Abstract
Background
Static hyperinflation is known to be increased during moderate acute exacerbations of chronic obstructive pulmonary disease (COPD) (AECOPD), but few data exist in patients with severe exacerbations of COPD. The role of dynamic hyperinflation during exacerbations is unclear.
Methods
In a prospective, observational cohort study, we recruited patients admitted to hospital for AECOPD. The following measurements were performed upon admission and again after resolution (stable state) at least 42 days later: inspiratory capacity (IC), body plethysmography, dynamic hyperinflation by metronome-paced IC measurement, health-related quality of life and dyspnea.
Results
Forty COPD patients were included of whom 28 attended follow-up. The IC was low at admission (2.05±0.11 L) and increased again during resolution by 15.6%±23.1% or 0.28±0.08 L (mean ± standard error of the mean, p<0.01). Testing of metronome-paced changes in IC was feasible, and it decreased by 0.74±0.06 L at admission, similarly to at stable state. Clinical COPD Questionnaire score was 3.7±0.2 at admission and improved by 1.7±0.2 points (p<0.01), and the Borg dyspnea score improved by 2.2±0.5 points from 4.4±0.4 at admission (p<0.01).
Conclusion
Static hyperinflation is increased during severe AECOPD requiring hospitalization compared with stable state. We could measure metronome-paced dynamic hyperinflation during severe AECOPD but found no increase.
Keywords: COPD, exacerbations of COPD, static hyperinflation, dynamic hyperinflation, severe acute exacerbations of COPD, COPD exacerbation, chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is currently the fourth leading cause of death and predicted to become the third by 2020.1 Chronic airflow limitation is the defining characteristic of COPD. This limitation is caused by a mix of small airways disease and parenchymal destruction caused by chronic inflammation.1
Important in the clinical course of COPD are episodes with worsening of respiratory symptoms from the stable state and beyond normal day-to-day variations, which require additional treatment.1 These exacerbations are associated with viral or bacterial airway infections in the majority of cases. Most exacerbations are of mild or moderate severity; only about 4% is categorized as severe.2 Severe exacerbations require hospital admission and are associated with increased mortality, morbidity, and health care costs.3–5
Apart from classification by severity (level of care) and infectious cause, little has been done to categorize exacerbations of COPD. Lopez-Campos and Agusti proposed a dual axes system for categorizing and thereby for treating exacerbations, classifying exacerbations on an axis of severity and of infectious or eosinophilic inflammation.6 We believe that hyperinflation is another important component.7 Hyperinflation is a better predictor of symptoms than most of our physiological parameters and also is a predictor of mortality in stable state.8,9 Furthermore different treatment strategies can be considered for hyperinflated patients.
Static hyperinflation is caused by entrapment of air during expiration, due to peripheral airway obstruction. This can be observed especially by destruction of alveolar attachments to small airways when the disease becomes more severe. Hyperinflation is characterized by increased functional residual capacity (FRC) and reduced inspiratory capacity (IC), resulting in increased dyspnea and limitation of exercise capacity.1,10–12
During tachypnea and exercise, hyperinflation can increase further and this is called dynamic hyperinflation. Dynamic hyperinflation is at least partly caused by a shortening expiration time, thus preventing patients to exhale completely and thereby causing air trapping.13–15 Dynamic hyperinflation of the lungs is known to limit exercise capacity in stable COPD and to impact on the perception of dyspnea.10,16,17
Only a few groups have attempted to study the course of hyperinflation during exacerbations. Parker et al included 7 hospitalized patients and 13 outpatients with moderate exacerbations.18 They measured dyspnea and lung volumes with plethysmography. They found that after resolution of the exacerbation, some COPD patients showed an increase in IC (therefore decrease of hyperinflation) and improvements in dyspnea. Stevenson et al studied admitted patients.19 They measured symptoms with a Borg dyspnea score and volumes with spirometry but lacked direct measurements of hyperinflation for instance with body plethysmography. Although both studies reported dyspnea changes in subgroup analyses, these studies did not analyze the temporal relation between changes in symptoms and changes in hyperinflation. These two studies provide a strong direction of thoughts regarding static hyperinflation. However, they did not completely answer the question whether and to what degree static hyperinflation is present during severe acute exacerbations of COPD and whether increased static hyperinflation is associated with more symptoms. Moreover, they did not assess dynamic hyperinflation.
This study was designed to confirm the presence of static hyperinflation in severe acute exacerbations and to analyze this in more depth with body plethysmography. Secondly, this study was aimed to assess dynamic hyperinflation. Furthermore, we hypothesized that improvement in dyspnea and quality of life during and after admission for an acute COPD exacerbation is closely related to changes in both static and dynamic hyperinflation.
