Abstract
Patients with diabetes mellitus (DM) or those experiencing the neurotoxic effects of chemotherapeutic agents may develop sensation disorders due to degeneration and injury of small-diameter sensory neurons, referred to as small fiber neuropathy. Present animal models of small fiber neuropathy affect both large- and small-diameter sensory fibers and thus create a neuropathology too complex to properly assess the effects of injured small-diameter sensory fibers. Therefore, it is necessary to develop an experimental model of pure small fiber neuropathy to adequately examine these issues. This protocol describes an experimental model of small fiber neuropathy specifically affecting small-diameter sensory nerves with resiniferatoxin (RTX), an ultrapotent agonist of transient receptor potential vanilloid type 1 (TRPV1), through a single dose of intraperitoneal injection, referred to as RTX neuropathy. This RTX neuropathy showed pathological manifestations and behavioral abnormalities that mimic the clinical characteristics of patients with small fiber neuropathy, including intraepidermal nerve fiber (IENF) degeneration, specifically injury in small-diameter neurons, and induction of thermal hypoalgesia and mechanical allodynia. This protocol tested three doses of RTX (200, 50, and 10 µg/kg, respectively) and concluded that a critical dose of RTX (50 µg/kg) is required for the development of typical small fiber neuropathy manifestations, and prepared a modified immunostaining procedure to investigate IENF degeneration and neuronal soma injury. The modified procedure is fast, systematic, and economic. Behavioral evaluation of neuropathic pain is critical to reveal the function of small-diameter sensory nerves. The evaluation of mechanical thresholds in experimental rodents is particularly challenging and this protocol describes a customized metal mesh that is suitable for this type of assessment in rodents. In summary, RTX neuropathy is a new and easily established experimental model to evaluate the molecular significance and intervention underlying neuropathic pain for the development of therapeutic agents.
Keywords: Neuroscience, Issue 132, Resiniferatoxin (RTX), von Frey hair filament, hot plate test, mechanical allodynia, thermal hypoalgesia, transient receptor potential vanilloid type 1 (TRPV1), small fiber neuropathy, nerve injury, activating transcription factor-3 (ATF3)
Introduction
Small fiber neuropathy involving neuropathic pain, which is evident by the degeneration of IENFs, is common in various types of conditions, such as DM, and as a result of the neurotoxic effects of chemotherapeutic agents1,2,3,4,5. IENFs are the peripheral terminals of small-diameter neurons located in the dorsal root ganglia (DRG), and are affected in parallel in cases of IENF degeneration6. For example, the altered upstream genetic transcription of neuronal somata has been demonstrated by the upregulation of activating transcription factor-3 (ATF3)6,7. Moreover, the evaluation of IENFs innervation with skin biopsy is useful for the diagnosis of small fiber neuropathy5,8,9. Traditionally, the profiles of the IENFs on the skin biopsy have depended on immunohistochemical demonstration of protein gene product 9.5 (PGP 9.5)1,10,11. Taken together, the pathological profiles of DRG and IENFs reflect the functional condition underlying small fiber neuropathy and may be an indicator for the functional consequences of this type of neuropathy on small-diameter neurons.
Previously, several experimental models have addressed the issue of IENF degeneration in cases of chemotherapy-induced neuropathy12,13 and nerve injury caused by compression or transection14,15,16. These experimental models also affected large-diameter nerves; it was, therefore, not possible to exclude the contribution of affected large-diameter nerves in the observed small fiber neuropathy; for instance, the examination of thermosensation disorder by noxious withdrawal depends on functional motor nerve fibers17,18,19. Thus, establishing a pure small fiber neuropathy model and systematically investigating the pathological status of both neuronal somata and their peripheral cutaneous nerve fibers in small-diameter neurons are necessary and imperative.
RTX is a capsaicin analogue and a potent agonist to transient receptor potential vanilloid receptor 1 (TRPV1), which mediates nociceptive processing20,21,22. Recently, peripheral RTX treatment relieved neurogenic pain23,24,25 and an intraganglionic injection of RTX induced irreversible loss of DRG neurons22. The effect of peripheral RTX administration is dose-dependent20,26,27, which resulted in the transient desensitization or degeneration of IENFs. Intriguingly, systematic high-dose RTX treatment led to neuropathic pain28, a symptom of small fiber neuropathy. These findings suggest that the treatment mode and dose of RTX produce distinct pathological effects and neuronal responses; to wit, peripheral administration prevented pain transmission by local effects29 and affected the neuronal somata that developed neuropathic behavior6. Collectively, these findings indicate that RTX has a multipotency effect and raised the issue whether there is a specific dose of RTX that could systematically affect the peripheral nerves, such as the peripheral IENFs and central neuronal somata. If so, RTX might be a potential agent to specifically affect small-diameter neurons and mimic small fiber neuropathy in the clinic. For example, DM in the clinic is a complicated issue including metabolic disorder and neuropathology of peripheral nerves, which are the main characteristics of small fiber neuropathy. The mechanisms of DM-associated small fiber neuropathy could not exclude the contribution of metabolic disorder that may not be the main agent affecting peripheral nerves. Therefore, DM-associated small fiber neuropathy requires a pure animal model that could exclude the effects of systematic metabolic disorder. This protocol describes the working dose of RTX to develop a typical small fiber neuropathy model, including IENF degeneration and small-diameter neuron injury, as demonstrated by modified immunostaining analysis.
