Abstract
Portable neuroimaging approaches provide new advances to the study of brain function and brain development with previously inaccessible populations and in remote locations. This paper shows the development of field functional Near Infrared Spectroscopy (fNIRS) imaging to the study of child language, reading, and cognitive development in a rural village setting of Côte d'Ivoire. Innovation in methods and the development of culturally appropriate neuroimaging protocols allow a first-time look into the brain's development and children's learning outcomes in understudied environments. This paper demonstrates protocols for transporting and setting up a mobile laboratory, discusses considerations for field versus laboratory neuroimaging, and presents a guide for developing neuroimaging consent procedures and building meaningful long-term collaborations with local government and science partners. Portable neuroimaging methods can be used to study complex child development contexts, including the impact of significant poverty and adversity on brain development. The protocol presented here has been developed for use in Côte d'Ivoire, the world's primary source of cocoa, and where reports of child labor in the cocoa sector are common. Yet, little is known about the impact of child labor on brain development and learning. Field neuroimaging methods have the potential to yield new insights into such urgent issues, and the development of children globally.
Keywords: Neuroscience, Issue 132, fNIRS, field methods, neuroimaging, cognition, neuroscience, child development, global literacy, language, reading, Ivory Coast, sub-Saharan Africa
Introduction
Portable fNIRS imaging provides the ability to study brain function and development outside the laboratory, in previously inaccessible settings or with understudied populations. Much of the knowledge in the domain of cognitive neuroscience comes from imaging studies conducted in university or hospital laboratory settings, in predominantly Western countries. By design, this contributes to a seldom-spoken-of problem in research: much of what is known about the brain is based on studies with participants for whom laboratory settings in (mostly) Western countries are accessible. That is, most neuroimaging research involves participants who live in reasonable proximity to a neuroimaging laboratory and have both the time and resources necessary to participate in a study. As a discipline, cognitive neuroscience aims to understand the brain and the factors that shape its development—including the powerful effects of a child's environment and their early-life experiences1,2,3. Methods that advance the field's capacity to study development in a fuller range of human experience can dramatically advance the understanding of the complex relation between brain development and the life experiences that shape it.
This paper presents a protocol for field neuroimaging, which was developed for use in rural sub-Saharan Africa, specifically southern Côte d'Ivoire. The aim of this field neuroimaging research program was to understand children's reading development in an environment with a high-risk of illiteracy. Côte d'Ivoire's youth (15-24 years) literacy rate is 53%, despite 93% primary school enrollment rates4. Côte d'Ivoire is the world's primary source of cocoa, and there are an estimated 1.3 million child laborers in the cocoa agricultural sector5. Yet, little is known about the impact of child labor on brain development and learning, specifically learning to read. Applying the latest tools of cognitive neuroscience, i.e., portable neuroimaging methods, can yield valuable insights into children’s learning outcomes. For example, field neuroimaging with fNIRS can allow the identification of neurodevelopmental periods during which targeted educational programs or interventions may have maximal impacts on children's learning outcomes.
fNIRS neuroimaging is well-suited for field research. Similar to functional magnetic resonance imaging (fMRI), fNIRS measures the brain's hemodynamic response6. However, fNIRS uses a series of light emitting optodes and light detectors rather than generating electromagnetic fields. There are no restrictions on metal in or near the testing area, and no electric shielding is necessary, as in the case for electroencephalography (EEG). A key advantage of fNIRS is its portability (i.e., some systems may fit in a suitcase) and ease of use. fNIRS is also easy to use with children; the child is comfortably seated in a chair during the experiment and the fNIRS system tolerates movement well compared to fMRI. Compared with fMRI, fNIRS also provides separate measures of deoxygenated (HbR) and oxygenated hemoglobin (HbO) during recording, compared to fMRI which yields a combined blood oxygen level density (BOLD) measure. fNIRS has superior temporal resolution to fMRI: sampling rates can vary between ~ 7-15 Hz. fNIRS has good spatial resolution: the fNIRS' depth of recording in the human cortex is less than fMRI, measuring about 3 to 4 cm in depth, which is well-suited for studying cortical functions, especially with infants and children who have thinner skulls than adults3,7,8,9,10.
This field neuroimaging protocol outlines considerations for traveling with and setting up a portable neuroimaging laboratory in low-resource contexts. The protocol also highlights the essential nature of meaningful, long-term collaborations with local science partners and ways by which this approach serves to build local science capacity. The neuroimaging protocol for collecting and analyzing fNIRS brain data from a battery of language, reading, and cognitive tasks, is demonstrated including recommendations for creating culturally appropriate informed consent procedures for imaging research. While this protocol is designed for cognitive development research with primary school aged children in rural Côte d'Ivoire, the protocol is highly relevant for any field neuroimaging study in challenging, low-resource environments, and can be adapted for novel contexts.
Protocol
All methods described here have been approved by the Institutional Review Board (IRB) of the University of Delaware.
