Abstract
Background: The malaria vector Anopheles merus occurs in the Mpumalanga Province of South Africa. As its contribution to malaria transmission in South Africa has yet to be ascertained, an intensification of surveillance is necessary to provide baseline information on this species. The aim of this study was therefore to map An. merus breeding sites in the Ehlanzeni District of Mpumalanga Province and to assess qualitative trends in the distribution and relative abundance of this species over a 9-year period.
Methods: The study was carried out during the period 2005–2014 in the four high-risk municipalities of Ehlanzeni District. Fifty-two breeding sites were chosen from all water bodies that produced anopheline mosquitoes. The study data were extracted from historical entomological records that are captured monthly.
Results: Of the 15 058 Anopheles mosquitoes collected, 64% were An. merus. The abundance and distribution of An. merus increased throughout the four municipalities in Ehlanzeni District during the study period.
Conclusion: The expanded distribution and increased abundance of An. merus in the Ehlanzeni District may contribute significantly to locally acquired malaria in Mpumalanga Province, likely necessitating the incorporation of additional vector control methods specifically directed against populations of this species.
Keywords: malaria transmission, Anopheles gambiae complex, An. arabiensis, minor vector, An. merus
Abstract
Contexte : Le vecteur du paludisme, Anopheles merus, sévit dans la province de Mpumalanga en Afrique du Sud. Comme sa contribution à la transmission du paludisme en Afrique du Sud reste à vérifier, une intensification de la surveillance est nécessaire afin de fournir des informations de départ sur cette espèce. Le but de cette étude a donc été de cartographier les sites de reproduction de An. merus dans le district d'Ehlanzeni de la province de Mpumalanga et d'évaluer les tendances qualitatives de la distribution et de l'abondance relative de cette espèce sur une période de 9 ans.
Méthodes : Cette étude a été réalisée pendant la période de 2005 à 2014 dans les quatre municipalités à risque élevé du district d'Ehlanzeni. Cinquante-deux sites de reproduction ont été choisis dans tous les plans d'eau qui ont produit des moustiques de l'espèce anophèle. Les données de l'étude ont été extraites de registres entomologiques historiques qui sont saisis chaque mois.
Résultats : Sur les 15 058 moustiques Anopheles recueillis, 64% ont été An. merus. L'abondance et la distribution d'An. merus ont augmenté dans les quatre municipalités du district d'Ehlanzeni pendant la période d'étude.
Conclusion: La distribution en expansion et l'abondance accrue d'An. merus dans le district d'Ehlanzeni peut contribuer significativement au paludisme acquis localement dans la province de Mpumalanga et nécessite l'incorporation de méthodes de lutte vectorielle supplémentaires spécifiquement dirigées contre les populations de cette espèce.
Abstract
Marco de referencia: Anopheles merus, vector del paludismo, está presente en la provincia de Mpumalanga de Suráfrica. Puesto que no se ha determinado su contribución a la transmisión del paludismo en el país, es necesario intensificar la vigilancia, con el fin de aportar información de referencia sobre esta especie. El objetivo del estudio fue cartografiar los criaderos de An. merus en el distrito de Ehlanzeni de la provincia de Mpumalanga y evaluar la evolución cuantitativa de la distribución y la abundancia relativa de esta especie durante un período de 9 años.
Métodos: El estudio se llevó a cabo del 2005 al 2014 en cuatro municipios de alto riesgo de transmisión del distrito de Ehlanzeni. Se escogieron 52 criaderos de todas las masas de agua productoras de mosquitos anófeles. Los datos del estudio se extrajeron de los registros entomológicos históricos que se captan cada mes.
Resultados: De los 15 058 mosquitos anófeles recogidos, el 64% correspondía a An. merus; su abundancia y distribución aumentó en los cuatro municipios del distrito de Ehlanzeni durante el período del estudio.
Conclusión: La ampliación de la distribución y el aumento de la presencia de An. merus en el distrito de Ehlanzeni pueden contribuir de manera significativa a los casos de paludismo adquiridos localmente en la provincia de Mpumalanga, y es probable que sera necesario incorporar otros métodos de control de vectores dirigidos específicamente contra las poblaciones de esta especie.
