Abstract
Induction of interferons (IFNs) is a central event of antiviral innate immunity. As crucial posttranscriptional regulators, microRNAs (miRNAs) are important for IFN-mediated host defense. Although screening has indicated a substantial number of miRNAs to be differentially expressed after IFN stimulation, the detailed mechanisms of these miRNAs in the antiviral response are underexplored and of great significance. Here, we show that hsa-miR-1225-3p is specifically down-regulated by type I IFN through the IFN/JAK/STAT signaling pathway. Silencing endogenous miR-1225-3p inhibited infection by multiple IFN-susceptible viruses, including hepatitis C virus, Sendai virus, and Newcastle disease virus. In contrast, overexpression of miR-1225-3p impaired the antiviral effect of IFNs and facilitated viral infection. Regarding the mechanism, we identified growth factor receptor–bound protein 2–associated binding protein 3 (GAB3) as a direct target of miR-1225-3p. GAB3 expression was up-regulated by IFN, and overexpression of GAB3 demonstrated potent antiviral effects through enhancing IFN response and virus-triggered innate immune activation. Taken together, our findings reveal the biological function of miR-1225-3p for the first time and propose a novel antiviral regulation pathway in which miRNA and GAB3 participate. This study contributes to the understanding of host miRNA participation in antiviral processes of IFN.
Keywords: virus, interferon, microRNA (miRNA), infection, host-pathogen interaction, GAB3, Growth factor receptor-bound protein 2-associated binding protein 3, miR-1225–3p
Introduction
Chronic and emerging viral infection is a serious threat to public health. The host innate immune system is the first line of defense against viral invasion or replication before the more specific adaptive immune system is established. Host pattern recognition receptors, including Toll-like receptors (TLRs),3 retinoic acid–inducible gene I (RIG-I)–like receptors, and nucleotide-binding oligomerization domain–like receptors, recognize pathogenic components and activate intracellular signaling. Signaling through members of the TLR and RIG-I–like receptor families activates immune response via the cascades that involves NF-κB, IFN-regulatory factor 3 (IRF3), and IRF7 to induce proinflammatory cytokines and type I IFN production (1, 2). IFNs play a key role in the control of initial virus spread and have been approved for treating a variety of diseases, such as hepatitis C and B virus infection (3, 4). IFNs inhibit viral propagation through distinct mechanisms, although the most widely accepted mechanism is the transcriptional regulation of hundreds of IFN-stimulated genes (ISGs). These ISGs control viral infection by directly targeting pathways either required for viral cell entry, replication, translation, and egress or leading to cell death under serious infection conditions.
Beyond conventional protein-coding ISGs, contemporary genome-wide and high-throughput approaches have identified a large number of IFN-regulated noncoding RNAs that participate in antiviral innate immunity through positive and negative mechanisms (5–8). MicroRNAs (miRNAs) are an abundant class of short (18–25 nucleotides), highly conserved noncoding RNAs that function as posttranscriptional regulators to suppress gene expression by binding to their target mRNAs (9). By modifying miRNA transcription or activity, IFNs may regulate host epigenetic mechanisms in the regulation of viral infection through either the direct recognition of viral sequences, which affects only the infection process of the targeted virus, or the mechanism of targeting components at many steps of the antiviral pathway, which induces a nonvirus-specific antiviral state (7, 10).
miRNAs that regulate host antiviral mechanisms are significant and promising candidates for a broad-spectrum antiviral strategy. For example, IFN-β–induced miR-155 targeted suppressor of cytokine signaling 1, which is a negative regulator of JAK/STAT signaling, and enhanced the innate immune response (11, 12). Moreover, IFN-regulated miRNAs can act as repressors of antiviral pathways, which introduces an additional layer of self-regulation for IFN signaling. IFN-γ–induced miR-29a inhibited the antiviral response by targeting the IFN α and β receptor subunit 1 (IFNAR1) (13, 14). IFN–down-regulated miR-27a inhibited the antiviral innate response by up-regulating two negative regulators of IFN production, namely sialic acid–binding Ig-like lectin 1 (Siglec1) and E3 ubiquitin ligase tripartite motif–containing protein 27 (TRIM27) (15). Significantly, increasing evidence suggests that the important impact of miRNA expression may occur through mutual regulation between the innate and adaptive responses (16). Given the importance of host noncoding RNAs in the antiviral response, we and other researchers profiled host miRNA expression patterns in response to multiple types of IFN stimuli through high-throughput screening (17, 18). Overlapping and distinct data were obtained from different laboratories, but further functional investigations of these identified IFN-regulated miRNAs are obviously urgent and of great significance. Our previous screening showed that hsa-miR-1225-3p might be a novel IFN-regulated miRNA (18). Hsa-mir-1225 was reported as a “mirtron,” which is formed by a short intron of PDK1 with hairpin potential (19). However, mir-1225 is processed through noncanonical mirtron biogenesis (20). miR-1225-3p may be related to microphthalmia-associated transcription factor dysfunction in melanocytes (21).
Growth factor receptor–bound protein 2 (Grb2)-associated binding (GAB) scaffolding/adapter proteins are a family of three members, including mammalian GAB1, GAB2, and GAB3 (22). GABs participate in multiple signaling pathways by recruiting enzymes into the signaling network or placing the enzymes close to substrates (22). In particular, GABs have been found to play important roles in immune signaling. GAB1 is needed for T/B cell receptor signal transduction and TLR– and RIG-I–triggered innate immune responses (22, 23). A study showed that GAB3 had high sequence similarity to GAB1 and facilitated macrophage differentiation upon stimulation with macrophage colony-stimulating factor (24). Up to now, the functions of GAB3 in antiviral immunity signaling have not been reported.
In this study, we confirmed the modulation of miR-1225-3p expression by IFNs and further elucidated the roles and mechanisms of miR-1225-3p in host antiviral regulation. Our findings highlight the role of an IFN–down-regulated miRNA in the positive regulatory mechanism for innate antiviral response.