1. Mobile Laboratory Transport and Setup
- Traveling with the fNIRS equipment
- Transport fNIRS equipment. NOTE: fNIRS equipment can be transported as checked-luggage on a major international airline, but it is imperative to confirm with the given airline. Equipment restrictions may vary by origin or destination country. Alternatively, fNIRS equipment can be shipped.
- Know the procedures for importing or traveling with fNIRS equipment for the destination country, and if applicable, obtain appropriate import approval documentation.
- Prepare for inspections. NOTE: Authorities (e.g., Transportation Security Administration) reserve the right to inspect checked-baggage. Fragile fNIRS fiber optics may be damaged during inspections. Arrange for appropriate documentation to accompany all equipment.
- Essential laboratory equipment in the field
- Prepare for climate conditions in the field. NOTE: Temperature and humidity conditions in the field can vary significantly from laboratory setting and may affect equipment function and longevity, as well as participant comfort during experimentation. Electronics operating in high humidity conditions, generally above 60%, are more susceptible to corrosion as excessive moisture can settle on parts and react with metal components. Humidity levels in an indoor lab (e.g., inside a university building) are generally between 30-50%. Humidity in southern Côte d'Ivoire can be 80-95%. Set up a portable air conditioning unit with low wattage demands.
- Ensure adequate electrical supply. Since electrical supply may not be available in rural settings, or may function only intermittently or with insufficient wattage, use portable solar generators to power small to medium sized electronics. Make available a diesel generator as backup power. Employ a local electrician who is familiar with electrical supply challenges in rural contexts.
- Prepare a suitable laboratory structure with minimal setup time such as a large customized tent with opaque and waterproof roof and walls. NOTE: Facilities (e.g., classroom at local school) are unlikely to be available, or provide waterproof and quiet testing space.
- Setting up the portable laboratory (Figure 1)
- Assemble the mobile laboratory (e.g., customized tent). Ensure that the laboratory is large enough to accommodate seating for the participant at a desk, seating for two experimenters, stimulus presentation computer, fNIRS data collection computer, fNIRS portable unit, three-dimensional (3D) digitizer, and portable air conditioner.
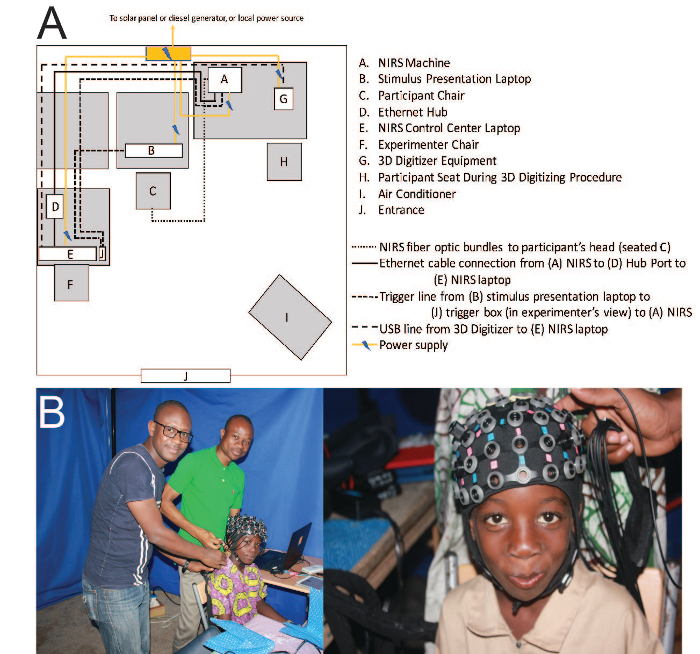
Figure 1. Schematics. (A) Schematic of laboratory setup. (B) Preparing the participant for data collection. Please click here to view a larger version of this figure.
2. Local Research Teams and Science Partners
Invest into formation of scientific collaborations and provide opportunities within the research framework to local researchers.
Establish partnership with local scientific institutions for the purpose of inclusivity. Gaining recognition from peers at the local level is important to communicating eventual research finding in the region.
Consult relevant local authorities before any research activities to receive authorization and license to operate. Familiarize with ethical review procedures in the target country, and make appropriate accommodations if no formal scientific review procedure is in place. NOTE: For example, direct communication with and approval from representatives in the Ministry of Higher Education and Research (or comparable government body) may be done in lieu of an ethical review procedure.
3. Informed Consent and Child Assent
Develop a consent procedure that is culturally appropriate and ensures that participants, their families, and communities are informed about research and their decision to participate in the study.
- Review local customs and history in typical protocol development and include members of the group with whom research needs to be conducted.
- Make sure to obtain a clear consent from local leader(s) (e.g., village chief, community elders, etc.) before proceeding with research. NOTE: This may be expressed by an ancestral benediction or by any other means typical to the community. After consent from the village chief, cultural customs may include the pouring of wine onto the earth and asking the ancestors to approve and bless the research activities.
At a more formal level, seek the consent of parent and educator groups which are responsible for making decision concerning children's participation in school approved activities. For example, the parent-teacher group ('Comité de Gestion d'Ecoles - COGES' in Cote d'Ivoire) is a key stakeholder in the national primary educational system consisting of members appointed by student's parents to defend their interests in decision making and in all other aspects related to the education of their children.
