Abstract
Adiponectin is an adipocyte-derived hormone with antidiabetic activities that include increasing the sensitivity of cells to insulin. Adaptor protein containing pleckstrin homology domain, phosphotyrosine-binding domain, and leucine zipper motif (APPL1) stimulates adiponectin signaling and promotes adiponectin's insulin-sensitizing effects by binding to two adiponectin receptors, AdipoR1 and AdipoR2, and the insulin receptor. In this study, we report an alternative splicing variant of APPL1 (APPL1sv) that is highly expressed in mouse liver, pancreas, and spleen tissues. The expression levels of APPL1sv in liver tissues were enhanced in a mouse model of obesity and diabetic dyslipidemia (i.e. db/db mice) and reduced in calorie-restricted mice compared with ad libitum–fed mice. APPL1sv overexpression or suppression inhibited or enhanced, respectively, adiponectin-stimulated phosphorylation of AMP protein kinase (AMPK) in mouse hepatocytes. We also found that APPL1sv binds to AdipoR1 and AdipoR2 under basal conditions and that adiponectin treatment reduces this binding. Overexpression of APPL1sv blocked adiponectin-induced interactions of APPL1 with the adiponectin receptors. Moreover, adenovirus-mediated and short hairpin RNA–based suppression of APPL1sv greatly reduced high fat diet–induced insulin resistance and hepatic glucose production in mice. Our study identifies a key suppressor of hepatic adiponectin signaling and insulin sensitivity, a finding that may shed light on identifying effective therapeutic targets for treating insulin resistance and type 2 diabetes.
Keywords: adiponectin, alternative splicing, insulin resistance, AMP-activated kinase (AMPK), liver, metabolism, diabetes, APPL1, APPL1sv, metabolic disorder
Introduction
Adiponectin is an adipocyte-derived hormone with antidiabetic and anti-inflammatory functions (1, 2). Several adiponectin receptors, including AdipoR1, AdipoR2, and T-cadherin, have been reported to mediate adiponectin signaling in various tissues (3, 4). A number of molecules have been identified as mediators of adiponectin signaling in cells and in vivo (5, 6).
The adaptor protein APPL1 (adaptor protein containing pleckstrin homology (PH)3 domain, phosphotyrosine binding (PTB) domain, and leucine zipper motif) acts as a scaffold protein that binds directly with adiponectin receptors (AdipoR1 and AdipoR2) and positively mediates adiponectin signaling in insulin target tissues (7, 8). In addition to binding with adiponectin receptors, APPL1 can recruit insulin receptor substrates 1 and 2 (IRS1/2) to the insulin receptor (IR) in response to adiponectin or insulin stimulation, which promotes insulin sensitivity (9). A recent study indicates that point mutations in the appl1 gene are associated with a risk of type 2 diabetes in humans (10). Consistent with these findings, overexpression of APPL1 in mouse liver potentiates insulin-suppressed hepatic glucose production and alleviates diabetes, whereas suppressing APPL1 expression in mouse liver leads to glucose intolerance (11). In addition, systemic knockout of the appl1 gene in mice results in impairment of insulin and adiponectin signaling in insulin target tissues, leading to insulin resistance and mitochondrial dysfunction (9, 12).
APPL2 is an isoform of APPL1 and shares a high level of amino acid sequence homology (∼54% similarity) with the APPL1 protein (13). Similar to APPL1, APPL2 contains an N-terminal Bin–Amphiphysin–Rvs (10) domain, a central PH domain, and a C-terminal PTB domain. Despite these similarities, APPL2 acts in the form of two homodimers, in contrast to a single homodimer form for APPL1 (14), and antagonizes APPL1 action on adiponectin signaling, acting in Yin-Yang regulation (15).
In this study, we report a splicing variant of APPL1 (APPL1sv) that is specifically expressed in mouse liver, pancreas, and spleen tissues. We found that the levels of APPL1sv protein expression in liver tissue are inversely correlated with insulin sensitivity in mouse models. Additionally, we demonstrate an inhibitory role of this splicing variant in regulating adiponectin signaling and function in hepatic cells and in vivo. Our study reveals, for the first time, a splicing variant of APPL1 that antagonizes adiponectin signaling and action in liver.
Results
Identification of APPL1sv
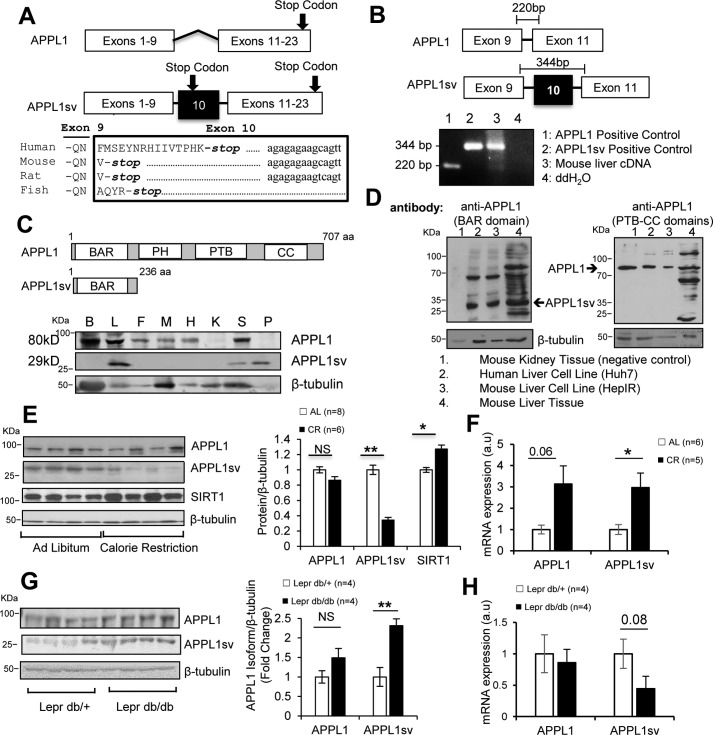
To understand the mechanism regulating adiponectin action in cells, we conducted yeast two-hybrid screening to identify signaling molecules that interact with adiponectin receptors. In addition to APPL1 (7), we obtained a candidate gene that contains an extra exon (exon 10) in conventional APPL1 cDNA (Fig. 1A), which results in a long form of APPL1 cDNA containing a total of 23 exons. To confirm the existence of this extra exon in the genome, we performed a sequence alignment analysis through the Basic Local Alignment Search Tool (BLAST), available from the National Center for Biotechnology Information (http://blast.ncbi.nlm.nih.gov/Blast.cgi), and found that exon 10 is conserved in the appl1 gene among humans, mice, rats, and zebrafish (Fig. 1A). Interestingly, there is a premature stop codon after a few residues within exon 10, which suggests a truncated form of APPL1 protein expression (Fig. 1A). Additionally, the genomic regions downstream of this premature stop codon are also highly conserved among humans, rats, and mice but not in fish, indicating that the premature stop is important to evolution and that the regulatory mechanism of exon 10 expression should be common in mammals. Based on our findings, we registered human svAPPL1 and mouse svAPPL1 in the GenBankTM/EBI Data Bank under accession numbers MG569951 for human and MF113398.1 for mouse.
Figure 1.
