Abstract
Settings: Swaziland is striving to achieve sustainable malaria elimination. Three preventive interventions are vital for reaching this goal: 1) effective household utilisation of long-lasting insecticide nets (LLINs), 2) indoor residual spraying (IRS), and 3) provision of chemoprophylaxis for those travelling to malaria-endemic areas.
Objectives: To assess the uptake of preventive intervention among confirmed malaria cases.
Design: A longitudinal study using nation-wide programme data from 2010 to 2015. Data on malaria cases from health facilities were sourced from the Malaria Surveillance Database System.
Results: Of a total 2568 confirmed malaria cases in Swaziland, 2034 (79%) had complete data on case investigations and were included in the analysis. Of 341 (17%) individuals who owned LLINs, 169 (8%) used them; 338 (17%) had IRS and 314 (15%) slept in sprayed structures. Of 1403 travellers to areas at high malaria risk, 59 (4%) used any form of malaria prevention, including chemoprophylaxis.
Conclusion: The uptake of all three key malaria prevention interventions is low, and could threaten the progress made thus far toward malaria elimination. Efforts to improve this situation, including qualitative research to understand the reasons for low uptake, are urgently needed.
Keywords: malaria surveillance, malaria chemoprophylaxis, malaria prevention, malaria case classification
Abstract
Contextes : Le Swaziland s'efforce de parvenir à l'élimination pérenne du paludisme. Trois interventions préventives sont vitales pour atteindre ce but : l'utilisation efficace de moustiquaires imprégnées d'insecticide rémanent (LLIN) ; la pulvérisation d'insecticide à effet rémanent (IRS) ; et la fourniture de chimioprophylaxie pour les personnes voyageant en zone d'endémie palustre.
Objectifs : Evaluer la couverture des interventions préventives parmi les cas confirmés de paludisme.
Schéma : Une étude longitudinale basée sur les données du programme national de 2010 à 2015. Les données des cas de paludisme des structures de santé proviennent de la base de données du système de surveillance du paludisme.
Résultats : Il y a eu un total de 2568 cas confirmés de paludisme au Swaziland, dont 2034 (79%) ont eu une investigation complète et ont été inclus dans l'analyse. Parmi eux, 341 (17%) disposaient de LLIN et 169 (8%) les utilisaient; 338 (17%) avaient bénéficié d'une IRS et 314 (15%) dormaient dans des structures vaporisées. Il y a eu 1403 voyageurs dans des zones à risque de paludisme, dont 59 (4%) ont utilisé une forme de prévention du paludisme incluant la chimioprophylaxie.
Conclusion : La couverture des trois interventions clés de prévention du paludisme est faible et peut menacer les progrès réalisés à ce jour vers l'élimination du paludisme. Les efforts visant à améliorer cette situation, notamment l'utilisation de recherche qualitative pour comprendre les raisons de cette faible couverture, sont requises d'urgence.
Abstract
Marco de referencia: Swazilandia se esfuerza por alcanzar una eliminación sostenible del paludismo. Existen tres intervenciones preventivas esenciales con miras a cumplir esta meta, a saber: 1) la utilización efectiva de mosquiteros impregnados de insecticidas de larga duración (LLIN); 2) la fumigación de interiores con insecticidas de efecto residual (IRS); y 3) la provisión de quimioprofilaxis a las personas que se desplazan hacia las zonas donde el paludismo es endémico.
Objetivos: Apreciar la aceptación de la intervención preventiva en los casos confirmados de paludismo.
Método: Un estudio longitudinal a partir de los datos del programa nacional del 2010 al 2015. Los datos sobre los casos de paludismo de los centros de atención de salud se obtuvieron de la Base de Datos del Sistema de Vigilancia del Paludismo.