Protocol
All the procedures described are in accordance with ethical guidelines for laboratory animals30, and the protocol has been approved by the Animal Committee of Kaohsiung Medical University, Kaohsiung, Taiwan.
1. Establishment of RTX Neuropathy
CAUTION: RTX is neurotoxic and hazardous. On contact, it acts as an irritant to the eyes, mucous membranes, and upper respiratory tract. Avoid inhalation and wear lab eyeglasses and coats during RTX preparation. Rinse with plenty of water in case of skin contact or after handling.
Add 1 mg of RTX powder to a 200 µL of mixture of equal volume of Tween 80 and absolute ethanol (100 µL for each solvent).
Aliquot the RTX solution (12 µL/vial) and store at -20 °C, for up to 3 months. This constitutes the RTX stock; discard the remaining RTX solution at expiration.
Dilute the RTX stock with normal saline to a final volume of 600 µL. The final concentration of the RTX solution should be 0.01%, which equals 1 µg RTX in 10 µL vehicle solution.
Use 8-week-old adult male ICR mice (35-40 g) as experimental animals and administer a single dose of RTX solution (dose: 200, 50, and 10 µg/kg, respectively) intraperitoneally (i.p.) with a microinjection syringe to the mice. Mice was anesthetized by inhaler with 5 % isoflurane for deep anesthesia. If mice showed withdrawal action of extremities during RTX injection, mice should take longer inhalation of anesthesia. Example: If the mouse weighs 40 g, then it will receive 20 µL of the RTX solution, representing the dose of 50 µg/kg.
Give one group of mice an equal volume of vehicle (10% Tween 80 and 10% absolute ethanol in saline solution), as a control.
After RTX injection, return the mice to a plastic cage on a 12-h light/12-h dark cycle and provide food and water ad libitum.
2. Evaluation of Neuropathic Behavior
NOTE: Maintain the animals in a comfortable environment (step 1.6) to allow recovery after injection. At day 7 post RTX injection (D7), each animal performs the hot plate and von Frey hair filament tests on the same day to reduce time bias and promote the efficiency of the behavioral tests. Bring the animals to a quiet room that is maintained at a stable humidity (40%) and temperature (27 °C) for optimizing animal acclimatization and reducing environmental effects during the behavioral testing. Do not disturb the animals during test periods; the behavioral tests are scheduled weekly.
- Measurement of thermal latencies with the hot plate test
- Place the animal gently on a metal hot plate (27 cm × 29 cm) with a transparent Plexiglas cage (length × width × height: 22 cm × 22 cm × 25 cm; Figure 1A). Set the temperature of the metal hot plate to 52 °C.
- Start measuring the duration of the animal's thermal latency on the hot plate with the step-on, built-in timer of the hot plate once the animal's hindpaws touch the hot plate, and observe the responses of the animal's hindpaws. If the animal shows shaking, licking of hindpaws, or jumping while on the hot plate, remove it and record the duration that the animal remained on the hot plate. This time duration defines the thermal latency of an individual animal. Record the thermal latency to the nearest 0.1 s.
- For each test session, perform three trials with 30-min intervals for response normalization after the last hot plate test. If the animal shows no response on the hot plate, stop the session after 25 s to avoid potential tissue damage.
- Measurement of mechanical threshold with the von Frey hair filament test
- Put the animal on the customized metal mesh (mesh size: 5 mm × 5 mm) with a semi-transparent cylinder Plexiglas cage (diameter: 13 cm; height: 12 cm) (Figure 1B) for acclimatization for at least 2 h.
- Apply the different calibers of the von Frey hair filaments to the plantar region of the hindpaw with the up-and-down method31. Start initial application from the middle force of a set of von Frey hair filaments for a duration of filament application of 5-8 s.