Methods
Subjects
Patients admitted with an acute COPD exacerbation were eligible for the study. The inclusion criteria we employed were as follows: 40 years or older, doctor’s diagnosis of COPD based on an incompletely reversible airflow obstruction defined as 1) a post-bronchodilator forced expiratory flow in 1 second (FEV1)/forced vital capacity (FVC) <70% and 2) post-bronchodilator FEV1<80% predicted. An exacerbation was defined as a worsening of respiratory symptoms from the stable state and beyond normal day-to-day variations, which requires additional treatment. Excluded were patients with an X-ray-confirmed pneumonia, an indication for (non)invasive ventilation, admission to an intensive care unit, unstable angina pectoris or other clinically important cardiac comorbidity requiring admission to a cardiology ward. Additionally, we excluded patients who received any investigational new drug within the last 4 weeks prior to admission. In concordance with the Dutch law and approved by our ethics committee (Medisch Ethische Toetsingscommissie Universitair Medisch Centrum Groningen), informed consent was obtained verbally within the first 24 hours of admission, and written informed consent was obtained in the first 48 hours after the patient had had time and energy to read the paperwork. Data of patients who did not finally provide written informed consent were excluded from the study.
Design
This trial was registered in the World Health Organization approved International Clinical Trials Registry Platform, the Netherlands Trial Registry (NTR 4600). The study was conducted in the emergency room and pulmonary ward of a university teaching hospital in the Netherlands. Participants were tested after inclusion, prior to discharge, and after discharge in stable state at day 42 or later. Patients were allowed entry into the trial only once. Baseline characteristics were obtained, including X-ray, medication use, differential blood count, cultures and swabs for viral polymerase chain reaction. Treatment according to local guidelines included steroids (between 30 and 40 mg of prednisolone), antibiotics, antivirals, oxygen and bronchodilators, all as needed. Individual doses of each were titrated, and the decisions about admittance and discharge were made by the treating physician who was not involved with the study team.
The primary outcomes were the changes in static hyperinflation (as measured by IC via spirometry [IC]) during resolution of the COPD exacerbation and changes in health-related quality of life (primary: Clinical COPD Questionnaire [CCQ] and dyspnea [Borg score]).
The secondary outcomes were the changes in dynamic hyperinflation (as measured by IC during the metronome-paced dynamic hyperinflation test) during resolution of the COPD exacerbation, changes in health-related quality of life by COPD Assessment Test and Modified Medical Research Council dyspnea score (mMRC), changes in other static hyperinflation volumes such as FRC, residual volume, total lung capacity (TLC) as well as changes in FEV1 and FVC during the resolution of the exacerbation.
Procedures
Spirometry was performed on working days during admittance, post-medication; no pre-bronchodilator lung function was attempted. Once during admission and once in stable state, a body plethysmography (Jaeger MasterScreen, CareFusion) was performed as per European Respiratory Society (ERS)/American Thoracic Society (ATS) criteria.20 When measuring static hyperinflation, the lung function technician aimed to measure the volumes at an elastic recoil pressure of the respiratory system of zero.
Metronome-paced hyperinflation was performed once during admission and stable state. Subjects were requested to breathe at a metronome-paced frequency of 40/minute during 30 seconds. Before increased pacing and immediately afterwards an IC maneuver was performed. Subjects were coached to maintain as much as possible a stable tidal volume. After at least 2 minutes, this measurement was repeated. Acceptability criteria of <150 mL and/or <5% were used (Oxycon Jaeger, CareFusion).
Pulmonary function testing during acute exacerbations is difficult for both patients and staff. If patients failed to produce a reliable value, the test was disregarded and was treated as missing value.
The diagnosis of an infection was established if either the culture or the nose swab tested positive. The swab used a polymerase chain reaction with primers for the 15 most common respiratory viruses in the Netherlands with a cutoff cycle threshold of 40.
Statistical analyses
Data were analyzed with IBM SPSS 24. Normally distributed data with 2 time points were assessed with paired t-tests. Variables with multiple time points were first assessed by ANOVA to obtain an f ratio. If the f ratio indicated a significant difference, a paired t-test was performed to assess the difference between admission and stable state. Unpaired t-tests were used to compare unpaired means. Bivariate correlations by Pearson were calculated to assess correlations between two variables.
No formal power calculation was deemed possible since no relevant data were identified adequately reflecting our target population. Based on previous studies we anticipated dropouts; our ambition to perform reliable measurements based on the ATS/ERS criteria would only increase that. Aiming to measure a difference of 100 mL of change in IC, we roughly estimated the necessity of 25 evaluable patients. This estimated sample size was approved by the ethics committee.
Results
From September 2014 till April 2016 patients admitted to the respiratory ward from our tertiary university hospital with an acute exacerbation of COPD were recruited. Forty-four patients provided their verbal informed consent upon admittance, 41 of them provided written informed consent within the first 24 hours; one of them developed a pneumothorax and had to be excluded from the analysis, leaving 40 subjects to be included in the study. Of 12 patients, no follow-up was obtained largely due to not being in stable state or not able to attend the follow-up. A flowchart of the study is provided in Figure 1. Baseline characteristics are provided in Table 1.
Figure 1.