Resultados: Ocurrieron 2568 casos confirmados de paludismo en Swazilandia, de los cuales 2034 (79%) contaban con datos completos sobre las investigaciones del caso y se incluyeron en el análisis. De estas personas, 341 poseían LLIN (17%) y 169 lo utilizaban (8%); el domicilio de 338 personas había sido fumigado con un IRS (17%) y 314 dormían en estructuras fumigadas (15%). Se contabilizaron 1403 viajeros a zonas con riesgo de transmisión del paludismo, de los cuales 59 utilizaron alguna forma de prevención, incluida la quimioprofilaxis (4%).
Conclusión: La utilización de las tres intervenciones esenciales de prevención del paludismo es muy baja y podría poner en peligro los logros alcanzados hasta ahora, en materia de eliminación de la enfermedad. Es urgente ejecutar medidas que mejoren esta situación, entre otras, la realización de investigaciones cualitativas que ayuden a comprender las razones de la baja utilización.
Malaria, caused by Plasmodium falciparum and transmitted by the female Anopheles mosquito species, remains a major cause of morbidity and mortality in sub-Saharan Africa. The region is home to 88% of all global malaria cases. There were an estimated 214 million malaria cases in 2014, and 438 000 deaths.1 The disease is also a major impediment to health and economic development in the sub-region.
In sub-Saharan Africa, eight countries (Angola, Zimbabwe, Zambia, Mozambique, Botswana, Namibia, South Africa and Swaziland), commonly referred to as the Elimination 8 (E8) countries, have committed to work toward malaria elimination. The latter four (E4) countries have low malaria transmission, and are currently considered front-runners for achieving malaria elimination by the year 2018.2 ‘Malaria elimination’ is defined as the interruption of local mosquito-borne malaria transmission in a defined geographical area, leading to zero incidence of locally contracted cases. Imported cases may continue to occur, and continued intervention measures are required.3,4
Although Swaziland has reached the elimination milestone of <1 case per 1000 population at risk, there are outbreak-prone (receptive) regions—parts of Hhohho, which borders South Africa, and Lubombo, which borders Mozambique—where about 30% of the Swazi population resides. Nearly all local (indigenous) malaria cases occur in these two areas because they have sufficient Anopheles mosquito vectors and other favourable ecological and climatic factors for vector breeding and malaria transmission. The majority of cases in Swaziland are imported from malaria-endemic countries.5
Three preventive interventions have been shown to reduce malaria incidence. These include targeted distribution of long-lasting insecticide nets (LLINs), indoor residual spraying (IRS) with insecticides for those living in high malaria risk areas, and provision of chemoprophylaxis for those travelling to outbreak-prone regions or malaria-endemic countries.3 Although Swaziland has intensified the implementation of these interventions, their effectiveness depends on the level of uptake in the community. Community attitudes towards preventive interventions may influence uptake, and this could be of added relevance, as malaria is no longer perceived as a major morbidity in Swaziland.6 For example, individuals may not perceive the need to own LLINs or may not use them appropriately, they may or may not sleep in a structure that has IRS, and they may or may not use recommended anti-malarial chemoprophylaxis when travelling to malaria-endemic areas.
Swaziland has engaged in intensified community activities for malaria prevention since 2009, including mass media campaigns and community outreach, which target at-risk population groups.7 Although malaria incidence has decreased by 76%, the majority of new malaria cases result from limited use of prevention methods despite intensified efforts, making it difficult to reach zero malaria cases. This stagnation necessitates further research into the uptake of specific prevention methods. Although prevention has been identified as an operational research priority for malaria elimination, there have been no published studies from Swaziland and the E4 countries to date on this subject.8 We aimed to assess the uptake of three key preventive interventions—LLIN, IRS and malaria prophylaxis—among confirmed malaria cases in Swaziland.
The specific objectives, for all of Swaziland, were to determine 1) the number of confirmed malaria cases, their demographic characteristics, which administrative region they came from and their case classification, i.e., local or imported; 2) the number and proportion of cases who owned and used LLINs, had household structures sprayed and regularly slept in sprayed structures; and 3) the number who had taken any form of preventive method during recent travel to malaria-risk areas within or outside the country.
