Abstract
Tissue engineering is a promising field, focused on developing solutions for the increasing demand on tissues and organs regarding transplantation purposes. The process to generate such tissues is complex, and includes an appropriate combination of specific cell types, scaffolds, and physical or biochemical stimuli to guide cell growth and differentiation. Microcarriers represent an appealing tool to expand cells in a three-dimensional (3D) microenvironment, since they provide higher surface-to volume ratios and mimic more closely the in vivo situation compared to traditional two-dimensional methods. The vascular system, supplying oxygen and nutrients to the cells and ensuring waste removal, constitutes an important building block when generating engineered tissues. In fact, most constructs fail after being implanted due to lacking vascular support. In this study, we present a protocol for endothelial cell expansion on recombinant collagen-based microcarriers under dynamic conditions in spinner flask and bioreactors, and we explain how to determine in this setting cell viability and functionality. In addition, we propose a method for cell delivery for vascularization purposes without additional detachment steps necessary. Furthermore, we provide a strategy to evaluate the cell vascularization potential in a perfusion bioreactor on a decellularized biological matrix. We believe that the use of the presented methods could lead to the development of new cell-based therapies for a large range of tissue engineering applications in the clinical practice.
Keywords: Bioengineering, Issue 132, Recombinant collagen I peptide, macroporous microcarriers, Cellnest, cell expansion, spinner flasks, BioVaSc, perfusion bioreactor, sprouting, vascularization
Introduction
One general problem in tissue engineering applications is to yield a high cell mass with the correct differentiation phenotype at the location of need. The application of microcarriers to address this issue started in 1967 with increasing significance to date in fields such as orthopaedic tissue engineering for large-scale generation of skin, bone, cartilage, and tendons1. They allow the handling of adherent cultures in ways similar to that of suspension cultures2 by expanding cells on microscale three-dimensional (3D) substrates. Thereby cells experience a homogeneous nutrient supply and cell-matrix interactions that lead to better maintenance of in vivo3,4 differentiation which is often lost over time in 2D approaches5. A higher surface-to-volume ratio - eventually leading to higher cell yields6,7, higher gas and nutrient exchange rates comparing to static systems8, the possibility to regulate and subject the culture to physical stimuli9, and the potential for scaling up of the expansion process7 are further advantages. Several features such as diameter, density, porosity, surface charge, and adhesion properties10,11 distinguish the different commercially available micro- and macro-carriers. However, one of the main advantage is their delivery potential as microtissues to site defect or demand.
For applications of the microcarrier technology in bone tissue engineering, we illustrated in a previous report12 the production of a new microcarrier type constituted of a recombinant collagen I peptide (RCP, commercially available as Cellnest). This new microcarrier allows the GMP-compliant up scaling of scaffold and cell production, as needed for cell delivery in a clinical scenario. In this context, tuning of scaffold stability, degradation rate, and surface properties through proper choice of a suited crosslinking strategy allows to adapt the technique to the selected application, cell type of interest or target tissue13. In particular, the potential employment of this microcarrier as an injectable cell delivery system for therapeutic application14 makes them particularly interesting in a clinical setting.
In this paper, we therefore illustrate the culturing procedure for the isolation and expansion of human bone marrow-derived mesenchymal stromal cells (hBMSCs) and human dermal microvascular endothelial cells (HDMECs) on collagen-I-based recombinant peptide-based microcarriers, and their preparation for delivery in a clinical setting. Furthermore, we describe additional protocols useful for the maintenance of cell viability upon implantation.
Cell viability after implantation is in fact strongly dependent on vascularization15,16,17, which ensures exchange of oxygen and nutrients and facilitates waste removal. Bioreactors constitute one approach to overcome vascularization challenges in tissue engineering and maintain cell viability, through perfusion of culture medium providing thereby oxygen and nutrients18. Here, we illustrate an in vitro method to evaluate the migration capability of microvascular endothelial cells from the RCP microcarriers to a biomatrix and their ability to contribute to the de novo vascularization and angiogenesis. This biomatrix is a decellularized segment of porcine jejunum termed BioVaSc (Biological Vascularized Scaffold), rich in collagen and elastin and with preserved vascular structures, which includes a feeding artery and a draining vein19 that has been applied for implantation issues20.
Protocol
hBMSCs were isolated from the femur head of osteoarthritis patients undergoing femur head replacement surgery. The procedure was performed under the approval of the Local Ethics Committee of the University of Wuerzburg and informed consent of the patients. Primary microvascular endothelial cells were isolated from foreskin biopsies of juvenile donors. Their legal representative(s) provided full informed consent in writing. The study was approved by the local ethical board of the University of Wuerzburg (vote 182/10).
1. Isolation of hBMSCs and HDMECs
- Human bone marrow-derived mesenchymal stromal cells (hBMSCs)
- Remove spongy bone from the femur head by using a sterile spoon. NOTE: The spongy bone appears as a loose and granular substance inside the cortical bone, which is hard and rigid. It may be necessary to scratch parts of the spongy bone from the cortical bone by using a scalpel.
- Insert the removed spongy bone in two 50 mL tubes. The volume of the spongy bone can vary from 5 to 10 mL according to the dimension of the removed femur head during the hip replacement surgery.
- Wash with DMEM-F12 (1:1 mixture of DMEM and Ham F12 medium), filling the tube with approx. 30 mL of medium.