Previously considered as only a minor, or even unimportant malaria vector, potentially unable to sustain transmission alone,1 Anopheles merus has now been identified as an ‘unexpectedly’ important vector species along the Tanzanian coast and in Mozambique.2,3 It has also been implicated in malaria transmission in Kenya and Madagascar.4–6 Originally referred to as a ‘salt water An. gambiae’ variant, it is now characterised as a member of the An. gambiae species complex.7 Other members in this complex include An. gambiae sensu stricto, An. coluzzii and An. arabiensis which, together with An. funestus, are recognised as the major African malaria vector species.8
As opposed to the fresh-water variants of the An. gambiae complex, An. merus mostly breeds along the eastern coastal salt-water areas of Africa. However, it has also been isolated further inland in both saline and fresh-water larval habitats in Mozambique, Zambia, Zimbabwe, Swaziland and South Africa.9–14
In the Ehlanzeni District of Mpumalanga Province in South Africa, an inland district with mostly fresh-water bodies, An. arabiensis is likely the major vector responsible for malaria transmission.7,8,15 However, An. merus also occurs in this district, and it has been shown that, in sufficient numbers, An. merus can contribute significantly to the transmission of malaria.2,3 Its increased breeding in habitats previously dominated by other mosquito species therefore has the potential to affect localised malaria epidemiology, and may also affect vector control strategies that are aligned to vector population localities, relative abundance, vulnerability and receptivity patterns of the vectors. Vector control in this region is based on indoor residual insecticide (IRS) spraying coupled with larval source management in selected areas.
It has been noted anecdotally that since its first identification in 1997 in the Ehlanzeni district,12 An. merus has been increasing in terms of geographical range and relative abundance. This is thought to be a possible contributory factor to the persistence of malaria cases still reported in the district.16
Sporozoite infectivity rates and blood feeding index are markers used to incriminate Anopheles populations in malaria transmission. In Tanzania, An. merus was shown to have high sporozoite infectivity rates of up to 11.6%.2 Two Mozambican studies also demonstrated a sharp increase in An. merus sporozoite infectivity rates, from 0.067% to 4.2%, between 2007 and 2009.3,17 This species has also been incriminated as a vector of lymphatic filariasis in coastal East Africa.18
Although vector control primarily based on IRS, when coverage is sufficiently high, has been demonstrated to interrupt the malaria transmission cycle, successful implementation of vector control interventions is also somewhat dependent on precise knowledge of the ecology and behaviour of target species. With South Africa aiming to eliminate malaria by 2020, targeted vector control strategies need to be enhanced. Detailed knowledge of the breeding sites and abundance of An. merus populations will help in these interventions, given the encroachment of this species into new, fresh-water areas and its potential malaria infectivity. Recording the changes in the distribution over time of this species in inland districts of South Africa will help to elucidate the bionomics of this species. The aim of the present study was therefore to map An. merus breeding sites in the Ehlanzeni District and to assess qualitative trends in the distribution and relative abundance of this species over a 9-year period.
METHODS
Study design
This was a qualitative, descriptive cross-sectional study using routinely collected entomological data over a 9-year period, from July 2005 to June 2014, in Ehlanzeni District, Mpumalanga Province, South Africa.
Setting
General setting
Mpumalanga Province is one of the malaria-endemic provinces of South Africa. It is bordered by Gauteng and Limpopo Provinces in the west and north, respectively. It is estimated that over 1.7 million people, 43% of Mpumalanga Province's population, live in the low-lying areas and are at risk of contracting malaria. The disease is endemic in Ehlanzeni District, which is further subdivided into five municipalities, four of which are considered malaria-endemic.
Specific site
This study was carried out in the four high-risk municipalities of Ehlanzeni District: Bushbuckridge, Mbombela, Nkomazi and Umjindi. In these municipalities malaria transmission is unstable, seasonal and greatly influenced by climatic factors such as rainfall, temperature and relative humidity. In Mpumalanga Province, the malaria season typically starts after the first rains in October, peaks in December and January, and wanes in April–May.
