Abstract
Background
Continuous-flow left ventricular assist devices (CF-LVADs) have become a standard treatment choice in advanced heart failure patients. We hypothesized that practice patterns with regards to CF-LVAD utilization vary significantly among transplant centers and impact waitlist outcomes.
Methods and Results
The UNOS registry was queried to identify adult patients who were waitlisted for heart transplantation between 2008 and 2015. Each patient was assigned a propensity score based upon likelihood of receiving a durable CF-LVAD before or while waitlisted. The primary outcomes of interest were death or delisting for worsening status and heart transplantation at 1 year. 22,863 patients from 92 centers were identified. Among these, 9013 (39.4%) were mechanically supported. CF-LVAD utilization varied significantly between and within UNOS regions. Freedom from waitlist death or delisting was significantly lower in propensity-score matched patients who were mechanically supported versus medically managed (83.5% vs. 79.2%, p<0.001). However, cumulative incidence of heart transplantation was also lower in mechanically supported patients (53.3% v. 63.6%, p<0.001). Congruous mechanical and medical bridging strategies based on clinical risk profile were associated with lower risk of death or delisting (HR 0.88, p=0.027) and higher likelihood of heart transplantation (HR 1.14, p<0.001).
Conclusion
CF-LVAD utilization may lower waitlist mortality at the expense of lower likelihood of heart transplantation. Decision to utilize CF-LVAD and timing of transition should be individualized based on patient-, center-, and region-level risk factors in order to achieve optimal outcomes.
Introduction
Heart transplantation (HT) remains the gold standard of treatment in patients with end-stage heart failure. Although the number of transplants performed annually is on the rise, overall donor supply falls far short of demand1. As a result, an increasing number of patients require mechanical circulatory support as a bridge-to-transplantation (BTT). In 2000, the International Society for Heart Transplantation reported that 19.1% of transplant recipients were mechanically bridged; a figure which increased to 45.0 % in 20122. This was accompanied by major advances in the device design evolving from previous generation pulsatile-flow left ventricular assist devices to new generation continuous-flow left ventricular assist devices (CF-LVADs). Prospective clinical trials have demonstrated excellent durability, complication profile, and survival in patients supported with CF-LVADs for the BTT indication3,4.
Mechanical bridging with CF-LVAD remains a reasonable strategy for majority of waitlisted patients, particularly for those with evidence of clinical decompensation, hemodynamic instability, or worsening end-organ function on medical therapy. However, certain patient populations are considered poor candidates for CF-LVAD therapy due to their underlying physiology (restrictive disease), bleeding diathesis, or hypercoagulable states. CF-LVAD implantation may also positively or negatively impact transplant priority and/or listing status depending on clinical course of the patient. Moreover, region- and center-level factors such as waitlist time and physician/surgeon preference may impact the decision to utilize CF-LVAD. The purpose of this study was 1) to determine the patient-level risk factors associated with CF-LVAD utilization in HT candidates, 2) to investigate region- and center-level differences in CF-LVAD utilization for BTT indication, 3) to assess the impact of mechanical vs. medical bridging strategies on HT waitlist outcomes.
Methods
Study Design, Variables, and Definitions
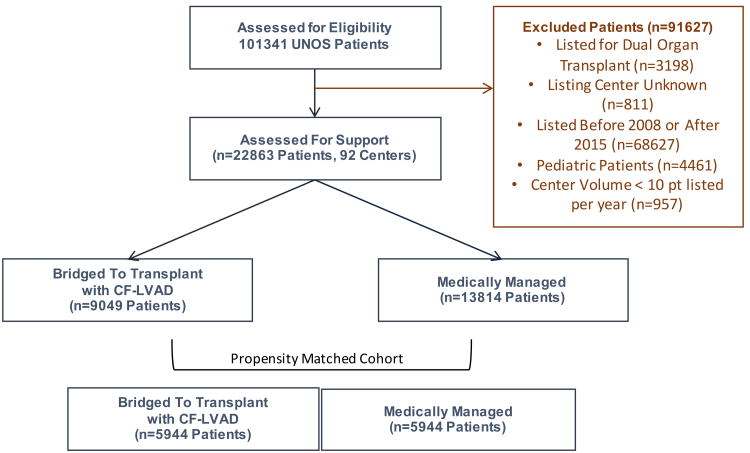
The UNOS database was queried to identify adult patients (≥18 years old) who were listed for HT between 2008 and 2015. Patients without a designated listing center, as well as those who were listed for multi-organ transplant, were excluded from the current analysis (Figure 1). Center waitlist volume was calculated by identifying the total number of patients listed for HT at a given center during the study period. Patients from centers who listed less than 10 patients per year during the study period (<80 patients overall) were excluded from the analysis. Patient-level baseline characteristics analyzed included demographics, etiology of heart failure, comorbid conditions, functional status, and UNOS status at listing. Patient who were less likely to receive a CF-LVAD based on their physiology including those with hypertrophic or restrictive cardiomyopathy, congenital abnormalities, or cardiac allograft failure were categorized under a non-dilated myopathy group. Bridging strategy was considered “mechanical” for patients who received a durable CF-LVAD before listing or while listed, and “medical” for those who did not receive a CF-LVAD. Primary outcome of the study was freedom from death or delisting for worsening status. Secondary outcome of the study was cumulative incidence of heart transplantation.