Approve all research activities by local authorities, for example, the Ivorian Ministry of Education or the Ministry of Higher Education and Research. The country that the project will take place in may not have a formal procedure for ethical approval through an IRB. Check regulations to ensure that the correct protocols for obtaining ethical approval for research are being followed. NOTE: At the time of obtaining approval, Côte d'Ivoire did not have a formal IRB review process. In lieu of this, the research team proceeded by preparing documentation akin to an IRB application to submit to the Ministry of Education. Multiple meetings were arranged with the Ministry of Education, Ministry of Higher Education, and research officials where the research team presented the proposed research plan to all officials, followed by group discussion and question and answer sessions. Ethical approval was obtained directly from the Ministry of Education in the form of a signed document granting authorization to conduct research with children at specific schools. This study received ethical approval from the University of Delaware IRB.
Explain the research purpose in simple words to participating children in a child assent procedure. The local community may highly value a child's obedience, in which case a child may assent to participate or continue participating in a study despite their unwillingness to do so because of cultural expectations. Ensure that the assent procedure carefully communicates voluntary participation in the research.
Clearly define how the research will benefit the participants and if they will receive compensation for their participation in the research. Ensure the compensation is appropriate both culturally and for the participants.
Conduct all consent and assent procedures in the local or preferred language of the participant by a trained member of the research team who is also a member of the language and cultural group.
4. fNIRS Scalp Placement and Measurement
- Collecting head measurements
- Direct the participant to sit on a chair, and explain the process to be expected during head measurement.
- Using a standard tape measure, measure the distances between: (1) the nasion and inion around the head, (2) the nasion and inion over top of the head through the midline central (Cz)11, and (3) the distance between the left and right ear tragus over the top of the head through Cz.
- Placement of the fNIRS cap and optodes on the participant's head 3 8 9 12
- Place the fNIRS optode holder cap onto the participant's head, aligning the cap to the international 10-20 system for scalp locations11. Ensure that the cap position is identical for all participants. Align points on the cap (e.g., probe holder) with scalp positions. NOTE: For example, center the front of the cap on the head to the frontopolar (FP) position. This position corresponds to 10% of the nasion-inion over top distance dorsal to nasion position.
- Secure the cap with a strap and ensure the participant is comfortable.
- 3D digitizer measurement
- Once the cap is in position, instruct the participant to sit still in the position for obtaining a 3D digitizer measurement of the key 10-20 system scalp positions11 and each optode place holder.
- Arrange the 3D digitizer equipment. Place one sensor on the participant's head at Cz and affixed securely (i.e., using an elastic or hair accessory), and place the second block sensor behind the participant. Let the participant be seated in a chair with their back to a table. Place the second sensor on the table directly behind the participant's head. Ensure that neither sensor move during the course of obtaining the 3D digitizer measurement.
- Open the Brainstorm software13 on the data collection computer. Make sure the 3D digitizer system is in communication with the Brainstorm software through the appropriate COM port.
- Move the 3D digitizer stylus to each probe location and across the key 10-20 system positions (nasion, inion, left ear, right ear, Cz). At each location, obtain position data through the Brainstorm function on the data collection computer.
- Placing light emitting optodes and detectors on the scalp
- After the 3D digitizer data are collected, direct the participant to be seated comfortably in front of the stimulus presentation computer.
- Using the fNIRS built-in software, select the probe arrangement that corresponds to the experiment design. fNIRS probes can be placed to cover the entire head (i.e., full head coverage), or alternatively, an array can be placed over general regions of interest. For example, this protocol used a 10 x 3 probe array (30 probes arranged in 3 rows of 10 probes each). This probe arrangement was placed to maximally overlay left hemisphere language areas and their right hemisphere homologues, as well as the frontal lobe (Figure 2).
- Ensure that each probe (emitter and detector) is numbered and the numbering system corresponds to the probe arrangement map.
- Using the optode map in the fNIRS inbuilt software as a guide, place each optode in the appropriate optode opening on the cap. The optode map indicates the location of each optode in the array (e.g., 10 x 3).
- Move any hair out of the way to ensure direct contact between the tip of the optode and participant's scalp.
- After all optodes are in position, check for signal quality using the fNIRS system built-in software.
- Adjust individual probes as necessary until sufficient signal quality is achieved. Once all optodes' signal quality checks have passed, proceed with experimental tasks.

5. Experimental Tasks
Design each neuroimaging task with the appropriate number of trials and conditions in line with the research goals. Understand that the neuroimaging tasks will vary depending on the research aims. For example, three tasks were used in this protocol: (1) a language processing and reading task, (2) a rhyme judgment task, and (3) a cognitive flexibility task. NOTE: The procedure (and representative results) of the rhyme judgment task are highlighted.
