Abstract
Setting: Larviciding has potential as a component of integrated vector management for the reduction of malaria transmission in Botswana by complementing long-lasting insecticide nets and indoor residual sprays.
Objective: To evaluate the susceptibility of local Anopheles to commonly used larvicides.
Design: This field test of the efficacy of Bacillus thuringiensis subsp. israliensis vs. Anopheles was performed by measuring larval density before treatment and 24 h and 48 h after treatment in seven sites of Bobirwa district, eastern Botswana, in 2012 and 2013. Vector density and malaria cases were compared between Bobirwa and Ngami (northwestern Botswana), with no larviciding in the control arm.
Results: Larviciding reduced larval density by 95% in Bobirwa in 2012, with two cases of malaria, while in 2013 larval density reduction was 81%, with 11 cases. Adult mosquito density was zero for both years in Robelela village (Bobirwa), compared to respectively four and 26 adult mosquitoes per room in Shorobe village (Ngami) in 2012 and 2013. There were no cases of malaria in Robelela in either year, but in Shorobe there were 20 and 70 cases, respectively, in 2012 and 2013.
Conclusion: Larviciding can reduce the larval density of mosquitoes and reduce malaria transmission in Botswana. Large-scale, targeted implementation of larviciding in districts at high risk for malaria is recommended.
Keywords: Bacillus thuringiensis, larval density, Anopheles, larval source management
Abstract
Contexte : Les opérations larvicides ont, en tant qu'élément de la gestion intégrée des vecteurs, le potentiel de réduire la transmission du paludisme au Botswana en complétant les moustiquaires imprégnées d'insecticide rémanent et la pulvérisation d'insecticide a effet rémanent. Objectif : Evaluer la sensibilité des Anophèles locaux aux larvicides généralement utilisés.
Schéma : Le test de terrain de l'efficacité du Bacillus thuringiensis sous-espèce israeliensis a été réalisé vis-à-vis d'Anopheles en mesurant la densité larvaire avant traitement et 24 h et 48 h après traitement dans sept sites du district de Bobirwa (est du Botswana) en 2012 et 2013. La densité vectorielle et les cas de paludisme ont été comparés à Bobirwa et à Ngami (nord-ouest du Botswana), le district témoin sans opérations larvicides.
Résultats : Les opérations larvicides ont réduit la densité larvaire de 95%, avec deux cas en 2012, tandis qu'en 2013 la réduction de la densité larvaire a été de 81%, avec 11 cas à Bobirwa. La densité de moustiques adultes a été de zéro pour les deux années dans le village de Robelela (Bobirwa), comparé à quatre et à 26 adultes par pièce dans le village de Shorobe (Ngami) en 2012 et 2013, respectivement. Il n'y a pas eu de cas de paludisme à Robelela au cours des deux années, mais respectivement 20 et 70 cas sont survenus à Shorobe en 2012 et 2013.
Conclusion: Les opérations larvicides peuvent réduire la densité larvaire des moustiques et réduire la transmission du paludisme au Botswana. La mise en œuvre à grande échelle et ciblée d'opérations larvicides dans les districts à haut risque de paludisme est recommandée.
Abstract
Marco de referencia: La aplicación de larvicidas podría convertirse en un componente del plan integrado de control de los vectores destinado a disminuir la transmisión del paludismo en Botswana, que complemente la utilización de mosquiteros impregnados de insecticidas de larga duración y la fumigación de interiores con insecticidas de acción residual.
Objetivo: Evaluar la susceptibilidad de los anófeles locales a los larvicidas más utilizados.
Método: Se llevó a cabo un ensayo sobre el terreno de la eficacia de Bacillus thuringiensis subespecie israliensis contra el género Anopheles, mediante la medición de la densidad larvaria antes del tratamiento y 24 h y 48 h después del mismo, en siete lugares del distrito de Bobirwa (Botswana oriental) en el 2012 y el 2013. Se compararon la densidad del vector y los casos de paludismo en Bobirwa y en Ngami (Botswana nororiental), que constituyó la rama testigo del estudio sin tratamiento larvicida.