Results
IFN down-regulates miR-1225-3p expression
In our previous attempt to identify IFN-regulated noncoding RNAs in Huh7 hepatoma cells using microarrays, miR-1225-3p was one of the top-ranked hits among the differentially expressed miRNAs (18). In this study, to further investigate the biological significance of miR-1225-3p during viral infection, we first validated the down-regulation of miR-1225-3p by type I IFN (IFN-α2b) in Huh7 cells using quantitative real-time RT-PCR (qRT-PCR) (Fig. 1, A and B). Because virus infection induces IFN production, we determined whether miR-1225-3p was down-regulated after viral infection. We found that infection with three RNA viruses, hepatitis C virus (HCV), Newcastle disease virus (NDV), and Sendai virus (SeV), also induced miR-1225-3p down-regulation in Huh7 or 293T cells (Fig. 1, C–G). To evaluate the abundance of endogenous miR-1225-3p, we used absolute quantification RT-PCR to amplify miR-1225-3p in four hepatoma cell lines, primary fetal liver cells, and 293T cells and found that miR-1225-3p could be readily detected (Fig. 1H). These results verified the inhibition of miR-1225-3p by IFN stimulation and virus infection.
Figure 1.
IFN treatment and virus infection down-regulate miR-1225-3p expression. Huh7 cells were treated with various concentrations of IFN-α2b for 8 h (A) or treated with 0.5 ng/ml IFN-α2b for different times (B). Shown is relative quantitation RT-PCR analysis of endogenous miR-1225-3p in Huh7 cells infected with JFH1-HCVcc (MOI of 20) for different time courses (C) or in 293T cells infected with various MOI of SeV (D) or NDV (F) for 8 h or infected with SeV (MOI of 5) (E) or NDV (MOI of 1) (G) for different time courses. H, absolute quantitative RT-PCR analysis of the endogenous miR-1225-3p levels in various cell lines. PFLC, primary fetal liver cells, Error bars, S.D. from at least three independent experiments. ctrl, control; ns, no significance. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
IFN/JAK/STAT pathway is required for miR-1225-3p down-regulation
Binding to membrane receptors (IFNAR1/2) and activating the JAK/STAT pathway are required for IFN-α2b to trigger downstream signaling and antiviral effects. To investigate cell membrane receptor usage specificity by IFN-α2b to suppress miR-1225-3p expression, we performed a receptor knockdown assay. We transfected Huh7 cells with siRNAs targeting IFNAR1 or IFNAR2 and then treated the cells with IFN-α2b before testing endogenous miR-1225-3p levels. We found that IFN-α2b treatments of mock-transfected or negative control (NC) siRNA–transfected cells led to a significant reduction in miR-1225-3p expression. However, transfection with siRNAs targeting IFNAR1 or IFNAR2 could rescue the miR-1225-3p expression from 40% inhibition to 8% inhibition in the presence of IFN-α2b (Fig. 2A). The knockdown of IFNAR1 or IFNAR2 by specific siRNAs was confirmed (Fig. 2B). The stimulation of OAS1 and IFIT1 by IFN-α2b was partially abolished in the absence of IFNAR1 and IFNAR2 (Fig. 2C). Using the small-molecule inhibitor fludarabine, we found that inhibition of STAT1 activation efficiently blocked IFN-α2b–induced miR-1225-3p down-regulation (Fig. 2D). Consistently, phosphorylation of STAT1 and expressions of OAS1 and IFIT1 were also suppressed by fludarabine (Fig. 2, E and F). Therefore, these results demonstrate that IFN down-regulates miR-1225-3p expression mainly through the IFN/JAK/STAT pathway.
Figure 2.
IFN down-regulates miR-1225-3p expression mainly through the IFN/JAK/STAT pathway. Huh7 cells were transfected with 50 nm siRNAs, as indicated, and incubated for 48 h. The transfected cells were then treated with IFN-α2b (0.5 ng/ml) or PBS as a control for 8 h. Quantitative RT-PCR and Western blotting were performed to quantify expression levels of miR-1225-3p (A), IFNAR1 and IFNAR2 (B), or OAS1 and IFIT1 (C). Huh7 cells pretreated with fludarabine (10 nm) or DMSO as a control for 1 h were stimulated with IFN-α2b (0.5 ng/ml) in the presence of fludarabine or DMSO for 6 h. Then quantitative RT-PCR of miR-1225-3p (D), Western blotting of STAT1 phosphorylation (p-STAT1) (E), and quantitative RT-PCR of OAS1 and IFIT1 (F) were performed. The data represent mean ± S.D. (error bars) (n = 3; *, p < 0.05; **, p < 0.01; ***, p < 0.001).
Silencing miR-1225-3p inhibits viral infection
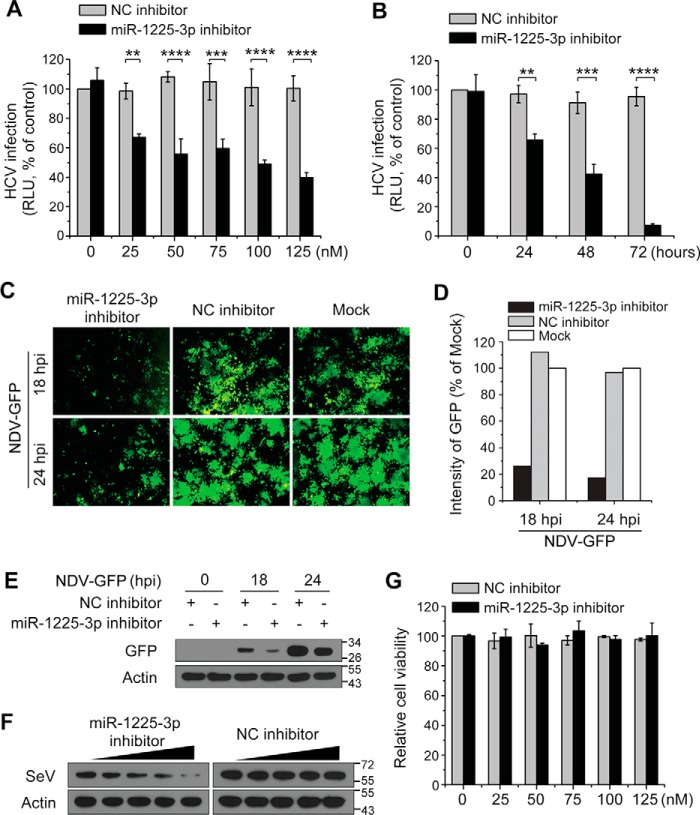
To determine whether the down-regulation of miR-1225-3p by IFN affects viral infection, we transfected miR-1225-3p inhibitors and analyzed the antiviral effects of silencing miR-1225-3p using HCV, a positive single-strand RNA virus susceptible to IFN treatment, as the indicator virus. We observed that miR-1225-3p down-regulation significantly inhibited HCV infection (Fig. 3, A and B), although the overexpression of miR-1225-3p per se had no effect on HCV (data not shown). Significant decreases in infection by NDV at both 18 h post-infection (hpi) and 24 hpi (Fig. 3, C–E) were observed. SeV infection was inhibited in a dose-dependent manner after miR-1225-3p was silenced (Fig. 3F). The transfection of miRNAs did not induce any cell viability changes (Fig. 3G). The inhibition of viral infection by miRNAs might occur by either directly targeting viral RNA or regulating host genes that mediate an antiviral state. To eliminate the possibility that miR-1225-3p targets virus genome, sequence alignments between miR-1225-3p and the genomic RNAs of HCV, NDV, and SeV were performed, and no putative miR-1225-3p target sites were found in viral RNA. These data elucidate that down-regulation of miR-1225-3p shows significant antiviral effects, probably through modulating host targets as the mechanism.