DESIGN, METHODS AND STUDY POPULATION
Study design
This was a longitudinal study using routine national programme data.
General setting
Swaziland is a landlocked country located in south-eastern Africa between Mozambique and South Africa, with an estimated population of 1.2 million. About one third (n = 400 000) of the population is at risk for malaria infection. The country experiences seasonal malaria, with the transmission season spanning from November to May (the rainy season), with a peak in January due to high cross-border travel during the holiday season. P. falciparum is responsible for over 99% of malaria cases, and An. arabiensis is the main vector. Malaria transmission in Swaziland is unstable and closely related to rainfall patterns, with major outbreaks occurring in the last two decades. Swaziland has about 1.2 million travellers passing across its borders every year.9
Preventive malaria interventions: LLINs, IRS, chemoprophylaxis
Key malaria preventive interventions include LLINs, IRS and chemoprophylaxis. LLINs are expected to last for about 3 years, and mass distribution is thus repeated along this time-frame. In each household, a maximum of two people are meant to share a bed net, and the entire at-risk population should receive bed nets. There is no specific monitoring of the continued ownership of LLINs by household or of their appropriate use.9,10
All households in malaria transmission areas are supposed to receive free IRS treatment prior to the start of the malaria season. Traditional structures are treated with dichlodiphenyltrichloethane (DDT), and modern structures are treated with pyrethroid insecticide.11
According to Swaziland's national malaria elimination policy (2010),11 it is recommended that all residents travelling to areas and countries with moderate-to-high malaria transmission take malaria chemoprophylaxis. The first-line drug is mefloquine, to be taken 1 tablet/week beginning 2 weeks before travel, continued during the period of stay and continued for 4 weeks following the traveller's return. Second-line prophylaxis (only for non-pregnant, non-elderly adults) is doxycycline or atovaquone/proguanil.11 Robust information, education and communication (IEC) activities at community level, including mass media campaigns, community dialogues and other activities targeting mobile populations, should encompass these preventive interventions.9
Study sites
The study sites included all health facilities in Swaziland that reported malaria cases.
Study population and period
The study population included all investigated malaria cases with information available on the use of preventive interventions for the period from August 2010 to August 2015. The study was conducted between June 2015 and January 2016.
Data variables, sources of data and validation
Data variables related to the study objectives were sourced from the Malaria Surveillance Database System, which contains detailed information on the uptake of preventive interventions that is entered onto case investigation forms by trained malaria surveillance agents.
Data entry and analysis
Data was entered into EpiData software for data entry and analysis (v. 3.1 for entry and v. 2.2.2.183 for analysis, EpiData Association, Odense, Denmark). The data were analysed descriptively, and differences between groups were compared.
Ethics
Ethical approval was received from the Ministry of Health Scientific and Ethics Committee (Mbabane, Swaziland) and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease (Paris, France). As this study used routine anonymised data, informed patient consent was not necessary.
RESULTS
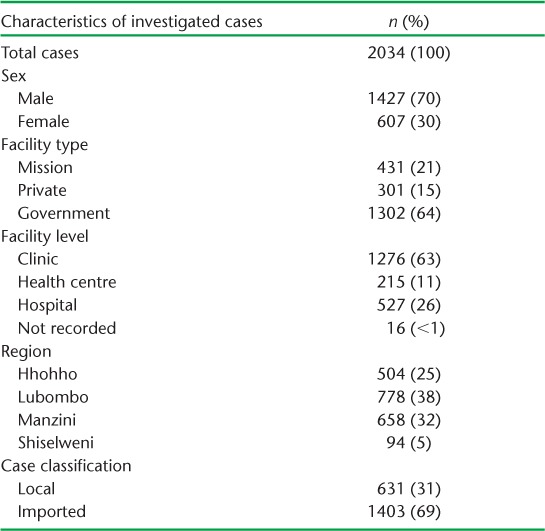
Of the 2568 total malaria cases in Swaziland, 534 (21%) did not undergo case investigations. The remaining 2034 cases with complete data were included in the analysis.