- Shake the tube manually, in a longitudinal way, for 5 s to let adipose cells out of the spongy bone. Centrifuge at 300 x g and 22 °C for 5 min.
- Aspirate with a graduated plastic pipette, the fat-cell layer and approx. 20 mL of the remaining supernatant. Let enough medium to cover the spongy bone.
- Fill the tubes with 10 ml of DMEM-F12 and shake vigorously longitudinally by hand, for 10 - 15 s, to let the cells out.
- Transfer 10 - 15 mL of cell suspension with a graduated plastic pipette from the tubes to a new 50 mL tube.
- Repeat 2 - 3 times from step 1.1.6 pooling together the obtained cell suspension until the spongy bone collected in the 50 mL tube is white.
- Transfer the cell suspension with a graduated plastic pipette to two new tubes, avoiding aspirating the collagen pellet deposited on the bottom of the 50 mL tubes.
- Centrifuge the cell suspension at 300 x g and 22 °C for 5 min. Discard the supernatant by aspirating it with a graduated plastic pipette.
- Add 40 mL of DMEM-F12 10% FCS 1% Pen/Strep 50 µg/mL Ascorbate-2-phosphate in a third tube. Transfer 5 mL of this medium to each tube containing the cell pellet. Re-suspend the cell pellets by flickering the bottom of the tubes vigorously and transfer the cell suspensions to the tube containing only medium.
- OPTIONAL: Dilute 100 µL of cells suspension for cell counting, diluting first 1:100 with PBS and then 1:10 in trypan blue, by adding 15 µL of the first dilution to 15 µL of DPBS and 30 µL Trypan Blue to obtain a DPBS: Trypan Blue ratio of 1:1. Count the cells with a standard Neubauer chamber.
- Seed the cells at 109 cells/175 cm2 T-Flask in 25 mL of medium as described in 1.1.11. Alternatively, divide the whole cell suspension in up to ten 175 cm2 cell culture flasks without counting. At this stage, hBMSCs are not distinguishable from the erythrocytes, which greatly outnumber them. hBMSCs will appear as sparse colonies (1 - 2/flask) over the course of the first 7 days.
- Change medium after 4 days from seeding to allow interaction of the blood cells with the hBMSCs. After the first medium exchange, change the medium every 2 - 3 days. Perform the medium exchange by completely removing the culture medium with a graduated plastic pipette, washing two times with DPBS (by adding 10 mL of DPBS to the adherent cells and removing completely the DPBS with a graduated plastic pipette) and then adding 20 mL of fresh medium, as described in 1.1.11.
- Human dermal microvascular endothelial cells (HDMECs)
- Treat the skin biopsies and isolate the HDMECs according to previously published protocols21. Briefly, after separation of dermis from epidermis, incubate the dermis in trypsin for 40 min at 37 °C and 5% CO2. After incubation time, transfer the dermis to a Petri dish containing culture medium, and scratch each piece with the use of a scalpel. Transfer the obtained cell suspension to a 50 mL plastic tube and centrifuge for 5 min at 300 x g and 22 °C. Count the cell suspension using a Neubauer chamber and seed at a density of 1.2 x 104 cells/cm2. Change the medium completely every other day.
2. Set-up and sterilization of spinner flasks, RCP microcarriers, and bioreactor
- Spinner flasks and RCP microcarriers
- One day before the experiments, set-up the spinner flasks and prepare them for sterilization by autoclave. NOTE: Leave the caps of the spinner flasks loose and, when possible, sterilize them in an upright position.
- Wrap the spinner flasks with aluminum foil around the side caps separately in order to avoid contamination during unwrapping. Wrap the flasks with 1 - 2 layers of aluminum foil for autoclaving in order to minimize contamination risks after sterilization.
- Weigh the required amount of RCP macroporous microcarriers in 20 mL of DPBS. NOTE: 30 mg is required per spinner flask. Let them hydrate for at least 1 h before heat sterilization by autoclave (121 °C for 15 min).
- Perfusion bioreactor
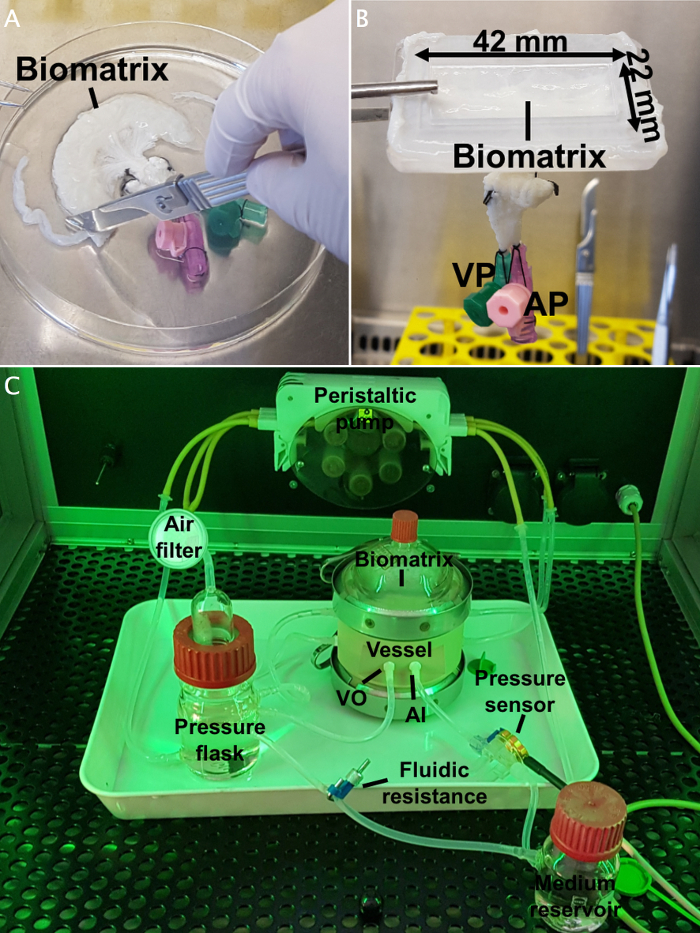
- Use the bioreactor system described to produce vascularized tissue equivalents20. This bioreactor consists of a vessel, in which the biomatrix is placed, a medium reservoir and a pressure bottle.