Figure 3.
Silencing miR-1225-3p inhibits viral infection. Huh7 cells were transfected with various concentrations of miR-1225-3p inhibitor and incubated for 48 h (A) or transfected with 100 nm miR-1225-3p inhibitor and incubated for different times (B), followed by infection with Jc1-luciferase reporter HCVcc (MOI = 0.2). The infected cells were cultured for an additional 48 h, and the luciferase reporter assay was carried out to measure HCV propagation. C, 293T cells were transfected with a 100 nm concentration of the indicated miRNA inhibitors. At 36 h after transfection, the cells were infected with NDV-GFP (MOI = 0.5). At 18 and 24 hpi, NDV-GFP infection was determined by visualized GFP expression using fluorescence microscopy (original magnification, ×100). D, the intensity values of GFP in C were quantified and presented as relative values. E, the GFP expression levels of cell lysates from the samples described in C were analyzed using Western blotting to determine NDV infection level. F, the 293T cells that had been transfected with various concentrations of NC or miR-1225-3p inhibitor (0, 25, 50, 75, or 100 nm; wedges) for 36 h were infected with SeV (MOI = 2). The infected cells were cultured for an additional 18 h, and infection with SeV was determined by Western blotting using anti-SeV antibody. G, cytotoxicity assays of miRNA inhibitor transfection were performed at 72 h after miRNA transfection. The detection of β-actin was used to control for sample loading in all Western blotting assays. The data represent mean ± S.D. (error bars) (n ≥ 3); **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Overexpression of miR-1225-3p attenuates the antiviral effect of IFN
To further determine whether the reduction of miR-1225-3p by IFN participates in the IFN-mediated inhibition of viral infection, we counteracted the IFN-elicited decrease in miR-1225-3p expression by transfecting miR-1225-3p mimic into cells and then analyzed the antiviral effect of IFN. As shown in Fig. 4 (A and B), IFN-α2b treatment markedly suppressed viral replication, but virus suppression was rescued by the overexpression of miR-1225-3p. Furthermore, overexpression of miR-1225-3p attenuated the induction and phosphorylation of STAT1, the activation of interferon-stimulated response element (ISRE), and the stimulations of IFIT1 and OAS1 by IFN (Fig. 4, C–E). These findings suggest that the down-regulation of miR-1225-3p might contribute to the antiviral effect and JAK/STAT signaling mediated by IFN-α2b.
Figure 4.
Overexpression of miR-1225-3p attenuates the antiviral effect of IFN. A, Jc1-luciferase reporter HCVcc (MOI = 0.1)–infected Huh7 cells were transfected with 50 nm miR-1225-3p mimic or NC mimic 24 h before treatment with 0.2 ng/ml IFN-α2b. The luciferase activity was measured at 24 h after IFN treatment to determine HCVcc infection. B, 293T cells were transfected with 50 nm miRNA mimics and then infected with SeV (MOI = 0.5) or NDV-GFP (MOI = 0.5) for 24 h. Cells were treated with 0.2 ng/ml IFN-α2b 18 h before measurement. Infection with SeV or NDV-GFP was determined by Western blotting. Huh7 cells were transfected with 50 nm miR-1225-3p mimic or NC mimic 24 h before treatment with 0.2 ng/ml IFN-α2b. Cells were harvested to evaluate their STAT1 and phosphorylated STAT1 (p-STAT1) protein levels by Western blotting (C) and to detect the mRNA levels of two ISGs, OAS1 and IFIT1, by qRT-PCR (E). D, before IFN treatment at 36 h, Huh7 cells were transfected with 200 ng of ISRE luciferase reporter plasmid together with 100 nm miRNA mimics. Luciferase activity was measured after 18 h from IFN treatment. The data represent mean ± S.D. (error bars) (n ≥ 3). *, p < 0.05; ***, p < 0.001.
GAB3 is one of the targets of miR-1225-3p
An online search of the miRWalk 2.0 database revealed hundreds of predicted targets of miR-1225-3p. Several predicted targets ranked in the top were verified. We found that the 3′-UTR of GAB3 mRNA contained two putative miR-1225-3p seed-matching sites, suggesting that GAB3 may be a potential target for miR-1225-3p (Fig. 5A). To verify the bioinformatics predication, the wildtype or mutant of GAB3 3′-UTR was constructed and inserted into the pmirGLO reporter vector (Fig. 5A). A luciferase reporter assay was then performed. As shown in Fig. 5B, transfection of cells with the miR-1225-3p mimic significantly decreased the luciferase activity of the co-transfected WT GAB3 mRNA reporter construct, whereas the stepwise addition of binding site mutations to the GAB3 mRNA 3′-UTR progressively restored luciferase activity, with a complete reversal of the inhibition of luciferase activity achieved when all binding sites were mutated. To further establish the direct interaction between miR-1225-3p and the GAB3 3′-UTR, we created mutant miR-1225-3p (defined as mut-1225-3p) in which seed match sites for the 3′-UTR of GAB3 mRNA were abolished but a perfect match for the mutant GAB3 3′-UTR was obtained (Fig. 5A). In contrast to miR-1225-3p, mut-1225-3p mimic had no significant effect on the luciferase activity of WT GAB3 mRNA reporter construct but significantly inhibited the reporter activity in the cells transfected with pmirGLO-GAB3–3′-UTR-Mut, with a progressive enhancement of the inhibition of luciferase activity appearing with the stepwise addition of complementary mutations to the GAB3 mRNA 3′-UTR (Fig. 5B). Furthermore, silencing endogenous miR-1225-3p resulted in an increase of the luciferase activity from the WT reporter construct but not the mutant construct (Fig. 5C). To experimentally confirm the regulatory effect of miR-1225-3p on GAB3, we transfected Huh7 cells with miR-1225-3p mimics and observed significant GAB3 decreases on both the mRNA and protein levels; in contrast, miR-1225-3p inhibitors increased the expression of GAB3 (Fig. 6, A–F). These findings suggest that GAB3 is one of the target genes of miR-1225-3p.