- Use a 2 min interval between filament applications to optimize animal normalization. Change the applied filament force based on the animal's last response. NOTE: A set of von Frey hair filaments consists of 0.064, 0.085, 0.145, 0.32, 0.39, 1.1, and 1.7 g force application. For example, if hindpaw withdrawal occurred with an initial force of 0.32 g, then apply 0.145 g. In the absence of paw withdrawal, a 0.39 g force is then applied. Then four additional filaments of varying forces are applied based on the preceding responses and the mechanical threshold is calculated according to a published formula31.
- For each test session, include the bilateral hindpaws. Perform three trials for each hindpaw. Express the average of these six mechanical thresholds as the mean mechanical threshold (mg) of each animal.
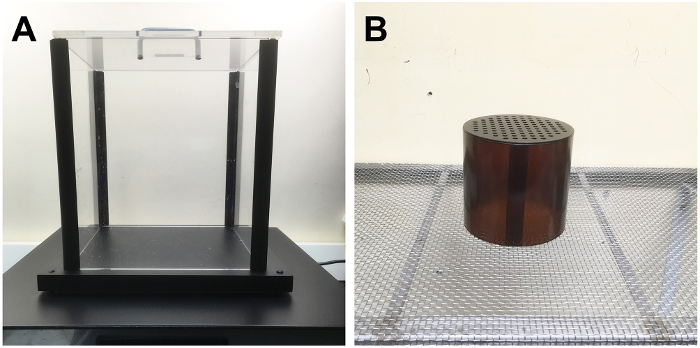
Figure 1. Custom-made Plexiglas cage and metal mesh for the evaluation of neuropathic pain in the mouse model of resiniferatoxin (RTX)-induced small fiber neuropathy. (A, B) These graphs show the equipment used to measure (A) the thermal latencies by a metal hot plate (27 cm × 29 cm) with a transparent Plexiglas cage (length × width × height: 22 cm × 22 cm × 25 cm) and evaluate (B) the mechanical threshold by a customized metal mesh (mesh size: 5 mm × 5 mm) with a semi-transparent cylinder Plexiglas cage (diameter: 13 cm; height: 12 cm) in the mice with RTX-induced small fiber neuropathy. Please click here to view a larger version of this figure.
3. Skin Biopsy Preparation and Evaluation of IENFs Innervation
After behavior testing, anesthetize the animals with 5% isoflurane and sacrifice the animals by intracardiac perfusion with 0.1 M phosphate buffer (PB) (pH 7.4) followed by 4% paraformaldehyde (4P) in 0.1 M PB.
Cut the first footpads of the two hindpaws after perfusion and post-fixate them in 4P for another 6 h. Transfer the footpad tissue to 0.1 M PB at 4 °C for long-term storage.
Cryoprotect footpads with 30% sucrose in PB overnight and cut in a vertical to plantar surface manner in 30-µm thick slices. Label the footpad sections sequentially, and then store in antifreeze at -20 °C. NOTE: The antifreeze composition is as follows: distilled water, ethylene glycerol, glycerol, and 2x PB in a 3:3:3:1 ratio.
- To ensure adequate sampling, select every third section of the footpad.
- Put the chosen footpad sections on coated glass slides and air-dry them.
- Cover a plastic coverplate on the slide and process with standard immunostaining procedures.
- Quench the footpad sections with 1% H2O2 in methanol for 30 min and block with 0.5% nonfat dry milk and 0.1% Triton X-100 in 0.5 M Tris buffer (Tris) for 1 h.
- Incubate footpad sections with antisera against pan axonal marker, PGP 9.5 (raised in rabbit; 1:1,000), overnight at 4 °C.
- Incubate footpad sections with a biotinylated goat anti-rabbit IgG secondary antibody at room temperature (RT) for 1 h, and then incubate with the avidin-biotin complex at RT for 45 min.
- Visualize the reaction product with 0.05% 3,3'-diaminobenzidine (DAB) solution for 45 s. Then wash footpad sections with distilled water and air-dry them for mounting. NOTE: The primary and secondary antisera are diluted with 0.5% nonfat dry milk in 0.5 M Tris.
4. DRG Section Preparation and Evaluation of Injured Small-diameter Neurons
Dissect the 4th and 5th lumbar DRG and post-fix for another 2 h.
Cryoprotect DRG tissues with 30% sucrose in PB overnight and cut to an 8-µm thickness sequentially, place on microscope slides, and label. Store DRG sections in a -80 °C freezer.
- Immunostain DRG sections in 80 µm-intervals to ensure adequate sampling.
- Perform DRG immunostaining procedures as those of the footpad sections, except for the double-labeling immunofluorescent procedures. Alternatively, include ATF3 (raised in rabbit; 1:100), an injury marker, and peripherin (raised in mouse; 1:800), a small-diameter neuronal marker in the primary antisera.