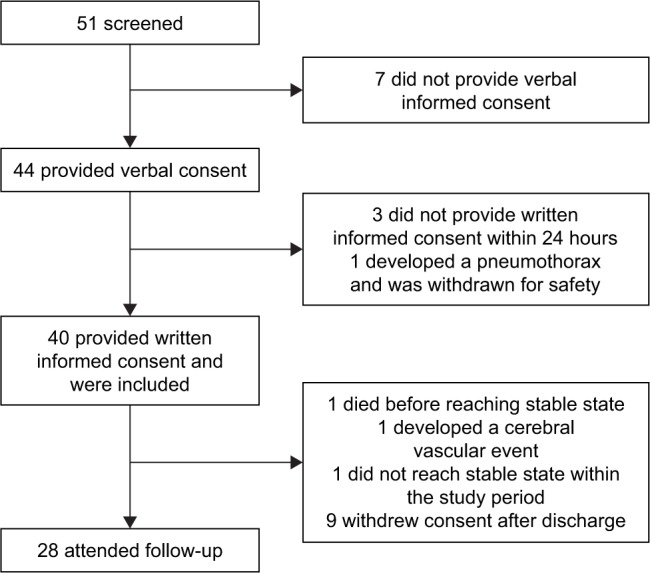
Flowchart of the trial.
Table 1.
Baseline characteristics
| Characteristics | |
|---|---|
| Number of patients | 40 |
| Age, years | 66 (10) |
| Sex, % male | 52.5 |
| Former smoker, % | 70 |
| Current smoker, % | 30 |
| Number of pack-years | 49 (35) |
| Body mass index, kg/m2 | 24.9 (4.8) |
| Post-bronchodilator FEV1 (% predicted) in stable state | 51 (18) |
| Post-bronchodilator FEV1 (L) in stable state | 1.3 (0.6) |
| Viral infection, % | 47.5 |
| Bacterial infection, % | 42.5 |
| Blood eosinophils, 10E9/L | 0.15 (0.19) |
| Blood neutrophils, 10E9/L | 8.6 (3.7) |
| pH on admission | 7.43 (0.04) |
| PaO2 (kPa) on admission | 8.6 (2.1) |
| PaCO2 (kPa) on admission | 5.4 (1.0) |
| Antibiotics % of usage during admission | 62.5 |
| Antiviral medication % of usage during admission | 7.5 |
| Days to discharge | 5 (2) |
| 6-month readmission rate, % | 40 |
Note: Values are presented as mean (SD) unless stated otherwise.
Abbreviation: FEV1, forced expiratory flow in one second.
The primary endpoint, change in static hyperinflation measured by IC, showed an improvement of 0.28±0.08 L or 15.6%±23.1% (mean±standard error of the mean) from admission to stable state (p<0.01). This was accompanied by an improvement in CCQ of −1.7±0.2 points and in Borg score of −2.2±0.5 points. No correlation between the change in IC and change in CCQ (r=0.12, p=0.58) or change in Borg (r=−0.2, p=0.36) was found. The relative changes in the IC of each individual participant are plotted in Figure 2.
Figure 2.
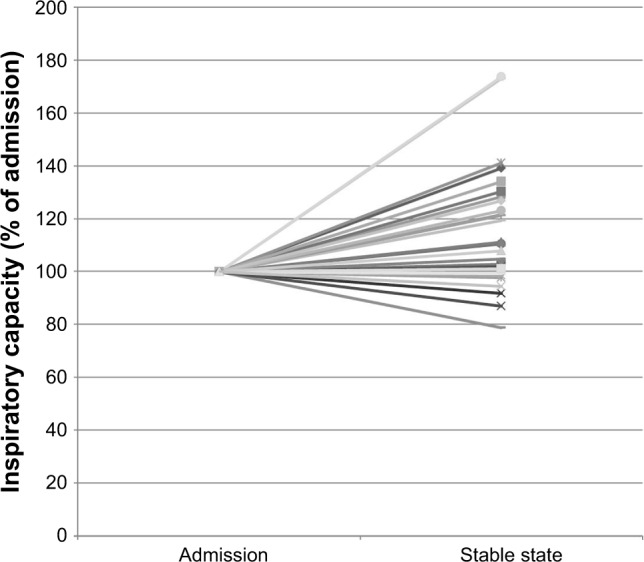
The relative changes in the inspiratory capacity of each individual participant.
Note: Y axis: percentage of inspiratory capacity change for each individual within this study.
Of the secondary endpoints, static hyperinflation, measured as change in FRC by body plethysmography, improved significantly by 334±102 mL (p<0.01). Residual volume decreased significantly by 501±140 mL or as percentage predicted 22%±6% (p<0.01). As expected, TLC did not change during the resolution of the exacerbation. Dynamic hyperinflation (as measured by change in IC during a metronome paced test) did not change significantly during the recovery from the exacerbation. Symptoms improved during the resolution of the exacerbation in all questionnaires (Table 2). The change in dynamic hyperinflation did not correlate with the change in symptoms.
Table 2.
