Abstract
Human small intestinal enteroids are derived from the crypts and when grown in a stem cell niche contain all of the epithelial cell types. The ability to establish human enteroid ex vivo culture systems are important to model intestinal pathophysiology and to study the particular cellular responses involved. In recent years, enteroids from mice and humans are being cultured, passaged, and banked away for future use in several laboratories across the world. This enteroid platform can be used to test the effects of various treatments and drugs and what effects are exerted on different cell types in the intestine. Here, a protocol for establishing primary stem cell-derived small intestinal enteroids derived from neonatal mice and premature human intestine is provided. Moreover, this enteroid culture system was utilized to test the effects of species-specific breast milk. Mouse breast milk can be obtained efficiently using a modified human breast pump and expressed mouse milk can then be used for further research experiments. We now demonstrate the effects of expressed mouse, human, and donor breast milk on the growth and proliferation of enteroids derived from neonatal mice or premature human small intestine.
Keywords: Medicine, Issue 132, Enteroids, intestinal stem cells, mini-guts, organoids, necrotizing enterocolitis, breast milk, milking
Introduction
Necrotizing enterocolitis (NEC) is the leading cause of death from gastrointestinal disease in premature infants, affecting nearly 1 in 10 infants born before 29 weeks gestation1,2,3. Half of the infants with NEC progress to the most severe form, where survival is only 10 - 30%4,5. In the United States, an estimated 2 - 3 billion USD/year are spent treating infants with NEC6,7, yet neither the survival rate nor therapy has changed over the past 30 years. The pathogenesis of NEC is characterized by intestinal injury and impaired mucosal healing8,9,10,11, however, the signaling pathways leading to an exacerbated inflammatory response and mechanisms to reverse the inflammation remain incompletely understood.
The administration of human breast milk has been found to be the only protective strategy against NEC for premature infants. We have previously shown that breast milk protects against NEC development through inhibition of the innate immune receptor toll-like receptor 4 (TLR4) in the intestinal epithelium via the epidermal growth factor receptor (EGFR) signaling pathway11. Supplementation of breast milk to an experimental NEC formula attenuated the inflammatory response seen in NEC as demonstrated by inhibition of enterocyte apoptosis and restoration of enterocyte proliferation in a manner that was dependent on epidermal growth factor (EGF) and EGFR11. In another study, it was shown that nitrate, another component of breast milk, contributes to its protective nature by modulating intestinal perfusion, as compared to infant formula, which is lacking nitrate and may contribute to the increased frequency of NEC in formula fed infants12,13. Other compounds present in breast milk that have been shown to be involved in the protection against NEC include human milk oligosaccharides, L-arginine, glutamine, and lactoferrin14,15,16,17,18,19. These beneficial elements of breast milk reveal the necessity of its use in the prevention of NEC, but also stress the importance of studying the mechanisms, signaling pathways, and cellular effects involved in how breast milk is mediating the protection against NEC.
In order to further study the protective properties of breast milk in a mouse model of NEC, we developed a novel, easy to use technique by which mouse breast milk can be extracted from an anesthetized dam using an electric human breast pump11,12. This strategy of acquiring mouse breast milk is advantageous, not only because human breast pumps are readily available and efficient in the procurement of breast milk, but also because this method allows for species-specific breast milk analyses. As a result, we can compare the effects of mouse breast milk with those of expressed human breast milk as well as pasteurized human donor milk from a milk bank in species-specific models. This technique allows for the study of breast milk components in relation to their contribution towards NEC prevention. Other investigators have developed breast milk extraction methods, however, these techniques are manual and typically require more than one lab member20,21,22. Here an easy technique that can be utilized by modifying a human electric breast pump to collect milk from a mouse is presented. This technique can also be applied to other species.