- Connect the vessel with the medium reservoir and the pressure bottle through silicon tubes (see Figure 3C). Leave the caps loose and place it in an autoclave plastic bag, close well, and place this in a second autoclave plastic bag. Sterilize by autoclave.
3. Culture of hBMSCs and HDMECs on RCP macroporous microcarriers
NOTE: The HDMECs used to seed the RCP microcarriers were labeled with RFP by lentiviral transduction. This protocol was modified after a previously published protocol5.
Let the RCP macroporous microcarriers settle to the bottom and wash them with 10 mL of culture medium. Repeat 3 times.
Wash spinner flasks with 10 mL of culture medium.
Add 30 mg of RCP macroporous microcarriers per spinner flask in 8 mL of culture medium. Equilibrate the spinner flasks containing the RCP microcarriers at 37 °C and 5% CO2 for 30 min, before seeding the cells. NOTE: This is based on approximate dry weight of RCP microcarriers before sterilization.
Seed 5 x 105 hBMSCs or HDMECs (passage 3) per spinner flask in 2 mL of culture medium.
Place the spinner flasks on the stirrer plate and start stirring at 90 rpm for 5 min and rest for 55 min, at 37 °C and 5% CO2, for a total of 1 h static/dynamic incubation. Repeat 4 times.
Add 10 mL of cell culture medium per spinner flask and then start continuous stirring at 95 rpm. Incubate at 37 °C and 5% CO2.
With the use of a micropipette take samples at days 1, 4 and 7: 333 µL for DNA content (~ 0.5 mg), 1000 µL for SEM analysis (~ 1.5 mg) and 333 µL for live/dead staining (~ 0.5 mg). NOTE: This is based on approximate dry weight of RCP microcarriers before sterilization.
Change 10 mL of culture medium every other day. For this, wait until the RCP microcarriers settle to the bottom and very carefully aspirate 10 mL from the spinner flask, with the use of a 10 mL pipette, and then add 10 mL of fresh culture medium.
Keep the cultures on the stirrer plate set at 95 rpm, at 37 °C and 5% CO2 for 7 days.
4. DNA content, SEM analysis, live dead staining, and sprouting assay
- DNA content
- With the use of a micropipette, collect approx. 0.5 mg of microcarriers from each spinner flask (contained in 333 µL of suspension) and transfer to a 2 ml plastic tube. NOTE: This is based on approximate dry weight of RCP microcarriers before sterilization.
- Wash once with 1 mL of DPBS, then aspirate supernatant.
- Remove remaining DPBS in a lyophilizer and weight the lyophilized microcarriers for later normalization.
- Freeze samples at -80 °C for at least 6 h.
- Incubate samples at 60 °C overnight with 300 µL papain 5 U/mL.
- Establish the appropriate titration curves following the manufacturer´s instructions of the quantitation assay (e.g., PicoGreen dsDNA assay) kit and perform measurement of the fluorescent signal at 520 nm with a luminescence detector.
- Scanning electron microscopy (SEM) analysis
- Collect ca. 1 mg of microcarriers from each spinner flask and transfer to a 2 mL plastic tube.
- Wash microcarriers once with 1 mL of DPBS.
- Remove DPBS and incubate in 1 mL of 70% ethanol for 1 h.
- Remove supernatant and incubate in 1 mL of 80% ethanol for 1 h.
- Remove supernatant and incubate in 1 mL of 90% ethanol for 1 h.
- Remove supernatant and incubate in 1 mL of 100% ethanol for 1 h.
- Remove supernatant and incubate in 1 mL of hexamethyldisilazane for 10 min.
- Open cap and let the samples dry overnight under a chemical hood.
- Fix samples on appropriate support and proceed to SEM analysis according to established protocols.
- DAPI and Live/Dead staining
- At day 2, 4, and 7 after cell seeding on the microcarriers collect 0.5 mg of microcarriers from each spinner flask and transfer to a 2 mL plastic tube. Wash once with 1 mL of DPBS.
- DAPI
- Fix samples for 10 min in enough 4% paraformaldehyde to cover them.
- Wash once with 1 mL of DPBS.
- Incubate at room temperature with the staining solution for 45 min on a rocking shaker.
- Detect DAPI fluorescent signal on a fluorescence microscope with a 420 nm emission filter and acquire images.