Fifty-two breeding sites were chosen from water bodies that have produced members of both the An. gambiae complex and An. funestus group during the period under review.
Laboratory techniques and procedures
Larval sampling
Field collection teams visited each breeding site once a month. The anopheline larvae collected were transferred into labelled plastic cups and transported to the Driekoppies insectary (Mpumalanga Province), where they were reared to adults.
Morphological identification
The adult mosquitoes were killed by freezing and separated according to sex. Female mosquitoes were identified and then separated into An. gambiae complex and An. funestus groups using the morphological identification key of Gillies and Coetzee.8 The mosquitoes were then individually placed in tubes containing silica gel desiccant and stored for molecular studies. The prepared specimens were assigned unique identifier numbers. Data on each specimen were recorded electronically in a separate MS Excel spreadsheet (Microsoft Corp, Redmond, WA, USA).
Molecular identification
Specimens belonging to the An. gambiae complex were identified to species level using the polymerase chain reaction (PCR) method of Scott et al.19 Those belonging to the An. funestus group were identified using the PCR method of Koekemoer et al.20 These procedures were conducted at the Vector Control Reference Laboratory of the National Institute for Communicable Diseases (NICD; Johannesburg, South Africa).
Data collection
Historical entomological data were extracted from the entomology database by two data assistants using standardised data abstraction forms. Data variables included a unique species identification per record, date of PCR identification, latitude and longitude coordinates, district, municipality, sector and locality where the collections were conducted, and total number of specimens collected by species. The extracted data were captured on the developed EpiData entry file (v3.1, EpiData Association, Odense, Denmark) during August and November 2015.
The Driekoppies entomology team verified the accuracy of the recorded global positioning system (GPS) coordinates for each An. merus breeding site identified.
Data analysis
The GPS coordinates were converted into degrees. Descriptive analyses were conducted and results presented as proportions. The recorded data files were converted to database files and then imported to ArcView software (Esri, Redlands, CA, USA) and visualised using an Ehlanzeni District shape file. Through the verified GPS coordinates for An. merus breeding sites, three sets of maps were produced.
Ethics approval
Ethical approval for the study was obtained from the Provincial Health and Research Ethics Committee of Mpumalanga Province and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease (Paris, France), and Médecins Sans Frontières (Geneva, Switzerland).
RESULTS
The numbers of Anopheles mosquito breeding sites in each municipality are shown in Table 1. Nkomazi municipality had the most Anopheles breeding sites. The numbers and relative abundance of anopheline species collected in each of the municipalities over the 9-year period are shown in Table 2. An. merus predominated in all municipalities except Bushbuckridge.
TABLE 1.
Number of water bodies identified as Anopheles mosquito breeding sites in each municipality, Ehlanzeni District, Mpumalanga Province, South Africa, 2005/2006–2014

TABLE 2.
Numbers and relative abundance (percentages) of Anopheles mosquitoes collected by species by municipality, Ehlanzeni District, Mpumalanga Province, South Africa, 2005/2006–2014

The distribution trends over time showed that An. merus and An. quadriannulatus continuously increased in relative abundance (based on absolute numbers and proportions of specimens collected per season/year) from 2010/2011 to 2013/2014 (Figure 1). This trend was most pronounced in An. merus. The numbers collected and the relative abundance of the other species remained comparatively stable during the review period. Figures 2A–2C show the progressively increasing geographical distribution and relative densities of An. merus by municipality from 2005/06 to 2014.
FIGURE 1.