Resultados: La aplicación del larvicida en Bobirwa disminuyó un 95% la densidad larvaria y se presentaron dos casos de paludismo en el 2012, pero en el 2013 esta disminución fue del 81% y se presentaron 11 casos. La densidad de mosquitos adultos fue cero en ambos años en la localidad de Robelela (Bobirwa), en comparación con cuatro a 26 adultos por pieza en Shorobe (Ngami) en el 2012 y el 2013, respectivamente. No se presentaron casos de paludismo en Robelela en estos dos años, pero en Shorobe ocurrieron 20 casos en el 2012 y 70 en el 2013.
Conclusión: La aplicación de larvicidas puede disminuir la densidad de mosquitos y la transmisión del paludismo en Botswana. Se recomienda una aplicación de larvicida dirigida en gran escala en los distritos con alto riesgo de transmisión de paludismo.
Integrated vector management (IVM) was adopted as a key malaria strategy recommended for World Health Organization African region (WHO AFRO) countries in 2004.1 IVM is defined as targeted use of different vector control methods in combination to reduce human-vector contact while addressing sustainability issues.2,3 The three most effective IVM methods employed include indoor residual spraying (IRS), use of long-lasting insecticide-treated nets (LLINs) and larval source management (LSM); these approaches include environmental, mechanical, biological or chemical control.4 IVM has been shown to be both efficacious and cost-effective.5 Furthermore, implementation of IVM has resulted in increased coverage and utilisation of vector control interventions, and has been shown to significantly reduce malaria transmission and burden in other African countries.6,7
Botswana adopted IVM in 2007 as a new strategic approach to vector control consistent with WHO recommendations.8 The main IVM strategies used in Botswana are IRS and LLINs.9,10 When optimally implemented, IRS and LLINs significantly reduce malaria parasite prevalence in low-transmission settings.11 The major challenge for IRS and LLINs is the ability of mosquitoes to develop resistance to the insecticides used in these interventions.12,13 In addition, use of only IRS and LLIN in combination may confer less protection, as they target indoor biting and resting mosquitoes.12,14 Larviciding can complement IRS+L-LIN in eliminating any remaining low-level transmission and reduce the possibility of mosquitoes developing resistance to insecticides. This is because microbial larvicides are not used to kill adult mosquitoes and are therefore not affected by the resistance mechanisms associated with adult mosquitoes.15 Studies performed elsewhere have shown that, unlike chemicals, the microbial larvicides Bacillus sphaericus (Bs) and B. thuringiensis subsp. israelensis are specific and therefore provide environmentally friendly, efficient management of major vectors for malaria in Africa.6,16
In contrast with studies undertaken elsewhere, larviciding in Botswana was carried out during the winter season, when malaria transmission is lowest, to reduce the density of adult mosquitoes before the rainy season began. This is in line with the WHO Global Malaria Programme (GMP) interim recommendation that larviciding should be considered for malaria control (with or without other interventions) only in areas where the breeding sites are few, fixed and findable.17 Although Anopheles gambiae and An. funestus complexes have been reported in Botswana,18 An. arabiensis is the most widespread vector of Plasmodium falciparum in Botswana, and was the species targeted for microbial larviciding. Application during winter reduced the number of water bodies requiring treatment, and was therefore likely to be less costly.
Despite benefits shown elsewhere in Africa, larviciding as a vector control intervention and its potential for larval source reduction has not been studied or implemented in Botswana. The present research was conducted to investigate whether larviciding, if implemented in addition to other vector control interventions, would contribute to a reduction in the malaria burden in a selected district in Botswana. The study focused on 1) evaluating the effectiveness of B. thuringiensis subsp. israliensis on larval density, 2) comparing household mosquito density in larviciding areas vs. areas without larviciding, and 3) comparing the frequency of malaria cases in larviciding areas vs. areas without larviciding.