To adequately interrogate the signaling pathways involved with NEC, model systems are needed to evaluate all of the different cell types known to be affected in the disease process. Here, we discuss one such model system - enteroids - and their establishment from mouse and human small intestine. Human intestinal enteroids (HIEs) in particular provide significant promise, because they offer an innovative, genetically diverse ex vivo human model to aid in the study of pathophysiological processes that take place in the gastrointestinal tract23. Enteroids have been found to be cultured long-term and can be frozen for later use23, and unlike Human Intestinal Organoids (HIOs), whose cultures are developed from inducible pluripotent stem cells, enteroids are generated from stem cells within isolated intestinal crypts24. Enteroids require less maintenance, can be infected quickly25, and can be established easily since intestinal crypts are more differentiated than HIOs23. Therefore, HIEs offer many advantages over existing techniques because they can be developed to exhibit region-specific compositional and functional properties of the human gastrointestinal epithelium23. The use of enteroids is a highly effective choice when in need of a human model of the intestine, with adherence to region-specific limitations and ease-of-use. Here we demonstrate the technique of isolating and maintaining primary stem cell-derived small intestinal enteroids from mice and premature human infants.
Protocol
All animal procedures in this study were approved by either the Washington University in St. Louis Institutional Animal Care and Use Committee (Protocol 20160187) or University of Pittsburgh Institutional Animal Care and Use Committee (Protocol 14103918). Human fetal small intestine at less than 24-week gestation was obtained in accordance with the University of Pittsburgh anatomical tissue procurement guidelines after Institutional Review Board approval (protocol PRO14100537) from the University of Pittsburgh Health Sciences Tissue Bank through an honest broker system. De-identified human breast milk or pasteurized donor breast milk was obtained with a waiver of consent from the University of Pittsburgh Institutional Review Board.
1. Crypt Isolation and Establishment of Enteroids from Neonatal Mice or Premature Human Small Intestine
- Media preparation NOTE: Media should be prepared in the hood the day before crypt isolation.
- Prepare 1.11 L Culture Media. Begin with 1 L Dulbecco's Modified Eagle's medium (DMEM) with 4.5 g/L glucose and L-glutamine and add 110 mL Fetal Bovine Serum (FBS) (final concentration 10%), 11 mL Penicillin-Streptomycin (final concentration 1%), and 1.1 mL sterile insulin (final concentration 0.1%).
- Prepare 500 mL PBS-Antibiotic Mixture. Begin with 500 mL 1x Dulbecco's phosphate-buffered saline (DPBS) with 10% FBS and add 5 mL Gentamicin (final concentration 1%), 1 mL Amphotericin B (final concentration 0.2%), and 5 mL Penicillin-Streptomycin (final concentration 1%).
- Prepare 30 mL Cell Disruption Media #1. First prepare 30 mL Culture Media. Add 600 µL 0.5 M Ethylenediaminetetraacetic acid (EDTA) (final concentration 10 mM), 300 µL Gentamicin (final concentration 1%), and 60 µL Amphotericin B (final concentration 0.2%).
- Prepare 30 mL Cell Disruption Media #2. First prepare 30 mL Culture Media. Add 300 µL 0.5 M EDTA (final concentration 5 mM), 300 µL Gentamicin (final concentration 1%), and 60 µL Amphotericin B (final concentration 0.2%).
- Prepare 50 mL Crypt Culture Media Without Growth Factors. Measure 33.4 mL 1x Advanced DMEM/F-12 and add 10 mL FBS (final concentration 20%).
- Add 500 µL Penicillin-Streptomycin (final concentration 1%), 500 µL L-glutamine (final concentration 1%), 500 µL Gentamicin (final concentration 1%), 100 µL Amphotericin B (final concentration 0.2%), 2.5 µL 1 M N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES) (final concentration 0.05 mM), 500 µL 100 mM N-Acetylcysteine (final concentration 1 mM), 500 µL 100x N-2 supplement, 1 mL 50x serum-free supplement (e.g., B-27) minus Vitamin A, and 500 µL 1 mM 100x Y-27632.
- Prepare 1 mL Crypt Culture Media With Growth Factors. First prepare 1 mL of Crypt Culture Media Without Growth Factors. Add 2.5 µL 40 µg/mL Wnt3a (final concentration 100 ng/mL), 1 µL 100 µg/mL Noggin (final concentration 100 ng/mL), 2 µL 250 µg/mL R-Spondin (final concentration 500 ng/mL), and 0.5 µL 100 µg/mL EGF (final concentration 50 ng/mL).