Recovery from severe acute exacerbations of chronic obstructive disease measured at admission, discharge and in stable state
| Admission
|
Discharge
|
Stable state
|
Change
|
|||||
|---|---|---|---|---|---|---|---|---|
| Subjects n | mean±SD | Subjects n | mean±SD | Subjects n | mean±SD | Subjects n | mean±SD | |
| Spirometry | ||||||||
| FEV1 (L) | 39 | 1.05±0.52 | 37 | 1.10±0.52 | 28 | 1.35±0.57 | 28 | 0.28±0.37* |
| FVC (L) | 36 | 2.61±0.85 | 36 | 2.98±0.85 | 28 | 3.29±0.88 | 26 | 0.58±0.68* |
| Static hyperinflation | ||||||||
| IC (L) | 36 | 2.06±0.65 | 34 | 2.13±0.67 | 28 | 2.40±0.70 | 27 | 0.28±0.41* |
| IC (% predicted) | 36 | 74±20 | 34 | 76±20 | 28 | 88±20 | 27 | 10±17* |
| IC relative to admission (%) | 36 | 100±0 | 31 | 104.4±17.8 | 27 | 115.6±23.1 | 27 | 15.6±23.1* |
| FRC (L) | 28 | 4.86±1.64 | 25 | 4.36±1.29 | 22 | −0.33±0.48* | ||
| RV (L) | 28 | 3.76±1.34 | 25 | 3.09±1.08 | 22 | −0.50±0.66* | ||
| TLC (L) | 28 | 6.82±1.72 | 25 | 6.72±1.47 | ||||
| Dynamic hyperinflation | ||||||||
| IC after metronome (L) | 25 | 1.3±0.59 | 25 | 1.48±0.50 | ||||
| IC change after metronome (L) | 25 | −0.74±0.31 | 25 | −0.82±0.43 | ||||
| Symptoms | ||||||||
| CCQ | 39 | 3.7±0.9 | 38 | 2.7±1.0 | 25 | 1.9±1.1 | 25 | −1.7±1.1* |
| Borg | 39 | 4.4±2.2 | 38 | 2.7±1.7 | 25 | 1.7±1.7 | 25 | −2.2±2.4* |
| mMRC | 39 | 3.4±1.0 | 38 | 3.2±0.9 | 25 | 2.4±1.3 | 25 | −1.1±1.2** |
| CAT | 25 | 26.2±7.0 | 25 | 20.8±6.0 | 25 | 16.5±9.0 | 25 | −8.1±8.1* |
Notes: Data are presented as mean ± standard deviation or number.
p<0.01 significant improvement from admission to stable state by paired t-test.
Significant improvement from admission to stable state by nonparametric Wilcoxon rank test.
Abbreviations: FEV1, forced expiratory flow in one second; FVC, forced vital capacity; IC, inspiratory capacity; FRC, functional residual capacity; RV, residual volume; TLC, total lung capacity; CCQ, Clinical COPD Questionnaire; Borg, borg dyspnea score; mMRC, Modified Medical Research Council; CAT, COPD Assessment Test.
We compared patients with additional hyperinflation during the exacerbation with patients without additional hyperinflation (Table 3). The additionally hyperinflated group was defined as those patients whose IC improved 100 mL or more after recovery. The hyperinflated group had larger ICs and lower mMRC dyspnea scores (both significant) in stable state and additionally tendencies for greater improvement in mMRC and Borg dyspnea scores (both nonsig-nificant) during resolution. Interestingly, the group without additional hyperinflation during exacerbation had a higher number of eosinophils in peripheral blood at admission and a lower body mass index.
Table 3.
Differences between patients with additionally increased hyperinflation during their exacerbation versus patients without additional hyperinflation
| Additional hyperinflation (IC change >100 mL)
|
No additional hyperinflation (IC change <100 mL)
|
|||
|---|---|---|---|---|
| Number | Mean±SD | Number | Mean±SD | |
| Number of patients | 15 | 12 | ||
| Sex (number of males) | 8 | 6 | ||
| Admission time (days) | 4.87±1.68 | 5.00±1.91 | ||
| Age (years) | 65.87±5.79 | 62.92±9.48 | ||
| BMI (kg/m2) | 27.11±3.58 | 22.82±5.06* | ||
| Pack-years | 55.42±39.23 | 49.00±41.18 | ||
| pH (kPa) | 7.42±0.05 | 7.44±0.03 | ||
| PaCO2 (kPa) | 5.43±0.85 | 5.35±0.63 | ||
| PaO2 (kPa) | 7.82±2.94 | 8.61±3.69 | ||
| Neutrophils admission (10e9/L) | 8.26±1.75 | 9.41±2.86 | ||
| Eosinophils admission (10e9/L) | 0.10±0.06 | 0.27±0.29* | ||
| Positive viral PCR | 8 | 5 | ||
| Positive bacterial culture | 4 | 7* | ||
| Lung function | ||||
| FEV1 in stable state (L) | 1.43±0.66 | 1.24±0.48 | ||
| Change in FEV1 (L) | 0.40±0.26 | 0.18±0.44 | ||
| IC during admission (L) | 2.10±0.72 | 2.12±0.53 | ||
| IC stable state (L) | 2.68±0.74 | 2.03±0.49* | ||
| Change in IC (L) | 0.58±0.28 | −0.09±0.18* | ||
| FRC plethysmograph admission | 4.63±1.56 | 4.70±1.55 | ||
| FRC plethysmograph stable | 4.23±1.29 | 4.64±1.32 | ||
| Change in FRC (L) | −0.47±0.50 | −0.22±0.41 | ||
| RV plethysmograph admission (L) | 3.65±1.39 | 3.43±1.11 | ||
| RV plethysmograph stable state (L) | 2.92±1.13 | 3.33±1.05 | ||
| Change in RV (L) | −0.80±0.72 | −0.17±0.37* | ||
| TLC plethysmograph stable state (L) | 6.70±1.62 | 6.83±1.40 | ||
| CO diffusion in stable state (mmol/min/kPA) | 4.39±1.18 | 3.72±1.60 | ||
| Dynamic hyperinflation admission (L) | −0.74±0.41 | −0.70±0.23 | ||
| Dynamic hyperinflation stable (L) | −0.86±0.53 | −0.80±0.32 | ||
| Symptoms | ||||
| CCQ (admission) | 3.4±0.7 | 4.0±0.9 | ||
| CCQ (stable state) | 1.6±1.0 | 2.3±1.2 | ||
| Change in CCQ | −1.8±1.3 | −1.6±0.8 | ||
| CAT (admission) | 22.4±7.3 | 28.1±6.9* | ||
| CAT (stable state) | 14.3±7.6 | 17.3±9.8 | ||
| Change in CAT | −7.5±7.8 | −10.7±8.2 | ||
| mMRC (admission) | 3.1±1.1 | 3.8±0.5 | ||
| mMRC (stable state) | 1.8±1.2 | 3.0±1.2** | ||
| Change in mMRC | −1.5±1.3 | −0.8±1.0 | ||
| Borg (admission) | 4.2±2.1 | 4.1±2.4 | ||
| Borg (stable state) | 1.2±1.2 | 2.1±2.2 | ||
| Change in Borg | −2.6±1.6 | −1.9±3.3 | ||
Notes:
p<0.05 in unpaired t-tests.