Figure 1. Study Population.
We first compared waitlisted patients who received mechanical vs. medical bridging strategy to identify patient-level determinants of CF-LVAD utilization. Based on these variables, a propensity score was generated for each patient predicting the likelihood of receiving a CF-LVAD before or while waitlisted– also termed “VAD Likelihood Score” (Supplemental Figure 1A). Variables included in the propensity score were gender, ethnicity, history of diabetes, smoking history, functional status, AICD, blood type O, high BSA, non-dilated myopathy, UNOS region, UNOS status at listing, and listing center. For example, Patient A who has a large BSA, blood type O, dilated myopathy, and listed Region 6 would have a high VAD likelihood score there by an increased chance of receiving a CF-LVAD, as opposed to Patient B who has a normal BSA, blood type AB, hypertrophic cardiomyopathy, and listed in Region 5 consistent with a low VAD likelihood score. Propensity score matching was then utilized to identify patients with similar VAD likelihood scores. Impact of mechanical vs. medical bridging strategies on transplant waitlist outcomes was assessed in the propensity-score matched patient cohort.
Next, we determined whether bridging strategy (mechanical vs. medical) was consistent with VAD likelihood score of each individual on the transplant waitlist (Supplemental Figure 1B). Patients with a high VAD likelihood score (> 1 SD above the mean) were considered to have congruous bridging strategy if mechanical support was chosen and considered to have incongruous bridging strategy if medical management was utilized. Conversely, patients with a low VAD likelihood score (> 1 SD below the mean) were considered to have congruous bridging strategy if medical management was chosen and considered to have incongruous bridging strategy if mechanical support was utilized. Patients who have a VAD likelihood score within 1 SD around the mean were considered to have a congruous bridging strategy irrespective of the strategy used. Waitlist outcomes were then assessed in four groups of patients: congruous mechanical bridging (anticipated CF-LVAD and received), congruous medical bridging (did not anticipate CF-LVAD and did not receive), incongruous mechanical bridging (did not anticipate CF-LVAD but received one), and incongruous medical bridging (anticipated CF-LVAD but did not receive one).
To determine region-level differences in CF-LVAD utilization for transplant eligible patients, we calculated CF-LVAD utilization percentage (VAD %) by dividing the number of patients implanted with CF-LVAD (before or during the waitlist period) by the total number of waitlisted patients for each center using the propensity matched cohort. Variability in mechanical vs. medical bridging strategies was then assessed at the center-level among and within UNOS regions. The current study was approved by the Columbia University Institutional Review Board. The data utilized in the study is available to other researchers for purposes of reproducing the results or replicating the procedure via data request from the UNOS/OPTN network.
Statistical Analysis
Descriptive analyses were conducted for all baseline variables and are presented as means and standard deviations for continuous variables and numbers and percentages for categorical variables. Differences among medical vs. mechanical bridging strategy groups were quantified using independent Student's t-test and chi-square where appropriate. Propensity scores for receiving durable CF-LVAD before listing or while listed (also termed as VAD Likelihood Score) were generated using multivariable logistic regression analysis. Propensity matching was performed using one to one nearest neighbor matching with specified caliper distance of 0.001. To ensure balance in propensity matched cohort, absolute standard differences (ASD) were assessed prior to and after matching, with <10% ASD considered acceptable. Kaplan Meier survival estimates were utilized to assess the impact of bridging strategies on freedom from death or delisting and cumulative incidence of transplant, respectively, in the propensity matched cohort with log-rank test used for comparisons between mechanical versus medical bridging groups. As secondary analysis, cumulative incidence curves were generated to visualize competing event rates of death or delisting versus heart transplantation, comparing both medical and mechanical and congruous and incongruous bridging strategies. Multivariable Cox-regression model was used to determine whether congruous utilization predicted waitlist outcomes. All p-values were reported as two-sided tests with p<0.05 considered statistically significant. STATA version 13.1 (Stata corp., College Station, TX) was used to perform statistical analysis.