- Crypt isolation
- Place solubilized basement membrane extract (see Table of Materials) on ice to thaw before beginning. NOTE: Keep basement membrane on ice or it will polymerize.
- In compliance with Institutional Animal Care and Use Committee protocol for mice greater than 14 days of age, euthanize mice with two methods of euthanasia with the second being a physical procedure to ensure death. NOTE: For mice under 14 days of age, decapitation is the standard procedure using scissors.
- Using scissors and forceps, make a vertical incision down the midline of the abdomen, through the skin and peritoneum, for the entire length of the abdomen. Excise the small intestine from the stomach to the cecum with scissors and remove the mesentery with forceps. NOTE: Two-week-old male and female C57BL/6J mouse pups, each weighing 5.5 - 7 g, were used for crypt isolation. Any age or size mouse can be used for crypt isolation.
- To prepare freshly harvested small intestine for crypt isolation, position intestine straight, and cut the intestine longitudinally along its entire length using scissors. NOTE: Mouse and human intestine can be prepared similarly.
- Clean stool out of intestine by gently shaking the intestine back and forth in a PBS-Antibiotic Mixture-filled Petri dish using forceps.
- Add 30 mL of Cell Disruption Media #1 to a 50 mL conical tube. Cut the intestine into 0.5 cm length pieces directly into the tube. Set the tube in an ice bucket and gently agitate on shaker at lowest speed for 15 min.
- While the tissues are on the shaker, obtain 24- or 48-well plates to grow crypts if the enteroids are to be used for RNA, DNA, or protein isolation. Use 8-well chamber slides to grow crypts if the enteroids are to be used for whole mount confocal imaging.
- In the hood, add 15 µL of basement membrane to the bottom of each well of the 24- or 48-well plate. If using chamber slides, add 9 µL of basement membrane to the bottom of each chamber. NOTE: Adjust volume of basement membrane according to the size of plate if needed.
- Quickly spread the basement membrane with the pipette tip so that the basement membrane forms a thin layer on the bottom of the well. Place the plate at 37 °C in incubator for 30 min to allow the basement membrane to polymerize. NOTE: The crypts will not attach if basement membrane is not a thin layer or if basement membrane contains air bubbles.
- After 15 min on a shaker, filter the tissue through a 100 µm strainer. Discard the flow through.
- Add 30 mL Cell Disruption Media #2 to a new 50 mL conical tube. Remove tissue from strainer and add to the tube.
- Set the tube in an ice bucket and gently agitate on shaker at lowest speed for 15 min.
- After 15 min on the shaker, filter tissue through a 100 µm strainer. Keep flow through at this step, in case yield is low in later steps. NOTE: All flow through tubes must be kept on ice.
- Add 15 mL DMEM with 4.5 g/L glucose and L-glutamine to a new 50 mL conical tube. Remove tissue from strainer and add to the tube. Vigorously shake the tube by hand for 10 s. Filter through a 100 µm strainer and collect flow through. Repeat until flow through contains no particulates.
- In the hood, filter the flow through from previous step into a new 50 mL conical tube using 70 µm strainers for mouse tissue or 100 µm strainers for human tissue. NOTE: Have extra strainers on standby, and pour slowly as strainers will clog with debris.
- Centrifuge the tube at 200 x g for 8 min at 4 °C and place directly in an ice bucket in the hood. Remove the supernatant by pipette without disturbing the pellet. Resuspend pellet with total desired amount of Crypt Culture Media Without Growth Factors. NOTE: The enriched crypts are the 'sandy' layer of the pellet. Each well requires 250 µL of Crypt Culture Media Without Growth Factors.
- Establishment of enteroids
- Plate the crypt media mixture directly on the basement membrane.
- Check crypts with a microscope to ensure isolation process was successful. NOTE: Cells and balls of cells are desired. Approximately 100 individual crypts are plated per well.
- Incubate the plate at 37 °C for 3 h to allow crypts to adhere to the basement membrane. NOTE: This incubation is with Crypt Culture Media Without Growth Factors.