Place noise-cancelling headphones on the participant's head, being mindful not to interfere with fNIRS probe placement. Ensure that the headphones will deliver auditory speech stimuli to the participant, as well as block any ambient noise. NOTE: Laboratory testing typically takes place in a sound attenuated room. Field laboratory testing does not provide the same degree of noise control, and noise-cancelling headphones can ensure quiet testing conditions for all participants.
Instruct the participant to face the computer monitor and fixate on the cross in the middle of the screen, and to remain still during the experiment. Present all experimental tasks on a computer screen.
- Rhyme judgment task
- Instruct the participant to listen to the word pairs presented aurally through headphones. Ask the participant to indicate whether word pairs rhymed or not (e.g., 'cat'-'hat' or 'cat'-'log') with a button press on the keyboard.
- In this example, use an event related design. Let participants complete 12 non-rhyming and 12 rhyming trials separated by jittered inter-stimulus intervals of between 8 and 17 s. NOTE: Tasks should be created in a manner suitable for the participant. In the study referenced here, researchers were investigating language, cognitive, and reading development in children who were very poor readers. The reading neuroimaging task developed with words that would be appropriate for a child with minimal literacy skills. As well, children were selected for the neuroimaging paradigm based on scores obtained on a reading assessment.
Dim lights and begin recording the participant on the built-in video camera.
Begin fNIRS data recording on the fNIRS command computer, and commence tasks on the stimulus presentation computer.
Monitor participant performance throughout all tasks. Provide breaks between tasks and runs.
Ensure that triggering from the experimental stimuli presentation computer is received by the fNIRS command computer.
At the end of all tasks, stop collecting video and fNIRS data.
6. Post-experimental Task Measurements
Remove each optode from the optode holder cap.
Without disrupting the position of the optode holder cap on the participant's head, direct the participant to sit in a position to obtain a second 3D digitizer measurement.
Repeat the 3D digitizer measurement as in the fNIRS Scalp Placement and Measurement Section 4 to ensure that any disruptions to the scalp probe position during the experiment can be detected by comparing the two position files.
Remove the optode holder cap from the participant's head.
At the end of the experiment, provide participants with a small gift (e.g., books and school supplies) and the acknowledgements of the research team for their participation.
7. Plan for Disseminating Data
Share the research findings with community members and relevant local authorities for their eventual translation into policy addressing the investigated issue. NOTE: Participants may not benefit directly from the experiment.
Make plans for follow-up visits to the participating communities. Prepare reports and tools that local educators can use. For example, any assessments created in local languages should be made available to school officials in the region. Prepare the members of the research team who speak the local language to meet with community leaders to communicate study findings.
Make plans to publish study findings in regional academic journals and in the language of the region, if applicable. For example, study findings should be disseminated in French if research was conducted in Francophone countries.
Make plans to meet with and deliver reports of the study findings to the government branch that granted approval for the research program.
8. Backup Data
Ensure that data are exported and backed up to multiple portable hard drives, as internet access for online data storage is unlikely to be available. Transfer the data to online data storage as sufficient internet connectivity is available.
9. Data Analysis
NOTE: Multiple data analysis packages exist for fNIRS14. Statistical Parametric Mapping for Near-Infrared Spectroscopy (NIRS-SPM)15 , Homer216 (widely used), and the fNIRS toolbox 17,18 (new and gaining popularity) are used for fNIRS data analysis. This protocol reviews data analysis methods using NIRS-SPM, but it is to the discretion of the researcher to select preferred method of analysis.
Analyze data from the fNIRS system using NIRS-SPM, Version 415,19. This toolbox for the neuroimaging suite SPM8 (http://www.fil.ion.ucl.ac.uk/spm) analyzes NIRS data with a general linear model based analysis approach and allows for the creation of activation maps with super-resolution localization.
- Data conversion to HbO and HbR
- Use the modified Beer-Lambert equation (with NIRS-SPM) to convert optical density values into concentration changes in HbO and HbR response.
- Data preprocessing
- Use one of the multiple options which exist for preprocessing fNIRS data. NOTE: Huppert et al.17 propose very rigorous methods for different sources of noise16. These include eigenvector-based reduction of motion artifacts, bandpass filtering techniques, and eigenvector-based reduction of spatial covariance for physiological interference in data (e.g., respiration, blood pressure, heart rate). They also share a thorough commentary on sources of noise in fNIRS research and implications for statistical analysis. The fNIRS researcher must investigate preprocessing applications that are most appropriate for a given study. Below, an analysis approach modeled after Worsely and Friston20 and Jang et al.19 is presented.
- Decompose changes in HbO and HbR concentrations using a Wavelet-Minimum Description Length (MDL) detrending algorithm in order to remove global trends resulting from breathing, blood pressure variation, vasomotion, or participant movement artifacts and to improve the signal-to-noise ratio19.
- Apply a low-pass filter with the shape of the hemodynamic response function to the data and use the Worsely and Friston20 precoloring method to remove temporal correlations.