Figure 5.
GAB3 is one of the targets of miR-1225-3p. A, schematic of the reporter gene construction and the two putative seed matches between miR-1225-3p and the GAB3 3′-UTR. The GAB3 3′-UTR was constructed and inserted into the luciferase reporter pmirGLO vector. The two seed match sites for miR-1225-3p in the construct pmirGLO-GAB3–3′-UTR are indicated. The wildtype and mutant of the seed match of miR-1225-3p and the GAB3 3′-UTR are shown. B, Huh7 cells in 48-well plates were co-transfected with 100 ng of the indicated reporter constructs and a 50 nm concentration the indicated miRNA mimics. Firefly and Renilla luciferase activity levels were measured at 24 h posttransfection. The activity of firefly luciferase was normalized to that of Renilla luciferase. C, Huh7 cells were co-transfected with 100 ng of the indicated reporter constructs and a 100 nm concentration of the indicated miRNA inhibitors. The activity of luciferase was measured and normalized as described in B. The data represent mean ± S.D. (error bars) (n ≥ 2; *, p < 0.05; **, p < 0.01; ****, p < 0.0001). RLU, relative luciferase units.
Figure 6.
The endogenous expression of GAB3 is regulated by miR-1225-3p and IFN-α2b. Huh7 cells were transfected with various concentrations of miRNA mimics or inhibitors and incubated for 48 h (A, C, and E) or transfected with 50 nm miRNA mimics or 100 nm miRNA inhibitors and incubated for different lengths of time as indicated (B, D, and F). Cells were harvested, and the mRNA and protein levels of endogenous GAB3 were then quantified using qRT-PCR and Western blotting. The data represent mean ± S.D. (error bars) (n ≥ 2; *, p < 0.05; **, p < 0.01). G, Huh7 cells were treated with various concentrations of IFN-α2b and incubated for 48 h (left) or treated with 1 ng/ml IFN-α2b and incubated for different lengths of time as indicated (right). The protein levels of endogenous GAB3 were then quantified using Western blotting. H, Huh7 cells were transfected with 50 nm miR-1225-3p mimic or NC mimic 24 h before treatment with 2 ng/ml IFN-α2b. Cells were treated for 48 h by IFN-α2b and harvested to evaluate their GAB3 protein levels by Western blotting.
GAB3 expression is down-regulated following IFN treatment
Based on our confirmation of GAB3 as a miR-1225-3p target and on the observation that miR-1225-3p could be down-regulated by IFN-α2b, we hypothesized that IFN-α2b might regulate GAB3 expression. As expected, IFN-α2b was able to increase GAB3 expression (Fig. 6G). Overexpressed miR-1225-3p attenuated the up-regulation of GAB3 by IFN-α2b (Fig. 6H).
Overexpressed GAB3 shows antiviral activity
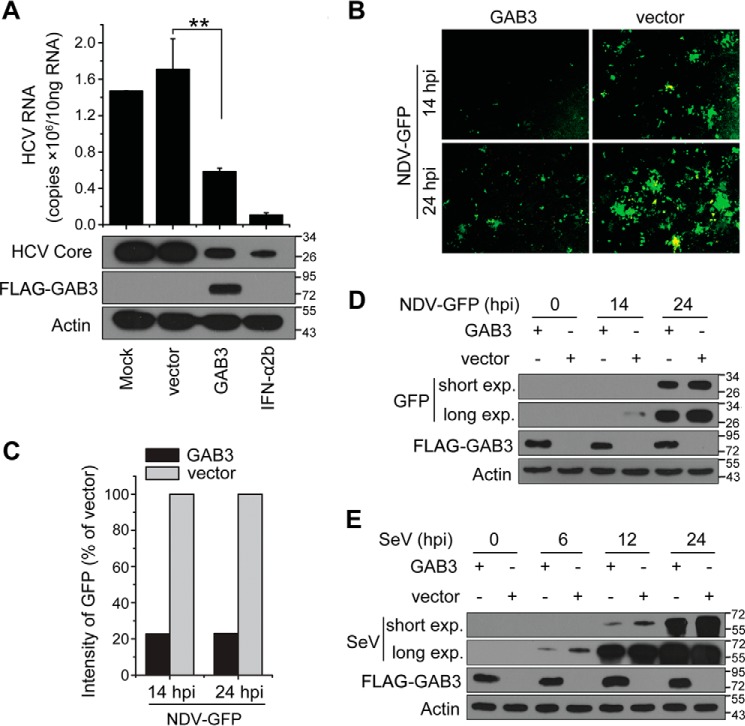
The fact that silencing miR-1225-3p inhibited viral infection (Fig. 3) but up-regulated GAB3 (Figs. 5 and 6) suggests the involvement of GAB3 in the antiviral effect. To verify this hypothesis, we measured the antiviral ability of GAB3. As expected, the overexpressed GAB3 inhibited HCV infection at the levels of viral RNA abundance and viral protein expression (Fig. 7A). Aside from HCV, we showed that infections with NDV at both 14 and 24 hpi (Fig. 7, B–D) and with SeV at 6, 12, and 24 hpi (Fig. 7E) were suppressed significantly by GAB3 overexpression. These results highlight the antiviral activity of GAB3.
Figure 7.