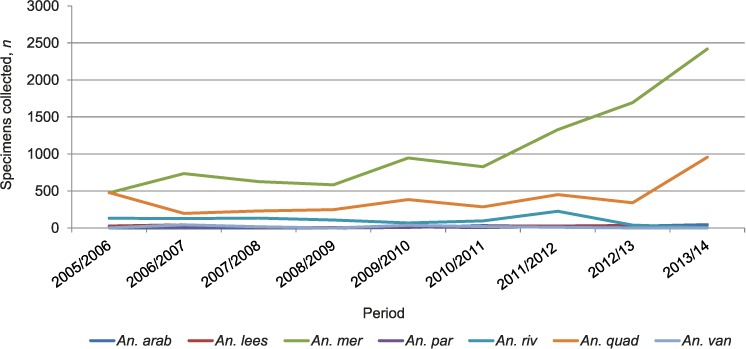
Trends in numbers of Anopheles mosquitoes collected by species over time in Ehlanzeni District, Mpumalanga Province, South Africa, 2005/2006–2014. An. arab = An. arabiensis; An. lees = An. leesoni; An. mer = An. merus; An. par = An. parensis; An. riv = An. rivulorum; An. quad = An. quadriannulatus; An. van = An. vaneedeni.
FIGURE 2.
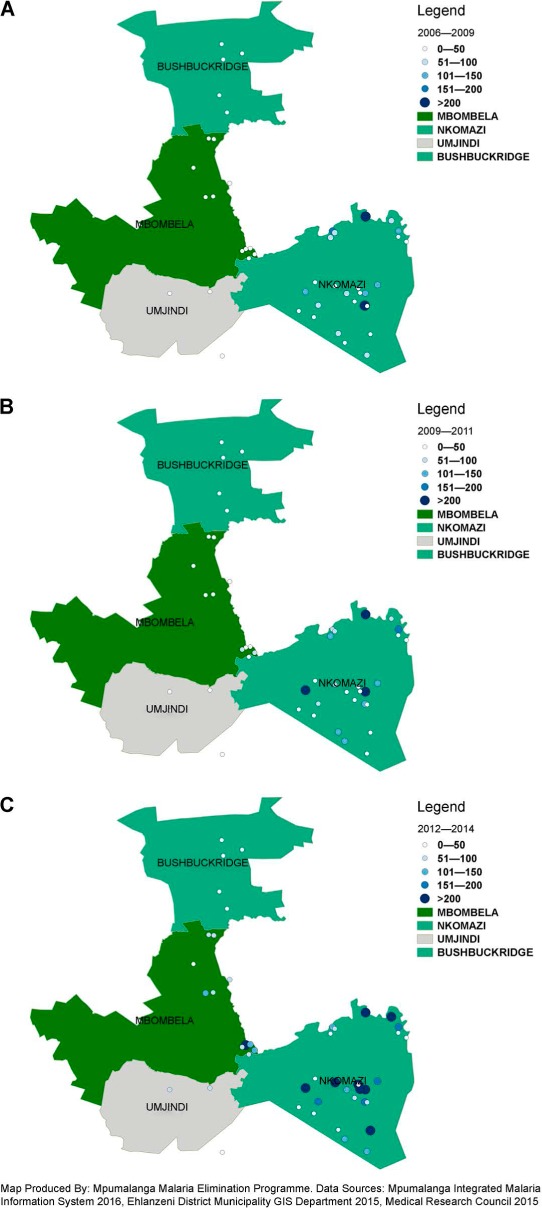
Distribution and relative abundance of identified Anopheles merus breeding sites by municipality in Ehlanzeni District, Mpumalanga Province, South Africa. A) During the period 2005/2006–2008. B) During the period 2009–2011. C) During the period 2012–2014.
During the period 2005/2006–2008, An. merus was confined to Nkomazi municipality, which had the highest mosquito density compared to the other three municipalities (Figure 2A). In the following years, this species spread to Mbombela and Nkomazi Districts, and finally to Bushbuckridge and Umjindi (Figures 2B and 2C).
DISCUSSION
This entomological survey spans a 9-year period and specifically focuses on the abundance and spatial distribution of An. merus in Ehlanzeni District. Of the 52 breeding sites identified for An. merus, half were in one municipality, Nkomazi. During the period under review, the spatial distribution of this species increased to include all the municipalities in the study. In addition, the preponderance of An. merus also increased with time in most of the municipalities.