- Model generation and statistical analysis
- Generate models for HbO and HbR containing experimental regressors convolved with the corresponding hemodynamic response function with time derivatives21. NOTE: The hemodynamic response function can have greater variability in higher cortical regions and across participants. These types of variability can be accommodated in analysis models by expanding the HRF to include temporal derivatives. Use a temporal derivative to model differences in the time to peak hemodynamic response21.
- Set up experimentally relevant t-test or F-test contrasts to test for the effect of one (or several) regressors (given the design matrix) on modulation of the fNIRS time series data.
- Visualizing results
- Perform spatial registration of NIRS channels to the Montreal Neurological Institute (MNI) space using data from a 3D digitizer.
- Load activation maps onto an appropriate brain template. For example, the recent Haskins Pediatric Brain Atlas provides a standardized template for children between 6-12 years of age24.
Representative Results
Probe position data obtained by the 3D digitizer (Figure 2) can be visualized on a standard brain template. Register fNIRS channels to MNI space using NIRS-SPM's stand-alone registration function25. The spatial registration function generates MNI coordinates, anatomical labels, and Brodmann areas maximally represented by each channel.
Figure 2. Data collection. (A) Placement of the fNIRS cap on the participant's head and collection of position data using the 3D digitizer. (B) International 10-20 system used to guide placement of the cap on the participant's head. (C) Spatial localization algorithm plotting x, y, z coordinate data on the MNI brain template. The image generated during stand-alone NIRS registration using 3D digitizer data in NIRS-SPM15,19,25. Please click here to view a larger version of this figure.
Probe position data can also be visualized over cortex surface template or anatomical MRI template using Brainstorm software (Figure 3).
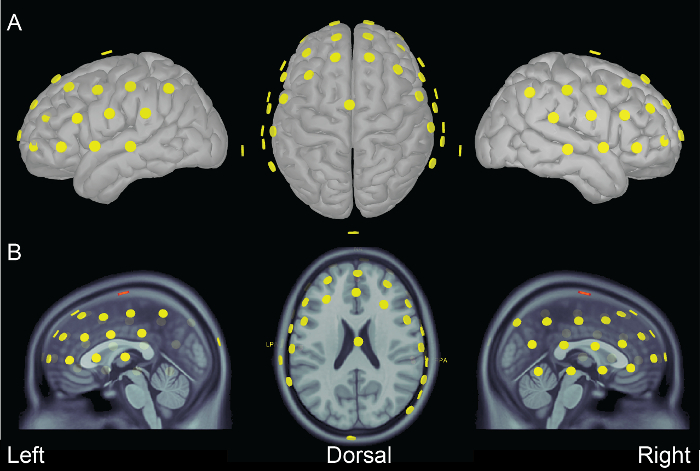
Figure 3. fNIRS probes. fNIRS probes visualized on (A) the surface of the cortex and (B) the MNI anatomical MRI template. Left, dorsal, and right views are presented. Images generated using Brainstorm software13. Please click here to view a larger version of this figure.
Here, representative data from the rhyme judgment task are shown (Figure 4). Participants completed two identically-structured runs of this task. Each run contained 13 trials; rhyming and non-rhyming trials were randomly ordered.
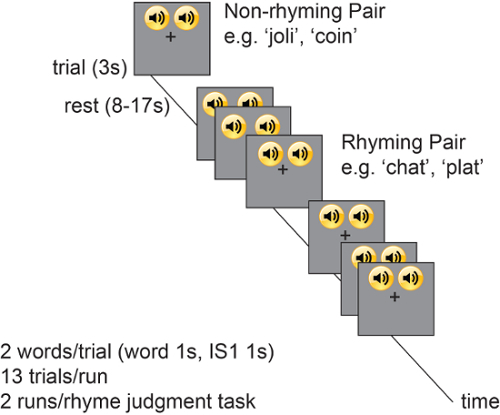
Figure 4. Task design. The rhyme judgment task scheme is shown. Participants continuously viewed a fixation cross while periodically listening to French rhyming or non-rhyming word pairs. The task was completed in two runs, each comprised of 13 trials. 13 rhyming and 13 non-rhyming trials were randomly presented. Each trial lasted 3 s; 1 s per word with a 1 s ISI. Jittered presentation of rest periods between trials, which lasted 8-17 s. Please click here to view a larger version of this figure.
The 3D position data and experimental design data were combined with fNIRS time-series data (Figure 5) for analysis in order to map experiment-related significant neural activation patterns on a standard brain template (Figure 6). Representative single subject data and results are shown in Figure 5 and Figure 6.

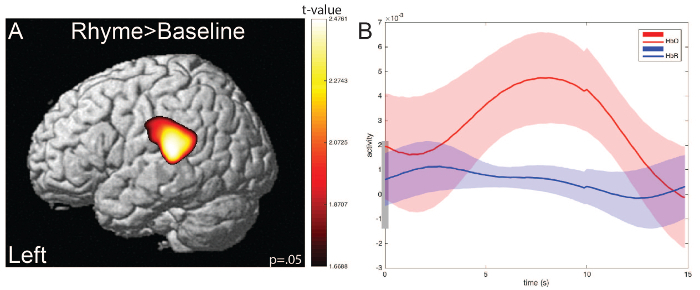
Figure 5. Representative time-series data from one fNIRS channel. (A) Raw time-series data corresponding to entire task length (rhyme judgment task; first run), not normalized. (B) Filtered time-series data using wavelet detrending. Rhyming and non-rhyming trials are indicated by solid and dashed box-car, respectively. Please click here to view a larger version of this figure.
