):6030–3. doi: 10.1021/acs.orglett.5b03030. [DOI] [PubMed] [Google Scholar]
- 145.Gillis EP, Burke MD. Multistep synthesis of complex boronic acids from simple MIDA boronates. Journal of the American Chemical Society. 2008;130(43):14084–5. doi: 10.1021/ja8063759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lv WX, Zeng YF, Li Q, Chen Y, Tan DH, Yang L, Wang H. Oxidative Difunctionalization of Alkenyl MIDA Boronates: A Versatile Platform for Halogenated and Trifluoromethylated alpha-Boryl Ketones. Angewandte Chemie (International ed in English) 2016;55(34):10069–73. doi: 10.1002/anie.201604898. [DOI] [PubMed] [Google Scholar]
- 147.Uno BE, Gillis EP, Burke MD. Vinyl MIDA boronate: a readily accessible and highly versatile building block for small molecule synthesis. Tetrahedron. 2009;65:3130–3138. [Google Scholar]
- 148.Lee CF, Holownia A, Bennett JM, Elkins JM, St Denis JD, Adachi S, Yudin AK. Oxalyl Boronates Enable Modular Synthesis of Bioactive Imidazoles. Angewandte Chemie (International ed in English) 2017;56(22):6264–6267. doi: 10.1002/anie.201611006. [DOI] [PubMed] [Google Scholar]
- 149.St Denis JD, Zajdlik A, Tan J, Trinchera P, Lee CF, He Z, Adachi S, Yudin AK. Boron-containing enamine and enamide linchpins in the synthesis of nitrogen heterocycles. Journal of the American Chemical Society. 2014;136(50):17669–73. doi: 10.1021/ja510963k. [DOI] [PubMed] [Google Scholar]
- 150.Zajdlik A, Wang Z, Hickey JL, Aman A, Schimmer AD, Yudin AK. alpha-Boryl isocyanides enable facile preparation of bioactive boropeptides. Angewandte Chemie (International ed in English) 2013;52(32):8411–5. doi: 10.1002/anie.201302818. [DOI] [PubMed] [Google Scholar]
- 151.Struble JR, Lee SJ, Burke MD. Ethynyl MIDA boronate: a readily accessible and highly versatile building block for small molecule synthesis. Tetrahedron. 2010;66:4710–4718. doi: 10.1016/j.tet.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Close AJ, Kemmitt P, Mark Roe S, Spencer J. Regioselective routes to orthogonally-substituted aromatic MIDA boronates. Organic & biomolecular chemistry. 2016;14(28):6751–6. doi: 10.1039/c6ob01141a. [DOI] [PubMed] [Google Scholar]
- 153.Eberlin L, Carboni B, Whiting A. Regioisomeric and Substituent Effects upon the Outcome of the Reaction of 1-Borodienes with Nitrosoarene Compounds. The Journal of organic chemistry. 2015;80(13):6574–83. doi: 10.1021/acs.joc.5b00593. [DOI] [PubMed] [Google Scholar]
- 154.Cornil J, Echeverria PG, Phansavath P, Ratovelomanana-Vidal V, Guerinot A, Cossy J. Heck coupling using a vinyliodo-MIDA boronate: an efficient and modular access to polyene frameworks. Organic letters. 2015;17(4):948–51. doi: 10.1021/acs.orglett.5b00042. [DOI] [PubMed] [Google Scholar]
- 155.Quiclet-Sire B, Zard SZ. Radical Instability in Aid of Efficiency: A Powerful Route to Highly Functional MIDA Boronates. Journal of the American Chemical Society. 2015;137(21):6762–5. doi: 10.1021/jacs.5b03893. [DOI] [PubMed] [Google Scholar]
- 156.Trinchera P, Corless VB, Yudin AK. Synthesis of Previously Inaccessible Borylated Heterocycle Motifs Using Novel Boron-Containing Amphoteric Molecules. Angewandte Chemie (International ed in English) 2015;54(31):9038–41. doi: 10.1002/anie.201504271. [DOI] [PubMed] [Google Scholar]
- 157.Adachi S, Liew SK, Lee CF, Lough A, He Z, St Denis JD, Poda G, Yudin AK. Condensation-Driven Assembly of Boron-Containing Bis(Heteroaryl) Motifs Using a Linchpin Approach. Organic letters. 2015;17(22):5594–7. doi: 10.1021/acs.orglett.5b02741. [DOI] [PubMed] [Google Scholar]
- 158.Denis JDS, He Z, Yudin AK. Amphoteric α-Boryl Aldehyde Linchpins in the Synthesis of Heterocycles. ACS Catalysis. 2015;5:5373–5379. [Google Scholar]