Identification of a novel APPL1 splicing variant (APPL1sv). A, schematic of APPL1 and APPL1sv transcripts (top panel) and alignment of the APPL1sv amino acid sequence through different species (bottom panel). Exon 10 contains a premature stop codon (bold letters) and the downstream conserved nucleotide sequence (lowercase letters). B, detection of exon 10 in the mouse liver APPL1 transcript. The presence of exon 10 was detected via RT-PCR with primers designed to flank exon 10. ddH2O, double-distilled H2O. C, schematics of APPL1 and APPL1sv protein domain structures (top panel) and tissue distribution of APPL1sv (bottom panel). Mouse brain (B), liver (L), fat (F), skeletal muscle (M), heart (H), kidney (K), spleen (S), and pancreas (P) tissues were collected from C57BL/6J male mice (3 months old). The levels of APPL1 were detected by Western blot analysis with APPL1 C-terminal antibody, whereas APPL1sv was detected by APPL1 N-terminal antibody. aa, amino acids. D, APPL1sv protein is present in mouse liver tissues as well as the human liver cell line. APPL1 protein was detected with antibodies specific to APPL1 (APPL1 PTB-CC domain antibody) and APPL1sv (APPL1 N-terminal antibody). E, APPL1 isoform expression in calorie-restricted mouse livers. Liver tissues were isolated from mice fed either ad libitum or calorie-restricted for 4 months. F, detection of APPL1 and APPL1sv mRNA expression in calorie-restricted mouse liver. RNA was isolated from calorie-restricted (CR) or ad libitum (AL) liver tissues. a.u., arbitrary units. G, APPL1 isoform expression in diabetic mouse livers. Liver tissues were isolated form db/db mice or control mice (db/+). H, detection of APPL1 and APPL1sv mRNA expression in db/db mouse liver. RNA was isolated from db/db or db/+ mouse liver tissues. The statistical analyses in E and F were performed by one-way ANOVA. Error bars, ± S.E. *, p < 0.05; **, p < 0.01; NS, no significant difference.
To determine whether exon 10 is retained in APPL1 transcripts, we isolated total mRNAs from the liver tissue of C57BL/6J mice and performed a reverse transcriptase reaction to generate a liver cDNA pool. The presence of exon 10 in the liver transcripts was determined by PCR, with the forward and reverse primers located on exon 9 and exon 11, respectively (Fig. 1B). The APPL1 plasmid and the APPL1 plasmid containing exon 10 were used as controls for PCR fragments lacking and containing exon 10, respectively. The cDNA pool generated from mouse liver tissue was subjected to PCR analysis with the abovementioned primer set, and two bands of 220 bp (lacking exon 10) and 344 bp (containing exon 10) were detected by agarose gel electrophoresis (Fig. 1B), indicating the presence of a novel splicing variant of APPL1 (hereafter referred to as APPL1sv) in liver tissues in addition to conventional APPL1.
We next examined whether APPL1sv protein is expressed in human or mouse cells and tissues. Given that exons 1–10 of the appl1 gene encode 236 amino acids, the predicted molecular mass of APPL1sv should be 29 kDa (Fig. 1C). With the antibody specific against the N-terminal BAR domain but not the C-terminal part of APPL1, we found that APPL1sv protein is expressed in both mouse and human hepatic cell lines (Fig. 1D). Unlike APPL1, which is widely expressed in various mouse tissues (7), APPL1sv is highly expressed in the liver and, to a lesser extent, in the pancreas and spleen (Fig. 1C). Under calorie restriction, which significantly increased SIRT1 levels as in previous reports (14), both the mRNA (Fig. 1F) and protein levels (Fig. 1E) of APPL1sv, but not of APPL1, were significantly reduced in the liver tissues. In addition, the protein levels of APPL1sv were significantly higher in the livers of db/db mice compared with controls (Fig. 1G), although the mRNA levels were reduced (Fig. 1H). Collectively, these data suggest that APPL1sv expression is inversely correlated with insulin sensitivity in mouse models.
Suppression of APPL1sv expression in liver tissues enhances insulin sensitivity in diet-induced obese mice
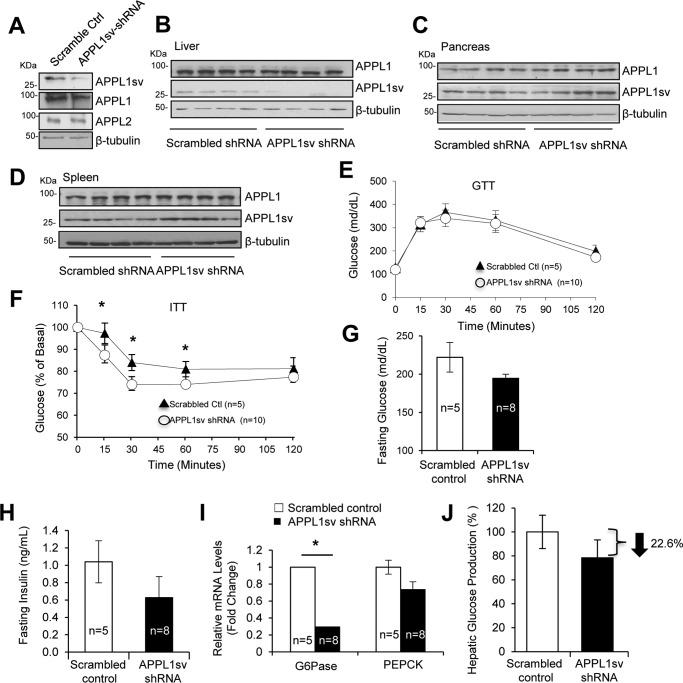
To understand the role of hepatic APPL1sv in vivo, we generated adenoviruses containing GFP plus APPL1sv shRNA that target exon 10 in APPL1 transcripts or GFP plus the scrambled control sequence. Infection of primary mouse hepatocytes with adenoviruses containing the shRNA led to suppression of APPL1sv expression, but not of APPL1 and APPL2 expression, compared with cells transfected with the viruses containing scrambled control (Fig. 2A). Male C57BL/6J mice fed with a high-fat diet (HFD) were injected with adenovirus containing APPL1sv shRNA or the scrambled control via tail vein injection. Fourteen days after injection, APPL1sv expression was selectively suppressed by the shRNA in the liver (Fig. 2B) but not in the pancreas (Fig. 2C) or the spleen tissues (Fig. 2D). Suppression of APPL1sv expression had no effect on glucose tolerance (Fig. 2E). However, insulin tolerance tests showed that suppression of hepatic APPL1sv expression led to a significant improvement of insulin sensitivity in diet-induced obese mice (Fig. 2F). Consistent with this finding, APPL1sv shRNA injection reduced fasting glucose (Fig. 2G) and insulin levels (Fig. 2H) in mice; the mRNA levels of two key gluconeogenic enzymes, glucose-6-phosphatase (Glc-6-Pase) and phosphoenolpyruvate carboxykinase (PEPCK), were also suppressed (Fig. 2I). In agreement with reduced gluconeogenic gene expression, hepatic glucose production was decreased in primary hepatocytes infected with an adenovirus containing APPL1sv shRNA compared with controls (Fig. 2J). Taken together, these results indicate that suppression of hepatic APPL1sv expression in HFD-fed mice leads to improvement of insulin sensitivity and reduction of hepatic glucose production and gluconeogenic gene expression, suggesting an inhibitory role of APPL1sv in regulating hepatic insulin sensitivity and function in vivo.