This subject showed greater activation in the left hemisphere Superior Temporal Gyrus (STG) during rhyming trials compared with rest (baseline fixation cross). Averaged HbO and HbR responses for rhyming trials show a canonical hemodynamic response: increasing HbO concentrations and corresponding decreasing HbR concentrations following stimulus presentation.
Figure 6. Representative single-subject results. (A) Greater activation is observed for rhyming trials versus baseline (fixation cross) in the left hemisphere, overlaying the posterior portion of the superior temporal gyrus (STG). Image generated during NIRS results steps using NIRS-SPM15,19,25. (B) Averaged event-related waveforms for HbO (red) and HbR (blue) during rhyming trials (rhyming word pair stimuli). Image generated using Xu Cui's plot average function26. Please click here to view a larger version of this figure.
Single-subject results differed between participants (see Figure 7). This individual variation may reflect underlying functional differences or developmental differences in the organization of specific brain networks. For example, subject 1 showed greater activation in the left inferior frontal gyrus region during non-rhyming versus rhyming trials; whereas subject 2 showed greater activation in the left STG region during the same experimental contrast (non-rhyming versus rhyming trials).

Figure 7. Representative single-subject results from two different participants for identical contrast. Greater activation for non-rhyming versus rhyming trials in the left hemisphere is shown in both A and B. (A) Subject 1 showed greater activation in the left inferior frontal gyrus. (B) Subject 2 showed greater activation the left superior temporal gyrus. Please click here to view a larger version of this figure.
Discussion
This paper presented a field neuroimaging protocol suitable for low-resource contexts in remote locations. The key advance of this field neuroimaging protocol is the first-time ability to study brain function and its development in understudied (or never-before studied) contexts. Critical steps in this protocol include traveling with and setting up a mobile laboratory suitable for quality data collection in tropical climates without electricity or available facilities. This protocol provides a general guide to forming strong partnerships with local scientific, educational, and government institutions, and we highlight the reciprocal knowledge transfer that occurs when successful long-term partnerships are formed between local and visiting scientists. Guidelines for the development of culturally-appropriate informed consent procedures and testing protocols are discussed with the aim of incorporating multiple cultural perspectives in research methods. Finally, this protocol provides detailed steps for field data collection and data analysis.
Local Science Engagement and Opportunities for Capacity Building:
One of the main challenges that local, particularly junior, researchers in Côte d'Ivoire are faced with when they complete their studies is the lack of opportunity for hands-on research experience with experienced researcher mentors and/or international collaborators. For this purpose, researchers should make all efforts to establish robust collaboration with local researchers from relevant disciplinary backgrounds, and include trainees at all levels (undergraduate, graduate, postdoctoral). Trainees can leverage the insight gained from this experience to work independently and further research. This experience can also be a stepping stone to build their capacities as researchers and develop their competitiveness at the international level in writing research proposals and papers and applying for grants. A research team excluding local researchers may have a reduced chance of success as local researchers will best know the local social and cultural values and systems, the local languages spoken in addition to the geographic knowledge of the area. Their contribution is therefore extremely important in understanding the local realities and designing culturally-appropriate protocols for the successful research projects.
Culturally-appropriate Research Methods:
The development of informed consent protocols to conduct research in rural settings specifically in Cote d'Ivoire is critical and failure to adopt the appropriate approach can inhibit the successful achievement of the research even though well-intentioned and scientifically robust27,28,29,30,31. Generally, in rural settings in Côte d'Ivoire, asking a villager to read a consent form and sign it can break any trust building between the researcher and the participant. In fact, the perceived formality of this procedure may create a psychological distance and a feeling of insecurity in the participant's mind. This may result in a clear or unexpressed unwillingness to collaborate. This attitude can be explained by many factors including a long history of oral tradition whereby communication is more oral than written and high rates of illiteracy that may be found in target communities. Communities in rural settings trust their chief and rely on his decision-making power. Therefore, the protocol presented incorporates the consent of the chief of the village at the community level. This is arguably more culturally important than individual consent. Additionally, participants and community members may have had limited or no exposure to neuroimaging technology or computers. Therefore, researchers need to take into consideration that the informed consent procedure, and instructions, may be misunderstood. The function of the fNIRS system should be communicated in lay terms and appropriate language easily understandable by child participants and community members who may have had very limited exposure to technology. These considerations can strongly influence the comfort and confidence of all community members involved in a field neuroimaging research project.