Results
Patient Population and Nationwide Trends in Durable Device Utilization
A total of 22,863 adult patients from 92 centers were identified as having been listed for single-organ HT during the study period. The number of waitlisted patients who received CF-LVAD has steadily increased from 2008 through 2014 (Supplemental Figure 2A). In parallel with this increase, median time spent on the waitlist has also increased (Supplemental Figure 2B). The baseline characteristics of study population were summarized in Table 1. Among these, 9,049 (39.4%) received a mechanical bridging strategy (Figure 1). Patients who received a mechanical bridging strategy were more likely to be male, and more likely to have a history of diabetes and tobacco use. Patients with larger BSA and blood type O were more likely to receive mechanical support (Table 1). Only 10% of patients overall had congenital heart disease, were listed for retransplantation, or had restrictive or hypertrophic cardiomyopathy. These patients only represented 3% of mechanically supported patients versus 15% of medically managed patients. Most patients were UNOS Status 1B at the time of listing, though both UNOS Status 1A and 1B were more common among mechanically supported patients.
Table 1. Patient Characteristics Based on Bridging Strategy.
| Overall N= 22863 | Mechanical Bridging N= 9049 | Medical Bridging N= 13814 | p-value* | |
|---|---|---|---|---|
| Clinical Characteristics | ||||
| Age > 60 | 13333 (58.3%) | 5245 (58.2%) | 8088 (58.4%) | 0.76 |
| Male Gender | 16967 (74.2%) | 7112 (78.9%) | 9855 (71.2%) | <0.001 |
| Ethnicity | <0.001 | |||
| White | 15362 (67.2%) | 5916 (65.6%) | 9446 (68.2%) | |
| Black | 4884 (21.4%) | 2192 (24.3%) | 2696 (19.4%) | |
| Hispanic | 1701 (7.4%) | 592 (6.6%) | 1109 (8.0%) | |
| Other | 916 (4.0%) | 313 (3.5%) | 603 (4.4%) | |
| Diabetes | 6547 (28.7%) | 2836 (31.5%) | 3711 (26.9%) | <0.001 |
| CVA | 1265 (5.6%) | 531 (6.0%) | 734 (5.4%) | 0.06 |
| AICD | 17687 (78.0%) | 7039 (78.7%) | 10648 (77.5%) | 0.033 |
| Smoking | 11041 (48.3%) | 4763 (52.9%) | 6278 (45.4%) | <0.001 |
| Blood Type O | 10059 (44.0%) | 4423 (49.1%) | 5636 (40.7%) | <0.001 |
| BSA > 2.25 | 4119 (8.0%) | 2050 (22.7%) | 2069 (14.9%) | <0.001 |
| Heart Failure Diagnosis | <0.001 | |||
| Dilated Myopathy | 20471 (89.5%) | 8732 (96.9%) | 11739 (84.8%) | |
| Non-Dilated Myopathy | 2392 (10.5%) | 281 (3.1%) | 2111 (15.2%) | |
| UNOS status @ Listing | <0.001 | |||
| Status 1A | 5168 (23.2%) | 2373 (27.5%) | 2795 (20.5%) | |
| Status 1B | 9678 (43.5%) | 4575 (53.0%) | 5103 (37.5%) | |
| Status 2 | 7411 (33.3%) | 1688 (19.6%) | 5723 (42.0%) | |
| Functional Status | 10490 (45.9%) | 4065 (45.1%) | 6425 (46.4%) | <0.001 |
| Excellent | 6919 (31.0%) | 2737(31.1%) | 4182 (31.0%) | |
| Moderate | 12099 (54.2%) | 4642 (52.7%) | 7457 (55.2%) | |
| Poor | 3305 (14.8%) | 1436 (16.3%) | 1869 (13.8%) | |