- Slowly remove the media and excess crypts that failed to attach using a P-200 pipette. NOTE: Do not disturb the basement membrane as the attached crypts can become dislodged.
- Slowly add 250 µL of Crypt Culture Media With Growth Factors to each well. NOTE: For 24-, 48-well plates, or chamber slides, 250 µL of Crypt Culture Media With Growth Factors is sufficient.
- Replace the media every 2 days with fresh Crypt Culture Media With Growth Factors for 5 days prior to treatments to allow for optimal attachment and growth.
2. Mouse Breast Milk Collection
Separate a C57BL/6J lactating dam at postpartum 7 - 12 days from its pups for 6 h prior to milking.
Anesthetize the lactating dam with isoflurane via a nose cone and administer a subcutaneous injection of oxytocin at 0.15 IU/kg body weight. Wait 3 min before attempting milking procedure.
Clean teats with 70% ethanol prior to milking procedure and let dry.
Extract milk from teats one at a time using an electric human breast pump with silicone tubing sized to fit mouse teats into a 5 mL collection tube. NOTE: Optimal breast pumps have two settings, one for speed and one for suction. It is best to start with the lowest settings of each and increase both slowly until milk is extracted. Ideally, total yield will be ~ 500 µL - 1 mL of mouse breast milk per lactating dam.
Immediately aliquot breast milk and store at -80 °C. NOTE: Avoid freeze/thaw cycles.
Treat enteroids on day 5 with 50 µL/mL of mouse breast milk per mL of Crypt Culture Media with Growth Factors for 24 h at 37 °C.
3. Whole Mount Staining of Enteroids
- Preparation of reagents
- Prepare 16 mL 1x PBS per 8 wells.
- Prepare 2 mL 4% paraformaldehyde (PFA) in 1x PBS per 8 wells.
- Prepare 2 mL 0.1% Triton X-100 in 1x PBS per 8 wells.
- Prepare 24 mL PBS Tween (PBST is 0.1% Tween 20 in 1x PBS) per 8 wells.
- Prepare 2 mL 10% normal donkey serum (NDS) in 0.1% PBST (10% NDS/PBST) per 8 wells.
- Prepare 4 mL 1% NDS in 0.1% PBST (1% NDS/PBST) per 8 wells.
- Staining Day 1
- Aspirate off treatments and wash enteroids with PBS. Add 250 µL 4% PFA per well. Place on rotator for 1 - 2 h at 4 °C. Remove 4% PFA with pipette.
- Wash 4 times with 250 µL/well 1x PBS. Place on rotator for 10 min at room temperature (RT). NOTE: It is possible to store plate for 1 - 2 days at 4 °C if needed.
- Add 250 µL/well 0.1% Triton X-100. Incubate for 1 h at RT. Remove 0.1% Triton X-100 with pipette.
- Wash 4 times with 250 µL/well 1x PBS. Place on rotator for 15 min at RT.
- Add 250 µL/well 10% NDS/PBST. Incubate for 45 min at RT. Remove 10% NDS/PBST with pipette.
- Add 250 µL/well primary antibody, diluted 1/250 in 1% NDS/PBST, and incubate overnight at 4 °C. NOTE: The optimal antibody dilution varies according to the manufacturer.
- Staining Day 2
- Wash at least 5 times for 4 - 5 h with 250 µL/well PBST. Place on rotator at 4 °C between each wash.
- Add 250 µL/well secondary antibody, diluted 1/1,000 in 1% NDS/PBST. Wrap the plate in foil and incubate overnight at 4 °C.
- Staining Day 3
- Add 250 µL/well nuclear stain 4',6-diamidino-2-phenylindole (DAPI) for 15 min. NOTE: Keep the plate in the dark.
- Wash 6 times with 250 µL/well PBST. Place on rotator for 10 min at 4 °C between each wash.
- Mount coverslip on chamber slide with mounting media.
- Take images with a 40X objective confocal microscope and equally render in a raster graphics editor (see Table of Materials).