Table 1 shows the number and demographic characteristics of investigated malaria cases. The majority (70%) were male, and 40% were aged between 20 and 45 years. Individuals with malaria primarily presented to government facilities (64%), and especially primary care clinics (63%). Imported cases from the malaria risk areas of Swaziland or other endemic countries represented 69% of all malaria cases.
TABLE 1.
Number of investigated malaria cases and their characteristics in Swaziland, 2010–2015
Tables 2 and 3 show the uptake of the three key prevention interventions (number and proportion of individuals with malaria who owned and used LLINs, had and regularly slept in sprayed household structures and used chemoprophylaxis when travelling) among malaria cases in Swaziland. Overall uptake was low across all interventions, reaching 17% at best.
TABLE 2.
Uptake of preventive interventions among investigated malaria cases in Swaziland, 2010–2015
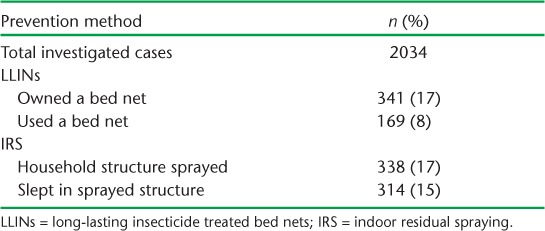
TABLE 3.
Uptake of specific preventive interventions among investigated malaria cases with a history of recent travel within or outside Swaziland, 2010–2015
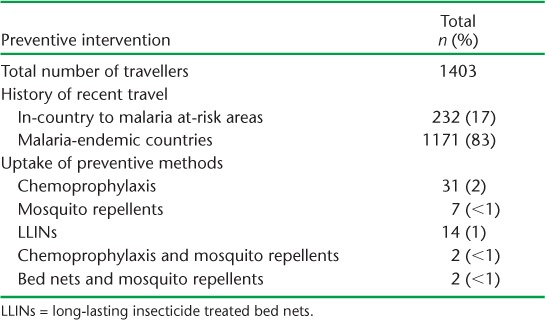
Table 3 shows the type of preventive intervention used by those who reported having travelled to high malaria risk areas of Swaziland or malaria-endemic countries. Of 1171 (83%) travellers who had been to malaria-endemic countries, only 2% had used chemoprophylaxis. Uptake of any form of preventive method was low, at 4%.
DISCUSSION
This is the first study from a front-runner country for malaria elimination in southern Africa to assess the uptake of preventive interventions for malaria. The results reveal a worrying gap in case investigations and, significantly, among investigated cases, low overall uptake of all the preventive interventions.
From a public health perspective, low uptake of preventive interventions considerably increases the risk of onward malaria transmission and the potential for malaria epidemics. Furthermore, as the great majority of malaria cases were imported from other endemic countries, there is a risk of spreading artemisinin-resistant malaria strains and therefore negatively impacting on the goal of eliminating malaria in Swaziland and the region.12–15 This study thus highlights the need for policy makers and implementers to make serious efforts to increase the current uptake of preventive interventions at the community level. There might also be a need for legislation that makes screening and follow-up mandatory for all travellers from malaria-endemic countries.
The strengths of this study are that it included all health facilities nationwide and over a 5-year period; the data source was the Malaria Surveillance Database System, the reference database for malaria control in the country; and the study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational research.16 The study is also in line with an identified operational research priority for the southern African region.8
The study limitations are that approximately 20% of malaria cases were not investigated and there were data missing on some of the variables, although this was minimal. Significantly, the study did not explore the reasons behind the low uptake of preventive interventions. This aspect merits specific qualitative research studies.