| Creatinine > 2 | 1421 (6.2%) | 555 (6.2%) | 866 (6.3%) | 0.73 |
| PRA* | 542 (29.3%) | 272 (29.1%) | 270 (29.5%) | 0.82 |
| UNOS Region | <0.001 | |||
| 1 | 2926 (12.8%) | 1122 (12.5%) | 1804 (13.0%) | |
| 2 | 1102 (4.8%) | 437 (4.9%) | 665 (4.8%) | |
| 3 | 2565 (11.2%) | 771 (4.9%) | 1794 (13.0%) | |
| 4 | 2558 (11.2%) | 849 (9.4%) | 1709 (12.3%) | |
| 5 | 3237 (14.2%) | 968 (10.7%) | 2269 (16.4%) | |
| 6 | 654 (2.9%) | 378 (4.2%) | 276 (2.0%) | |
| 7 | 2086 (9.1%) | 1011 (11.2%) | 1075 (7.8%) | |
| 8 | 1383 (6.1%) | 516 (5.7%) | 867 (6.3%) | |
| 9 | 1611 (7.1%) | 719 (5.7%) | 892 (6.4%) | |
| 10 | 1917 (8.4%) | 923 (10.2%) | 994 (7.2%) | |
| 11 | 2824 (12.4%) | 1319 (14.6%) | 1505 (10.9%) | |
Categorical variables represented as n (%)
CVA, cerebrovascular accident; AICD, automatic internal cardiac defibrillator; Dilated Myopathy, includes ischemic and nonischemic dilated cardiomyopathies as well as myocarditis; Non-Dilated Myopathy, includes restrictive, hypertrophic, and congenital cardiomyopathy. Also includes retransplant; PRA, panel reactive antibodies present; UNOS, United Network for Organ Sharing; WL, waitlist.
Predictors of Bridge to Transplant with CF-LVAD and Propensity Score
In an effort to identify factors which contributed to CF-LVAD utilization at the patient level, we performed univariable and multivariable logistic regression analyses utilizing baseline patient characteristics. Male gender, ethnicity, diabetes, smoking history, functional status, blood type O, high BSA, etiology of heart failure, UNOS status at listing, and UNOS regions were strongly associated with CF-LVAD utilization as BTT (Table 2). When the variables were utilized to generate a propensity score, a propensity matched cohort of 11,888 patients (5944 mechanically supported, 5944 medically bridged) was identified. Propensity scores ranged from 0.024 to 0.869 and showed a normal distribution within the study population (Supplemental Figure 1A). Baseline characteristics between patients who received mechanical versus medical bridging strategies were well balanced after propensity score matching (Supplemental Table 1).
Table 2. Predictors of CF-LVAD Utilization on Waitlist.
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
|
| ||||
| Risk Factors | Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value |
| Patient characteristics | ||||
| Male Gender | 1.52 (1.42 – 1.61) | <0.001 | 1.40 (1.30 – 1.51) | <0.001 |
| Ethnicity | <0.001 | <0.001 | ||
| White | (Reference) | (Reference) | ||
| African American | 1.30 (1.22 – 1.39) | 1.15 (1.06 – 1.24) | ||
| Hispanic | 0.85 (0.77 – 0.95) | 0.90 (0.79 – 1.03) | ||
| Other | 0.63 (0.60 – 0.65) | 0.85 (0.72 – 1.00) | ||
| Diabetes | 1.25 (1.18 – 1.33) | <0.001 | 1.07 (0.99 – 1.14) | 0.071 |
| Smoking | 1.35 (1.28 – 1.43) | <0.001 | 1.19 (1.12 – 1.27) | <0.001 |
| Function Status | <0.001 | <0.001 | ||