- Incubate DRG sections with the mixture of primary antisera overnight at 4 °C.
Incubate DRG sections with either Texas red or fluorescein isothiocyanate (FITC)-conjugated secondary antisera (1:200), corresponding to the appropriate primary antisera at RT for 1 h, and then mount for quantification.
Representative Results
This protocol describes a novel mouse model of RTX neuropathy, which specifically affects small-diameter neurons, including IENF degeneration, associated with sensory disorders (Figure 2). Following the protocol described herein, animals exhibited thermal hypoalgesia and mechanical allodynia at D7 post RTX injection. To establish this small fiber neuropathy model, three doses of RTX: 200, 50, and 10 µg/kg were administered by the i.p. route. The RTX dose (50 µg/kg) was deemed critical and the preliminary study showed that high-dose RTX (200 µg/kg) caused high mouse lethality (Figure 3).
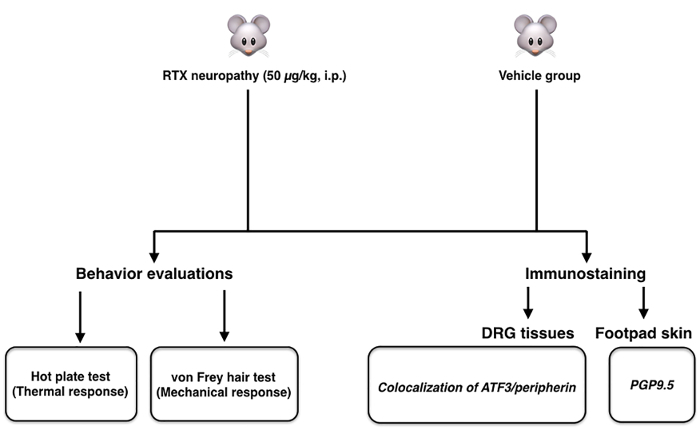
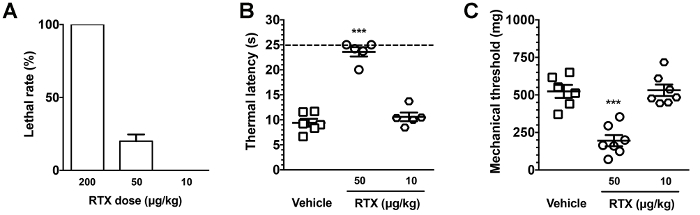
Figure 2. Scheme of the mouse model of resiniferatoxin (RTX)-induced small fiber neuropathy. The scheme shows the protocol of established RTX-induced small fiber neuropathy. For systematic assessment, behavior evaluation, and neuropathological examination, it included the hot plate and von Frey tests, and double-labeled immunostaining studies, respectively. Please click here to view a larger version of this figure.
Figure 3. Dose-effect of resiniferatoxin (RTX) on animal lethality and behavioral dysfunction. (A) Diverse doses of RTX were administered by intraperitoneal (i.p.) injection. The lethality of the dose effect was dose-dependent; for example, a high-dose of RTX (200 µg/kg) caused 100% lethality. (B, C) Thermal latencies and mechanical thresholds were evaluated with the hot plate (B) and von Frey filament tests (C), respectively. A 50 µg/kg dose of RTX induced thermal hypoalgesia and mechanical allodynia compared to the vehicle and the 10 µg/kg-administered group. Open square, vehicle; open circle, 50 µg/kg; open diamond, 10 µg/kg. Dashed line in (B), cutoff time point of hot plate test. *** p < 0.001. Please click here to view a larger version of this figure.
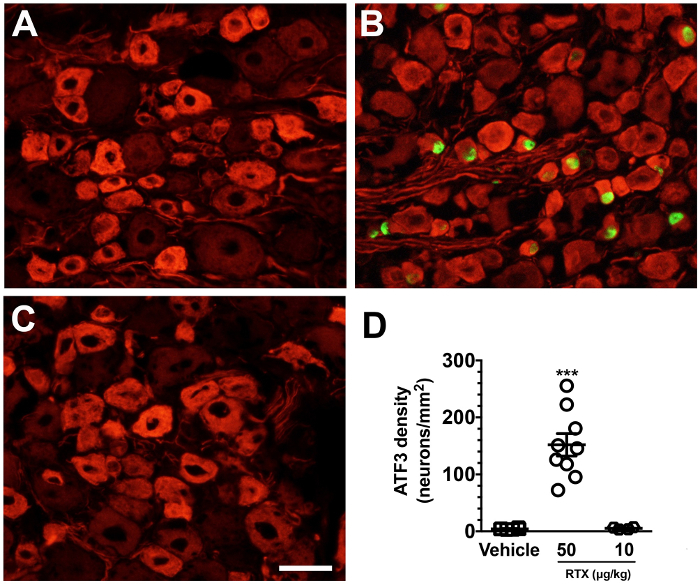
Pathologically, there was IENF degeneration and marked ATF3 induction. Double-labeling studies showed that injured neurons were specifically peripherin(+) small-diameter neurons. In contrast, the low-dose of RTX (10 µg/kg) did not establish small fiber neuropathy, including no changes in IENF innervation (Figure 4) and no neuronal injury (ATF3 induction) (Figure 5). Accordingly, this protocol considers the 50 µg/kg dose critical to establishing the mouse model of small fiber neuropathy.