p<0.05 for nonparametric independent sample test. Data are presented as mean±standard deviation. Increased hyperinflation was defined as a difference in IC of at least 100 mL between exacerbation and stable state.
Abbreviations: BMI, body mass index; PCR, polymerase chain reaction; FEV1, forced expiratory flow in one second; IC, inspiratory capacity; FRC, functional residual capacity; RV, residual volume; TLC, total lung capacity; CCQ, Clinical COPD Questionnaire; CAT, COPD Assessment Test; mMRC, Modified Medical Research Council; Borg, borg dyspnea score.
No difference in dynamic hyperinflation between the groups was detected. Subdividing the groups based on IC/TLC greater or lesser than 0.25 (instead of on decreased IC) yielded similar results, independent of whether exacerbation or stable state data were used.
An exacerbation is often associated with a viral or bacterial airway infection. To assess the hyperinflation in relation to these infections, hyperinflation was analyzed in the subgroups based on the presence or absence of a viral or bacterial infection (Table 4). A significant change in static hyperinflation was observed in patients with a culture-positive bacterial infection, whereas no change was detected in the group without bacterial infection; these changes, however, did not statistically differ from one another (p=0.32). No difference was found in change in hyperinflation in patients with versus without a viral infection. No difference in dynamic hyperinflation was observed between any of the subgroups.
Table 4.
Differences in hyperinflation between exacerbations with and without a viral or bacterial infection
| Static hyperinflation
|
DH
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IC admission (l) | IC discharge (l) | IC stable state (l) | IC change (l) | p-value | DH admission (IC change in l) | DH stable state (IC change in l) | p-value | |
| Bacterial infection | 2.3±0.7 | 2.5±0.7 | 2.7±0.8 | −0.4 | <0.01* | −0.8±0.3 | −1.0±0.5 | 0.4 |
| No bacterial infection | 2.0±0.5 | 2.0±0.6 | 2.1±0.5 | −0.2 | 0.29 | −0.7±0.3 | −0.7±0.3 | 0.9 |
| Viral infection | 2.1±0.7 | 2.3±0.7 | 2.4±0.7 | −0.2 | 0.02* | −0.8±0.3 | −0.8±0.3 | 1.0 |
| No viral infection | 2.0±0.6 | 1.9±0.6 | 2.4±0.7 | −0.3 | 0.03* | −0.7±0.3 | −0.8±0.5 | 0.2 |
Notes: Data are presented as mean±standard deviation. p-value: difference between admission and stable state by paired t-test.
p<0.05 in paired t-test.
Abbreviations: IC, inspiratory capacity; DH, dynamic hyperinflation.
Discussion
This study showed a more complete picture of the course of static and dynamic hyperinflation in patients hospitalized for acute severe exacerbations of COPD, and its resolution toward stable state. Static hyperinflation is increased during acute severe exacerbations compared with stable state. We were able to measure dynamic hyperinflation during the exacerbation but found no further increase (more than the increase in static hyperinflation). No correlation between change in hyperinflation and symptoms was found.