| Excellent | (Reference) | (Reference) | ||
| Moderate | 0.95 (0.89 – 1.01) | 1.31 (1.21 – 1.43) | ||
| Poor | 1.17 (1.08 – 1.28) | 1.68 (1.51 – 1.88) | ||
| AICD | 1.07 (1.01 – 1.14) | 0.033 | 0.96 (0.89 – 1.05) | 0.390 |
| Blood Type O | 1.40 (1.33 – 1.48) | <0.001 | 1.62 (1.52 – 1.73) | <0.001 |
| BSA > 2.25 | 1.68 (1.57 – 1.79) | <0.001 | 1.52 (1.39 – 1.65) | <0.001 |
| Diagnosis | <0.001 | <0.001 | ||
| Dilated Myopathy | (Reference) | (Reference) | ||
| Non-Dilated Myopathy | 0.18 (0.16 – 0.20) | <0.001 | 0.19 (0.17 – 0.22) | |
| UNOS Status @ Listing | <0.001 | <0.001 | ||
| Status 1A | (Reference) | (Reference) | ||
| Status 1B | 1.06 (0.99 – 1.13) | 0.71 (0.66 – 0.78) | ||
| Status 2 | 0.85 (0.80 – 0.90) | 0.21 (0.19 – 0.23) | ||
| UNOS Region | <0.001 | <0.001 | ||
| 1 | 1.06 (0.92 – 1.22) | 1.00 (0.63 – 1.58) | ||
| 2 | (Reference) | (Reference) | ||
| 3 | 0.69 (0.62 – 0.77) | 0.38 (0.24 – 0.60) | ||
| 4 | 0.80 (0.71 – 0.89) | 0.78 (0.51 – 1.18) | ||
| 5 | 0.69 (0.62 – 0.76) | 2.41 (1.50 – 3.86) | ||
| 6 | 2.20 (1.86 – 2.62) | 3.57 (2.17 – 5.89) | ||
| 7 | 1.51 (1.35 – 1.69) | 3.79 (2.42 – 5.94) | ||
| 8 | 0.96 (0.84 – 1.09) | 1.73 (1.11 – 2.68) | ||
| 9 | 1.30 (1.15 – 1.47) | 1.22 (0.86 – 1.72) | ||
| 10 | 1.49 (1.33 – 1.68) | 3.18 (1.84 – 5.50) | ||
| 11 | 1.41 (1.27 – 1.57) | 3.75 (2.42 – 5.80) | ||
Nationwide Variability in Center Level Utilization of CF-LVAD as BTT
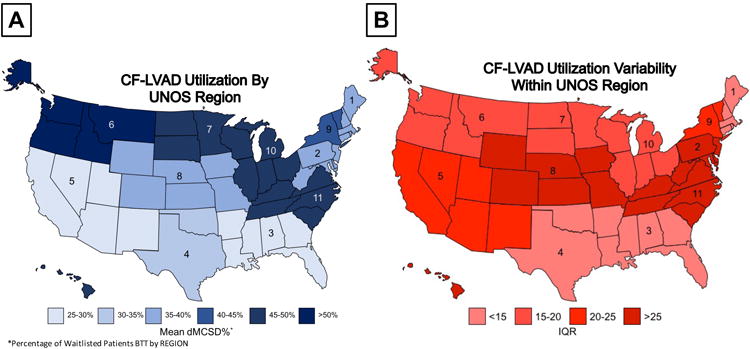
After analyzing patient-level determinants of CF-LVAD utilization, we turned our attention to region- and center- specific utilization patterns. In the overall cohort, mean center CF-LVAD utilization was 38.2% and highly variable among centers with a range of 8.1% to 77.4%. The variability among UNOS regions was displayed using a color-coded map in Figure 2A, where the mean VAD% of each region is represented. Significant variability not only existed among regions, but also within regions, where Figure 2B demonstrates the variation in size of interquartile range from the mean center-specific VAD% within each region. We hypothesized that, in part, variability in center-to-center CF-LVAD utilization may be explained by wait list times. To this end, we correlated overall wait list days and days as UNOS 1A, 1B, and Status 2 with VAD% in each of the 93 centers. In analyzing overall wait list days, we found a weakly positive correlation (R-Squared = 0.16) with VAD% (Supplemental Figure 3A). While the correlation was similar when UNOS status 1A days were analyzed (R-Squared = 0.13, Supplemental Figure 3B), the correlation was stronger between UNOS 1B days and VAD% (R-Squared = 0.25, Supplemental Figure 3C). There was no correlation between UNOS status 2 days and VAD%.
Figure 2. Nationwide Variability in Device Utilization in Heart Transplantation Candidates (A) Among UNOS Regions (B) Within UNOS Regions.