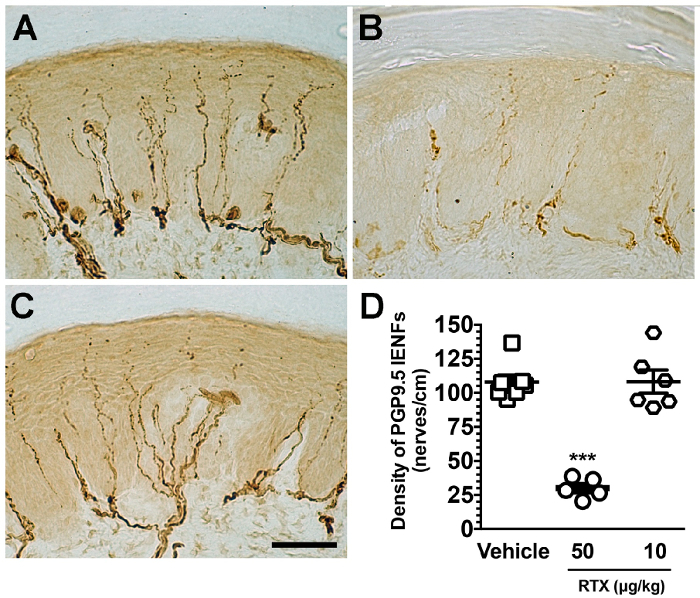
In summary, systematic RTX administration with a 50 µg/kg dose specifically affected small nerve fibers. For instance, it led to neuronal soma injury and peripheral IENF degeneration, which are associated with sensory disorders.
Figure 4. Degeneration of intraepidermal nerve fibers (IENFs) in resiniferatoxin (RTX) neuropathy. (A-C) Tissue sections from the footpad skin of mice were immunostained with anti-protein gene product 9.5 (PGP 9.5) antisera in the vehicle (A), 50 µg/kg- (B), and 10 µg/kg-administered (C) groups. PGP 9.5(+) IENFs arise from the subepidermal nerve plexus with a typical varicose appearance. PGP 9.5 (+) IENFs are markedly reduced in the 50 µg/kg, but not in the 10 µg/kg group. (D) IENFs were quantitated according to the immunohistochemical results of A-C. Open square, vehicle; open circle, 50 µg/kg; open diamond, 10 µg/kg. *** p < 0.001 compared to the vehicle group. Scale bar, 50 µm. Please click here to view a larger version of this figure.
Figure 5. Specificity of small-diameter neuron injury in resiniferatoxin (RTX) neuropathy. (A-C) Double-labeling immunofluorescent staining was performed with anti-activating transcription factor-3 (ATF3; A-C, in green) and peripherin (A-C, in red) in the vehicle (A), 50 µg/kg- (B), and 10 µg/kg-administered (C) groups. (D) The diagram indicates the density changes of ATF3(+) neurons. ATF3(+) neurons were increased in the 50 µg/kg, but not in the vehicle and 10 µg/kg groups. Open square, vehicle; open circle, 50 µg/kg; open diamond, 10 µg/kg. *** p < 0.001 compared to the vehicle group. Scale bar, 25 µm. Please click here to view a larger version of this figure.
Discussion
Efficacious therapy of small fiber neuropathy in the clinic is required for promoting the functional recovery and life quality of patients. Currently, there is lack of a therapeutic guide targeting sensory disorder associated with small fiber neuropathy due to lack of comprehensive understanding of the molecular mechanisms underlying small-diameter neuronal injury. Previous models of neuropathy usually affected both large- and small-diameter sensory nerves; for instance, the models of chemotherapy-induced neuropathy12,32,33 and mechanical-induced neuropathy34,35. Thus, the contribution of motor weakness and large-diameter sensory nerve damage could not be completely excluded in the behavioral testing of these neuropathy models. The present protocol describes a new model of small fiber neuropathy in mice, which only affects small-diameter sensory nerves by providing pathological and functional evidence of IENFs degeneration.