Characterization of the antiviral activity of GAB3. A, Huh7 cells were transfected with a GAB3 overexpression vector or an empty control vector and cultured for 24 h, followed by infection with JFH-1 HCVcc (MOI = 0.4). The infected cells were cultured for an additional 48 h. The IFN-α2b treatment was used as a positive control. Total cellular RNA was isolated using TRIzol reagents for qRT-PCR analysis of HCV RNA (top). Data are shown as mean ± S.D. (n = 3; **, p < 0.01). Cell lysates from the samples were analyzed by Western blotting to determine HCV core protein levels (bottom). B, cultured 293T cells were transfected with a GAB3 overexpression vector or an empty control vector and incubated for 24 h, followed by infection with NDV-GFP (MOI = 0.5). NDV infection was determined by fluorescence microscopy of GFP (original magnification, ×100). C, quantification of the intensity values of GFP in B. Data are presented as relative values. D, the GFP expression of cell lysates from the samples described in B was analyzed using Western blotting to determine NDV infection level. E, cultured 293T cells transfected with the indicated vector were infected with SeV (MOI = 2). Infection with SeV was determined by Western blotting after different durations of infection. β-Actin detection served as an equal loading control.
GAB3 enhances the activation of IFN signaling
The GAB family has been identified as scaffolding adapter molecules contributing to antiviral immune signal transduction (22, 25). Judging from the fact that miR-1225-3p affects the activation of IFN signaling (Fig. 4), GAB3, as a target of miR-1225-3p, might regulate the IFN signal transduction. To test this hypothesis, we measured the regulation of IFN/JAK/STAT cascade by GAB3. As shown in Fig. 8, IFN-α2b–activated STAT1, ISRE, and ISG expression (IFIT1 and OAS1) were further enhanced by GAB3 overexpression.
Figure 8.
overexpression of GAB3 enhances IFN signaling. Huh7 cells were transfected with GAB3 overexpression or control vector followed by treatment with 0.2 ng/ml IFN-α2b for 12 h. Cells were harvested to evaluate their STAT1 and phosphorylated STAT1 (p-STAT1) protein levels by Western blotting (A) and to detect the mRNA levels of two ISGs, OAS1 and IFIT1, by qRT-PCR (C). B, before 0.2 ng/ml IFN-α2b treatment at 36 h, Huh7 cells were transfected with 200 ng of ISRE luciferase reporter plasmid and GAB3 overexpression plasmid. Luciferase activity was measured 12 h after treatment. Data are shown as mean ± S.D. (n ≥ 3; **, p < 0.01; ***, p < 0.001).
Suppression of miR-1225-3p or overexpression of GAB3 enhances virus-induced innate immune activation
To further investigate the biological significance of GAB3 in antiviral immunity, we tested whether GAB3 affects virus-triggered innate immune activation. Considering the importance of IFNs and ISGs in antiviral immunity, we evaluated the mRNA levels of IFN-β, IFN-λ3, and regulated on activation normal T cell expressed and secreted (RANTES) in SeV-infected cells. As shown in Fig. 9A, the induction of IFN-β, IFN-λ3, and RANTES was significantly enhanced by GAB3 overexpression at multiple time points after SeV infection. Using IFN-β and ISRE reporter plasmids, we found that SeV-activated reporter gene expressions were enhanced in GAB3-overexpressing cells, compared with those in control vector-transfected cells (Fig. 9, B and C). These results suggest that GAB3 enhances virus-triggered IFN and ISG production. In accordance with the antiviral effect of miR-1225-3p suppression and its regulation of GAB3, we detected the effect of miR-1225-3p inhibitor on virus-triggered innate immune activation. Similar to the role of GAB3, miR-1225-3p inhibition effectively enhanced SeV-induced expression of IFNs and ISG and activation of IFN-β and ISRE reporter genes (Fig. 9, D–F). These data suggest that miR-1225-3p and its targeting to GAB3 might be involved in host antiviral innate immunity.
Figure 9.
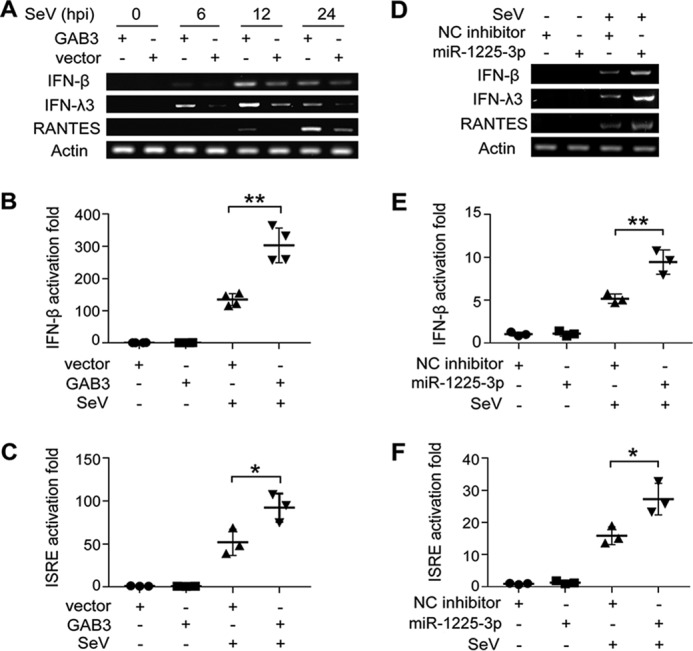
Suppression of miR-1225-3p or overexpression of GAB3 enhances virus-induced innate immune activation. A, GAB3-transfected 293T cells were infected with or without SeV (MOI = 2) for the indicated time courses, and the expression levels of IFN-β, IFN-λ3, RANTES, and β-actin were analyzed by RT-PCR. B and C, before SeV infection at 24 h, 293T cells were transfected with 100 ng of IFN-β or ISRE luciferase reporter plasmid and 20 ng of pRL-actin-Renilla-luciferase plasmid, together with GAB3 overexpression plasmid. Luciferase activity was measured 18 h after infection and normalized by Renilla luciferase activity. D, 293T cells transfected with 100 nm miR-1225-3p inhibitors were infected with SeV for 24 h. The mRNA levels of IFN-β, IFN-λ3, RANTES, and β-actin were analyzed by RT-PCR. E and F, 293T cells transfected with 100 nm miR-1225-3p and the indicated reporter plasmids were infected by SeV for 18 h. Luciferase activity was measured. Data from RT-PCR were visualized by agarose gel electrophoresis. Data are shown as mean ± S.D. (n ≥ 3; *, p < 0.05; **, p < 0.01).