There are a number of policy and practice implications that can be derived from the study. First, the low uptake of preventive interventions may be due to limited availability and/or acceptability of various preventive methods. Only two of 10 households, for example, owned a bed net, and even when they owned one, only half used it. These findings are in agreement with those of a knowledge, attitude and practice study undertaken in Lubombo region in 2007.17 Uptake may also have been influenced by the current government policy of restricting bed net distribution to only those areas at risk for malaria. With malaria cases being reported in all regions of the country, it would seem logical to expand the distribution of bed nets to all those at risk, including migrant workers and cross-border traders residing in other regions, for use when travelling.
The acceptability of IRS may depend upon the type of insecticide used. Supply and human resource shortages for spraying teams may also be an important factor. In recent studies, the main reason for household structures not being sprayed was the unavailability of spray operators.17 An overriding issue is the lack of available funding for community sensitisation and engagement, in a country where the malaria caseload is low and therefore not perceived to be a public health problem at community level. In low-burden settings, complacency should be avoided through enhanced community engagement and social mobilisation.18,19 A paradigm shift is thus required to increase and sustain motivation and adherence to prevention recommendations. Specific operational research in these areas would be merited. Meanwhile, as a first step, it would seem worthwhile to review the level of emphasis placed on community engagement and mobilisation.
Second, although all health workers should be included in efforts to promote the uptake of preventive measures among confirmed cases, government facilities and primary health centres should be prioritised. Better collaboration between community health workers at primary health care facilities and malaria surveillance agents could improve collaboration in case investigation and malaria prevention. An enhanced dynamic is likely to have the added benefit of improving the continuum between health facilities and the community.19
Third, the great majority of malaria cases were imported from other endemic countries, with fewer than 2% of travellers using any form of chemoprophylaxis. The low uptake of chemoprophylaxis could be due to the accessibility and availability of these drugs. Although the drugs have side-effects, these are not so severe as to deter travellers from taking them.20 Making chemoprophylaxis drugs more available and accessible to mobile populations is an urgent requirement for countries in the pre-elimination stage. This aside, there is an overall need to raise awareness in the general population and among travellers to improve the use of prevention methods. The general population could be targeted through effective mass media messaging. As Swaziland and neighbouring countries are known to have significant cross-border migration, a critical recommendation would be to make preventive methods available at all border posts. This should be coupled with active health promotion by port health officers.
In conclusion, countrywide assessment of uptake of key malaria prevention methods in Swaziland is unsatisfactory, and could threaten the progress made so far toward malaria elimination. Steps need to be taken to rectify this situation.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR, Geneva, Switzerland). SORT IT programmes include a teaching component developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union, Paris, France) and Médecins Sans Frontières (MSF, Geneva, Switzerland). The specific SORT IT programme that resulted in this publication was implemented by WHO/TDR; WHO Global Malaria Programme (GMP); WHO/African Region (AFRO); Operational Research Unit (LuxOR), MSF, Brussels Operational Centre, Luxembourg; the Centre for Operational Research, The Union; University of Nairobi (Nairobi, Kenya), Global AIDS Interfaith Alliance (San Rafael, CA, USA), Academic Model Providing Access to Healthcare (AMPATH, Eldoret, Kenya) and Johns Hopkins University (Baltimore, MD, USA). The programme was funded by WHO/TDR, WHO GMP and WHO/AFRO. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Conflicts of interest: none declared.