The protocol presented here also highlighted the importance of sharing research findings with community members and government partners. Partnerships built on continued dialogue aid in the eventual translation of research findings into policy. It is imperative to arrange post data collection field visits to disseminate research findings and deliver reports and, possibly, share any tools that resulted from the study (e.g., assessments in local languages). Participating communities in rural settings may never otherwise receive information about study completion and findings given lack of internet service and/or computers. Likewise, researchers in the country may have limited access to academic journal subscriptions and poor internet connectivity at regional universities. Published results should be shared in a regional forum, and made available in an accessible language.
Limitations and Potential Challenges:
This field neuroimaging protocol should be modified to suit the planned data collection sites. The protocol presented here has been developed for research with primary school aged children in rural Cote d'Ivoire. However, the methods outlined here may not be suitable, specifically with respect to informed consent procedures, in other countries or even other regions of Cote d'Ivoire. Researchers who aim to conduct field neuroimaging must first carefully research local customs and incorporate local perspectives into study design. Therefore, a research team working on study designs must include members from the local cultural groups.
Field neuroimaging has limitation in comparison with laboratory methods. Importantly, control of the testing environment is considerably reduced in the field. Field neuroimaging researchers should plan extended data collection trips. Tropical rains, risk of contracting tropical diseases, civil strikes, and political unrest may significantly impact research plans. Researchers need to ensure security levels in the region are sufficient and monitor for updates to any situations that may affect security levels. Continuous communication between team members, specifically with respect to security levels, may mitigate potential risks.
Future Applications and Relevance to Existing Methods:
The use of this field neuroimaging method can be applied to evaluating the impact of early risk on infant and child development in global health settings. Researchers have begun using this approach to study child development in rural Gambia and an urban slum in Bangladesh32. In an urban slum in Dhaka, researchers are using fNIRS to examine how factors such as nutrition and sanitation contribute to growth and brain development33. In rural Gambia, researchers have used fNIRS to study cognitive function of infants, and have demonstrated that fNIRS is a viable imaging tool in resource-poor settings34,35. Such work promises to reveal new insights into the development of children in the developing world, who are disproportionately affected by malnutrition and poor sanitation. Portable neuroimaging technologies continue to become more accessible and applicable for research in low-resource environments, thus highlighting the need for rigorous methods for field neuroimaging.
Conclusion:
Portable neuroimaging systems with the capability to function on battery-supplied power have recently become available. As these techniques are relatively new, advances to battery systems will provide ongoing improvements. Diverse communities of international scientists developing research programs using these tools will undoubtedly optimize mobile laboratory settings to provide increased control of the testing environment. Meaningful engagement between international and local scientists and local communities can ensure that members of study populations have active roles in the development of research programs and represent the interests of their communities. Only such collaborative and integrated research teams would be well-positioned to apply field neuroimaging methods to study all human brain development, and reveal both theoretically- and practically-relevant information aimed at understanding the most urgent child development issues.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This research was made possible through the Jacobs Foundation Early Career Fellowship to K. Jasinska (Fellowship Number: 2015 118455). The authors also wish to acknowledge Axel Blahoua, Fabrice Tanoh, Ariane Amon, Brice Kanga, and Yvette Foto for their assistance in data collection and field support. Special thanks to the families and children of Moapé, Ananguié, Affery, and Becouefin for their participation in this research program and the villages' warm hospitality.
References
- Dawson G, Ashman SB, Carver LJ. The role of early experience in shaping behavioral and brain development and its implications for social policy. Dev Psychopathol. 2000;12(4):695–712. doi: 10.1017/s0954579400004089. [DOI] [PubMed] [Google Scholar]
- Blair C, Raver CC. Poverty, Stress, and Brain Development: New Directions for Prevention and Intervention. Acad Pediatr. 2016;16(3 Suppl):S30–S36. doi: 10.1016/j.acap.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasińska KK, Petitto LA. How age of bilingual exposure can change the neural systems for language in the developing brain: A functional near infrared spectroscopy investigation of syntactic processing in monolingual and bilingual children. Dev Cogn Neurosci. 2013;6c:87–101. doi: 10.1016/j.dcn.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics UIf. Côte d'Ivoire. 2017.