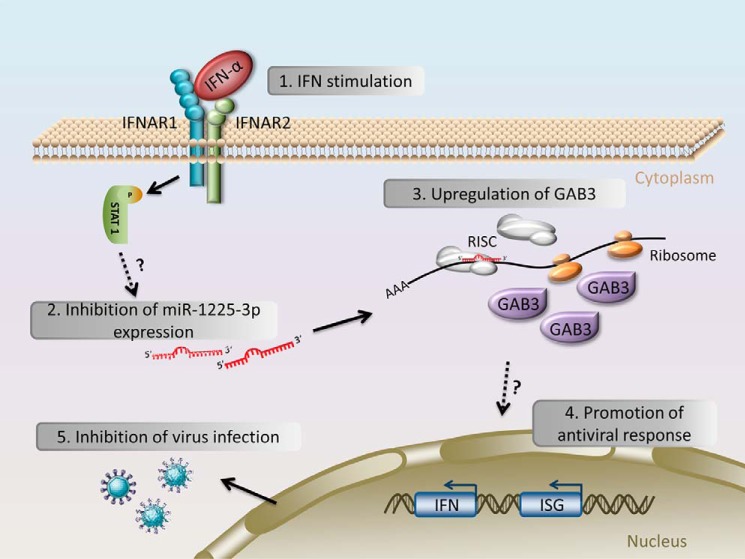
Taken together, our findings describe a novel antiviral regulation pathway. IFN-stimulated miR-1225-3p down-regulation inhibits viral infection by releasing the suppression of its target gene, GAB3, allowing this scaffolding adapter protein to be up-regulated and consequently to enhance host antiviral response (Fig. 10).
Figure 10.
Schematic representation of the mechanism by which the IFN–down-regulated miR-1225-3p inhibits viral infection via up-regulating GAB3 and promoting the antiviral response.
Discussion
Once IFN binds to its receptors, a series of events are activated to transduce signals and transcribe ISGs (26, 27). It is the nature of these genes and their magnitude, duration, and cellular context that will determine the outcome of the IFN response. In recent years, there has been great progress in understanding the role of ISGs in the innate immune system, which has evolved to include noncoding RNA as well as ISGs (18, 28). Several miRNAs have been reported to respond to IFN stimulation and contribute to IFN-dependent antiviral immunity (13–15, 18). miR-1225-3p attracts much attention from us because of its down-regulation, with inducibility by type I IFN, and the significant anti-HCV ability that it shows (Figs. 1 (A and B) and 3 (A and B)). Mechanistic analysis revealed that IFN–down-regulated miR-1225-3p increases GAB3, which promotes an antiviral immune response, thus constituting a positive loop for IFN response (Fig. 10). Zheng et al. (15) reported that IFN–down-regulated miR-27a can up-regulate Siglec1 and TRIM27 expression, which inhibits type I IFN production, thus constituting a negative loop for innate antiviral response. This study and our present report highlight the importance of epigenetic modification in immune response by IFN–down-regulated miRNAs. As a special class of ISGs, IFN–down-regulated miRNAs, through their posttranscriptional regulation of the immune system, are worthy of more attention and research (29). Furthermore, overexpression of miR-1225-3p results in partial alleviation of the antiviral effects of IFN (Fig. 4, A and B), suggesting that IFN-regulated gene networks involve many factors and complex and dynamic processes (30).
miRNAs are important elements of regulatory networks, providing positive or negative feedback between functional responses. Over the past decade, the number of miRNAs implicated in immune system function has increased dramatically (29, 30). Importantly, multiple miRNAs can act in either a cooperative or antagonistic fashion, and a single miRNA may regulate several targets in a gene network (31). We had tested other predicted targets of miR-1225-3p, such as IFNAR1, IFNLR1, IRF1, STAT1, STAT2, JAK3, PTPA, and SOCS7 (data not shown). No evidence showed that these genes were regulated by miR-1225-3p. It remains to be determined whether additional unidentified targets mediate the antiviral effects of miR-1225-3p down-regulation.
The GAB proteins occupy a potentially pivotal position in receptor tyrosine kinase (RTK)-mediated signaling pathways. GAB proteins contain binding sites for the Src homology region 2 (SH2) and SH3 domains. Through its tyrosine phosphorylation by RTKs, the GAB family may generate an interacting platform for proteins with SH2 and SH3 domains, such as phosphatidylinositol 3-kinase and SH2 domain-containing phosphatase 2, and may transfer these molecules to the plasma membrane, thereby contributing to their activation and RTK-mediated signal transduction (22, 25). Thus, it is not surprising to find GABs involved in innate immune processes. Supporting this view, we find that GAB3 plays a positive role in antiviral immune response (Figs. 7–9). The specific molecular mechanism of GAB3 in immune signaling, which is not discussed in the present study, needs to be researched in further detail. It has been reported that GAB1 enhances TLR3/4– and RIG-I–triggered innate responses by promoting activation of the phosphatidylinositol 3-kinase/Akt pathway through direct binding to p85 (23). The high conservation of functional domains among GAB1, GAB2, and GAB3 prompts speculation that GAB3 may utilize a similar molecular mechanism to regulate immune response (24). Given the apparently central role played by GAB signaling via many receptors, delineating the precise mechanism(s) of GAB-mediated signaling is critical to understanding how cytokines mediate a variety of biological activities.
Among the growing family of GAB proteins, GAB3 has a unique and overlapping pattern of expression compared with GAB1 and GAB2 (24). The expression of GAB3 is highest in the hematopoietic system and lymphocytes during the steady state but is detectable at low levels within the kidney, lung, and other tissues. Because of our discovery that GAB3 is targeted by miR-1225-3p in hepatocytes (Figs. 5 and 6), we deduce that posttranscriptional modification by miRNA is one of the mechanisms contributing to the low expression of GAB3 in nonhematopoietic tissues. Some stimuli, such as cytokines, relieve the restriction of GAB3 expression by regulating the relative miRNA expression, thus changing the expression pattern of GAB3 in originally low-expression cells.