METHODOLOGY
Study design
This was an interventional, analytical study of microbial larviciding in selected villages in Bobirwa district. A comparison of malaria cases was made between two selected villages: Robelela (Bobirwa district) with microbial larviciding and Shorobe (Ngami district) with no larviciding.
Setting
Botswana is a landlocked country, with a total population of approximately 2 million. Bobirwa sub-district is in the eastern part of the central district in Botswana, bordered by Zimbabwe to the north-east and South Africa to the south-east. Average annual rainfall in the district ranges from 300 to 400 mm.19
The study was conducted between the months of August and early October in 2012 and 2013, just after the last month of winter, which starts in May and lasts until the end of July. The area was selected due to its high prevalence of malaria cases and significant amounts of stagnant water, which met the set criteria.20 Before larviciding, stagnant bodies of water were located and characterised. Pools with larval densities that met predefined thresholds were mapped and assigned for larviciding. As the topography of the area varied, potential breeding sites were characterised as permanent or semi-permanent to expedite the frequency of routine follow-up required. The communities in the operational areas were informed about the larviciding initiative to obtain their support and participation.
The two villages included in the study, Robelela and Shorobe, are located in riverine ecosystems where patches of water are found that are used by mosquitoes for breeding. Both villages lie in epidemiological zone B, an intermediate malaria transmission area in which 18.8% of the population living there are almost equally at risk for malaria.10 As the malaria vector in both villages is An. arabiensis, this will not confound the results observed. Microclimatic data that could confound the results were not measured. The major confounding factor could be the different levels of LLIN and IRS interventions: IRS coverage in the two districts falls short of the target of 80% set by the National Malaria Programme (NMP) (Figure).10
FIGURE.
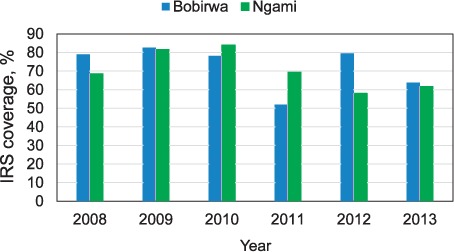
IRS coverage, 2008–2013. IRS = indoor residual spraying.
Method of application
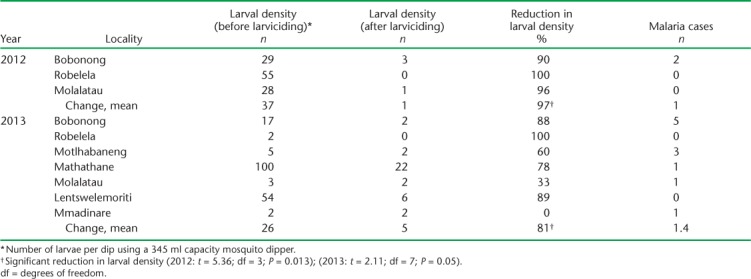
The microbial larvicide used for this project is B. thuringiensis subsp. israliensis, applied as a liquid formulation (VectoBac® 12AS, Bayer AG, Leverkusen, Germany), at 1200 international toxin units (ITU) in 2012. In 2013, a granular formulation (VectoBac G, GS, 200 ITU) was used. The liquid formulation was applied at a rate of 2 l/hectare using a knapsack sprayer, while granules of B. thuringiensis subsp. israliensis were applied at 2.0 g/m2 of water. In smaller areas that were relatively easy to access, a hand spreader was used for application. For larger water bodies that were difficult to access, hand broadcasting was utilised. The team took 5 days to treat the water bodies in selected locations in Bobirwa district (Table 1).
TABLE 1.
Effects of bio-larvicide on Anopheles fourth instar larval abundance and malaria cases in Bobirwa sub-district, 2012–2013
Measurement of larval density
Larval density was determined using a 354 ml dipper to collect water. Ten dips were collected and the average number of larvae per dip was recorded.16 This was done before treatment and at 24 h and 48 h after treatment at all locations in Bobirwa sub-district to provide an objective performance measurement of the microbial larvicides.