- 159.Seath CP, Wilson KL, Campbell A, Mowat JM, Watson AJ. Synthesis of 2-BMIDA 6,5-bicyclic heterocycles by Cu(i)/Pd(0)/Cu(ii) cascade catalysis of 2-iodoaniline/phenols. Chemical communications (Cambridge, England) 2016;52(56):8703–6. doi: 10.1039/c6cc04554e. [DOI] [PubMed] [Google Scholar]
- 160.Chan JM, Amarante GW, Toste FD. Tandem Cycloisomerization/Suzuki Coupling of Arylethynyl MIDA Boronates. Tetrahedron. 2011;67(24):4306–4312. doi: 10.1016/j.tet.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.He Z, Yudin AK. Amphoteric alpha-boryl aldehydes. Journal of the American Chemical Society. 2011;133(35):13770–3. doi: 10.1021/ja205910d. [DOI] [PubMed] [Google Scholar]
- 162.St Denis JD, He Z, Yudin AK. Chemoselective palladium-catalyzed alpha-allylation of alpha-boryl aldehydes. Organic & biomolecular chemistry. 2012;10(39):7900–2. doi: 10.1039/c2ob26503f. [DOI] [PubMed] [Google Scholar]
- 163.Li J, Burke MD. Pinene-derived iminodiacetic acid (PIDA): a powerful ligand for stereoselective synthesis and iterative cross-coupling of C(sp3) boronate building blocks. Journal of the American Chemical Society. 2011;133(35):13774–7. doi: 10.1021/ja205912y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yoon JM, Lee CY, Jo YI, Cheon CH. Synthesis of Optically Pure 3,3′-Disubstituted-1,1′-Bi-6-Methoxy-2-Phenol (BIPhOL) Derivatives via Diastereomeric Resolution. The Journal of organic chemistry. 2016;81(18):8464–9. doi: 10.1021/acs.joc.6b01645. [DOI] [PubMed] [Google Scholar]
- 165.Lee SJ, Gray KC, Paek JS, Burke MD. Simple, efficient, and modular syntheses of polyene natural products via iterative cross-coupling. Journal of the American Chemical Society. 2008;130(2):466–8. doi: 10.1021/ja078129x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee SJ, Anderson TM, Burke MD. A simple and general platform for generating stereochemically complex polyene frameworks by iterative cross-coupling. Angewandte Chemie (International ed in English) 2010;49(47):8860–3. doi: 10.1002/anie.201004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Woerly EM, Cherney AH, Davis EK, Burke MD. Stereoretentive Suzuki-Miyaura coupling of haloallenes enables fully stereocontrolled access to (−)-peridinin. Journal of the American Chemical Society. 2010;132(20):6941–3. doi: 10.1021/ja102721p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fujii S, Chang SY, Burke MD. Total synthesis of synechoxanthin through iterative cross-coupling. Angewandte Chemie (International ed in English) 2011;50(34):7862–4. doi: 10.1002/anie.201102688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, Burke MD. Amphotericin primarily kills yeast by simply binding ergosterol. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(7):2234–9. doi: 10.1073/pnas.1117280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brun E, Bellosta V, Cossy J. Synthesis of the Acyclic Carbon Skeleton of Filipin III. The Journal of organic chemistry. 2016;81(18):8206–21. doi: 10.1021/acs.joc.6b01166. [DOI] [PubMed] [Google Scholar]
- 171.Fujita K, Matsui R, Suzuki T, Kobayashi S. Concise total synthesis of (−)-myxalamide A. Angewandte Chemie (International ed in English) 2012;51(29):7271–4. doi: 10.1002/anie.201203093. [DOI] [PubMed] [Google Scholar]
- 172.Dennis EG, Jeffrey DW, Johnston MR, Perkins MV, Smith PA. Procyanidin oligomers. A new method for 4-8 interflavan bond formation using C8-boronic acids and iterative oligomer synthesis through a boron-protection strategy. Tetrahedron. 2012;68:340–348. [Google Scholar]
- 173.Mohamed YMA, Hansen TV. Synthesis of methyl (5Z,8Z,10E,12E,14Z)-eicosapentaenoate. Tetrahedron Letters. 2011;52:1057–1059. [Google Scholar]