Despite decades of developments in IFN biology, we are still striving to understand IFN-regulated antiviral mechanisms. Numerous IFN-regulated miRNAs and relevant epigenetic events contribute to the antiviral state. Our findings provide the first example of an IFN–down-regulated miRNA, miR-1225-3p, playing a role in the positive regulation of antiviral immune response (Fig. 10). This discovery raises the question of whether more miRNAs will have roles in IFN responses. Furthermore, we need to continue building on and identifying an integrated and comprehensive network architecture of how multiple miRNAs interact with multiple mRNAs in the immune system, which will allow us to identify nodes that may be viable therapeutic targets.
Experimental procedures
Cells and reagents
Human embryonic kidney 293T cells, human primary fetal liver cells, and HepG2, Huh7.5.1, and Huh7 hepatoma cells have been described previously (18, 32, 33). Hep3B cells were obtained from ATCC and passaged in our laboratory. IFN-α2b was from Schering Plough (Kenilworth, NJ). Fludarabine, an inhibitor of STAT1 activation, was from Selleck (Shanghai, China). Antibodies were obtained from Abcam (anti-GAB3, ab129553; anti-IFNAR1, ab45172; anti-IFNAR2, ab56070; anti-STAT1, ab103813), Thermo Scientific (anti-HCV core, MA1-080), MBL (anti-SeV, PD029), Cell Signaling Technology (anti-pSTAT1, 7649; anti-STAT1, 9172), Abmart (anti-GFP, P30010), and Sigma (anti-FLAG, F1804; anti-β-actin, A5316). Horseradish peroxidase–conjugated secondary antibodies were from Jackson ImmunoResearch (West Grove, PA).
Viruses
The generation of cell culture–derived hepatitis C virus (strain JFH-1 HCVcc) and firefly luciferase reporter HCVcc (Jc1-Luc HCVcc) has been described previously (32, 33). JFH-1 HCVcc was concentrated using an Amicon ultracentrifugal filter (Millipore, 100 kDa). HCV infection was evaluated by measuring virus RNA abundance and core protein level of JFH-1 HCVcc or luciferase activity (Promega, Madison, WI) of Jc1-Luc HCVcc. SeV and NDV expressing GFP (NDV-GFP) were kindly provided by Dr. Zhen-Dong Zhao (Chinese Academy of Medical Sciences, Beijing, China). SeV replication was determined by Western blotting using anti-SeV antibody. NDV-GFP infection was evaluated based on GFP expression. Cells infected with NDV-GFP were imaged with a Nikon Eclipse TE2000-U fluorescence microscope and NIS-Elements BR 2.30 software. The intensity of the GFP fluorescence was quantified using NIH Image software and was normalized to the intensity of the control fluorescence.
Plasmid constructs and transfection
After cDNA encoding human GAB3 (NM_001081573.2) was amplified from cDNA made from Huh7 cells using the SuperScript III first-strand synthesis system (Invitrogen), it was cloned into the plasmid pMIR-cFLAG to generate FLAG-tagged protein. The cloning primer sequences were as follows: forward primer, 5′-CTTGGATCCAGTGCGGGCGACGCAGTGTG-3′; reverse primer, 5′-AAGGAAAAAAGCGGCCGCTACTTTGGATTGCCTTTCATC-3′ (BamHI and NotI double digestion). DNA transfection of Huh7 or 293T cells with Lipofectamine LTX (Invitrogen) was performed according to the manufacturer's instructions.
miRNA-mRNA target prediction
The potential mRNA targets of miR-1225-3p were predicted using miRWalk 2.0 (34). The predicted target mRNAs simultaneously identified by miRWalk, miRanda, miRDB, RNA22, RNAhybrid, and Targetscan algorithms were selected for next validation. The predicted targets that could be regulated by miR-1225-3p and inhibit viral infection by its overexpression were considered as targets of miR-1225-3p.
Reporter constructs and luciferase reporter assay
The pmirGLO vector (Promega) contains the firefly luciferase gene as the primary reporter to monitor mRNA regulation and the Renilla luciferase gene as a control reporter for normalization. There are two putative miR-1225-3p seed match sites at positions 40–47 and 222–228 of the GAB3 3′-UTR. A fragment consisting of bp 1–1476 from the WT 3′-UTR of GAB3 was inserted into pmirGLO to generate the pmirGLO-GAB3–3′-UTR-Wild reporter construct. Site-directed mutagenesis was performed using the QuikChange site-directed mutagenesis system (Stratagene, La Jolla, CA) using KOD -Plus- as the polymerase (Toyobo, Osaka, Japan) and the construct encoding the WT 3′-UTR of GAB3 as the template. As depicted in Fig. 4A, the pmirGLO-GAB3–3′-UTR-Mut-#1 construct contains a mutation at match site 1, pmirGLO-GAB3–3′-UTR-Mut-#2 contains a mutation at site 2, and pmirGLO-GAB3–3′-UTR-Mut-#1+#2 harbors mutations at both sites. All constructs were verified by restriction enzyme digestion and sequencing. IFN-β luciferase reporter plasmid, ISRE luciferase reporter plasmid, and pRL-actin-Renilla-luciferase plasmid were gifts from Dr. Zhen-dong Zhao. The luciferase reporter assay was performed using a protocol that has been described previously (18).
qRT-PCR
Total intracellular RNA was prepared using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. qRT-PCR analysis was performed with a QuantiFast SYBR Green RT-PCR kit (Qiagen, Düsseldorf, Germany) and a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). The preparation of total intracellular RNA and the performance of qRT-PCR have been described (18). The absolute quantification of HCV RNA has been described in detail previously (33). For the relative quantification of mRNA, primer pairs for GAB3 (forward, 5′-AATGGAGGAGCACCGAACAG-3′; reverse, 5′-GAAGTTTCTGCTGCATGGGG-3′), interferon-induced protein with tetratricopeptide repeats 1 (IFIT1) (forward, 5′-CCTCCTTGGGTTCGTCTACA-3′; reverse, 5′-AGTGGCTGATATCTGGGTGC-3′), IFNAR1 (forward, 5′-TCCAGTACATTGTATAAAGACCACAGT-3′; reverse, 5′-GTTCTGATTTTGGACACTGACTTC-3′), and IFNAR2 (forward, 5′-TAGCCTCCCCAAAGTCTTGA-3′; reverse, 5′-AAATGACCTCCACCATATCCA-3′) were designed. Primers used for qRT-PCR amplification of 2′–5′-oligoadenylate synthetase 1 (OAS1) and β-actin were described previously (18, 33). The expression of β-actin served as the endogenous control.