Representative Results
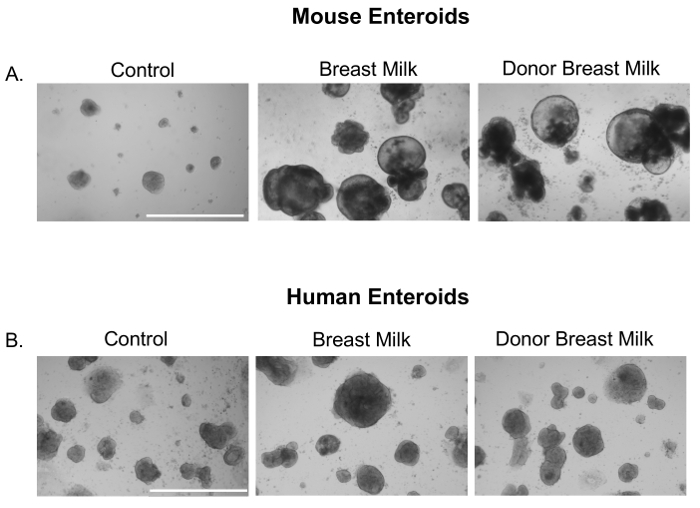
We first sought to investigate whether expressed human breast milk or pasteurized donor breast milk had an effect on small intestinal enteroids. Indeed, human breast milk and donor breast milk increased the growth of neonatal mouse (Figure 1A) and premature human derived enteroids (Figure 1B).
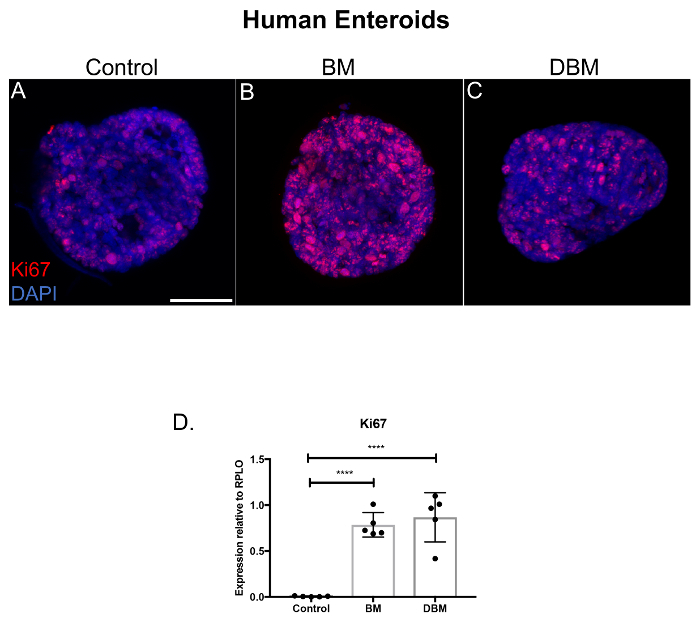
Since human breast milk increased the growth of small intestinal enteroids, we next investigated if breast milk had an effect on the proliferation of premature human enteroids. We demonstrate that human breast milk increases the proliferation of premature human enteroids as compared to control (Figure 2A-B, Ki67, red staining). Importantly, human donor breast milk also increased enteroid proliferation compared to control, but to a slightly lesser degree than non-pasteurized human breast milk (Figure 2B-C). We next evaluated the Ki67 mRNA expression in the premature human enteroids and discovered that Ki67 was increased in the enteroids treated with either breast milk or donor breast milk relative to control (Figure 2D).