The increasing abundance and distribution of An. merus in this region, for reasons unknown, is important because An. merus may be partially responsible for ongoing malaria transmission, which threatens South Africa's elimination programme.16 Although this species has never been directly implicated in malaria transmission within South Africa, it has been implicated in neighbouring southern Mozambique.3
A strength of this study is that it extended over a long period—9 years—allowing the gathering of sufficient entomological data to map the spread of An. merus. Standardised mosquito collection and identification methods were used in all municipalities during this period. Nevertheless, some limitations are recognised. These data cannot be adequately correlated with clinical and epidemiological indicators because the mosquito abundances obtained by the larval sampling method may differ from those of adult sampling methods, and the actual contribution of An. merus to malaria transmission in South Africa has not been established.21
The increasing abundance and distribution of An. merus populations may hamper efforts to eliminate malaria in South Africa, especially if the reservoir of Plasmodium parasites increases via immigration of infected persons from other endemic areas. Significant population migration between the Ehlanzeni District and neighbouring malaria-endemic regions, including Mozambique, Swaziland and Zimbabwe, was recently noted.22 The increasing distribution and abundance of An. merus, coupled with a possible increase in the Plasmodium parasite reservoir, may lead to an increase in the incidence of locally acquired malaria in Mpumalanga Province, necessitating the exploration of new vector control strategies specifically directed against populations of this species.
Ongoing entomological surveillance is necessary to establish the role of An. merus and other Anopheles species in malaria transmission. These surveillance activities should also include insecticide susceptibility monitoring of the incriminated vector populations. Furthermore, information concerning the feeding, resting and breeding habits of incriminated species will enable the design of secondary control measures—such as targeted winter larviciding, screening of houses and direct focal spraying—that can be used to enhance the effectiveness of the IRS programme.
CONCLUSION
The distribution and abundance of An. merus has evidently increased in the Ehlanzeni District, most notably in the Nkomazi municipality. This trend may lead to an increase in the incidence of locally acquired malaria in Mpumalanga Province, likely necessitating the incorporation of additional vector control methods specifically directed against populations of this species.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR, Geneva, Switzerland). SORT IT programmes include a teaching component developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union, Paris, France) and Médecins Sans Frontières (MSF, Geneva, Switzerland).
The specific SORT IT programme that resulted in this publication was implemented by WHO/TDR, WHO Global Malaria Programme (GMP), WHO/Africa Region (AFRO), the Operational Research Unit (LuxOR), Brussels Operational Centre, MSF (Luxembourg), the Centre for Operational Research, The Union, University of Nairobi (Nairobi, Kenya), Global AIDS Interfaith Alliance (San Rafael, CA, USA), Academic Model Providing Access to Healthcare (AMPATH, Eldoret, Kenya) and Johns Hopkins University (Baltimore, MD, USA). The programme was funded by WHO/TDR, WHO GMP and WHO/AFRO. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. MC is supported by a National Research Foundation-South African Research Chairs Initiative (NRF-SARChI) grant.
Footnotes
Conflicts of interest: none declared.