Surveillance of malaria vectors
Routine mosquito surveys were conducted by the NMP for entomological surveillance to monitor vector density, which guided the vector control activities. Adult mosquitoes were collected from houses using the pyre-thrum spray sheet technique.16 Mosquitoes were collected from a sample of 40 rooms per site between January and April each year, at least 2 months after larviciding. A fast commercial aerosol insecticide was sprayed inside the house and simultaneously around the eaves of the house from the outside. Dead mosquitoes were collected after 8 min from white sheets previously spread on the house floor, counted and classified as Anophelinae or Culicinae, and separated into male or female, as the females are the malaria vectors. Female mosquitoes were further categorised by abdominal condition. Mosquito density (mosquitoes per room) was classified as follows: absent = 0, low = 1–5, medium = 6–11, high = 12–19 and very high = 20–45+.
Malaria cases in larviciding vs. non-larviciding districts
Data on malaria cases were collected at Robelela in Bobirwa district, where larviciding was performed, and from Shorobe in Ngami district, where no larviciding was performed, for comparison. The NMP provided the data. The data collected were stratified according to location, year of application, treatment (before and after), and area under study (larviciding vs. no larviciding).
Analysis and statistics
Routine summary descriptive statistics were utilised to obtain frequencies, percentages and means. The t-test was used to compare the effects of microbial larviciding (before and after larviciding) on larval density. The χ2 test and correlation analyses were used to test significant associations between the number of malaria cases and the decrease in larval density between the villages of Robelela and Shorobe. We used EpiData software for data entry and analysis (v. 3.1 for entry and v. 2.2.2.182 for analysis, EpiData Association, Odense, Denmark).
Ethics
As this study involved the collection of routine programme data, consent was not required. Confidentiality was ensured through de-identification of any household and patient-level data. Data were stored and archived securely, and only the researchers had access. Because this was an interventional analytical study using programme data, no harm to study participants occurred. Ethical approval was obtained from the Botswana Ministry of Health research unit (Gaborone, Botswana) for ethical review by the research committee for clearance and the Ethics Advisory Group (EAG) of the International Union Against Tuberculosis and Lung Disease (The Union, Paris, France). Because the study involved analyses of routine programme data with no human subject, an exemption was issued by The Union's EAG (Study Approval no. 49/15).
RESULTS
Three study sites were selected for a larvicide effectiveness analysis in 2012; this was expanded to seven in 2013. Table 1 shows the results of larval density before/after treatment in the respective sites by year, including the rate of decrease in larval density and the reported number of malaria cases from the same areas in the respective sites, stratified by year. Significance testing using paired t-test comparing the effects of microbial larviciding (before/after larviciding) showed a significant decrease in larval density (Table 1).
Key findings include a mean larval density reduction of 95% in 2012 across the three study sites, and only two cases of reported malaria. In 2013, the mean total larval density reduction was lower, at 81%, for the seven target sites, but only 11 malaria cases were reported from these areas. There was no significant association between the number of malaria cases and the reduction in larval density after microbial bio-larviciding (Table 2). This was confirmed by correlation analysis, where no significance was observed (Table 2).
TABLE 2.