- Live/Dead Staining
- Incubate samples in 500 µL of Live/Dead dye solution. Keep the samples at 37 °C for 20 min, protected from light.
- Wash once with 1 mL of DPBS.
- Detect fluorescence signal of living cells with a 490 nm emission filter and of dead cells with a 545 nm emission filter on a fluorescence microscope. Acquire images.
- Sprouting assay for HDMECs
- Mix 330 µL of collagen R solution 0.4%, 170 µL of 0.1% acetic acid, and 50 µL of medium M199 in a 15 mL plastic tube. Keep on ice.
- Take ~0.375 mg of microcarriers (250 µL) with the use of a micropipette. Transfer to a 1.5 mL plastic tube, wait for the RCP microcarriers to settle to the bottom and remove the medium completely. NOTE: This is based on approximate dry weight of RCP microcarriers before sterilization.
- Add the volume of NaOH 0.2 N required to neutralize the collagen mixture and immediately mix it with the RCP microcarriers. NOTE: Determine the required amount of NaOH 0.2 N in advance by adding it to the mixture until change in pH (turn from yellow color to pink), and then placing the mixture in the incubator for 1 min, after which a gel must be formed.
- Plate the collagen gel-RCP microcarriers mixture in a well of a 24-well plate. Incubate at 37 °C and 5% CO2 for 30 min.
- Add 200 µL of the vascular endothelial growth factor (e.g., VascuLife VEGF-Mv culture medium). Incubate at 37 °C and 5% CO2 for 24 h.
- Observe under a inversed phase contrast microscope, using a 10X magnification object, and acquire images.
5. Biomatrix seeding and start of bioreactor system for HDMECs
- One day before starting the bioreactor cultures, cut open BioVaSc (the biomatrix) longitudinally on the antimesenteric side and fix it in a previously disinfected polycarbonate frame (see Figure 3A, B). NOTE: The BioVaSc is a biomatrix obtained from a decellularized porcine jejunal segment, prepared as previously published21. NOTE: As the polycarbonate frame cannot be sterilized by heat, disinfect it by 20 min incubation in sonic bath (40 kHz) followed by 3 times incubation in ethanol 70% for 25 min. Then, wash the frame 3 times with DPBS sterile.
- Place the biomatrix-frame system in a 100 mm Petri dish and fill it with the vascular endothelial growth factor + 1% Penicillin Streptomycin (Pen/Strep).
- Equilibrate the biomatrix by incubating overnight at 37 °C and 5% CO2.
After detaching and counting the cells, inject 10 x 107 HDMECs in 1000 µL of culture medium through the preserved vasculature of the biomatrix, with the use of a 2 mL syringe. NOTE: Inject ~700 µL through the arterial inlet and ~300 µL through the vein outlet.
Incubate at 37 °C and 5% CO2 for 3 h, to allow cell attachment.
Fill the bioreactor system with 350 mL of the vascular endothelial growth factor + 1% Pen/Strep and incubate at 37 °C and 5% CO2.
Place the frame inside the vessel of the bioreactor. Connect the arterial and venous pedicles to the vessel.
Start perfusion by connecting the bioreactor system to a Peristaltic pump. Set the pressure at 10 mmHg and amplitude ±1.
Increase the pressure stepwise every hour until reaching 100 mmHg of pressure, amplitude ±20.
Keep the bioreactor system for 7 days under these conditions at 37 °C and 5% CO2.
6. Addition of RFP-HDMECs to the biomatrix
Mix 330 µL of 0.4% collagen R solution, 170 µL of 0.1% acetic acid, and 50 µL of medium M199 in a 15 mL Falcon tube. Keep on ice.
Take the RCP microcarriers from the spinner flasks. Remove the culture medium completely.
Add the volume of NaOH 0.2 N required to neutralize the collagen mixture and immediately mix it with the RCP microcarriers.
Add the collagen gel-RCP microcarriers mixture on the lumen of the biomatrix. Allow gelation for 30 min.
In parallel, change the medium of the bioreactor by removing the medium completely and then add 350 mL of fresh, pre warmed, vascular endothelial growth factor + 1% Pen/Strep.
Connect the bioreactor to the Peristaltic pump and set up the pressure at 100 mmHg and amplitude ±20.
Change the medium every 7 days.
Keep the bioreactor in culture for 21 days.
7. Analysis and read-outs of bioreactor cultures
- Preparation of the samples
- Disconnect the biomatrix from the bioreactor and remove it from the polycarbonate frame.
- Cut the obtained piece into 6 sections: 2 for MTT, 2 for paraffin embedding/staining and 2 for SEM analysis.
- Paraffin embedding
- Place the sections in a Petri dish and wash with enough DPBS to cover them. Discard the DPBS.
- Fix the sections in 4% paraformaldehyde overnight.
- Prepare the cassettes for paraffin embedding and perform it according to standard protocols.
- Prepare the sections in paraffin blocks and cut samples of 3 µm for H&E staining, with the use of a microtome.
- H&E staining
- Stain rehydrated sections according to standard protocols32.
- Scanning electron microscopy (SEM) analysis
- Place the sections in a Petri dish and wash with enough DPBS to cover them. Discard the DPBS.
- Fix the sections with 4.75% glutaraldehyde overnight.