Figure 2.
The roles of APPL1sv in regulating hepatic glucose metabolism and insulin sensitivity. A, suppression of APPL1sv expression with shRNA in primary hepatocytes. Primary mouse hepatocytes were infected with an adenovirus containing GFP plus APPL1sv shRNA or GFP plus the scrambled control (Ctrl). B–D, injection of an adenovirus containing APPL1sv shRNA with GFP into mice selectively suppresses APPL1sv expression in liver tissue. Three-month-old male C57BL/6J mice fed a 45% high-fat diet for 15 weeks were treated with adenoviruses encoding APPL1sv-shRNA or scrambled control by tail vein injection (2 × 109 pfu/mouse). Fourteen days after injection, mice were sacrificed, and tissues were collected. APPL1 C-terminal and APPL1 N-terminal antibodies were used to detect APPL1 and APPL1sv protein expression in the liver (B), pancreas (C), and spleen (D). E, glucose tolerance test (GTT). Mice were fasted for 16 h and then injected intraperitoneally with glucose (2 g/kg). Tail vein blood glucose was checked at the indicated times. F, insulin tolerance test (ITT). Mice were fasted for 5 h and injected intraperitoneally with 0.75 units/kg insulin. Tail vein blood glucose was checked at the indicated times. G, fasting glucose concentration. Tail vein blood was collected in 5-h fasted mice 10 days after viral injection. H, fasting insulin concentration. Tail vein blood was collected in 5-h–fasted mice 10 days after viral injection. I, gluconeogenic gene expression. The levels of Glc-6-Pase and PEPCK expression were detected via real-time quantitative PCR using primers for Glc-6-Pase and PEPCK and normalized against β-actin. J, primary mouse hepatocytes were infected with adenoviruses encoding either scrambled control shRNA or APPL1sv-shRNA. Forty-eight hours after infection, the cells were incubated in glucose-free DMEM for 30 min and then treated with either 1 × PBS or 10 nm insulin for another 30 min, followed by treatment with gluconeogenic substrates, 1 mm sodium l-lactate and 1 mm sodium pyruvate, for 6 h. Glucose production was assessed using the Amplex Red glucose/glucose oxidase assay kit. Results were analyzed by Student's t test, and error bars are presented as ± S.E. from three independent experiments. Statistical analysis in E and F was performed by two-way ANOVA with repeated measures. Statistical analysis in G–I was performed by Student's t test. Results are presented as ± S.E. *, p < 0.05.
APPL1sv is a negative regulator of hepatic adiponectin signaling
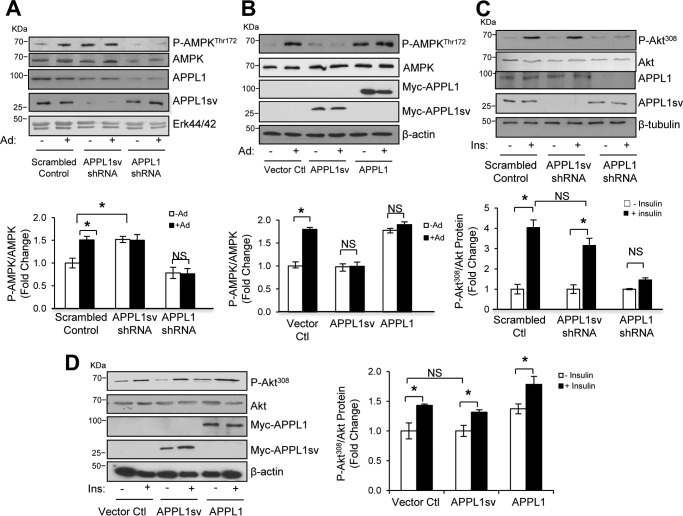
To investigate the mechanism underlying the inhibitory role of APPL1sv in insulin sensitivity, we suppressed APPL1sv and APPL1 expression with the respective shRNAs in mouse hepatocytes (Fig. 3A). Consistent with our previous reports that down-regulation of APPL1 expression reduces AMPK expression and blocks adiponectin-stimulated AMPK phosphorylation in muscle cells (7, 9), suppression of APPL1 expression in mouse hepatocytes reduced AMPK expression and blunted the stimulatory effect of adiponectin on AMPK phosphorylation (Fig. 3A). On the other hand, suppression of APPL1sv expression in hepatocytes significantly enhanced basal AMPK phosphorylation (Fig. 3A). To test whether up-regulation of APPL1sv is sufficient to inhibit adiponectin signaling, we expressed myc-tagged APPL1sv or APPL1 in hepatocytes. As shown in Fig. 3B, overexpression of APPL1sv blocked adiponectin-stimulated AMPK phosphorylation, which is opposite to the effect of overexpressing APPL1 on adiponectin signaling (7). Together, these results indicate that APPL1sv plays an inhibitory role in regulating adiponectin signaling in hepatic cells.
Figure 3.
The effects of APPL1sv on hepatic adiponectin and insulin signaling. A, suppressing APPL1sv expression enhances adiponectin signaling. Primary mouse hepatocytes infected with adenoviruses encoding either scrambled control shRNA, APPL1sv-shRNA, or APPL1-shRNA were serum-starved for 4 h and then treated with (+) or without (−) 1 μg/ml full-length adiponectin (Ad) for 15 min. Phosphorylation of AMPK at Thr172 as well as the protein levels of AMPK, APPL1, and APPL1sv were detected by Western blot analysis using specific antibodies as indicated. ERK 1/2 was used as a loading control. B, overexpressing APPL1sv inhibits adiponectin signaling. Mouse hepatocytes transfected with plasmids containing control vector, myc-tagged APPL1sv, or myc-tagged APPL1 were serum-starved for 4 h and treated with (+) or without (−) 1 μg/ml full-length adiponectin for 15 min. Phosphorylation of AMPK at Thr172 as well as the protein levels of AMPK, myc-tagged APPL1, and myc-tagged APPL1sv were detected by Western blot analysis using specific antibodies as indicated. β-Tubulin was used as a loading control. C, the effect of suppressing APPL1sv expression on insulin signaling. Primary mouse hepatocytes infected with adenoviruses encoding either scrambled control shRNA, APPL1sv-shRNA, or APPL1-shRNA were serum-starved for 4 h and treated with (+) or without (−) 10 nm insulin (Ins) for 5 min. Phosphorylation of Akt at Thr308 as well as the protein levels of Akt, APPL1, and APPL1sv were detected by Western blot analysis using specific antibodies as indicated. β-Tubulin was used as a loading control. D, the effect of overexpressing APPL1sv on insulin signaling. Mouse hepatocytes transfected with plasmids containing control vector, myc-tagged APPL1sv, or myc-tagged APPL1 were serum-starved for 4 h and treated with (+) or without (−) 10 nm insulin for 5 min. Phosphorylation of Akt at Thr308 as well as the protein levels of Akt, myc-tagged APPL1, and myc-tagged APPL1sv were detected by Western blot analysis using specific antibodies as indicated. β-Tubulin was used as a loading control. Statistical significance was determined by two-way ANOVA with Tukey's multiple correction tests. Results are presented as ± S.E. from three independent experiments. *, p < 0.05; NS, no significant difference.