In accordance with WHO's open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO licence (http://creativecommons.org/licenses/by/3.0/igo/legalcode) that permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1. World Health Organization. . World malaria report, 2015. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 2. Southern African Development Community. . Elimination 8 Strategic Plan 2015–2020: Working towards a malaria-free Southern Africa. Gaborone, Botswana: SADC, 2015. http://www.shrinkingthemalariamap.org/files/content/resource/attachment/E8%20Strategic%20Plan%20(2015-2020).pdf Accessed March 2018. [Google Scholar]
- 3. World Health Organization. . Disease surveillance for malaria elimination: an operational manual. Geneva, Switzerland: WHO, 2012. [Google Scholar]
- 4. Feachem R G A, Malaria Elimination Group. . Shrinking the malaria map: a guide on malaria elimination for policy makers. San Francisco, CA, USA: The Global Health Group, Global Health Sciences, University of California, San Francisco, 2009. http://www.shrinkingthemalariamap.org/sites/www.shrinkingthemalariamap.org/files/content/resource/attachment/AGuideonMalariaEliminationforPolicyMakers.pdf Accessed December 2017. [Google Scholar]
- 5. Cohen J M, Dlamini S, Novotny J M, Kandula D, Kunene S, Tatem A J.. Rapid case-based mapping of seasonal malaria transmission risk for strategic elimination planning in Swaziland. Malar J 2013; 12: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atkinson J A, Fitzgerald L, Toaliu H, . et al. Community participation for malaria elimination in Tafea Province, Vanuatu. Part I: Maintaining motivation for prevention practices in the context of disappearing disease. Malar J 2010; 9: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization. . Evaluation registry. Swaziland malaria programme performance review. Geneva, Switzerland: WHO, 2011. https://extranet.who.int/evaluationregistry/EvaluationView.aspx?id=22775 Accessed December 2017. [Google Scholar]
- 8. World Health Organization. . Planning meeting for operational research on malaria elimination. WHO/HTM/GMP/2014.5 Geneva, Switzerland: WHO, 2013. http://www.who.int/malaria/publications/atoz/meeting-rep-op-research-malaria-elimination-may2014.pdf Accessed December 2017. [Google Scholar]
- 9. National Malaria Control Programme. . National malaria control strategy, 2008–2015. Mbabane, Swaziland: Ministry of Health, 2008. [Google Scholar]
- 10. World Health Organization. . Malaria elimination–field manual for low and moderate endemic countries. Geneva, Switzerland: WHO, 2007. [Google Scholar]
- 11. National Malaria Control Programme. . Swaziland National Malaria Elimination Policy. Mbabane, Swaziland: Ministry of Health, 2010. [Google Scholar]
- 12. Tun K M, Imwong M, Lwin K M, . et al. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet Infect Dis 2015; 15: 415– 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pindolia D K, Garcia A J, Wesolowski A, . et al. Human movement data for malaria control and elimination strategic planning. Malar J 2012; 11: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wai K T, Kyaw M P, Oo T, . et al. Spatial distribution, work patterns, and perception towards malaria interventions among temporary mobile/migrant workers in artemisinin resistance containment zone. BMC Public Health 2014; 14: 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ashley E A, Dhorda M, Fairhurst R M, . et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2014; 371: 411– 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. von Elm E, Altman D G, Egger M, . et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344– 349. [DOI] [PubMed] [Google Scholar]
- 17. Hlongwana K, Mabaso M L H, Kunene S, Govender D, Maharaj R.. Community knowledge, attitudes and practices (KAP) on malaria in Swaziland: a country earmarked for malaria elimination. Malar J 2009; 8: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sleigh A, Xueming L, Jackson S, Huang K.. Eradication of schistosomiasis in Guangxi, China. Part 1: Setting, strategies, operations, and outcomes, 1953–92. Bull World Health Organ 1998, 76: 361– 372. [PMC free article] [PubMed] [Google Scholar]
- 19. Kateera F K, Hakizimana E, Mens P, . et al. Towards achieving malaria pre-elimination status in eastern Rwanda: use of an integrated community focused model. Int J Infect Dis 2014; 21 Suppl 1: 164. [Google Scholar]
- 20. Sumadhya D F, Chaturaka R, Senaka R.. Chemoprophylaxis in malaria: drugs, evidence of efficacy and costs. Asian Pac J Trop Med 2011; 4: 330– 336. [DOI] [PubMed] [Google Scholar]