- Perform dehydration chain with increasing concentrations of ethanol 50% - 100%.
- Cover the sections with hexamethyldisilazane (HMDS) for 10 min. Discard the used HMDS and add fresh HMDS.
- Allow the sections to dry overnight under fume hood, and then prepare the samples for imaging.
- 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
- Mix the MTT reagent with the vascular endothelial growth factor, in a concentration of 1 mg/mL.
- Incubate the sections in the MTT solution for 90 min at 37 °C and 5% CO2.
- Observe the vascular structures and cell-seeded microcarriers macroscopically or under a bright-field microscope, to detect metabolically active cells. Acquire images.
Representative Results
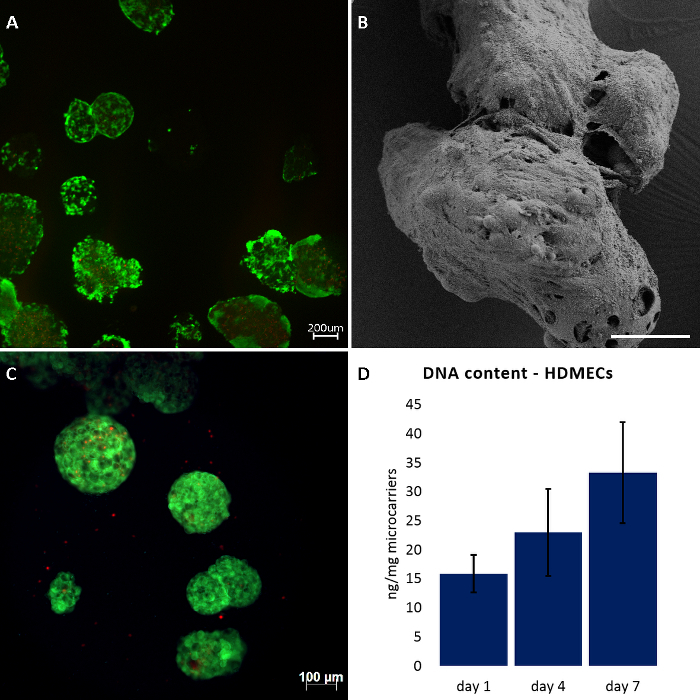
As shown in Figure 1A, we obtained high number of viable cells on the RCP microcarriers after 7 days of culture, determined by live/dead staining. Those results were confirmed by SEM analysis, in which completely colonized microcarriers were observed around the pores, partly overgrowing them (Figure 1B). On the other hand, experiments in which cells were not evenly seeded resulted in several empty microcarriers. Failed experiments are characterized by an abnormally high number of dead cells that should not be over 10% of the total cell amount (Figure 1C). Moreover, DNA content quantification of HDMECs showed an increase of dsDNA over time (Figure 1D).
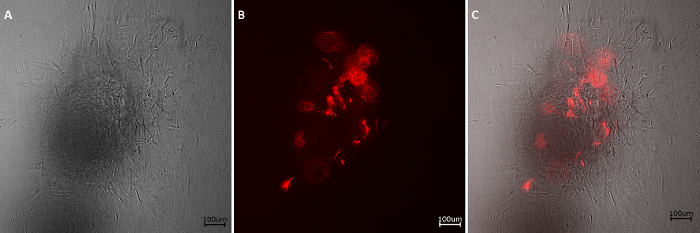
Additionally, RFP-HDMECs were able to maintain their functionality after culture on RCP microcarriers, as shown in Figure 2, where the cells adopted a sprouting phenotype when cultured in a collagen gel.
Furthermore, we used RFP-HDMECs-colonized RCP microcarriers to re-seed the lumen of the biomatrix BioVaSc. For this, we employed a bioreactor system for physiological perfusion of vascularized tissue equivalents22 (Figure 3C) in which we cut-open the biomatrix and placed it in a polycarbonate frame, so that the lumen was exposed in an even set-up (Figure 3B).
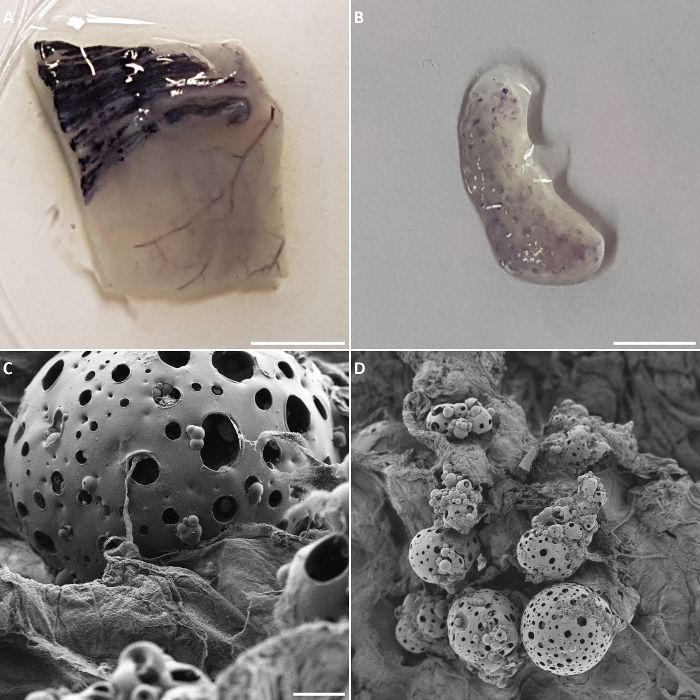
After 21 days of bioreactor cultures, metabolically active cells were present both on the preserved vasculature of the biomatrix as well as on the RCP microcarriers (Figure 4A andFigure 3B). Failed experiments or a wrong choice of slices during sectioning of the histological samples can lead to absence of colonizing endothelial cells in the lumen of the vessels of the biomatrix or on the microcarriers. These results could be confirmed by SEM analysis of the biomatrix sections, where it could be observed that some areas of the RCP microcarriers were still covered by cells and that the RCP microcarriers were in close proximity to the biomatrix (Figure 4C and 4D).