Robelela site (larviciding) vs. Shorobe site (no larviciding), total number of adult Anopheles, mosquito density and number of reported malaria cases, Botswana, 2012–2013
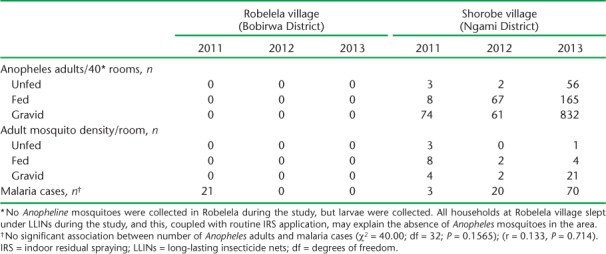
To compare the possible effects of larviciding in treated vs. non-treated areas, the sites of Robelela and Shorobe were selected (Table 2). Shorobe was chosen as the comparator due to similarities in environment, population and location to Robelela. Using the previously described mosquito sample techniques for both inside and outside the residences, mosquito density was found to be zero at Robelela at baseline (2011) and in both 2012 and 2013, compared to significant densities in Shorobe during both comparison years. There were no reported cases of malaria in Robelela, and respectively 20 and 70 cases of malaria in Shorobe in 2012 and 2013.
DISCUSSION
This study was initiated as a pilot project to pave the way for large-scale implementation of the larval source reduction that would complement the LLIN and IRS interventions already in place in high-risk malaria areas in Botswana. The introduction of this project was premised on the fact that microbial larviciding had never been attempted in Botswana and it was therefore necessary to pilot the approach under the semi-arid conditions of the country.
While larval control as a malaria intervention is used by at least 48 countries globally,21 its potential for larval source reduction has not been studied or implemented in Botswana. There was concern in Botswana that the impact of larviciding interventions had not been quantified. There were also arguments that it would add to and increase the budget, as it is an expensive exercise. No costing was performed during the current study, however; this needs to be done when microbial larviciding is rolled out to other districts in the next phase.
The results from this study confirmed that microbial larviciding using B. thuringiensis subsp. israelensis reduces larval density when applied to water bodies.22 Our premise was that winter larviciding would lower the density of indoor resting mosquito adults in summer and consequently reduce both disease transmission and the number of malaria cases. Following the application of B. thuringiensis subsp. israelensis larvicide, zero densities of indoor resting adults were found, and malaria cases were very low compared to Shorobe, where no larviciding was done.
It is probable that the lower number of malaria cases in Robelela was due to reduced adult mosquito density associated with winter larviciding. However, caution is required in drawing conclusions: IRS and LLIN interventions had been implemented in previous years in the villages in the study, and their residual effect could also have contributed to the lower populations of adult mosquitoes and the decrease in the number of malaria cases. Nevertheless, both interventions (IRS+LLIN) were implemented in the comparison village of Shorobe, which clearly had more reported malaria cases than Robelela during the post-larviciding monitoring period. The increase in malaria cases at Shorobe could be related to lower IRS coverage in Ngami district compared to Bobirwa district. In 2012, IRS coverage in Bobirwa was 79.5%, higher than in Ngami, where coverage was 58% (Figure). The lowest IRS coverage was in 2013, at respectively 63.8% and 61.6% in Bobirwa and Ngami. The lower coverage may have accounted for variations in the number of malaria cases found during the current study.
The variation in the rate of larval density reduction between 2012 and 2013 could be attributed to several factors associated with the application procedures and formulations used. However, studies have shown no significant differences in the mortality of mosquito larvae controlled by either liquid or granular formulations.23 The feeding rate of the larvae is also important, because the proteins ingested can be influenced by other factors, such as temperature during application, water depth, larval age, water temperature and the density of the larvae.
A possible behavioural change in mosquitoes was recently noted in some areas in Botswana, with a shift toward more outdoor biting.23 LSM with larvicides has the potential to overcome this problem, as reducing larval density will consequently reduce adult density, regardless of indoor and outdoor biting behaviours. Larviciding will therefore likely complement IRS and LLIN to eliminate the remaining low-level transmission in Botswana.