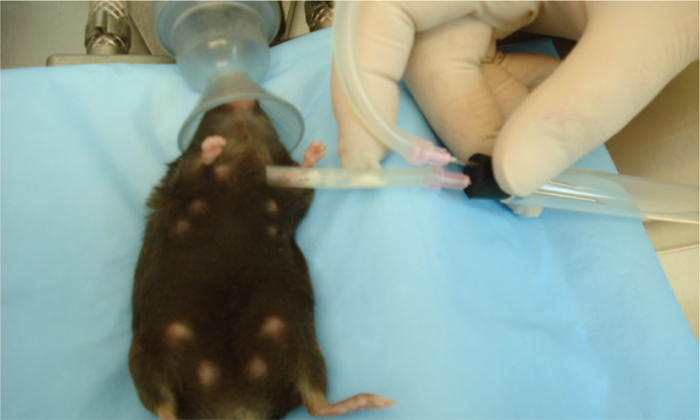
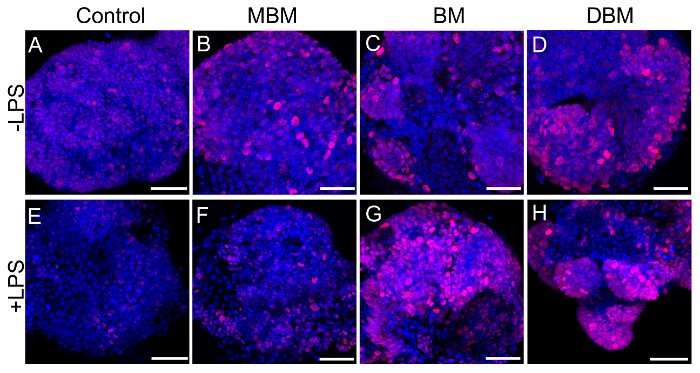
We next wanted to see if these effects were species-specific with regards to breast milk. We developed a way to collect breast milk from lactating mice using a modified commercially available human breast pump as in references11,12. Depicted is an anesthetized lactating dam undergoing milk collection using this device (Figure 3). The next series of experiments was performed on mouse enteroids and we evaluated the proliferation effects of expressed breast milk from either mouse, human, or donor breast milk. As shown in Figure 4, mouse, human, and pasteurized donor breast milk increased proliferation in the mouse enteroids as compared to control (Figure 4A-D). Proliferation in the mouse enteroids was decreased in the presence of the TLR4 ligand, lipopolysaccharide (LPS), compared to control (Figure 4A, E). Importantly, mouse, human, or donor breast milk all restored enteroid proliferation in the presence of LPS as demonstrated by increased Ki67 staining compared to LPS alone (Figure 4F-H).
From our experiments, we show that small intestinal enteroids derived from neonatal mice and premature human fetal intestine can be maintained in culture. We further provide a novel breast milk collection method that provides an efficient way to extract breast milk from mice, which can then be used for various experiments. We discovered that expressed breast milk from humans or mice increased the size, growth, and proliferation in mouse and human enteroids. Moreover, mouse, human, and donor breast milk restored the enteroid proliferation after LPS exposure. This suggests that breast milk can exert anti-inflammatory effects on the small intestine of mice and humans.
Figure 1. Breast milk and donor breast milk increase the size and growth of mouse and premature human enteroids. Photomicrographs of small intestinal enteroids from neonatal mice (p14) or premature human intestine treated with either media (Control), breast milk, or donor breast milk (50 µL/mL) for 24 h. Size bar: 1,000 µm. Please click here to view a larger version of this figure.
Figure 2. Breast milk and donor breast milk increase the proliferation of premature human enteroids. (A) Confocal micrographs of premature human enteroids cultured for 5 days, treated with human breast milk and pasteurized donor breast milk for 24 h and stained for the proliferation marker Ki67 (red) and DAPI (nuclear, blue). Size bar: 50 µm. (B) qRT-PCR for Ki67 expression of human enteroids relative to the housekeeping gene RPLO. n = 5 per group. **** p <0.0001 comparisons with Student t test compared to control. Data are mean ± standard deviation. Please click here to view a larger version of this figure.
Figure 3. Collection of milk from an anesthetized mouse using a modified human breast pump. Sterile human breast pump tubing placed on teats of anesthetized lactating dam after receiving oxytocin injection. Mouse milk is collected using the human breast pump on medium speed and power into a 5 mL collection tube. Collective volumes extracted from all teats range from 500 µL to 1 mL per mouse at postnatal days 7 - 12. Please click here to view a larger version of this figure.