Finally, H&E staining confirmed the presence of HDMECs on the biomatrix, specifically in the vascular structures (Figure 5A). When observed under fluorescence microscope, the cells colonizing the vascular structures of the biomatrix resulted to be RFP-HDMECs (Figure 5B), indicating the migration of the cells from the RCP microcarriers to the biomatrix. Some RFP-HDMECs were also observed on the RCP microcarriers (Figure 5C and 5D). In experiments in which cells did not survived due to altered culturing conditions (e.g. shorter culture times), they could not be detected inside the vascular structure with none of the previously reported techniques (Figure 5E and 5F).
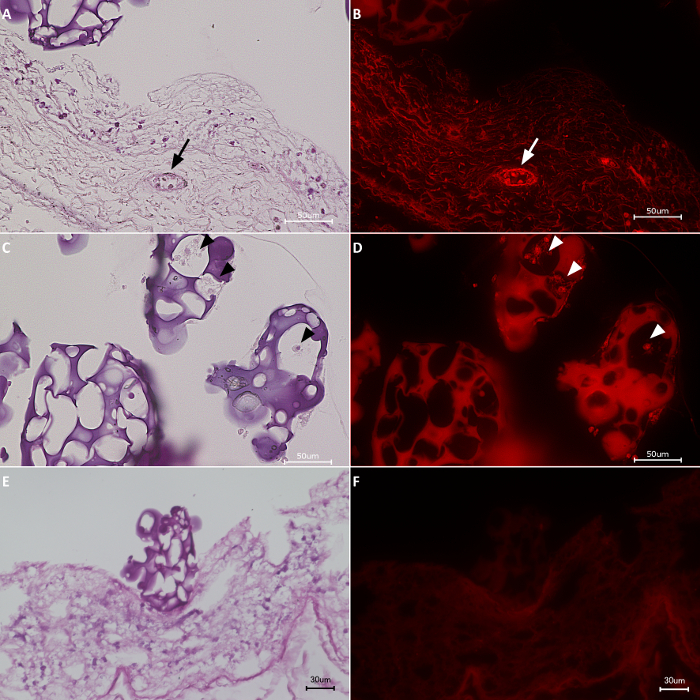
Figure 1: Culture of HDMECs and hBMSCs on RCP microcarriers.(A, C) live/dead staining after 7 days of dynamic cultures. (B) SEM analysis of HDMECs cultured on RCP microcarriers after 7 days of dynamic cultures. (D) DNA content determined by PicoGreen assay kit. Data expressed as mean ± standard deviation (n=3). Scale bars: 200 µm for A, 88 µm for B, and 100 µm for C. Please click here to view a larger version of this figure.
Figure 2: Sprouting assay. Sprouts of RFP-HDMECs can be observed under bright-field (A) and fluorescence microscopy (B), emerging from the colonized RCP microcarrier after 24 h of culture in a collagen gel. (C) Overlay image. Scale bars: 100 µm. Please click here to view a larger version of this figure.
Figure 3: Biomatrix and bioreactor set-up.(A) Preparation of the biomatrix and mounting on the polycarbonate frame (B). (C) Bioreactor set-up. The vessel is connected to the medium reservoir and the pressure bottle through silicon tubes, and the whole system is connected to a peristaltic pump. The pressure is measured through a pressure sensor. AI: arterial inlet; VO: venous outlet. This figure has been modified from Groeber et al.22 Please click here to view a larger version of this figure.
Figure 4: Bioreactor read-out. MTT assay performed on a biomatrix section after 21 days of culture, in which metabolically active cells were observed in the preserved vasculature of the biomatrix (A) as well as on the RCP microcarriers (B). (C, D) SEM images of RCP microcarriers on a biomatrix section. Scale bars: 0.28 cm for A, 0.45 cm for B, 47 µm for C, and 225 µm for D. Please click here to view a larger version of this figure.
Figure 5: H&E staining & fluorescence images.(A, C, E) H&E staining. (B, D, F) After 21 days of culture, RFP-HDMECs were observed inside of the vasculature of the biomatrix (arrows) as well as on the RCP microcarriers (arrow heads). Scale bars: 50 µm for A-D and 30 µm for E and F. Please click here to view a larger version of this figure.
Discussion
One main goal of microcarrier is the expansion of cells while maintaining their differentiation in order to deliver cells to the place of need. The represented method introduce RCP microcarriers where cells were able to attach, proliferate, and colonize the microcarriers with high cell density. This was observed by live/dead staining, in which more than 90% of viable cells were detected while only few dead cells were obtained after 7 days of dynamic cultures. Likewise, the SEM images confirmed that the cells covered the entire surface of the microcarriers after 7 days of cultures.