Our study is in agreement with other studies that have reported a decrease in malaria cases following larviciding.3 Additional studies have also demonstrated that larvicide reduced malaria vector mosquito larvae and adult females by 90% in targeted rural areas.7,24 Vector control with microbial larvicides was shown to be capable of enhancing the malaria control achieved using LLINs alone.4,7 Collectively, this and other studies indicate that multiple vector control strategies are likely synergistic when used in combination as an IVM plan.5,6
The strengths of this study include the rigorous approach to choosing application sites, larvicide application techniques and standardised follow-up mosquito density counts. Furthermore, the malaria case reporting systems in Botswana are quite robust, with support from the WHO. The primary weakness in some sampling sites for data collection includes the lack of follow-up in 2012 due to logistical problems within the Ministry of Health. This was secondary to rain in August and September, when larviciding was not practical. Furthermore, the data presented here are from a relatively small pilot project and need to be scaled up to cover more malaria endemic areas, with further evaluation.
Microbial larviciding as a component of LSM needs to be expanded to two epidemiological zone B districts, namely Tutume and Bobirwa. These two districts have manageable water bodies, most of which are fewer, fixed and findable.17 As microbial larviciding was able to reduce larval density to a minimum of two larvae per breeding site in four locations (Table 1), there is potential for this intervention to contribute to a reduction in the malaria burden in some parts of Botswana.25 A combination of IRS, LLINs and LSM can contribute to the effort of Botswana in its aim to eliminate malaria. Because resources are often limited, community larviciding at district level, working with environmental health teams, will help implement cost-effective larviciding in Botswana.
In conclusion, the main findings of this study are that larviciding can effectively reduce both the larval density of mosquitoes and the numbers of adult mosquitoes, and may be associated with a reduction in the transmission of malaria in the semi-arid context of Botswana. It is therefore plausible to supplement other vector control strategies with larviciding to delay the development of resistance and address shifting mosquito behaviours. We would propose the further scale-up of larviciding in Botswana's high-risk malaria regions to evaluate whether these outcomes are reproducible and to obtain sustainability outcome assessments.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR, Geneva, Switzerland). The SORT IT programmes include a teaching component developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union, Paris, France) and Médecins Sans Frontières (MSF, Geneva, Switzerland). The specific SORT IT programme that resulted in this publication was implemented by WHO/TDR, the WHO Global Malaria Programme (GMP), the WHO/African Region (AFRO), the Operational Research Unit (LuxOR), MSF, Brussels Operational Centre (Luxembourg), the Centre for Operational Research, The Union, the University of Nairobi (Nairobi, Kenya), the Global AIDS Interfaith Alliance (San Rafael, CA, USA), the Academic Model Providing Access to Healthcare (AMPATH, Eldoret, Kenya) and Johns Hopkins University (Baltimore, MD, USA). WHO/TDR, WHO GMP and WHO/AFRO funded the programme. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest: none declared.
In accordance with WHO's open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the ©right of this publication through a Creative Commons Attribution IGO license (http://creativecommons.org/licenses/by/3.0/igo/legalcode) that permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1. Manga L, Toure A, Shililu J.. Implementation of integrated vector management in the WHO-African region: progress report 2000–2003. Washington, DC, USA: US Agency for International Development, 2004. [Google Scholar]
- 2. World Health Organization Regional Office for Africa. . Report of a workshop on a framework for development and implementation of vector control interventions in the WHO African region. Harare, Zimbabwe: WHO Regional Office for Africa, 2001. [Google Scholar]
- 3. World Health Organization. . Global strategic framework for integrated vector management. WHO/CDS/CPE/PVC/2004.10 Geneva, Switzerland: WHO, 2004. [Google Scholar]