APPL1sv has no direct effect on hepatic insulin signaling
We and others have demonstrated that APPL1 potentiates insulin signaling via direct binding with Akt, IRS1/2, and IR (9, 15). We next investigated the role of APPL1sv in regulating insulin signaling by suppressing or overexpressing APPL1sv in hepatocytes. Although suppression or overexpression of APPL1 reduced or promoted insulin-stimulated Akt phosphorylation, respectively, as we reported previously in muscle cells (7), neither suppression (Fig. 3C) nor overexpression (Fig. 3D) of APPL1sv had any effect on insulin signaling, probably because this splicing variant lacks the PTB domain and CC motif (Fig. 1C), which are essential for binding with Akt, IRS1/2, or IR (9, 15). Nevertheless, our data suggest that APPL1sv suppresses insulin sensitivity via selectively regulating adiponectin signaling but not directly targeting the insulin signaling pathway.
APPL1sv allosterically prevents APPL1 binding to adiponectin receptors
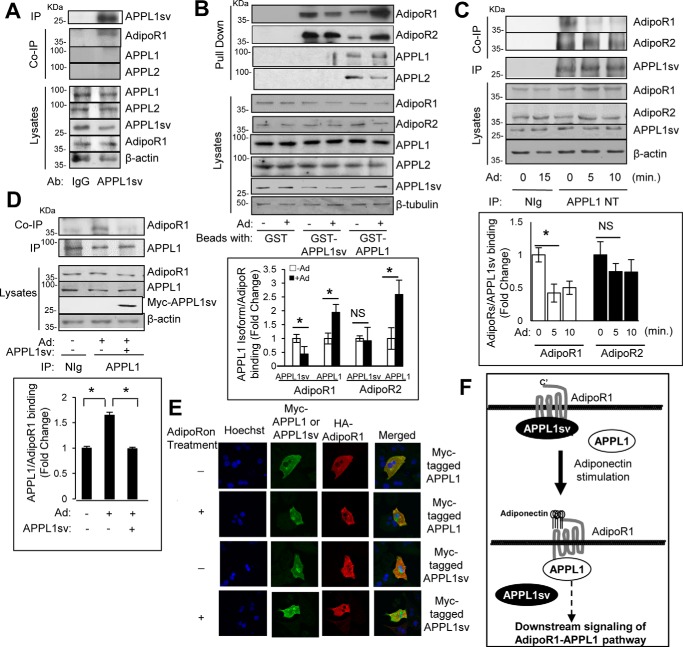
APPL1 promotes adiponectin signaling by binding directly with adiponectin receptors in muscle and liver cells (7). We therefore tested potential interactions of APPL1sv with signaling proteins in the adiponectin pathway. To this end, we generated an antibody using APPL1sv protein that contained the BAR domain of APPL1 as the antigen. Immunoprecipitation assays demonstrated that this homemade APPL1sv antibody specifically recognizes and binds to APPL1sv (Fig. 4A) but cannot recognize or bind to APPL1 and APPL2 in mouse hepatocytes (data not shown). Co-immunoprecipitation assays indicated that, unlike APPL1, APPL1sv does not interact with APPL1 or APPL2 in cells (Fig. 4A). On the other hand, APPL1sv, like APPL1, pulled down both AdipoR1 and AdipoR2 from hepatic cells under basal conditions (Fig. 4B). Consistent with the opposite roles of APPL1 and APPL1sv in regulating adiponectin signaling, adiponectin treatment induced significant dissociation of APPL1sv from AdipoR1 (Fig. 4B), whereas stimulation promoted the binding of APPL1 with adiponectin receptors, as we have reported previously (7). A time course study revealed that adiponectin stimulation induced rapid dissociation of APPL1sv from AdipoR1 (Fig. 4C), which is opposite to the effect of adiponectin on APPL1–AdipoRs interactions (7). Consistent with the in vitro binding study (Fig. 4B), there was a limited effect of adiponectin on the interaction of APPL1sv with AdipoR2 in hepatic cells (Fig. 4C), suggesting a selective effect of APPL1sv on the adiponectin-AdipoR1 signal pathway.
Figure 4.
APPL1sv inhibits adiponectin signaling via binding with adiponectin receptors. A, no interaction of endogenous APPL1sv with APPL1 and APPL2 in cells. Mouse hepatocyte lysates were incubated with immunoprecipitation (IP) beads containing IgG antibody (Ab) or homemade APPL1sv antibody. The bound endogenous AdipoR1 was detected with anti-AdipoR1 antibody. The endogenous APPL proteins APPL1, APPL1sv, and APPL2 were detected with antibodies specific for APPL1, APPL1sv, and APPL2, respectively. B, APPL1sv binds with adiponectin receptors in vitro. Mouse hepatocytes were serum-starved for 4 h and treated with (+) or without (−) 1 μg/ml full-length adiponectin (Ad) for 10 min. The cell lysates were incubated with beads containing GST, GST fused with APPL1 (GST-APPL1), or GST fused with APPL1sv (GST-APPL1sv). The bound endogenous AdipoR1, AdipoR2, APPL1, and APPL2 were detected with anti-AdipoR1, anti-AdipoR2, anti-APPL1, and anti-APPL2 antibodies, respectively. C, interaction of APPL1sv with adiponectin receptors in cells. Mouse hepatocytes were serum-starved for 4 h and treated with (+) or without (−) 50 μm adipoRon (Ad) for 0, 5, or 10 min. Immunoprecipitated APPL1sv and co-immunoprecipitated AdipoR1 and AdipoR2 were detected with the antibodies to the proteins. D, overexpression of APPL1sv blocks APPL1 binding with AdipoR1. Mouse hepatocytes were transfected with plasmids containing either control vector or myc-tagged APPL1sv for 24 h. The cells were serum-starved for 4 h and treated with (+) or without (−) 50 μm adipoRon (Ad) for 10 min. Immunoprecipitated APPL1 and co-immunoprecipitated AdipoR1 were detected with antibodies to the proteins. E, adipoRon stimulation dissociates the binding of APPL1sv protein from AdipoR1. Mouse primary hepatocytes were transfected with an HA-tagged AdipoR1 plasmid and a plasmid containing either myc-tagged APPL1 or myc-tagged APPL1sv for 48 h. The cells were serum-starved for 4 h and treated with (+) or without (−) 50 μm adipoRon (Ad) for 5 min. Myc-tagged APPL1 or APPL1sv (green, second column), stained with myc antibody and HA-tagged AdipoR1 (red, third column), and stained with HA antibody. The cell nuclei were stained with DAPI (blue, first column). F, schematic depicting APPL1sv regulation of adiponectin signaling. Statistical significance was determined by Student's t test in B–D. Error bars, ± S.E. from three independent experiments. *, p < 0.05; NS, no significant difference.