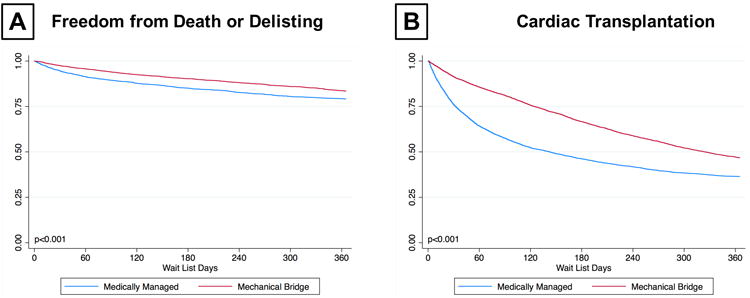
Waitlist Outcomes Based on Patient Level Device Utilization
In the propensity matched cohort, a total of 1,944 (16.4%) patients died or were delisted for worsening status during the first year on the wait list. Kaplan-Meier analysis demonstrated that patients who received mechanical support had significantly greater freedom from death or delisting as compared to patients who were managed medically (83.5% vs. 79.2%, p<0.001) (Figure 3A). On the contrary, mechanically supported patients had significantly lower rates of HT compared to those who were medically managed (53.3% v. 63.6%, p<0.001) (Figure 3B). Cumulative incidence curves treating death or delisting and transplantation as competing events for patients who had mechanical or medical bridging are represented in Supplemental Figure 4A.
Figure 3. Transplant Waitlist Outcomes of Patients with Medical versus Mechanical Bridging Strategy (A) Freedom from Waitlist Death or Delisting for Worsening Status (B) Cumulative Incidence of Heart Transplantation.
Impact of Congruous Bridging Strategy on Waitlist Outcome
Next, we assessed the impact of congruous bridging strategy on waitlist outcomes. Each patient was assigned one of four categories based on their VAD likelihood score and bridging strategy utilized (e.g. congruous mechanical bridging, congruous medical bridging, incongruous mechanical bridging, and incongruous medical bridging). We defined incongruous utilization based upon bridging strategy and propensity score greater than or less than one standard deviation from the mean. In the propensity matched cohort, we identified 2,065 patients (17.4%) who had an incongruous bridging strategy based upon their propensity score. Among these 1063 had incongruous medical bridging and 1,002 had incongruous mechanical bridging. When freedom from death or delisting at 1 year was assessed, patients who had congruous mechanical bridging had the best 1-year survival, while those who had incongruous medical bridging had the worst 1-year survival (Figure 4A). When cumulative incidence of transplant at 1 year was assessed, patients who had incongruous mechanical bridging had the lowest likelihood of transplantation. Those who had incongruous medical bridging also had a lower rate of transplant than those who had congruous medical bridging (Figure 4B). Congruous bridging was an independent predictor of death or delisting (HR 0.84 (0.75 – 0.95), p=0.004) and heart transplantation (HR 1.08 (1.01 – 1.15), p=0.026) (Supplemental Table 2). Taking into account both outcomes, congruous utilization improved rates of death or delisting and increased likelihood of transplant in both mechanical and medical bridging groups (Supplemental Figure 4B).
Figure 4.
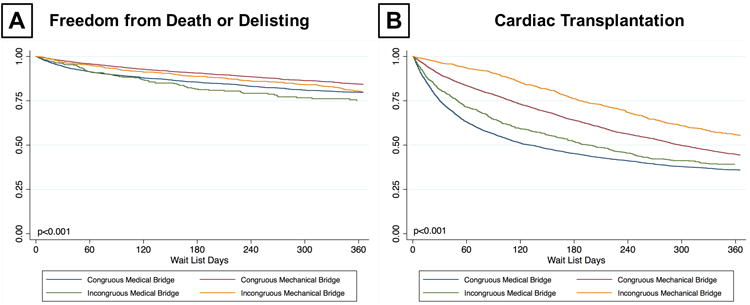
Transplant Waitlist Outcomes of Patients Based on Congruous versus Incongruous Utilization of Bridging Strategies (A) Freedom from Waitlist Death or Delisting for Worsening Status Based (B) Cumulative Incidence of Heart Transplantation
Discussion
The current study examines the impact of CF-LVAD utilization on patient-specific waitlist outcomes, specifically death or delisting and HT. The important findings of the present study are: 1) The number of heart transplant candidates who were implanted with a CF-LVAD has steadily increased nationwide following the approval of this technology for the BTT indication, 2) Significant variability exists in CF-LVAD utilization among and within UNOS regions 3) Both patient-level and center-level factors impact the decision to utilize CF-LVAD in transplant candidates 4) Bridging with a CF-LVAD appears to decrease the likelihood of waitlist mortality or delisting for worsening status at the expense of decreased likelihood of transplantation within the first year of listing 5) Improved waitlist outcomes can be achieved by congruous utilization of medical and mechanical bridging strategies based on region, center, and patient-level factors.