COPD exacerbations are the main cause for admissions of COPD patients, and hospital-related mortality and morbidity in COPD patients is high. Nevertheless, little is known about the physiology of such exacerbations,3,21–24 and there is not really a universally accepted clinical definition of a COPD exacerbation nor of strict criteria when to admit.3,25 Although efforts have been made to better define and prevent exacerbations in several recent trials, the treatment of an exacerbation in the hospital has remained mostly unchanged for the last 2 decades.1,26–29
Two previous studies assessed static hyperinflation in the setting of an acute exacerbation of COPD. Our results in inpatients are in line with the results of the study of Parker et al (n=20) who studied mostly outpatients with less severe exacerbations than in the current study.18 The study of Stevenson et al (n=22) did study the same group of patients as the current study but provided only the change in IC as measured by spirometry, without the confirmation of body plethysmography. Our data confirm their results and extend them with additional measurements.19
Assessment of dynamic hyperinflation has not been described earlier in the setting of an acute severe exacerbation of COPD to our knowledge. Patients were measured in this trial with a metronome-paced test aimed at changes in IC during tachypnea.12,30 No changes in dynamic hyperinflation between the exacerbation and stable state were found. Multiple explanations are possible for this result. It could indicate that patients with an exacerbation severe enough to require admission are limited by something else than hyperinflation, eg, airway resistance, mucus, hypercapnia or a change in ventilation/perfusion ratio. Another explanation could be that admitted patients already have a severely decreased inspiratory reserve capacity before admission and hardly have any room for further deterioration, as opposed to patients who do not need admission and in whom past data have shown decreasing ICs during exacerbations. Another explanation lies in the breathing frequency and tidal volume during the metronome-paced dynamic hyperinflation test. Due to ethical and practical concerns we imposed a frequency of 40, with a stable tidal volume during both tests. An alternative would have been to double their breathing frequency, which we deemed impossible for patients during severe exacerbations of COPD. A further increase in breathing frequency could have allowed to find an additional dynamic hyperinflation component. Next, measurement of static hyperinflation depends on being able to measure at zero elastic recoil level.13 Although every attempt was made to achieve this, it is more difficult to achieve during severe exacerbations. If this elastic recoil cannot be achieved for the first FRC of the dynamic measurement, ie, before increasing the breathing frequency, dynamic hyperinflation (decrease in IC) during metronome pacing will be underestimated. Finally, one could discuss whether the test used in the current trial is the optimal standard to detect dynamic hyperinflation.13,31 Based upon ethical arguments, the study team chose not to perform the more commonly used and better validated exercise test during the acute distress of severe exacerbation requiring admission.
Hyperinflation might provide a target for therapeutic strategies in patients with severe exacerbations. Patients admitted with a severe exacerbation of COPD are most commonly treated with short-acting bronchodilators via nebulizers. Long-acting bronchodilators, both anticholinergics and beta-2-mimetics, have been shown in stable state to provide larger reductions in hyperinflation compared to short-acting bronchodilators, alongside greater increases in flows.32–37 Long-acting bronchodilators, however, are not commonly available via nebulizers. A recent Cochrane review showed that after several decades of treatment with nebulizers, there is still no evidence to favor nebulizers over regular pressurised metered dose inhalers with good instruction.26 New studies should shed light on the potential of combined long-acting bronchodilators on reduction of hyperinflation during severe exacerbations requiring hospitalization. Such a trial could compare combined long-acting bronchodilators in currently available pressurised metered dose inhalers or dry powder inhalers versus short-acting bronchodilators by nebulizer, the latter being usual care in many hospitals. Based on the finding that hyperinflation is increased during exacerbations, we can speculate that the long-acting bronchodilators provide an early treatment for impending hyperinflation-predominant exacerbations of COPD, thus preventing some of them.38,39 Other strategies such as rehabilitation and noninvasive ventilation have been shown to reduce hyperinflation, while cognitive-behavioral strategies and perhaps even bronchoscopic lung volume reduction interventions could be further investigated as treatment of hyperinflation in selected patients during an acute event.7,40
Interestingly, patients who did hyperinflate during an exacerbation of COPD had higher ICs during stable state and fewer symptoms (CCQ, mMRC and Borg) both in stable state and during exacerbations. In other words, they had a better preserved inspiratory reserve capacity. This could perhaps also explain the lack of correlation between decrease in IC (worsening of hyperinflation) and increase in symptoms. One could argue that patients who do not hyperinflate during an exacerbation are those patients who in stable state already have a flow limitation and are less able to increase their IC. This could result in more symptoms both during and after the exacerbation. This explanation is supported by the nonsignificant observation that symptoms improve more during resolution in patients with additional hyperinflation during exacerbations.
Increased static hyperinflation was found in patients with a bacterial or viral infection. The patients without a cultured bacterial infection showed no increased static hyperinflation during their exacerbations. These changes, however, do not significantly differ. This might suggest that the presence or absence of increased hyperinflation is related with an infectious origin; however, more research and a larger sample size would be necessary before drawing conclusions from this subgroup analysis.
This study has several strengths but also has weaknesses that should be considered. A strong point of the study was that treatment decisions such as bronchodilator dose and discharge were made by the treating physician without influence from the trial team and without influence by the study measurement results. Another strong point of the trial is that we excluded patients with pneumonia. This will make the results from the trial more applicable toward exacerbations, since pneumonia might influence lung volumes. Patients who were in such distress that (non) invasive ventilation was required were also excluded in order to prevent bias due to inability to perform reliable pulmonary function tests. A weakness of the study is that it has been performed in only one center, potentially limiting the applicability of its results. Due to the severity of the exacerbation and disease, a relative high number of patients was not able or willing to provide reproducible pulmonary function tests or attend follow-up. Especially the intensive tests including the static and dynamic hyperinflation tests and spirometry repeatedly during the hospitalization were quite a burden on patients.
Static hyperinflation turned out to be an important feature of severe acute exacerbations of COPD in our population. We believe that this supports discussions whether the occurrence of hyperinflation should be incorporated in a new definition, since it is a common but not universal finding and opens a path toward a more precision medicine strategy in treatment. To our surprise, changes in hyperinflation were not directly correlated with symptoms although hyperinflated patients showed lower changes in symptoms. There must be other factors as well. Perhaps a model incorporating hyperinflation along with the current parameters of inflammation and respiratory infections will help to work on a future definition.