Figure 4. Mouse, human, and pasteurized donor breast milk enhance mouse enteroid proliferation. (A-H) Confocal micrographs of p14 small intestinal mouse enteroids (Control, A) treated with mouse breast milk (MBM, B), human breast milk (BM, C), or human donor breast milk (DBM, D) at 50 µL/mL of media for 12 h in the absence (A-D) or presence of lipopolysaccharide (LPS) at a dose of 25 µg/mL of media for 12 h, (E-H) and stained for the proliferation marker Ki67 (red) and DAPI (nuclear, blue). Size bar: 50 µm. Please click here to view a larger version of this figure.
Discussion
The intestinal epithelium is comprised of many cellular subtypes that are responsible for providing host defense against pathogens, maintaining gut barrier integrity, and can be breached in the pathogenesis of several diseases. While animal models can recapitulate some facets of the disease, the ex vivo model of enteroids derived from the small intestine of mice and humans provides a platform to test effects of various treatments. The significance of the enteroid model allows researchers to culture and differentiate intestinal stem cells into all epithelial cellular subtypes from naïve or diseased animals and humans that are not available with existing methods. This model system can be used for infections with human pathogens that may not be readily translatable into the mouse, such as enterovirus25 or norovirus26 among others. The enteroid model has several advantages and uses; however, care must be taken to ensure consistency in the reagents that are used to make the media and the specific exogenous growth factors that are required for the survival of enteroids.
Within the enteroid model, several steps are of critical importance to assure success in producing enteroids. To minimize cold ischemic time, tissue should be processed expediently. Enteroids may be produced up to 24 h after the tissue is harvested if the tissue is placed in Roswell Park Memorial Institute (RPMI) medium at 4 °C. This can facilitate the option of shipping or transportation if necessary. For the establishment of enteroids, the basement membrane must be a thin, flat layer at the bottom of each well for the crypts to attach and grow. If the basement membrane is not flat or has bubbles present, the crypts may fail to adhere and will be removed when the media is changed. After the enteroids are established, they may be passaged every 7 days or frozen in liquid nitrogen for long term storage. Removal and addition of Crypt Culture Media with Growth Factors and the administration of each treatment must be done slowly and to the edge of each well as to avoid disturbing the enteroids. Disruption of the enteroids can cause them to become detached and washed away when the media is changed. For the best results, care should be taken to preserve the integrity of the basement membrane and the attached enteroids.
In the current study, we report that enteroids derived from neonatal mice and premature infants can grow and proliferate in the presence of different types of breast milk. These studies advance our understanding of the effects of breast milk on the premature intestine. We provide a detailed method on how to establish enteroid cultures in the laboratory from humans and mice. In addition, we demonstrate an efficient method of collecting breast milk from a lactating mouse using a modified electric human breast pump. We show that mouse, human, and pasteurized donor breast milk enhance the growth and proliferation of enteroids from mice and humans. In addition, we show decreased enteroid proliferation in the presence of the TLR4 ligand LPS, which was restored when mouse, human, or donor breast milk was administered to the enteroids. Future applications may include personalized precision medicine with high throughput drug screens on enteroids obtained from infants after intestinal resection to see how certain medications can affect the particular infant. Taken together, we hope other laboratories will find potential success in utilizing these techniques in many different realms of pediatric diseases of the intestine, including NEC, where there is an urgent clinical need to develop new therapeutic strategies.
Disclosures
The authors have nothing to disclose and no conflicts of interest.
Acknowledgments
MG is supported by grants K08DK101608 and R03DK111473 from the National Institutes of Health, March of Dimes Foundation Grant No. 5-FY17-79, the Children's Discovery Institute of Washington University and St. Louis Children's Hospital and the Department of Pediatrics at Washington University School of Medicine, St. Louis. CJL is supported by R01DK104946 (PI: Silverman), the Children's Discovery Institute of Washington University and St. Louis Children's Hospital.