After recent improvements in durability and energy efficiency, CF-LVADs have become a mainstay of advanced heart failure therapy5, 6. Although recent ISHLT registry data suggests that the percent of HT recipients who are bridged to transplant with CF-LVAD are increasing, their data does not address the waitlist population1. The data in the current study suggests that, since 2008, the utilization of CF-LVADs has increased steadily in this population – likely reflective of increased wait list time and static organ supply in the contemporary era.
The next interesting finding of the current study was the dramatic variability in CF-LVAD utilization not only among, but also within, UNOS regions. Center-level analysis has demonstrated a correlation between longer transplant waitlist times and increased CF-LVAD utilization, suggesting that the average time to transplant at a given center may impact the decision to utilize CF-LVADs. However, this finding should be interpreted with caution, as CF-LVAD utilization may stabilize sick patients and prolong waitlist times by downgrading their status to UNOS 1B in absence of device complications. In addition, the strength of the correlation between waitlist times and CF-LVAD utilization was moderate at best, suggesting that other region- and center-level factors may contribute to the nationwide variability in utilization. For example, insurance coverage for CF-LVAD as BTT varies at the state level, which most certainly influences the way in which patients are cared for prior to transplant. In addition, physician and surgeon preference likely has a significant impact on the decision to utilize CF-LVAD, type of support device used, and the timing of device implantation in reference to patient's clinical status. Other center-level factors such as hospital resources, infrastructure, and referral patterns may certainly play a role in the observed variability. It is important to note that many of the aforementioned factors are inherently difficult to quantify and poorly represented in nationwide registries such as UNOS or INTERMACS. Nevertheless, future observational studies incorporating center-specific data at the granular level are required to identify precise reasons underlying nationwide variability in CF-LVAD utilization.
The decision to utilize a CF-LVAD as BTT is also related to patient-specific factors. We identified male gender, ethnicity, diabetes, history of smoking, current AICD, blood type O, large BSA, cardiomyopathy diagnosis, UNOS Status at listing, and UNOS region as patient level factors which were significantly associated with the decision to bridge a patient with a CF-LVAD. While prior studies have elucidated many of these patient characteristics as risk factors, the current study also takes into account UNOS region and listing center – which dictate organ availability and expected wait list time and, thus, contributes to decision making regarding CF-LVAD utilization in waitlisted patients.
When the primary outcomes were compared between medically and mechanically bridged patients among the propensity matched cohort, mechanically supported patients were less likely to die or be delisted, but also less likely to receive a transplant, than those who were medically managed. This survival benefit is consistent with data from the REMATCH trial, which demonstrate superior outcomes with CF-LVAD as compared to optimal medical management in the destination therapy population7. These results are also similar to those published by Trivedi et al., who studied the impact of BTT with Heartmate II as compared to medical management in a smaller, less contemporary cohort8. They found that BTT patients had lower waitlist mortality and a longer waiting time to transplant than those who were medically bridged. This study, however, only included patients who were listed at UNOS status 1A and 1B and excluded patients with other types of CF-LVADs from the analysis. Similarly, Wever-Pinzon et al. demonstrated that CF-LVAD patients in the contemporary era had improved freedom from death or delisting when compared to UNOS Status 1A and 1B patients who were medically managed9.
Lastly, we analyzed congruity of CF-LVAD utilization based upon patient's location and clinical profile and assessed its impact on waitlist outcomes. Those patients who, based upon their propensity scores and bridging method (mechanical v. medical management), had a congruous bridging strategy appeared to have superior outcomes as compared to those who had incongruous bridging. This suggests that improved patient selection – both for CF-LVAD utilization as BTT and medical therapy – could improve waitlist outcomes and increase the number of patients successfully and safely bridged to transplantation.
Importantly, the findings presented in the present study must be viewed in the context of the upcoming changes in the UNOS organ allocation system for heart transplantation, which will give higher priority to CF-LVADs than in the previous schema. By prioritizing transplants to CF-LVAD patients, the new system might attenuate some of the advantage to medically bridged patients with regards to likelihood of transplantation seen in the current study. However, it could also incentivize centers to utilize CF-LVAD more often as BTT, further highlighting the importance of appropriate utilization given the suboptimal outcomes seen in those patients who inappropriately received a CF-LVAD as BTT.