- 174.Weber A, Dehn R, Schlager N, Dieter B, Kirschning A. Total synthesis of the antibiotic elansolid B1. Organic letters. 2014;16(2):568–71. doi: 10.1021/ol403441c. [DOI] [PubMed] [Google Scholar]
- 175.Davoren JE, Lee CW, Garnsey M, Brodney MA, Cordes J, Dlugolenski K, Edgerton JR, Harris AR, Helal CJ, Jenkinson S, Kauffman GW, Kenakin TP, Lazzaro JT, Lotarski SM, Mao Y, Nason DM, Northcott C, Nottebaum L, O’Neil SV, Pettersen B, Popiolek M, Reinhart V, Salomon-Ferrer R, Steyn SJ, Webb D, Zhang L, Grimwood S. Discovery of the Potent and Selective M1 PAM-Agonist N-[(3R,4S)-3-Hydroxytetrahydro-2H-pyran-4-yl]-5-methyl-4-[4-(1,3-thiazol-4-yl)ben zyl]pyridine-2-carboxamide (PF-06767832): Evaluation of Efficacy and Cholinergic Side Effects. Journal of medicinal chemistry. 2016;59(13):6313–28. doi: 10.1021/acs.jmedchem.6b00544. [DOI] [PubMed] [Google Scholar]
- 176.Go EB, Wetzler SP, Kim LJ, Chang AY, Vosburg DA. Concise, diastereoconvergent synthesis of endriandric-type tetracycles by iterative cross-coupling. Tetrahedron. 2016;72:3790–3794. [Google Scholar]
- 177.Nishioka Y, Yano Y, Kinashi N, Oku N, Toriyama Y, Katsumara S, Shinada T, Sakaguchi K. Stereocontrolled Synthesis of Paracentrone. Synlett. 2017;28:327–332. [Google Scholar]
- 178.Li J, Ballmer SG, Gillis EP, Fujii S, Schmidt MJ, Palazzolo AM, Lehmann JW, Morehouse GF, Burke MD. Synthesis of many different types of organic small molecules using one automated process. Science. 2015;347(6227):1221–6. doi: 10.1126/science.aaa5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Woerly EM, Struble JR, Palyam N, O’Hara SP, Burke MD. (Z)-(2-bromovinyl)-MIDA boronate: a readily accessible and highly versatile building block for small molecule synthesis. Tetrahedron. 2011;67(24):4333–4343. doi: 10.1016/j.tet.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Grob JE, Dechantsreiter MA, Tichkule RB, Connolly MK, Honda A, Tomlinson RC, Hamann LG. One-pot C-N/C-C cross-coupling of methyliminodiacetic acid boronyl arenes enabled by protective enolization. Organic letters. 2012;14(21):5578–81. doi: 10.1021/ol302702q. [DOI] [PubMed] [Google Scholar]
- 181.Yoder RA, Johnston JN. A case study in biomimetic total synthesis: polyolefin carbocyclizations to terpenes and steroids. Chemical reviews. 2005;105(12):4730–56. doi: 10.1021/cr040623l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Heathcock CH, Piettre S, Ruggeri RB, Ragan JA, Kath JC. Daphniphyllum alkaloids. 12. A proposed biosynthesis of the pentacylic skeleton. proto-Daphniphylline. Journal of Organic Chemistry. 1992;57(9):2554–2566. [Google Scholar]
- 183.Monk BC, Goffeau A. Outwitting multidrug resistance to antifungals. Science. 2008;321(5887):367–9. doi: 10.1126/science.1159746. [DOI] [PubMed] [Google Scholar]
- 184.Wilcock BC, Endo MM, Uno BE, Burke MD. C2′-OH of amphotericin B plays an important role in binding the primary sterol of human cells but not yeast cells. Journal of the American Chemical Society. 2013;135(23):8488–91. doi: 10.1021/ja403255s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Davis SA, Vincent BM, Endo MM, Whitesell L, Marchillo K, Andes DR, Lindquist S, Burke MD. Nontoxic antimicrobials that evade drug resistance. Nature chemical biology. 2015;11(7):481–7. doi: 10.1038/nchembio.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brak K, Ellman JA. Total synthesis of (−)-aurantioclavine. Organic letters. 2010;12(9):2004–7. doi: 10.1021/ol100470g. [DOI] [PubMed] [Google Scholar]
- 187.Igarashi Y, Aoki K, Nishimura H, Morishita I, Usui K. Total synthesis of hydroxy-alpha- and hydroxy-beta-sanshool using Suzuki-Miyaura coupling. Chemical & pharmaceutical bulletin. 2012;60(8):1088–91. doi: 10.1248/cpb.c12-00382. [DOI] [PubMed] [Google Scholar]