- University T. 2013/14 Survey Research on Child Labor in West African Cocoa Growing Areas. School of Public Health and Tropical Medicine; 2015. [Google Scholar]
- Cui X, Bray S, Bryant DM, Glover GH, Reiss AL. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage. 2011;54(4):2808–2821. doi: 10.1016/j.neuroimage.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaresima V, Bisconti S, Ferrari M. A brief review on the use of functional near-infrared spectroscopy (fNIRS) for language imaging studies in human newborns and adults. Brain Lang. 2012;121(2):79–89. doi: 10.1016/j.bandl.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Jasińska KK, Berens MS, Kovelman I, Petitto LA. Bilingualism yields language-specific plasticity in left hemisphere's circuitry for learning to read in young children. Neuropsychologia. 2016;98:34–45. doi: 10.1016/j.neuropsychologia.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Jasińska KK, Petitto LA. Development of neural systems for reading in the monolingual and bilingual brain: new insights from functional near infrared spectroscopy neuroimaging. Dev Neuropsychol. 2014;39(6):421–439. doi: 10.1080/87565641.2014.939180. [DOI] [PubMed] [Google Scholar]
- Petitto L, et al. The "Perceptual Wedge Hypothesis" as the basis for bilingual babies' phonetic processing advantage: new insights from fNIRS brain imaging. Brain Lang. 2012;121(2):130–143. doi: 10.1016/j.bandl.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper HH. Report of the Committee on Methods of Clinical Examination in Electroencephalography. Electroencephalogr Clin Neurophysiol. 1958;10(2):370–371. [Google Scholar]
- Shalinsky MH, Kovelman I, Berens MS, Petitto LA. Exploring Cognitive Functions in Babies, Children & Adults with Near Infrared Spectroscopy. Journal of visualized experiments. 2009. [DOI] [PMC free article] [PubMed]
- Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci. 2011;2011:879716. doi: 10.1155/2011/879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak S, Ye JC. Statistical analysis of fNIRS data: A comprehensive review. Neuroimage. 2014;85, Part 1(0):72–91. doi: 10.1016/j.neuroimage.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Ye JC, Tak S, Jang KE, Jung J, Jang J. NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy. Neuroimage. 2009;44(2):428–447. doi: 10.1016/j.neuroimage.2008.08.036. [DOI] [PubMed] [Google Scholar]
- Huppert TJTJ, Diamond SGSG, Franceschini MAMA, Boas DADA. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl Opt. 2009;48(10):D280–D298. doi: 10.1364/ao.48.00d280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ. Commentary on the statistical properties of noise and its implication on general linear models in functional near-infrared spectroscopy. Neurophotonics. 2016;3(1):010401. doi: 10.1117/1.NPh.3.1.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso AL, et al. Neuroimaging of an attention demanding dual-task during dynamic postural control. Gait Posture. 2017;57:193–198. doi: 10.1016/j.gaitpost.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang KEKE, et al. Wavelet minimum description length detrending for near-infrared spectroscopy. Journal of Biomedical Optics. 2009;14(3):034004–034004. doi: 10.1117/1.3127204. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2(3):173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Josephs O, Rees G, Turner R. Nonlinear event-related responses in fMRI. Magn Reson Med. 1998;39(1):41–52. doi: 10.1002/mrm.1910390109. [DOI] [PubMed] [Google Scholar]
- Sun JY. Tail Probabilities of the Maxima of Gaussain Random-Fields. The Annals of Probability. 1993;21(1):34–71. [Google Scholar]
- Sun JY, Loader CR. Simultaneous Confidence Bands for Linear-Regression and Smoothing. The Annals of Statistics. 1994;22(3):1328–1345. [Google Scholar]
- Molfese PJ, Glen D, Mesite L, Pugh K, Cox R. Organization of Human Brain Mapping. Honolulu Hawaii: 2015. [Google Scholar]
- Singh AK, Okamoto M, Dan H, Jurcak V, Dan I. Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. Neuroimage. 2005;27(4):842–851. doi: 10.1016/j.neuroimage.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Cui X. Handy programs to visualize NIRS data (6): plotAverage. 2013. Available from: http://www.alivelearn.net/?p=1533.
- Krosin MT, Klitzman R, Levin B, Cheng J, Ranney ML. Problems in comprehension of informed consent in rural and peri-urban Mali, West Africa. Clinical Trials. 2006;3 doi: 10.1191/1740774506cn150oa. [DOI] [PubMed] [Google Scholar]
- Leach A. An evaluation of the informed consent procedure used during a trial of a Haemophilus influenzae type B conjugate vaccine undertaken in The Gambia, West Africa. Soc Sci Med. 1999;48 doi: 10.1016/s0277-9536(98)00317-7. [DOI] [PubMed] [Google Scholar]
- Molyneux CS, Peshu N, Marsh K. Understanding of informed consent in a low-income setting: three case studies from the Kenyan Coast. Soc Sci Med. 2004;59 doi: 10.1016/j.socscimed.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Oduro AR. Understanding and retention of the informed consent process among parents in rural northern Ghana. BMC Med Ethics. 2008;9(1):1–9. doi: 10.1186/1472-6939-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindana PO, Kass N, Akweongo P. The Informed Consent Process in a Rural African Setting:: A Case Study of the Kassena-Nankana District of Northern Ghana. IRB. 2006;28(3):1–6. [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, et al. fNIRS in Africa & Asia: an Objective Measure of Cognitive Development for Global Health Settings. The FASEB Journal. 2016;30(1 Supplement) [Google Scholar]
- Storrs C. Nature News. Nature Publishing Group; 2018. [Google Scholar]
- Lloyd-Fox S, et al. Functional near infrared spectroscopy (fNIRS) to assess cognitive function in infants in rural Africa. Sci Rep. 2014;4:4740. doi: 10.1038/srep04740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papademetriou MD, et al. Optical imaging of brain activation in Gambian infants. Adv Exp Med Biol. 2014;812:263–269. doi: 10.1007/978-1-4939-0620-8_35. [DOI] [PubMed] [Google Scholar]