RTX is an ultrapotent agonist of TRPV1 and a capsaicin analogue, which may cause loss of peptidergic DRG neurons in culture36 and in vivo systems18,19. Previous studies on RTX and capsaicin have mainly focused on the morphological or functional loss of DRG neuronal cell bodies, which revealed the role of TRPV1 in the thermal transmission response37,38,39. Moreover, a previous study demonstrated systematic high-dose RTX treatment (200 µg/kg) in rats, induced mechanical allodynia and thermal hypoalgesia, possibly due to pathology of large-diameter nerve fibers28. The dose of 200 µg/kg, however, is a lethal dose in mice and this current protocol developed a pure small fiber neuropathy model by reducing the RTX dose (50 µg/kg). This dose of RTX (50 µg/kg) is critical to establishing a pure small fiber neuropathy model, which is superior to that previously reported28, as it spares large fibers18. That is, it only affects small nerve fibers; to wit, only small-diameter neurons were injured, as confirmed by the induction of ATF3 upregulation6,40 on small-diameter DRG neurons and IENFs degeneration6,18,19,41, associated with sensory disorders. These pathological manifestations comprehensively mimic the clinical symptoms of small fiber neuropathy. Moreover, this current model induced the typical neuropathology and neuropathic pain profile of small fiber neuropathy and the effects lasted for 8 weeks post RTX treatment6,18,19. The durations of neuropathology and neuropathic pain were equivalent and could be reversed by promoting the synthesis of nerve growth factor (NGF)18,40,41. Collectively, this protocol both established a pure small fiber neuropathy model and highlighted the possible therapeutic potential of NGF.
Clinically, the gold standard for investigating neuropathies affecting small-diameter nociceptive nerves8,9 is biopsying limb skin for evaluating skin innervation. Our current report applied this technique to the footpad skin of experimental animals to evaluate the skin innervation of a small fiber neuropathy model, which could mimic the pathology of IENFs in the clinic, and also investigated the morphological profiles of DRG sections with the injury marker, ATF3, to reveal the pathological status of neuronal somata. Notably, the spatial distributions of IENFs within the epidermis are highly branching and the counting criteria are the major factor leading to statistical differences among groups. For example, our current protocol counted each IENF with branching points only in the dermis and IENFs with branching points within the epidermis as a single IENF14,18,19. This criterion may have caused a lower density of IENFs in our investigations than in those of other groups. We prepared and processed the skin and DRG sections of experimental animals in a systematic and bulk-evaluation fashion with our current modified protocol. Accordingly, these systematic investigations of IENF degeneration and neuronal injury could avoid the stereological bias of functional and pathological conditions of small-diameter neurons in small fiber neuropathy.
The functional evaluation of small-diameter nerves with behavioral testing, particularly with innoxious von Frey hair filament application, has been traditionally applied to patients' skin for diagnosing the mechanical sensitivity underlying small fiber neuropathy. The observation of mechanical allodynia in experimental animals is challenging due to foot grounding on the metal mesh, which is considered to be exogenous mechanical stimulation, and the animals are highly active during tests. The current protocol optimized a specific sized mesh wire floor (5 mm × 5 mm) in a semi-transparent plastic cage for the environmental adaptation of experimental animals for the behavioral tests. This size of the mesh floor could reduce the exogenous stimulation of foot grounding and avoid foot-dropping.
This RTX mouse model of neuropathy could be applied to different types of small fiber neuropathy, such as diabetes, which is associated with IENF degeneration1,42. However, this model remains limited. For example, the animal model of spinal nerve ligation43 with the characteristics of small fiber neuropathy, referred to as the radiculopathy in the clinic, may also affect the large fibers in the spinal rootlet.
Disclosures
The authors have nothing to disclose
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology (106-2320-B-037-024), Kaohsiung Medical University (KMU-M106028, KMU-S105034) and Aim for the Top Universities Grant (TP105PR15), Kaohsiung Medical University, Taiwan.