To investigate the mechanism underlying APPL1sv action on adiponectin signaling, we tested whether overexpressing APPL1sv is sufficient to block adiponectin-stimulated binding of APPL1 to AdipoR1. Endogenous APPL1 was immunoprecipitated in the absence or presence of exogenous APPL1sv in mouse hepatocytes. As shown in Fig. 4D, adiponectin-stimulated association of APPL1 with AdipoR1 was significantly reduced in cells overexpressing APPL1sv. In addition, we tested cellular localization of APPL1sv in response to adiponectin stimulation. Consistent with our previous report in myotubes (16), adiponectin induced APPL1 plasma membrane translocation and enhanced binding with AdipoR1 in mouse hepatocytes (Fig. 4E). In contrast, APPL1sv co-localized with AdipoR1 under unstimulated conditions, and adiponectin treatment induced APPL1sv cytosolic translocation and dissociation with AdipoR1 (Fig. 4E). Together, these data indicate that APPL1sv plays an inhibitory role in regulating hepatic adiponectin signaling by binding with adiponectin receptors and preventing the interaction of APPL1 with AdipoR1 in the liver.
Discussion
The adaptor protein APPL1 positively mediates adiponectin signaling by binding directly with adiponectin receptors (AdipoR1 and AdipoR2) in multiple cells (7, 8) and promotes insulin sensitivity and adiponectin signaling in vivo (9, 11). In this study, we report for the first time the existence of a splicing variant of APPL1 (APPL1sv) that plays an inhibitory role in regulating hepatic adiponectin signaling and action by binding with adiponectin receptors to block the interaction of APPL1 with AdipoR1 (Fig. 4F).
The expression of APPL1sv is mostly in the liver, with lower expression in the pancreas and spleen. Because liver tissue is one of the major target sites for insulin and adiponectin action (17, 18), the existence of APPL1sv in the liver implies its role in regulating hepatic insulin sensitivity and adiponectin signaling. Consistent with this, the expression levels of APPL1sv were significantly decreased in the livers of mice under calorie restriction but increased in db/db mice. Of interest, adiponectin and leptin, both associated with insulin sensitivity, are positively correlated with calorie restriction and inversely correlated with insulin resistance, as demonstrated in db/db mice (19, 20). The negative correlation of APPL1sv expression with insulin sensitivity in these mouse models suggests a functional role of this isoform in liver tissues. Consistent with this, suppression of APPL1sv expression in mouse liver reduced HFD-induced insulin resistance by suppressing gluconeogenic gene expression and hepatic glucose production. Mechanistically, APPL1sv exerts its inhibitory role in regulating adiponectin signaling by binding directly with adiponectin receptors, competitively inhibiting the binding of APPL1 to AdipoR1. Together with our finding that overexpression of APPL1sv inhibits adiponectin-stimulated AMPK phosphorylation in hepatic cells, and suppression of APPL1sv expression enhances such phosphorylation, we conclude that APPL1sv acts as a novel regulatory factor that negatively regulates hepatic insulin sensitivity in the liver.
Bioinformatic and genomic studies indicate that inclusion of an exon (exon 10) in the middle of the APPL1 transcript leads to a premature termination of APPL1 protein during translation, resulting in generation of a truncated protein (APPL1sv) comprised solely of a BAR domain. Although both APPL1 and APPL1sv contain a BAR domain, their conformations seem different. Crystal structure analysis reveals that the BAR domain in APPL1 forms a structure unit with the PH domain, which shows a tightly packed, crescent-like shape and provides a binding region for forming the APPL1 homodimer (21, 22). The features of the APPL1 structure leave the BAR domain with limited torsional flexibility but leave the PTB domain and coiled-coil region of APPL1 accessible for scaffolding to other proteins, such as adiponectin receptors (7) and the insulin receptor (9). In contrast to conventional APPL1, the BAR domain in APPL1sv is sufficient to bind with adiponectin receptors in vitro and in hepatocytes, suggesting greater flexibility of the APPL1sv structure. It is interesting to note that APPL2, an APPL1 isoform that inhibits adiponectin signaling by competing with APPL1 for binding with adiponectin receptors (16), has a distinct binding mechanism compared with APPL1 despite sharing a high degree of amino acid similarity and almost identical domain structures. APPL1 interacts with AdipoR1 and AdipoR2 via its C-terminal part, including the PTB domain and CC region (7), whereas APPL2 binds with the receptors solely via its BAR domain at the N terminus (16). This dissimilarity could be explained by their different conformations. Crystallization studies reveal that the BAR domain in APPL2 has an extra hinge structure, which provides more torsional flexibility for binding. Unlike APPL1, which forms a homodimer through its rigid BAR–PH domains (21, 22), APPL2 forms a tetramer via its BAR domain (23). This enhanced flexibility could be the mechanism by which APPL2 binds directly with adiponectin receptors via its BAR domain. Because APPL1sv contains only one domain (10), its flexibility in this domain is more similar to that of APPL2 but not APPL1. This could partly explain why the function of APPL1sv is more similar to that of APPL2 than APPL1 in regulating adiponectin signaling.
Identification of APPL1sv as an inhibitory factor in regulating hepatic adiponectin signaling and glucose metabolism is significant for understanding the cause and development of hepatic insulin resistance. Tightly regulated hepatic gluconeogenesis is a hallmark of hepatic insulin sensitivity, and adiponectin acts as an insulin sensitizer to promote insulin effects on suppressing gluconeogenesis (17, 24). Hepatic adiponectin signaling is essential for mediating the role of adiponectin in the liver (9, 25, 26), but little is known about the regulation of this pathway. This study indicates that APPL1sv expression is inversely associated with insulin sensitivity in vivo because calorically restricted mice have reduced and obese mice have enhanced APPL1sv expression in liver tissues compared with their control mice, suggesting that down-regulation of adiponectin signaling could contribute to the development of insulin resistance. The mechanism underlying the regulation of APPL1sv expression is currently unknown. We speculate that a change in nutritional status, as seen in calorie restriction, regulates the expression and activity of splicing factors that control APPL1sv expression. One of these factors could be the serine/arginine splicing factor (SRSF) family, a class of splicing factors that is known to be regulated, in part, by nutritional conditions (27, 28). Interestingly, insulin and growth factor signaling have been shown to directly regulate the expression and activity of SRSF proteins (29–31). In addition, we found that the junction region of exon 9/exon 10 in the APPL1sv transcript contains putative recognition sequences for several SRSF proteins, suggesting that SRSF proteins are reasonable candidate factors in regulating APPL1sv expression. Further investigation of these splicing factor(s) will yield the molecular mechanisms underlying the regulation of APPL1sv expression and hepatic insulin sensitivity.
Identification of APPL1sv as an inhibitory factor in regulating hepatic adiponectin signaling also provides evidence of adiponectin resistance in the liver. Because regulation of adiponectin serum levels is challenging because of its high concentration in serum (roughly three orders of magnitude higher than other hormones) and its complicated structure (32), intracellular regulation of adiponectin signaling plays a critical role in controlling insulin sensitivity in response to a change in nutrition status. In addition to identifying APPL1 as the first regulatory molecule promoting adiponectin signaling in cells and in vivo (7, 9), we have reported APPL2 as the first inhibitory factor in the adiponectin pathway, which prevents adiponectin-stimulated glucose uptake and fatty acid oxidation in skeletal muscle cells under serum starvation conditions (16). Subsequent in vivo studies that altered APPL2 expression in mouse skeletal tissue support the inhibitory role of APPL2 in regulating insulin sensitivity (33). We thus propose that APPL1 and APPL2 act as a “Yin–Yang” regulatory machinery to control the balance of adiponectin sensitivity and adiponectin resistance in insulin target tissues (16). Consistent with the view that dysregulation of adiponectin signaling contributes to adiponectin resistance, myocardial infarction–induced phosphorylation of the adiponectin receptor AdipoR1 has been shown to desensitize adiponectin signaling and block the cardioprotective effects of adiponectin in vivo (34). Our study reveals APPL1sv as another novel inhibitory factor in adiponectin signaling, demonstrating hepatic adiponectin resistance and its impact on insulin sensitivity and glucose metabolism.