- 4. Fillinger U, Lindsay S W.. Larval source management for malaria control in Africa: myths and reality. Malar J 2011; 10: 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chanda E, Masaninga F, Coleman M, . et al. Integrated vector management: the Zambian experience. Malar J 2008; 7: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fillinger U, Lindsay S W.. Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya. Trop Med Int Health 2006; 11: 1629– 1642. [DOI] [PubMed] [Google Scholar]
- 7. Fillinger U, Sonye G, Killeen G F, Knols B G, Becker N.. The practical importance of permanent and semipermanent habitats for controlling aquatic stages of Anopheles gambiae sensu lato mosquitoes: operational observations from a rural town in western Kenya. Trop Med Int Health 2004; 9: 1274– 1289. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization. . Handbook for integrated vector management. WHO/HTM/NTD/VEM/2012.3 Geneva, Switzerland: WHO, 2012. [Google Scholar]
- 9. Chihanga S, Moakofhi K, Mosweunyane T, . et al. Malaria control in Botswana, 2008–2012: the path towards elimination. Malar J 2013; 12: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Botswana Ministry of Health. . Botswana malaria indicator survey, 2012 report. Gaborone, Botswana: National Malaria Programme, MoH, 2012. [Google Scholar]
- 11. Geissbuhler Y, Kannady K, Chaki P P, . et al. Microbial larvicide application by a large-scale, community-based program reduces malaria infection prevalence in urban Dar Es Salaam, Tanzania. PLOS ONE 2009; 4: 5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mulla M S, Thavara U, Tawatsin A, Chomposri J, Su T.. Emergence of resistance and resistance management in field populations of tropical Culex quinquefasciatus to the microbial control agent Bacillus sphaericus. J Am Mosq Control Assoc 2003; 19: 39– 46. [PubMed] [Google Scholar]
- 13. Munhenga G, Masendu H T, Brooke B D, Hunt R H, Koekemoer L K.. Pyrethroid resistance in the major malaria vector Anopheles arabiensis from Gwave, a malaria-endemic area in Zimbabwe. Malar J 2008; 7: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fillinger U, Kannady K, William G, . et al. A tool box for operational mosquito larval control: preliminary results and early lessons from the Urban Malaria Control Program in Dar es Salaam, Tanzania. Malar J 2008; 7: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization. . Environ Health Criteria 217. Microbial pest control agent: Bacillus thuringiensis. Geneva, Switzerland: WHO, 1999. [Google Scholar]
- 16. World Health Organization. . Entomological field techniques for malaria control. Volume 1 Geneva, Switzerland: WHO, 1992. [Google Scholar]
- 17. World Health Organization. . Interim position statement. The role of larviciding for malaria control in sub-Saharan Africa. Geneva, Switzerland: WHO, 2012. [Google Scholar]
- 18. Tawe L, Ramatlho P, Waniwa K, . et al. Preliminary survey on Anopheles species distribution in Botswana shows the presence of Anopheles gambiae and Anopheles funestus complexes. Malar J 2017; 16: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kayombo B, Tsheko R, Semetsa S, Malepa D.. Documentation of indigenous knowledge and best-bet practices on use of animals and plants for sustainable natural resource management in Botswana. Bots J Agric Appl Sci 2014; 10: 3– 10. [Google Scholar]
- 20. World Health Organization. . Report of the WHO consultation on integrated vector management (IVM). WHO/CDS/NTD/VEM/2007.1 Geneva, Switzerland: WHO, 2007. [Google Scholar]
- 21. World Health Organization. . World malaria report, 2015. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 22. Fillinger U, Ndenga B, Githeko A, Lindsay S W.. Integrated malaria vector control with microbial larvicides and insecticide-treated nets in western Kenya: a controlled trial. Bull World Health Organ 2009; 87: 655– 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harwood J F, Farooq M, Turnwall B T, Richardson A G.. Evaluating liquid and granular Bacillus thuringiensis var. israelensis broadcast applications for controlling vectors of dengue and chikungunya viruses in artificial containers and tree holes. J Med Entomol 2015; 52: 663– 671. [DOI] [PubMed] [Google Scholar]
- 24. Majambere S, Lindsay S W, Green C, Kandeh B, Fillinger U.. Microbial larvicides for malaria control in The Gambia. Malar J 2007, 6: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fillinger U, Sombroek H, Majambere S, van Loon E, Takken W, Lindsay S W.. Identifying the most productive breeding sites for malaria mosquitoes in The Gambia. Malar J 2009, 8: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]