References
- Shun CT, et al. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain. 2004;127(Pt 7):1593–1605. doi: 10.1093/brain/awh180. [DOI] [PubMed] [Google Scholar]
- Polydefkis M, et al. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127(Pt 7):1606–1615. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- Holland NR, et al. Small-fiber sensory neuropathies: clinical course and neuropathology of idiopathic cases. Ann Neurol. 1998;44(1):47–59. doi: 10.1002/ana.410440111. [DOI] [PubMed] [Google Scholar]
- Chaudhry V, Rowinsky EK, Sartorius SE, Donehower RC, Cornblath DR. Peripheral neuropathy from taxol and cisplatin combination chemotherapy: clinical and electrophysiological studies. Ann Neurol. 1994;35(3):304–311. doi: 10.1002/ana.410350310. [DOI] [PubMed] [Google Scholar]
- Mellgren SI, Nolano M, Sommer C. The cutaneous nerve biopsy: technical aspects, indications, and contribution. Handb Clin Neurol. 2013;115:171–188. doi: 10.1016/B978-0-444-52902-2.00010-2. [DOI] [PubMed] [Google Scholar]
- Hsieh YL, Chiang H, Lue JH, Hsieh ST. P2X3-mediated peripheral sensitization of neuropathic pain in resiniferatoxin-induced neuropathy. Exp Neurol. 2012;235(1):316–325. doi: 10.1016/j.expneurol.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, et al. Re-evaluation of the phenotypic changes in L4 dorsal root ganglion neurons after L5 spinal nerve ligation. Pain. 2012;153(1):68–79. doi: 10.1016/j.pain.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Joint Task Force of the E, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. J Peripher Nerv Syst. 2010;15(2):79–92. doi: 10.1111/j.1529-8027.2010.00269.x. [DOI] [PubMed] [Google Scholar]
- Hsieh ST. Pathology and functional diagnosis of small-fiber painful neuropathy. Acta Neurol Taiwan. 2010;19(2):82–89. [PubMed] [Google Scholar]
- Kennedy WR, Wendelschafer-Crabb G. Utility of the skin biopsy method in studies of diabetic neuropathy. Electroencephalogr Clin Neurophysiol Suppl. 1999;50:553–559. [PubMed] [Google Scholar]
- Kennedy WR. Opportunities afforded by the study of unmyelinated nerves in skin and other organs. Muscle Nerve. 2004;29(6):756–767. doi: 10.1002/mus.20062. [DOI] [PubMed] [Google Scholar]
- Verdu E, et al. Physiological and immunohistochemical characterization of cisplatin-induced neuropathy in mice. Muscle Nerve. 1999;22(3):329–340. doi: 10.1002/(sici)1097-4598(199903)22:3<329::aid-mus5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Ko MH, Hu ME, Hsieh YL, Lan CT, Tseng TJ. Peptidergic intraepidermal nerve fibers in the skin contribute to the neuropathic pain in paclitaxel-induced peripheral neuropathy. Neuropeptides. 2014;48(3):109–117. doi: 10.1016/j.npep.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Hsieh ST, Chiang HY, Lin WM. Pathology of nerve terminal degeneration in the skin. J Neuropathol Exp Neurol. 2000;59(4):297–307. doi: 10.1093/jnen/59.4.297. [DOI] [PubMed] [Google Scholar]
- Tseng TJ, Hsieh YL, Ko MH, Hsieh ST. Redistribution of voltage-gated sodium channels after nerve decompression contributes to relieve neuropathic pain in chronic constriction injury. Brain Res. 2014;1589:15–25. doi: 10.1016/j.brainres.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Hsieh YL, Lin WM, Lue JH, Chang MF, Hsieh ST. Effects of 4-methylcatechol on skin reinnervation: promotion of cutaneous nerve regeneration after crush injury. J Neuropathol Exp Neurol. 2009;68(12):1269–1281. doi: 10.1097/NEN.0b013e3181c17b46. [DOI] [PubMed] [Google Scholar]
- Tseng TJ, Chen CC, Hsieh YL, Hsieh ST. Effects of decompression on neuropathic pain behaviors and skin reinnervation in chronic constriction injury. Exp Neurol. 2007;204(2):574–582. doi: 10.1016/j.expneurol.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Hsieh YL, Chiang H, Tseng TJ, Hsieh ST. Enhancement of cutaneous nerve regeneration by 4-methylcatechol in resiniferatoxin-induced neuropathy. J Neuropathol Exp Neurol. 2008;67(2):93–104. doi: 10.1097/nen.0b013e3181630bb8. [DOI] [PubMed] [Google Scholar]