In accordance with WHO's open access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the ©right of this publication through a Creative Commons Attribution IGO licence (http://creativecommons.org/licenses/by/3.0/igo/legalcode) that permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1. White G B. Anopheles gambiae complex and disease transmission in Africa. Trans R Soc Trop Med. Hyg 1974; 68: 278– 298. [DOI] [PubMed] [Google Scholar]
- 2. Temu E A, Minjas J N, Coetzee M: . The role of four anopheline species (Diptera: culicidae) in malaria transmission in coastal Tanzania. Trans R Soc Trop Med Hyg 1998; 92: 152– 158. [DOI] [PubMed] [Google Scholar]
- 3. Cuamba N, Mendis C.. The role of Anopheles merus in malaria transmission in an area of southern Mozambique. J Vec Borne Dis 2009; 46: 157– 159. [PubMed] [Google Scholar]
- 4. Mosha F W, Petrarca V.. Ecological studies on Anopheles gambiae complex sibling species on the Kenya coast. Trans R Soc Trop Med Hyg 1983; 77: 344– 345. [DOI] [PubMed] [Google Scholar]
- 5. Pock Tsy J-M L, Duchemin J-B, Marrama L, . et al. Distribution of the species of the Anopheles gambiae complex and first evidence of Anopheles merus as a malaria vector in Madagascar. Malar. J 2003; 2: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomson R C M. Studies on salt-water and fresh-water Anopheles gambiae on the East African Coast. Bull Entomol Res 1951; 41: 487– 502. [Google Scholar]
- 7. Sinka M E, Bangs M J, Manguin S, . et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors 2010; 3: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gillies M T, Coetzee M A.. Supplement to the Anophelinae of Africa south of the Sahara. Johannesburg: South Africa: Publications of the South African Institute for Medical Research, 1987. [Google Scholar]
- 9. Masendu H T, Hunt R H, Koekemoer L L, Brooke B D, Govere J, Coetzee M.. Spatial and temporal distributions and insecticide susceptibility of malaria vectors in Zimbabwe. Afr Entomol 2005; 13: 25– 34. [Google Scholar]
- 10. Coetzee M, Craig M, Le Sueur D.. Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol Today 2000, 16: 74– 77. [DOI] [PubMed] [Google Scholar]
- 11. Kloke R. New distribution record of Anopheles merus Donitz (Diptera: Culicidae) in Zambia. Afr Entomol 1997; 5: 361– 362. [Google Scholar]
- 12. Govere J, Durrheim D N, Coetzee M.. Captures of mosquitoes of the Anopheles gambiae complex (Diptera: Culicidae) in the Lowveld Region of Mpumalanga Province, South Africa. Afr Entomol 2000; 8: 91– 99. [Google Scholar]
- 13. Coetzee M, Hunt R H, Braack L, Davidson G.. Distribution of mosquitoes belonging to the Anopheles gambiae complex, including malaria vectors, south of latitude 15°S. S Afr J Sci 1993; 89: 227– 231. [Google Scholar]
- 14. La Grange J. Survey of anopheline mosquitoes (Diptera: Culicidae) in a malarious area of Swaziland. Afr Entomol 1995; 3: 217– 219. [Google Scholar]
- 15. Gillies M T, de Mellion B.. The Anophelinae of Africa south of the Sahara. Johannesburg: South Africa: Publications of the South African Institute for Medical Research, 1968. [Google Scholar]
- 16. Moonasar D, Morris N, Kleinschmidt I, . et al. What will move malaria control to elimination in South Africa? S Afr Med J 2013; 103 Suppl 2: 801– 806. [DOI] [PubMed] [Google Scholar]
- 17. Sharp B L, Kleinschmidt I, Streat E, . et al. Seven years of regional malaria control collaboration—Mozambique, South Africa, and Swaziland. Am J Trop Med Hyg 2007, 76: 42– 47. [PMC free article] [PubMed] [Google Scholar]
- 18. Bushrod F M. The Anopheles gambiae Giles complex and Bancroftian filariasis transmission in a Tanzanian coastal village. Ann Trop Med Parasitol 1981, 75: 93– 100. [DOI] [PubMed] [Google Scholar]
- 19. Scott J A, Brogdon W G, Collins F H.. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg 1993; 49: 520– 529. [DOI] [PubMed] [Google Scholar]
- 20. Koekemoer L L, Kamau L, Hunt R H, Coetzee M.. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg 2002; 66: 804– 811. [DOI] [PubMed] [Google Scholar]
- 21. Brooke B D, Koekemoer L L, Kruger P, Urbach J, Misiani E, Coetzee M.. Malaria vector control in South Africa. S Afr Med J 2013; 103: 784– 788. [DOI] [PubMed] [Google Scholar]
- 22. Raman J, Morris N, Frean J, . et al. Reviewing South Africa's malaria elimination strategy (2012–2018): progress, challenges and priorities. Malar J 2016; 15: 438. [DOI] [PMC free article] [PubMed] [Google Scholar]