To ensure cell survival in 3D models, it is important to maintain the supply of oxygen and nutrients to the cells and allow removal of waste substances. Blood vessels are the responsible structures of this exchange process, in which endothelial cells play a key role since they are the cells lining the inner part of blood vessels23. They have the capability to proliferate, migrate, adhere, sprout, and form vessel-like structures24. These properties were maintained after culture of RFP-HDMECs on the RCP microcarriers, since it was observed that the cells were able to adopt the sprouting phenotype (see Figure 2). We could prove here that these colonized RCP microcarriers were effectively delivering primary endothelial cells to re-seed the lumen of former vessels of the BioVaSc biomatrix. This suggests our system to be suitable to improve the vascularization of tissue engineered implants for clinical applications. Derivatives of this matrix have been successfully used in vascularization approaches25, as well as in in vitro tumor test sytems21,26,27,28 and for the production of skin equivalents as alternatives to animal experimentation29. Here it is used in an open, flattened set-up and placed in a perfusion bioreactor as applied for the generation of tissue equivalents22.
Bioreactors have been used in tissue engineering to produce tissue equivalents that could help ease the demand of organs while reducing the associated risks like rejection and morbidity30. However, producing tissue-like constructs is a very complex process that involves the use of tissue-specific cell types, a suitable scaffold and appropriate growth factors that allow the proper differentiation and assembling into 3D tissues. One major drawback of the engineered constructs is the lack of a proper vascular network that supports cells survival both in vitro and in vivo17. Here we combine the RCP-colonized microcarriers with a decellularized matrix in a bioreactor model, providing the cell type required, a scaffold that allows cell adhesion and vessel formation and a specific culture medium that provides the essential factors for the cells to proliferate and maintain their functional properties. A significant result of this model is the ability of the HDMECs to migrate from the RCP microcarriers to the scaffold without any additional detachment or digestion as necessary in other approaches31. Afterwards, they colonized specifically the vascular structures of the matrix, proving the concept that macroporous microcarriers can be used as cell delivery system for Regenerative Medicine purposes.
Altogether, this protocol represents a promising strategy in regenerative medicine for obtaining a maximized cell expansion and it could serve as cell delivery to implantation sites, where it could improve vascularization support for tissue engineered grafts.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The research leading to these results has received funding from the European Union Seventh Framework Programme FP7/2007-2013 under grant agreement n° 607051 (BIO-INSPIRE). We thank Carolien van Spreuwel-Goossens from Fujifilm Manufacturing Europe B.V., for the technical assistance during RCP manufacturing, and Werner Stracke from Fraunhofer Institute for Silicate Research ISC, for assistance with the SEM analysis.
References
- Li B, et al. Past, present, and future of microcarrier-based tissue engineering. Journal of Orthopaedic. 2015;3(2):51–57. doi: 10.1016/j.jot.2015.02.003. Translation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ME, Costa AR, Henriques M, Azeredo J, Oliveira R. Evaluation of solid and porous microcarriers for cell growth and production of recombinant proteins. Methods Mol Biol. 2014;1104:137–147. doi: 10.1007/978-1-62703-733-4_10. [DOI] [PubMed] [Google Scholar]
- Akhmanova M, Osidak E, Domogatsky S, Rodin S, Domogatskaya A. Physical, Spatial, and Molecular Aspects of Extracellular Matrix of In Vivo Niches and Artificial Scaffolds Relevant to Stem Cells Research. Stem Cells Int. 2015;2015:167025. doi: 10.1155/2015/167025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sart S, Tsai AC, Li Y, Ma T. Three-dimensional aggregates of mesenchymal stem cells: cellular mechanisms, biological properties, and applications. Tissue Eng Part B. Rev. 2014;20:365–380. doi: 10.1089/ten.teb.2013.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, Malhotra M, Curtin CM, Brien FJO, O'Driscoll CM. Life in 3D is never flat: 3D models to optimise drug delivery. Journal of Controlled Release. 2015;215:39–54. doi: 10.1016/j.jconrel.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Tan KY, Reuveny S, Oh SKW. Recent advances in serum-free microcarrier expansion of mesenchymal stromal cells: Parameters to be optimized. Biochemical and Biophysical Research Communications. 2016;473:769–773. doi: 10.1016/j.bbrc.2015.09.078. [DOI] [PubMed] [Google Scholar]
- de Soure AM, Fernandes-Platzgummer A, da Silva CL, Cabral JM. Scalable microcarrier-based manufacturing of mesenchymal stem/stromal cells. J Biotechnol. 2016;236:88–109. doi: 10.1016/j.jbiotec.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Schop D, et al. Expansion of human mesenchymal stromal cells on microcarriers: growth and metabolism. J Tissue Eng Regen. Med. 2010;4:131–140. doi: 10.1002/term.224. [DOI] [PubMed] [Google Scholar]