In summary, this study measured changes in static and dynamic hyperinflation during acute severe exacerbations of COPD requiring hospital admittance. The increases in static hyperinflation were anticipated based on two earlier studies, only partially performed in hospital with less severe exacerbations. They have now been confirmed with body plethysmography. We were bold enough to attempt at measuring dynamic hyperinflation during acute exacerbations in the hospital setting, but could not find a further increase over and above the change in static hyperinflation already induced by the exacerbation.
Acknowledgments
We thank Jantien Remmelink, Margot Klijnsma and Alice Niemeijer for the collection of data and for performing measurements. We thank Joost van den Aardweg for his support with some of the calculations. We would like to thank our complete pulmonary function team, especially Marga Star-Kroesen, Martijn Farenhorst, Margrietha Swieringavan der Veen, Yvonne Valkema-Tol, Wies Heins-Konigers and Jenny Stevens-van der Vinne for their time, flexibility and dedication to perform and schedule the pulmonary function tests during the study.
Footnotes
Disclosure
WHVG reports a grant for an investigator-initiated trial to University Medical Center Groningen from Novartis and an ERS short-term research fellowship, outside the submitted work. HAMK reports that his institution (University Medical Center Groningen) has received a fee per patient for recruitment in trials and a grant for investigator-initiated studies from GlaxoSmithKline, Novartis, and FLUIDDA. Additionally, his institution has received grants as well as consultancy fees from Novartis, AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline, all outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 2.Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi: 10.1056/NEJMoa1516385. [DOI] [PubMed] [Google Scholar]
- 3.Wedzicha JA, Calverley PMA, Albert RK, et al. Prevention of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;50(3):1602265. doi: 10.1183/13993003.02265-2016. [DOI] [PubMed] [Google Scholar]
- 4.Müllerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999–1007. doi: 10.1378/chest.14-0655. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson GC, Wedzicha JA. The causes and consequences of seasonal variation in COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2014;9:1101–1110. doi: 10.2147/COPD.S54475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Campos JL, Agusti A. Heterogeneity of chronic obstructive pulmonary disease exacerbations: a two-axes classification proposal. Lancet Respir Med. 2015;3(9):729–734. doi: 10.1016/S2213-2600(15)00242-8. [DOI] [PubMed] [Google Scholar]
- 7.van Geffen WH, Slebos DJ, Kerstjens HA. Hyperinflation in COPD exacerbations. Lancet Respir Med. 2015;3(12):e43–e44. doi: 10.1016/S2213-2600(15)00459-2. [DOI] [PubMed] [Google Scholar]
- 8.Moore AJ, Soler RS, Cetti EJ, et al. Sniff nasal inspiratory pressure versus IC/TLC ratio as predictors of mortality in COPD. Respir Med. 2010;104(9):1319–1325. doi: 10.1016/j.rmed.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Casanova C, Cote C, de Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(6):591–597. doi: 10.1164/rccm.200407-867OC. [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell DE, Elbehairy AF, Webb KA, Neder JA, Canadian Respiratory Research Network The link between reduced inspiratory capacity and exercise intolerance in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14(Suppl 1):S30–S39. doi: 10.1513/AnnalsATS.201610-834FR. [DOI] [PubMed] [Google Scholar]
- 11.Mahler DA, O’Donnell DE. Recent advances in dyspnea. Chest. 2015;147(1):232–241. doi: 10.1378/chest.14-0800. [DOI] [PubMed] [Google Scholar]
- 12.Cooper CB. The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function. Am J Med. 2006;119(10 Suppl 1):21–31. doi: 10.1016/j.amjmed.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Rossi A, Aisanov Z, Avdeev S, et al. Mechanisms, assessment and therapeutic implications of lung hyperinflation in COPD. Respir Med. 2015;109(7):785–802. doi: 10.1016/j.rmed.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 14.O’Donnell DE, Laveneziana P. The clinical importance of dynamic lung hyperinflation in COPD. COPD. 2006;3(4):219–232. doi: 10.1080/15412550600977478. [DOI] [PubMed] [Google Scholar]
- 15.O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(2):180–184. doi: 10.1513/pats.200508-093DO. [DOI] [PubMed] [Google Scholar]
- 16.Langer D, Ciavaglia CE, Neder JA, Webb KA, O’Donnell DE. Lung hyperinflation in chronic obstructive pulmonary disease: mechanisms, clinical implications and treatment. Expert Rev Respir Med. 2014;8(6):731–749. doi: 10.1586/17476348.2014.949676. [DOI] [PubMed] [Google Scholar]
- 17.Guenette JA, Webb KA, O’Donnell DE. Does dynamic hyperinflation contribute to dyspnoea during exercise in patients with COPD? Eur Respir J. 2012;40(2):322–329. doi: 10.1183/09031936.00157711. [DOI] [PubMed] [Google Scholar]