In summary, we have identified a novel APPL1 splicing variant, APPL1sv, that negatively regulates hepatic adiponectin signaling and insulin sensitivity. Our finding sheds light onto a new facet of APPL1 regulation in the post-transcription stages and identifies a new candidate target for preventing hepatic adiponectin resistance and type 2 diabetes.
Experimental procedures
Plasmids, adiponectin, chemicals, and antibodies
The cDNA encoding full-length human APPL1 was described previously (7). The cDNA encoding amino acids 1–236 of human APPL1sv was cloned by PCR from a human cDNA library and subcloned into the pcDNA 3.1 myc/His A plasmid (Invitrogen), in-frame at its C terminus with a sequence encoding a Myc tag.
Recombinant full-length adiponectin was produced and purified in-house. Briefly, the mouse adiponectin gene was cloned into the pcDNA 3.1 myc/His B plasmid and transfected into human embryonic kidney 293 cells. Single adiponectin-positive cells were selected by screening for neomycin resistance. The culture media containing secreted His-tagged adiponectin were collected from the human embryonic kidney 293–adiponectin–stable cells every 4 days and subjected to affinity purification using nickel-nitrilotriacetic acid–agarose beads (Qiagen, catalog no. 30210).
AdipoRon was purchased from MedChem Express (catalog no. HY-15848). Insulin was purchased from Sigma (catalog no. 91077C). Homemade APPL1, AdipoR1, AdipoR2, and Myc antibodies were used as described previously (7). The homemade APPL1sv antibody was generated in rabbits using GST-APPL1sv (GST fused with APPL1sv, amino acids 1–236). The monoclonal β-tubulin antibody was purified from hybridoma B lymphocytes (ATCC, catalog no. HB-11129). The APPL1 antibody against the N terminus of the protein (amino acids 1–205) was purchased from Abcam (catalog no. ab153809). Antibodies to phospho-AMPK at Thr172 and AMPK protein were purchased from Cell Signaling Technology (catalog nos. 2531 and 2532, respectively). The Akt antibody was purchased from Upstate (catalog no. 07-372). Akt308 and Akt473 phospho-antibodies were purchased from Cell Signaling Technology (catalog nos. 9275S and 9271S, respectively). The β-actin antibody was purchased from Abcam (catalog no. 6276-100). The Erk1/2 antibody was purchased from Upstate (catalog no. 06-182). The normal IgG antibody was purchased from Millipore (catalog no. 12-370).
Animal studies
C57BL/6J male mice from The Jackson Laboratory (stock no. 000664) were bred and housed at the University of Texas Health Science Center at San Antonio (UTHSCSA). Ad libitum–fed mice and calorie-restricted mice were bred and housed at the Barshop Institute (San Antonio, TX). Animal procedures and housing were carried out in adherence to National Institutes of Health, federal, state, and institutional guidelines at UTHSCSA. Protocols were approved by the Institutional Animal Care and Use Committee at UTHSCSA.
Cell culture, shRNA construct, and adenovirus generation
Primary mouse hepatocytes were isolated from 3- to 5-month-old male C57BL/6J mice. In brief, mice were anesthetized, and the liver was perfused in situ through the hepatic portal vein with EGTA solution (137 mm NaCl, 5 mm KCl, 0.33 mm Na2HPO4, 0.44 mm KH2PO4, 10 mm HEPES (pH 7.2), 0.5 mm EGTA, 5 mm glucose, and 4 mm NaHCO3) for 10 min, followed by perfusion with collagenase solution (0.05% collagenase type II (Sigma, catalog no. C6885), 137 mm NaCl, 5 mm KCl, 0.33 mm Na2HPO4, 0.44 mm KH2PO4, 10 mm HEPES (pH 7.5), 5 mm CaCl2, and 4 mm NaHCO3) until complete digestion. Mice were euthanized during perfusion. Livers were carefully removed and transferred into Petri dishes containing ice-cold HBSS/HEPES buffer (370 mm NaCl, 5 mm KCl, 0.33 mm Na2HPO4, 0.44 mm KH2PO4, and 10 mm HEPES (pH 7.4)). The liver tissue was cut into small pieces, with the liver fractions filtered through a 100-μm cell strainer, and washed three times with HBSS/HEPES buffer by low-speed centrifugation (500 rpm at 4 °C for 2 min). Freshly isolated hepatocytes were suspended in Williams' medium E (Thermo Fisher Gibco, catalog no. 12551-032), and viability (∼85–95%) was determined by trypan blue dye exclusion. The cells were then immediately plated on collagen type I–coated 6-well plates in William's medium E with 4% fetal bovine serum at a density of 7 × 105 cells/well. The shRNA of APPL1sv was chemically synthesized and ligated into pRNATin-H1.2/Neo (GenScript, Piscataway, NJ). The sense sequence corresponds to nucleotides 713–733 of mouse APPL1sv (5′-GAGAGAAAGCAGTTCCAATCA-3′). The adenovirus containing GFP plus APPL1sv shRNA or a scrambled control sequence was generated by recombination of the shRNA or control into the pAd Easy adenovirus backbone vector (AdEasy adenoviral vector system, Agilent, Santa Clara, CA).
Hepatic glucose production assay
Primary hepatocytes were isolated from C57BL/6J mice (3 months old) and infected with the adenovirus containing APPL1sv shRNA or the scrambled control (multiplicity of infection 100). Forty-eight hours after infection, the hepatocytes were incubated in glucose-free Dulbecco's modified Eagle's medium (Thermo Fisher Gibco, catalog no. D1050) for 30 min and then treated with either PBS or 10 nm insulin for another 30 min. The cells were then incubated with gluconeogenic substrates, 1 nm sodium l-lactate (Sigma, catalog no. L7022) and 1 mm sodium pyruvate (Sigma, catalog no. P2256) for 6 h. Culture media collected from the hepatocytes were used for measuring glucose content with the Amplex Red glucose/glucose oxidase assay kit (Invitrogen, catalog no. A22189) following the manufacturer's instructions.
Sequence alignment
To detect the APPL1sv sequence in the mouse genome, a sequence alignment via BLAST using mouse genomic DNA (NC_000080.6:c26971205–26918986) against the novel APPL1 exon 10 sequence was conducted. A blastn procedure was performed and optimized for “highly similar sequences (megablast).”
To detect the APPL1sv sequence within the human genome, we performed a sequence alignment via BLAST using human genomic DNA (NG_047003.1:5001–50735) against the novel APPL1 exon 10 sequence. A tblastn was performed and optimized for “somewhat similar sequences (blastn).”
To detect the APPL1sv sequence within the rat genome, we conducted a sequence alignment via BLAST using Rattus norvegicus genomic DNA (NC_005115.4:c2602035–2560103) against the novel APPL1 exon 10 sequence. A tblastn was performed and optimized for “highly similar sequences (megablast).”