It is also important to take the findings of the current study in the context of its many limitations. First the data was extracted from a large registry – which subjects the data to both missingness and error in data entry. Secondly, because data points are routinely collected at the time of listing and the time of transplant, a substantial amount of patient information regarding time while listed (awaiting HT e.g.) is not available. This is specifically challenging when it comes to capturing patients who received MCS during the waiting period, but who died or were delisted prior to transplant. Lastly, data regarding acuity of patients at the time of CF-LVAD implantation (such as INTERMACS profile) was not available, which may vary among centers and could impact outcomes analyzed in this study.
In conclusion, CF-LVAD utilization in transplant eligible patients has steadily increased in the United States with significant variability among and within UNOS regions. Both patient-, center-, and region-level factors impact the decision to utilize CF-LVAD. Based upon the discordance in outcomes seen in those congruously and incongruously bridged, the risks and benefits of CF-LVAD therapy, as well as timing of its initiation, must be carefully weighed in each individual. As CF-LVAD use as BTT becomes more widespread, and particularly in light of the new UNOS organ allocation system, ongoing research is necessary to identify which patients would derive the greatest benefit, and the least harm, from CF-LVAD utilization as BTT.
Supplementary Material
What Is New?
This study demonstrates that significant variation in the use of left ventricular assist devices as a bridge to heart transplant exists among and within UNOS regions.
The use of ventricular assist devices in waitlisted patients can decrease the likelihood of death or delisting in the first year, but may increase time to transplant.
Although overall wait list survival may improve with ventricular assist device use, patient, as well as center and region specific, characteristics must be considered in order to improve outcomes.
What Are The Clinical Implications?
Because of the higher priority given to ventricular assist devices in the new allocation system, time to transplant may be reduced. However, overtreating with ventricular assist devices, particularly in patients with unfavorable characteristics, could lead to worsening waitlist outcomes.
More research is needed to better delineate optimal selection of bridging strategy by analyzing patient, center, and regional level data.
Acknowledgments
Sources of Funding: This study was supported by Lisa and Mark Schwartz and the Program to Reverse Heart Failure at New York Presbyterian Hospital/Columbia University.
Footnotes
Disclosures: Dr. Naka received consulting fees from Abbott and Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Lund LH, Edwards LB, Dipchand AI, Goldfarb S, Kucheryavaya AY, Levvey BJ, Meiser B, Rossano JW, Yusen RD, Stehlik J International Society for H and Lung T. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Heart Transplantation Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2016;35:1158–1169. doi: 10.1016/j.healun.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Transplantation ISHLT. Adult Heart Transplantation Statistics. 2014;Slide 41 https://www.ishlt.org/registries/slides.asp?slides=heartLungRegistry&year=2014.11/2317. [Google Scholar]
- 3.Slaughter MS, Pagani FD, McGee EC, Birks EJ, Cotts WG, Gregoric I, Howard Frazier O, Icenogle T, Najjar SS, Boyce SW, Acker MA, John R, Hathaway DR, Najarian KB, Aaronson KD HeartWare Bridge to Transplant ATI. HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:675–83. doi: 10.1016/j.healun.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH HeartMate IICI. Use of a continuous-flow device in patients awaiting heart transplantation. The New England journal of medicine. 2007;357:885–96. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 5.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Ulisney KL, Baldwin JT, Young JB. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2010;29:1–10. doi: 10.1016/j.healun.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Ulisney KL, Baldwin JT, Young JB. Third INTERMACS Annual Report: the evolution of destination therapy in the United States. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30:115–23. doi: 10.1016/j.healun.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure Study G. Long-term use of a left ventricular assist device for end-stage heart failure. The New England journal of medicine. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 8.Trivedi JR, Cheng A, Singh R, Williams ML, Slaughter MS. Survival on the heart transplant waiting list: impact of continuous flow left ventricular assist device as bridge to transplant. The Annals of thoracic surgery. 2014;98:830–4. doi: 10.1016/j.athoracsur.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Wever-Pinzon O, Drakos SG, Kfoury AG, Nativi JN, Gilbert EM, Everitt M, Alharethi R, Brunisholz K, Bader FM, Li DY, Selzman CH, Stehlik J. Morbidity and mortality in heart transplant candidates supported with mechanical circulatory support: is reappraisal of the current United network for organ sharing thoracic organ allocation policy justified? Circulation. 2013;127:452–62. doi: 10.1161/CIRCULATIONAHA.112.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.