- 18.Parker CM, Voduc N, Aaron SD, Webb KA, O’Donnell DE. Physiological changes during symptom recovery from moderate exacerbations of COPD. Eur Respir J. 2005;26(3):420–428. doi: 10.1183/09031936.05.00136304. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson NJ, Walker PP, Costello RW, Calverley PM. Lung mechanics and dyspnea during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(12):1510–1516. doi: 10.1164/rccm.200504-595OC. [DOI] [PubMed] [Google Scholar]
- 20.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 21.Ko FW, Chan KP, Hui DS, et al. Acute exacerbation of COPD. Respirology. 2016;21(7):1152–1165. doi: 10.1111/resp.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawkins PE, Alam J, McDonnell TJ, Kelly E. Defining exacerbations in chronic obstructive pulmonary disease. Expert Rev Respir Med. 2015;9(3):277–286. doi: 10.1586/17476348.2015.1046438. [DOI] [PubMed] [Google Scholar]
- 23.Wedzicha JA, Singh R, Mackay AJ. Acute COPD exacerbations. Clin Chest Med. 2014;35(1):157–163. doi: 10.1016/j.ccm.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Hurst JR, Vestbo J, Anzueto A, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 25.Leidy NK, Wilcox TK, Jones PW, Roberts L, Powers JH, Sethi S, EXACT-PRO Study Group Standardizing measurement of chronic obstructive pulmonary disease exacerbations. Reliability and validity of a patient-reported diary. Am J Respir Crit Care Med. 2011;183(3):323–329. doi: 10.1164/rccm.201005-0762OC. [DOI] [PubMed] [Google Scholar]
- 26.van Geffen WH, Douma WR, Slebos DJ, Kerstjens HA. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst Rev. 2016;8:CD011826. doi: 10.1002/14651858.CD011826.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bathoorn E, Groenhof F, Hendrix R, et al. Real-life data on antibiotic prescription and sputum culture diagnostics in acute exacerbations of COPD in primary care. Int J Chron Obstruct Pulmon Dis. 2017;12:285–290. doi: 10.2147/COPD.S120510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brill SE, Wedzicha JA. Oxygen therapy in acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1241–1252. doi: 10.2147/COPD.S41476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Geffen WH, Bruins M, Kerstjens HA. Diagnosing viral and bacterial respiratory infections in acute COPD exacerbations by an electronic nose: a pilot study. J Breath Res. 2016;10(3):036001. doi: 10.1088/1752-7155/10/3/036001. [DOI] [PubMed] [Google Scholar]
- 30.Gelb AF, Gutierrez CA, Weisman IM, Newsom R, Taylor CF, Zamel N. Simplified detection of dynamic hyperinflation. Chest. 2004;126(6):1855–1860. doi: 10.1378/chest.126.6.1855. [DOI] [PubMed] [Google Scholar]
- 31.Klooster K, ten Hacken NH, Hartman JE, Sciurba FC, Kerstjens HA, Slebos DJ. Determining the role of dynamic hyperinflation in patients with severe chronic obstructive pulmonary disease. Respiration. 2015;90(4):306–313. doi: 10.1159/000439056. [DOI] [PubMed] [Google Scholar]
- 32.O’Donnell DE, Casaburi R, Frith P, et al. Effects of combined tiotropium/olodaterol on inspiratory capacity and exercise endurance in COPD. Eur Respir J. 2017;49(4):1601348. doi: 10.1183/13993003.01348-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahler DA, Kerstjens HA, Donohue JF, Buhl R, Lawrence D, Altman P. Indacaterol vs tiotropium in COPD patients classified as GOLD A and B. Respir Med. 2015;109(8):1031–1039. doi: 10.1016/j.rmed.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Kerstjens HA, Deslee G, Dahl R, et al. The impact of treatment with indacaterol in patients with COPD: a post-hoc analysis according to GOLD 2011 categories A to D. Pulm Pharmacol Ther. 2015;32:101–108. doi: 10.1016/j.pupt.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Powrie DJ, Wilkinson TM, Donaldson GC, et al. Effect of tiotropium on sputum and serum inflammatory markers and exacerbations in COPD. Eur Respir J. 2007;30(3):472–478. doi: 10.1183/09031936.00023907. [DOI] [PubMed] [Google Scholar]
- 36.O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832–840. doi: 10.1183/09031936.04.00116004. [DOI] [PubMed] [Google Scholar]
- 37.Casaburi R, Maltais F, Porszasz J, et al. 205.440 Investigators Effects of tiotropium on hyperinflation and treadmill exercise tolerance in mild to moderate chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(9):1351–1361. doi: 10.1513/AnnalsATS.201404-174OC. [DOI] [PubMed] [Google Scholar]
- 38.Wedzicha JA, Agusti A, Donaldson G, Chuecos F, Lamarca R, Garcia Gil E. Effect of aclidinium bromide on exacerbations in patients with moderate-to-severe COPD: a pooled analysis of five Phase III, randomized, placebo-controlled studies. COPD. 2016;13(6):669–676. doi: 10.3109/15412555.2016.1170111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169(12):1298–1303. doi: 10.1164/rccm.200310-1443OC. [DOI] [PubMed] [Google Scholar]
- 40.van Geffen WH, Kerstjens HAM, Slebos DJ. Emerging bronchoscopic treatments for chronic obstructive pulmonary disease. Pharmacol Ther. 2017;179:96–101. doi: 10.1016/j.pharmthera.2017.05.007. [DOI] [PubMed] [Google Scholar]