To detect the APPL1sv sequence with in the zebrafish genome, we conducted a sequence alignment via BLAST using Danio rerio (zebrafish) genomic DNA (NC_007122.7:42600804–42629365) against the novel APPL1 exon 10 sequence. A tblastn was performed and optimized for “highly similar sequences (megablast).”
Confocal microscopy
Plasmids containing HA-tagged pBex-AdipoR1 were co-transfected with either myc-tagged pcDNA-APPL1sv or myc-tagged pcDNA-APPL1 into mouse primary hepatocytes. Forty-eight hours after transfection, the cells were serum-starved for 4 h and stimulated with or without AdipoRon (50 μm) for 5 min. The fixed cells were incubated with anti-myc and anti-HA antibody overnight at 4 °C. After washing with BSA/PBS solution, cells were incubated with FITC-conjugated or rhodamine red–conjugated secondary antibodies. Hoechst33347 was used for nuclear staining.
Transfection, Western blotting, and statistical analyses
Plasmids containing myc-tagged pcDNA-APPL1sv were transfected into murine hepatocytes (35) with Lipofectamine 2000 (Invitrogen, catalog no. 11668-019) according to the manufacturer's instructions. The expression and phosphorylation levels of various proteins in cell lysates or immunoprecipitation/co-immunoprecipitation assays were detected by Western blot analysis with specific antibodies. Quantification of the relative intensity of protein phosphorylation (expressed as -fold change in basal phosphorylation, arbitrarily set as 1.0) was performed using the ChemiImaging 4400 program (Alpha Innotech Corp., San Leandro, CA) and was normalized for the amount of protein expressed in each experiment. Statistical evaluation of the data was done using Student's t test or one-way analysis of variance (ANOVA). A value of p ≤ 0.05 was considered statistically significant.
Author contributions
A. K. G.-D., J. R., and L. Q. D. data curation; A. K. G.-D., M. L., Q. D., and L. Q. D. formal analysis; A. K. G.-D., A. K., F. L., and L. Q. D. funding acquisition; A. K. G.-D., F. L., and L. Q. D. validation; A. K. G.-D., J. R., K. D., Y. X., Z. D., D. Z., Z. L., A. M. D., K. D. L., F. L., and L. Q. D. investigation; A. K. G.-D. and L. Q. D. visualization; A. K. G.-D., J. R., A. K., Q. D., F. L., and L. Q. D. methodology; A. K. G.-D. and L. Q. D. writing-original draft; A. K. G.-D., F. L., and L. Q. D. project administration; J. R., F. L., and L. Q. D. supervision; J. R., F. L., and L. Q. D. writing-review and editing; M. L., F. L., and L. Q. D. conceptualization; F. L. and L. Q. D. resources; L. Q. D. software.
Acknowledgment
We thank Derong Hu for expertise regarding cell culture.
This work was supported in part by NIDDK, National Institutes of Health Pre-Doctoral Fellowship PA-11-112 (to A. K. G.-D.); National Institutes of Health R01 Grant DK76902 (to F. L.), Veterans Affairs Merit Review Award 1I01BX001744-01 (to A. K.); and American Heart Association Research Award 15GRNT23230053, American Diabetes Association Grant 7-13-BS-043, and National Institutes of Health R01 Grant DK102965 (to L. Q. D.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) MG569951 and MF113398.1.
- PH
- pleckstrin homology
- PTB
- phosphotyrosine binding
- IR
- insulin receptor
- sv
- splicing variant
- cDNA
- complementary DNA
- HFD
- high-fat diet
- Glc-6-Pase
- glucose-6-phosphatase
- PEPCK
- phosphoenolpyruvate carboxykinase
- AMPK
- AMP-activated kinase
- SRSF
- serine/arginine splicing factor
- HA
- hemagglutinin
- ANOVA
- analysis of variance
- GST
- glutathione S-transferase
- HBSS
- Hank's balanced salt solution.
References
- 1. Yamauchi T., and Kadowaki T. (2013) Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 17, 185–196 10.1016/j.cmet.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 2. Turer A. T., and Scherer P. E. (2012) Adiponectin: mechanistic insights and clinical implications. Diabetologia 55, 2319–2326 10.1007/s00125-012-2598-x [DOI] [PubMed] [Google Scholar]
- 3. Yamauchi T., Kamon J., Ito Y., Tsuchida A., Yokomizo T., Kita S., Sugiyama T., Miyagishi M., Hara K., Tsunoda M., Murakami K., Ohteki T., Uchida S., Takekawa S., Waki H., et al. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762–769 10.1038/nature01705 [DOI] [PubMed] [Google Scholar]
- 4. Hug C., Wang J., Ahmad N. S., Bogan J. S., Tsao T. S., and Lodish H. F. (2004) T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl. Acad. Sci. U.S.A. 101, 10308–10313 10.1073/pnas.0403382101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deepa S. S., and Dong L. Q. (2009) APPL1: role in adiponectin signaling and beyond. Am. J. Physiol. Endocrinol. Metab. 296, E22–E36 10.1152/ajpendo.90731.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruan H., and Dong L. Q. (2016) Adiponectin signaling and function in insulin target tissues. J. Mol. Cell Biol. 8, 101–109 10.1093/jmcb/mjw014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mao X., Kikani C. K., Riojas R. A., Langlais P., Wang L., Ramos F. J., Fang Q., Christ-Roberts C. Y., Hong J. Y., Kim R. Y., Liu F., and Dong L. Q. (2006) APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat. Cell Biol. 8, 516–523 10.1038/ncb1404 [DOI] [PubMed] [Google Scholar]
- 8. Cheng K. K., Lam K. S., Wang Y., Huang Y., Carling D., Wu D., Wong C., and Xu A. (2007) Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes 56, 1387–1394 10.2337/db06-1580 [DOI] [PubMed] [Google Scholar]
- 9. Ryu J., Galan A. K., Xin X., Dong F., Abdul-Ghani M. A., Zhou L., Wang C., Li C., Holmes B. M., Sloane L. B., Austad S. N., Guo S., Musi N., DeFronzo R. A., Deng C., White M. F., et al. (2014) APPL1 potentiates insulin sensitivity by facilitating the binding of IRS1/2 to the insulin receptor. Cell Rep. 7, 1227–1238 10.1016/j.celrep.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prudente S., Jungtrakoon P., Marucci A., Ludovico O., Buranasupkajorn P., Mazza T., Hastings T., Milano T., Morini E., Mercuri L., Bailetti D., Mendonca C., Alberico F., Basile G., Romani M., et al. (2015) Loss-of-function mutations in APPL1 in familial diabetes mellitus. Am. J. Hum. Genet. 97, 177–185 10.1016/j.ajhg.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng K. K., Iglesias M. A., Lam K. S., Wang Y., Sweeney G., Zhu W., Vanhoutte P. M., Kraegen E. W., and Xu A. (2009) APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. Cell Metab. 9, 417–427 10.1016/j.cmet.2009.03.013 [DOI] [PubMed] [Google Scholar]