- 188.Lindsay AC, Sperry J. Extending the Utility of the Bartoli Indolization: Synthesis of Marinoquinolines C and E. Synlett. 2013;24:461–464. [Google Scholar]
- 189.Coluccini C, Manfredi N, Salamone MM, Ruffo R, Lobello MG, De Angelis F, Abbotto A. Quaterpyridine ligands for panchromatic Ru(II) dye sensitizers. The Journal of organic chemistry. 2012;77(18):7945–56. doi: 10.1021/jo301226z. [DOI] [PubMed] [Google Scholar]
- 190.Ronga L, Del Favero M, Cohen A, Soum C, Le Pape P, Savrimoutou S, Pinaud N, Mullie C, Daulouede S, Vincendeau P, Farvacques N, Agnamey P, Pagniez F, Hutter S, Azas N, Sonnet P, Guillon J. Design, synthesis and biological evaluation of novel 4-alkapolyenylpyrrolo[1,2-a]quinoxalines as antileishmanial agents–part III. European journal of medicinal chemistry. 2014;81:378–93. doi: 10.1016/j.ejmech.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 191.Llona-Minguez S, Hoglund A, Jacques SA, Johansson L, Calderon-Montano JM, Claesson M, Loseva O, Valerie NCK, Lundback T, Piedrafita J, Maga G, Crespan E, Meijer L, Moron EB, Baranczewski P, Hagbjork AL, Svensson R, Wiita E, Almlof I, Visnes T, Jeppsson F, Sigmundsson K, Jensen AJ, Artursson P, Jemth AS, Stenmark P, Berglund UW, Scobie M, Helleday T. Discovery of the First Potent and Selective Inhibitors of Human dCTP Pyrophosphatase 1. Journal of medicinal chemistry. 2016;59(3):1140–1148. doi: 10.1021/acs.jmedchem.5b01741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hornum M, Kumar P, Podsiadly P, Nielsen P. Increasing the Stability of DNA:RNA Duplexes by Introducing Stacking Phenyl-Substituted Pyrazole, Furan, and Triazole Moieties in the Major Groove. The Journal of organic chemistry. 2015;80(19):9592–602. doi: 10.1021/acs.joc.5b01577. [DOI] [PubMed] [Google Scholar]
- 193.Webster AM, Cobb SL. Synthesis of biaryl-linked cyclic peptoides. Tetrahedron Letters. 2017;58:1010–1014. [Google Scholar]
- 194.Godfrey AG, Masquelin T, Hemmerle H. A remote-controlled adaptive medchem lab: an innovative approach to enable drug discovery in the 21st Century. Drug discovery today. 2013;18(17-18):795–802. doi: 10.1016/j.drudis.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 195.Pastre JC, Browne DL, Ley SV. Flow chemistry syntheses of natural products. Chemical Society reviews. 2013;42(23):8849–69. doi: 10.1039/c3cs60246j. [DOI] [PubMed] [Google Scholar]
- 196.Adamo A, Beingessner RL, Behnam M, Chen J, Jamison TF, Jensen KF, Monbaliu JC, Myerson AS, Revalor EM, Snead DR, Stelzer T, Weeranoppanant N, Wong SY, Zhang P. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science. 2016;352(6281):61–7. doi: 10.1126/science.aaf1337. [DOI] [PubMed] [Google Scholar]
- 197.Peplow M. Organic synthesis: The robo-chemist. Nature. 2014;512(7512):20–2. doi: 10.1038/512020a. [DOI] [PubMed] [Google Scholar]
- 198.Made V, Els-Heindl S, Beck-Sickinger AG. Automated solid-phase peptide synthesis to obtain therapeutic peptides. Beilstein journal of organic chemistry. 2014;10:1197–212. doi: 10.3762/bjoc.10.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Iyer RP, Beaucage SL. Oligonucleotide Synthesis. In: Kool ET, editor. Comprehensive Natural Products Chemistry. Vol. 7. Elsevier; Amsterdam: 1999. pp. 105–152. [Google Scholar]
- 200.Hahm HS, Schlegel MK, Hurevich M, Eller S, Schuhmacher F, Hofmann J, Pagel K, Seeberger PH. Automated glycan assembly using the Glyconeer 2.1 synthesizer. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(17):E3385–e3389. doi: 10.1073/pnas.1700141114. [DOI] [PMC free article] [PubMed] [Google Scholar]