- Carmelo JG, Fernandes-Platzgummer A, Diogo MM, da Silva CL, Cabral JM. A xeno-free microcarrier-based stirred culture system for the scalable expansion of human mesenchymal stem/stromal cells isolated from bone marrow and adipose tissue. Biotechnol J. 2015;10:1235–1247. doi: 10.1002/biot.201400586. [DOI] [PubMed] [Google Scholar]
- Malda J, Frondoza CG. Microcarriers in the engineering of cartilage and bone. Trends Biotechnol. 2006;24:299–304. doi: 10.1016/j.tibtech.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Chen AK, Reuveny S, Oh SK. Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: achievements and future direction. Biotechnol Adv. 2013;31:1032–1046. doi: 10.1016/j.biotechadv.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Confalonieri D, La Marca M, van Dongen E, Walles H, Ehlicke F. An Injectable Recombinant Collagen I Peptide-Based Macroporous Microcarrier Allows Superior Expansion of C2C12 and Human Bone Marrow-Derived Mesenchymal Stromal Cells and Supports Deposition of Mineralized Matrix. Tissue Eng Part A. 2017. [DOI] [PubMed]
- Davidenko N, et al. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta biomaterialia. 2015;25:131–142. doi: 10.1016/j.actbio.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin GZ, Park JH, Seo SJ, Kim HW. Dynamic cell culture on porous biopolymer microcarriers in a spinner flask for bone tissue engineering: a feasibility study. Biotechnol Lett. 2014;36:1539–1548. doi: 10.1007/s10529-014-1513-6. [DOI] [PubMed] [Google Scholar]
- Bae H, et al. Building Vascular Networks. Science Translational Medicine. 2012;4(160):160ps23. doi: 10.1126/scitranslmed.3003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Wang J, Hou J, Xing W, Liu C. Vascularization and bone regeneration in a critical sized defect using 2-N, 6-O-sulfated chitosan nanoparticles incorporating BMP-2. Biomaterials. 2014;35(2):684–698. doi: 10.1016/j.biomaterials.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Novosel E, Kleinhans C, Kluger P. Vascularization is the key challenge in tissue engineering. Advanced Drug Delivery Reviews. 2011;63(4-5):300–311. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Cartmell SH, Porter BD, García AJ, Guldberg RE. Effects of medium perfusion on cell-seeded three-dimensional bone constructs in vitro. Tissue Engineering. 2004;9(6):1197–1203. doi: 10.1089/10763270360728107. [DOI] [PubMed] [Google Scholar]
- Schanz J, Pusch J, Hansmann J, Walles H. Vascularised human tissue models: a new approach for the refinement of biomedical research. Journal of biotechnology. 2010;148(1):56–63. doi: 10.1016/j.jbiotec.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Steinke M, Gross R, Walles H, Schütze K, Walles T. An engineered 3D human airway mucosa model based on a SIS scaffold. Biomaterials. 2014;35(26):7355–7362. doi: 10.1016/j.biomaterials.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Moll C, et al. Tissue Engineering of a Human 3D in vitro Tumor Test System. J. Vis. Exp. 2013. p. e50460. [DOI] [PMC free article] [PubMed]
- Groeber F, Kahlig A, Loff S, Walles H, Hansmann J. A bioreactor system for interfacial culture and physiological perfusion of vascularized tissue equivalents. Biotechnology Journal. 2013;8:308–316. doi: 10.1002/biot.201200160. [DOI] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cells. New York: 2002. Blood vessels and endothelial cells. [Google Scholar]
- Logsdon EA, Finley SD, Popel AS, Gabhann FM. A systems biology view of blood vessel growth and remodeling. Journal of Cellular and Molecular Medicine. 2014;18(8):1491–1508. doi: 10.1111/jcmm.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller K, Dally I, Hartmann N, Münst B, Braspenning J, Walles H. Upcyte® Microvascular endothelial cells repopulate decellularized scaffold. Tissue Engineering Part C: Methods. 2012;19(1):57–67. doi: 10.1089/ten.tec.2011.0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nietzer S, et al. Mimicking Metastases Including Tumor Stroma: A New Technique to Generate a Three-Dimensional Colorectal Cancer Model Based on a Biological Decellularized Intestinal Scaffold. Tissue Engineering Part C: Methods. 2016;22(7):621–635. doi: 10.1089/ten.tec.2015.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlich C, et al. A Combined 3D Tissue Engineered In Vitro/In Silico Lung Tumor Model for Predicting Drug Effectiveness in Specific Mutational Backgrounds. J. Vis. Exp. 2016. p. e53885. [DOI] [PMC free article] [PubMed]
- Stratmann AT, et al. Establishment of a human 3D lung cancer model based on a biological tissue matrix combined with a Boolean in silico model. Molecular oncology. 2014;8(2):351–365. doi: 10.1016/j.molonc.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeber F, et al. A first vascularized skin equivalent for as an alternative to animal experimentation. Altex. 2016;33(4):415–422. doi: 10.14573/altex.1604041. [DOI] [PubMed] [Google Scholar]
- Plunkett N, O'Brien FJ. Bioreactors in tissue engineering. Technology and Health Care. 2011;19(1):55–69. doi: 10.3233/THC-2011-0605. [DOI] [PubMed] [Google Scholar]
- Nienow AW, Rafiq QA, Coopman K, Hewitt CJ. A potentially scalable method for the harvesting of hMSCs from microcarriers. Biochemical Engineering Journal. 2014;85:79–88. [Google Scholar]
- Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols. 2008;2008(5):pdb-prot4986. doi: 10.1101/pdb.prot4986. [DOI] [PubMed] [Google Scholar]