- 12. Wang C., Li X., Mu K., Li L., Wang S., Zhu Y., Zhang M., Ryu J., Xie Z., Shi D., Zhang W. J., Dong L. Q., and Jia W. (2013) Deficiency of APPL1 in mice impairs glucose-stimulated insulin secretion through inhibition of pancreatic β cell mitochondrial function. Diabetologia 56, 1999–2009 10.1007/s00125-013-2971-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miaczynska M., Christoforidis S., Giner A., Shevchenko A., Uttenweiler-Joseph S., Habermann B., Wilm M., Parton R. G., and Zerial M. (2004) APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell 116, 445–456 10.1016/S0092-8674(04)00117-5 [DOI] [PubMed] [Google Scholar]
- 14. Rodgers J. T., Lerin C., Haas W., Gygi S. P., Spiegelman B. M., and Puigserver P. (2005) Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 10.1038/nature03354 [DOI] [PubMed] [Google Scholar]
- 15. Mitsuuchi Y., Johnson S. W., Sonoda G., Tanno S., Golemis E. A., and Testa J. R. (1999) Identification of a chromosome 3p14.3–21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene 18, 4891–4898 10.1038/sj.onc.1203080 [DOI] [PubMed] [Google Scholar]
- 16. Wang C., Xin X., Xiang R., Ramos F. J., Liu M., Lee H. J., Chen H., Mao X., Kikani C. K., Liu F., and Dong L. Q. (2009) Yin-Yang regulation of adiponectin signaling by APPL isoforms in muscle cells. J. Biol. Chem. 284, 31608–31615 10.1074/jbc.M109.010355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berg A. H., Combs T. P., Du X., Brownlee M., and Scherer P. E. (2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 7, 947–953 10.1038/90992 [DOI] [PubMed] [Google Scholar]
- 18. Combs T. P., Berg A. H., Obici S., Scherer P. E., and Rossetti L. (2001) Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Invest. 108, 1875–1881 10.1172/JCI14120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stern J. H., Rutkowski J. M., and Scherer P. E. (2016) Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 23, 770–784 10.1016/j.cmet.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yadav A., Kataria M. A., Saini V., and Yadav A. (2013) Role of leptin and adiponectin in insulin resistance. Clin. Chim. Acta 417, 80–84 10.1016/j.cca.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 21. Li J., Mao X., Dong L. Q., Liu F., and Tong L. (2007) Crystal structures of the BAR-PH and PTB domains of human APPL1. Structure 15, 525–533 10.1016/j.str.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 22. Zhu G., Chen J., Liu J., Brunzelle J. S., Huang B., Wakeham N., Terzyan S., Li X., Rao Z., Li G., and Zhang X. C. (2007) Structure of the APPL1 BAR-PH domain and characterization of its interaction with Rab5. EMBO J. 26, 3484–3493 10.1038/sj.emboj.7601771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. King G. J., Stöckli J., Hu S. H., Winnen B., Duprez W. G., Meoli C. C., Junutula J. R., Jarrott R. J., James D. E., Whitten A. E., and Martin J. L. (2012) Membrane curvature protein exhibits interdomain flexibility and binds a small GTPase. J. Biol. Chem. 287, 40996–41006 10.1074/jbc.M112.349803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scherer P. E., Williams S., Fogliano M., Baldini G., and Lodish H. F. (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 10.1074/jbc.270.45.26746 [DOI] [PubMed] [Google Scholar]
- 25. Bjursell M., Ahnmark A., Bohlooly Y. M., William-Olsson L., Rhedin M., Peng X. R., Ploj K., Gerdin A. K., Arnerup G., Elmgren A., Berg A. L., Oscarsson J., and Lindén D. (2007) Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes 56, 583–593 10.2337/db06-1432 [DOI] [PubMed] [Google Scholar]
- 26. Yamauchi T., Nio Y., Maki T., Kobayashi M., Takazawa T., Iwabu M., Okada-Iwabu M., Kawamoto S., Kubota N., Kubota T., Ito Y., Kamon J., Tsuchida A., Kumagai K., Kozono H., et al. (2007) Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 13, 332–339 10.1038/nm1557 [DOI] [PubMed] [Google Scholar]
- 27. Chalfant C. E., Mischak H., Watson J. E., Winkler B. C., Goodnight J., Farese R. V., and Cooper D. R. (1995) Regulation of alternative splicing of protein kinase C β by insulin. J. Biol. Chem. 270, 13326–13332 10.1074/jbc.270.22.13326 [DOI] [PubMed] [Google Scholar]
- 28. Walsh C. M., Suchanek A. L., Cyphert T. J., Kohan A. B., Szeszel-Fedorowicz W., and Salati L. M. (2013) Serine arginine splicing factor 3 is involved in enhanced splicing of glucose-6-phosphate dehydrogenase RNA in response to nutrients and hormones in liver. J. Biol. Chem. 288, 2816–2828 10.1074/jbc.M112.410803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patel N. A., Apostolatos H. S., Mebert K., Chalfant C. E., Watson J. E., Pillay T. S., Sparks J., and Cooper D. R. (2004) Insulin regulates protein kinase CβII alternative splicing in multiple target tissues: development of a hormonally responsive heterologous minigene. Mol. Endocrinol. 18, 899–911 10.1210/me.2003-0391 [DOI] [PubMed] [Google Scholar]
- 30. Patel N. A., Kaneko S., Apostolatos H. S., Bae S. S., Watson J. E., Davidowitz K., Chappell D. S., Birnbaum M. J., Cheng J. Q., and Cooper D. R. (2005) Molecular and genetic studies imply Akt-mediated signaling promotes protein kinase CβII alternative splicing via phosphorylation of serine/arginine-rich splicing factor SRp40. J. Biol. Chem. 280, 14302–14309 10.1074/jbc.M411485200 [DOI] [PubMed] [Google Scholar]
- 31. Blaustein M., Pelisch F., Tanos T., Muñoz M. J., Wengier D., Quadrana L., Sanford J. R., Muschietti J. P., Kornblihtt A. R., Cáceres J. F., Coso O. A., and Srebrow A. (2005) Concerted regulation of nuclear and cytoplasmic activities of SR proteins by AKT. Nat. Struct. Mol. Biol. 12, 1037–1044 10.1038/nsmb1020 [DOI] [PubMed] [Google Scholar]
- 32. Liu M., and Liu F. (2014) Regulation of adiponectin multimerization, signaling and function. Best Pract. Res. Clin. Endocrinol. Metab. 28, 25–31 10.1016/j.beem.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheng K. K., Lam K. S., Wang B., and Xu A. (2014) Signaling mechanisms underlying the insulin-sensitizing effects of adiponectin. Best Pract. Res. Clin. Endocrinol. Metab. 28, 3–13 10.1016/j.beem.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 34. Wang Y., Gao E., Lau W. B., Wang Y., Liu G., Li J. J., Wang X., Yuan Y., Koch W. J., and Ma X. L. (2015) G-protein-coupled receptor kinase 2-mediated desensitization of adiponectin receptor 1 in failing heart. Circulation 131, 1392–1404 10.1161/CIRCULATIONAHA.114.015248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riojas R. A., Kikani C. K., Wang C., Mao X., Zhou L., Langlais P. R., Hu D., Roberts J. L., Dong L. Q., and Liu F. (2006) Fine tuning PDK1 activity by phosphorylation at Ser163. J. Biol. Chem. 281, 21588–21593 10.1074/jbc.M600393200 [DOI] [PubMed] [Google Scholar]