References
- Patel RM, Denning PW. Therapeutic use of prebiotics, probiotics, and postbiotics to prevent necrotizing enterocolitis: what is the current evidence? Clin Perinatol. 2013;40(1):11–25. doi: 10.1016/j.clp.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan MS, Jilling T. New concepts in necrotizing enterocolitis. Curr Opin Pediatr. 2001;13(2):111–115. doi: 10.1097/00008480-200104000-00004. [DOI] [PubMed] [Google Scholar]
- Henry MC, Moss RL. Necrotizing enterocolitis. Annu Rev Med. 2009;60:111–124. doi: 10.1146/annurev.med.60.050207.092824. [DOI] [PubMed] [Google Scholar]
- Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368(9543):1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartick M, Reinhold A. The burden of suboptimal breastfeeding in the United States: a pediatric cost analysis. Pediatrics. 2010;125(5):e1048–e1056. doi: 10.1542/peds.2009-1616. [DOI] [PubMed] [Google Scholar]
- Bisquera JA, Cooper TR, Berseth CL. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics. 2002;109(3):423–428. doi: 10.1542/peds.109.3.423. [DOI] [PubMed] [Google Scholar]
- Leaphart CL, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179(7):4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- Gribar SC, et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol. 2009;182(1):636–646. doi: 10.4049/jimmunol.182.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M, et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proceedings of the National Academy of Sciences. 2012;109(28):11330–11335. doi: 10.1073/pnas.1200856109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M, et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 2015;8(5):1166–1179. doi: 10.1038/mi.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazji I, et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci U S A. 2013. [DOI] [PMC free article] [PubMed]
- Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- Bober-Olesinska K, Kornacka MK. Effects of glutamine supplemented parenteral nutrition on the incidence of necrotizing enterocolitis, nosocomial sepsis and length of hospital stay in very low birth weight infants. Med Wieku Rozwoj. 2005;9(3 Pt 1):325–333. [PubMed] [Google Scholar]
- Li N, et al. Glutamine decreases lipopolysaccharide-induced intestinal inflammation in infant rats. Am J Physiol Gastrointest Liver Physiol. 2004;286(6):G914–G921. doi: 10.1152/ajpgi.00493.2003. [DOI] [PubMed] [Google Scholar]
- Good M, et al. The human milk oligosaccharide 2'-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br J Nutr. 2016;116(7):1175–1187. doi: 10.1017/S0007114516002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantscher-Krenn E, et al. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut. 2012;61(10):1417–1425. doi: 10.1136/gutjnl-2011-301404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin IM, et al. Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T-regulatory cells. Am J Perinatol. 2014;31(12):1111–1120. doi: 10.1055/s-0034-1371704. [DOI] [PubMed] [Google Scholar]
- Amin HJ, et al. Arginine supplementation prevents necrotizing enterocolitis in the premature infant. J Pediatr. 2002;140(4):425–431. doi: 10.1067/mpd.2002.123289. [DOI] [PubMed] [Google Scholar]
- Rodgers CT. Practical aspects of milk collection in the rat. Lab Anim. 1995;29(4):450–455. doi: 10.1258/002367795780739980. [DOI] [PubMed] [Google Scholar]
- DePeters EJ, Hovey RC. Methods for collecting milk from mice. J Mammary Gland Biol Neoplasia. 2009;14(4):397–400. doi: 10.1007/s10911-009-9158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham K, et al. Milk collection methods for mice and Reeves' muntjac deer. J Vis Exp. 2014. [DOI] [PMC free article] [PubMed]
- Saxena K, et al. Human Intestinal Enteroids: a New Model To Study Human Rotavirus Infection, Host Restriction, and Pathophysiology. J Virol. 2015;90(1):43–56. doi: 10.1128/JVI.01930-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos NC, et al. Human Enteroids/Colonoids and Intestinal Organoids Functionally Recapitulate Normal Intestinal Physiology and Pathophysiology. J Biol Chem. 2016;291(8):3759–3766. doi: 10.1074/jbc.R114.635995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond CG, et al. Enteroviruses infect human enteroids and induce antiviral signaling in a cell lineage-specific manner. Proc Natl Acad Sci U S A. 2017;114(7):1672–1677. doi: 10.1073/pnas.1617363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettayebi K, et al. Replication of human noroviruses in stem cell-derived human enteroids. Science. 2016;353(6306):1387–1393. doi: 10.1126/science.aaf5211. [DOI] [PMC free article] [PubMed] [Google Scholar]