- Hsieh YL, et al. Role of Peptidergic Nerve Terminals in the Skin: Reversal of Thermal Sensation by Calcitonin Gene-Related Peptide in TRPV1-Depleted Neuropathy. PLoS One. 2012;7(11):e50805. doi: 10.1371/journal.pone.0050805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert JK, et al. Peripherally induced resiniferatoxin analgesia. Pain. 2003;104(1-2):219–228. doi: 10.1016/s0304-3959(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Almasi R, Petho G, Bolcskei K, Szolcsanyi J. Effect of resiniferatoxin on the noxious heat threshold temperature in the rat: a novel heat allodynia model sensitive to analgesics. Br J Pharmacol. 2003;139(1):49–58. doi: 10.1038/sj.bjp.0705234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karai L, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113(9):1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis A, et al. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology. 2005;65(2):400–405. doi: 10.1016/j.urology.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Helyes Z, et al. Antiinflammatory and analgesic effects of somatostatin released from capsaicin-sensitive sensory nerve terminals in a Freund's adjuvant-induced chronic arthritis model in the rat. Arthritis Rheum. 2004;50(5):1677–1685. doi: 10.1002/art.20184. [DOI] [PubMed] [Google Scholar]
- Kissin I, Bright CA, Bradley EL., Jr Selective and long-lasting neural blockade with resiniferatoxin prevents inflammatory pain hypersensitivity. Anesth Analg. 2002;94(5):1253–1258. doi: 10.1097/00000539-200205000-00038. table of contents. [DOI] [PubMed] [Google Scholar]
- Helyes Z, et al. Inhibitory effect of anandamide on resiniferatoxin-induced sensory neuropeptide release in vivo and neuropathic hyperalgesia in the rat. Life Sci. 2003;73(18):2345–2353. doi: 10.1016/s0024-3205(03)00651-9. [DOI] [PubMed] [Google Scholar]
- Kissin I. Vanilloid-induced conduction analgesia: selective, dose-dependent, long-lasting, with a low level of potential neurotoxicity. Anesthesia and analgesia. 2008;107(1):271–281. doi: 10.1213/ane.0b013e318162cfa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan HL, Khan GM, Alloway KD, Chen SR. Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats: mechanism of action. J Neurosci. 2003;23(7):2911–2919. doi: 10.1523/JNEUROSCI.23-07-02911.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola MJ, Mannes AJ. The vanilloid agonist resiniferatoxin for interventional-based pain control. Current topics in medicinal chemistry. 2011;11(17):2171–2179. doi: 10.2174/156802611796904942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, et al. Physiological characterization of Taxol-induced large-fiber sensory neuropathy in the rat. Ann Neurol. 1998;43(1):46–55. doi: 10.1002/ana.410430111. [DOI] [PubMed] [Google Scholar]
- Lipton RB, et al. Taxol produces a predominantly sensory neuropathy. Neurology. 1989;39(3):368–373. doi: 10.1212/wnl.39.3.368. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Ko MH, Hsieh YL, Hsieh ST, Tseng TJ. Nerve demyelination increases metabotropic glutamate receptor subtype 5 expression in peripheral painful mononeuropathy. Int J Mol Sci. 2015;16(3):4642–4665. doi: 10.3390/ijms16034642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeftinija S, Liu F, Jeftinija K, Urban L. Effect of capsaicin and resiniferatoxin on peptidergic neurons in cultured dorsal root ganglion. Regul Pept. 1992;39(2-3):123–135. doi: 10.1016/0167-0115(92)90534-2. [DOI] [PubMed] [Google Scholar]
- Caudle RM, et al. Resiniferatoxin-induced loss of plasma membrane in vanilloid receptor expressing cells. Neurotoxicology. 2003;24(6):895–908. doi: 10.1016/S0161-813X(03)00146-3. [DOI] [PubMed] [Google Scholar]
- Acs G, Biro T, Acs P, Modarres S, Blumberg PM. Differential activation and desensitization of sensory neurons by resiniferatoxin. J Neurosci. 1997;17(14):5622–5628. doi: 10.1523/JNEUROSCI.17-14-05622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiou A, et al. Vanilloid receptor agonists and antagonists are mitochondrial inhibitors: how vanilloids cause non-vanilloid receptor mediated cell death. Biochem Biophys Res Commun. 2007;354(1):50–55. doi: 10.1016/j.bbrc.2006.12.179. [DOI] [PubMed] [Google Scholar]
- Wu CH, Ho WY, Lee YC, Lin CL, Hsieh YL. EXPRESS: NGF-trkA signaling modulates the analgesic effects of prostatic acid phosphatase in resiniferatoxin-induced neuropathy. Mol Pain. 2016;12 doi: 10.1177/1744806916656846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao TH, Fu YS, Ho WY, Chen TH, Hsieh YL. Promotion of thermal analgesia and neuropeptidergic skin reinnervation by 4-methylcatechol in resiniferatoxin-induced neuropathy. Kaohsiung J Med Sci. 2013;29(8):405–411. doi: 10.1016/j.kjms.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Chao CC, et al. Pathophysiology of neuropathic pain in type 2 diabetes: skin denervation and contact heat-evoked potentials. Diabetes Care. 2010;33(12):2654–2659. doi: 10.2337/dc10-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]