For the quantification of miRNA, a mirVana miRNA isolation kit (Ambion, Austin, TX) was used to isolate total RNA. The reverse transcription reaction and quantitative PCR were then carried out using a TaqMan microRNA reverse transcription kit, TaqMan universal PCR master mix, and TaqMan microRNA assays (Applied Biosystems) according to the manufacturer's protocols. The expression level of the U6 gene was used as the endogenous control in relative qRT-PCR. The miRNA standards of miR-1225-3p for absolute quantification was purchased from RiboBio (Guangzhou, China).
RT-PCR
100 ng of total RNA extracted with TRIzol reagent was reverse-transcribed and PCR-amplified in one reaction with a PrimeScript One Step RT-PCR kit (Takara, Otsu, Japan). The induction of IFN-β, IFN-λ3, and RANTES was analyzed by One Step RT-PCR with 21, 27, and 18 cycles, respectively, of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 20 s. The specific primers for these cytokines were as follows: IFN-β (forward, 5′-CCAACAAGTGTCTCCTCCAA-3′; reverse, 5′-ATAGTCTCATTCCAGCCAGT-3′), IFN-λ3 (forward, 5′-ATGACCGGGGACTGCATGCC-3′; reverse, 5′-CGCTGGCAACACAATTCAGGTC-3′), RANTES (forward, 5′-CCTCGCTGTCATCCTCATTG-3′; reverse, 5′-GGTAGGATAGTGAGGGGAAG-3′). The expression level of β-actin served as the endogenous control and was detected at 15 cycles of PCR amplification.
RNAs and RNA transfection
The mimic and inhibitor for miRNA-1225-3p, the negative control mimic and inhibitor (cel-miR-67-3p), the mimic for customized mutant miR-1225-3p (defined as mut-1225-3p), and all of the siRNAs (siRNA) were obtained from RiboBio. The sequences for siRNAs were as follows: IFNAR1 siRNA, 5′-GCUUUCCUACUUCCUCCAGUCUUUA-3′; IFNAR2 siRNA, 5′-UGAACCACCAGAGUUUGAGAUUGUU-3′. Transfection of miRNA and siRNA was performed using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocol.
Western blotting
These experiments were conducted according to the standard procedures of our laboratory, as described elsewhere (33). The densitometries of the bands were quantified using ImageJ software and were normalized to the density of the control band.
Cytotoxicity assay
The cytotoxicity of miRNA transfection was evaluated via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide assay as described elsewhere (35).
Statistics
Statistical analyses of two-sample comparisons were performed by an independent sample t test. One-way analysis of variance and Dunnett's multiple-comparison test were made across multiple samples. Two-way analysis of variance and Sidak's multiple-comparison test were made in grouped graphs. A p value < 0.05 was considered statistically significant.
Author contributions
M.C., X.C., and W.Y. conceptualization; M.C., Y.N., and J.F. data curation; M.C., Y.N., and J.F. formal analysis; M.C. and W.Y. funding acquisition; M.C., Y.N., J.F., and X.C. visualization; M.C. writing-original draft; Y.N., X.L., and W.Y. resources; Y.N., J.F., X.C., and X.L. validation; Y.N. and J.F. methodology; J.F. software; X.C., X.L., and W.Y. supervision; X.L. and W.Y. investigation; W.Y. project administration; W.Y. writing-review and editing.
Acknowledgments
We thank Dr. T. Wakita for providing the JFH-1 strain and Dr. Z. Zhao for providing SeV and NDV viruses and reporter plasmids.
This work was supported by National Natural Science Foundation of China Grants 81401674, 81672030, and 81471954; National Basic Research Program of China Grant 2015CB554301; CAMS Initiative for Innovative Medicine Grant 2016-I2M-3-020; and National Science and Technology Major Project 2017ZX10304402-001-013. The authors declare that they have no conflicts of interest with the contents of this article.
- TLR
- Toll-like receptor
- IFN
- interferon
- miRNA
- microRNA
- IFNAR1/2
- interferon α and β receptor subunit 1/2
- Siglec1
- sialic acid–binding Ig-like lectin 1
- TRIM27
- E3 ubiquitin ligase tripartite motif–containing protein 27
- GAB1/2/3
- growth factor receptor–bound protein 2–associated binding protein 1/2/3
- RIG-I
- retinoic acid–inducible gene I
- SeV
- Sendai virus
- NDV
- Newcastle disease virus
- HCV
- hepatitis C virus
- IFIT1
- interferon-induced protein with tetratricopeptide repeats 1
- OAS1
- 2′–5′-oligoadenylate synthetase 1
- RANTES
- regulated on activation normal T cell expressed and secreted
- ISRE
- interferon-sensitive response element
- mut-1225-3p
- mutant miR-1225-3p
- RTK
- receptor tyrosine kinase
- SH2
- Src homology region 2
- ISG
- IFN-stimulated gene
- IRF
- IFN-regulatory factor
- Grb2
- growth factor receptor–bound protein 2
- qRT-PCR
- quantitative real-time RT-PCR
- JAK
- Janus kinase
- STAT
- signal transducers and activators of transcription
- IFNAR
- IFN α and β receptor subunit
- NC
- negative control
- hpi
- hours post-infection
- NDV-GFP
- NDV expressing GFP
- MOI
- multiplicity of infection.
References
- 1. Akira S., Uematsu S., and Takeuchi O. (2006) Pathogen recognition and innate immunity. Cell 124, 783–801 10.1016/j.cell.2006.02.015 [DOI] [PubMed] [Google Scholar]
- 2. Broz P., and Monack D. M. (2013) Newly described pattern recognition receptors team up against intracellular pathogens. Nat. Rev. Immunol. 13, 551–565 10.1038/nri3479 [DOI] [PubMed] [Google Scholar]
- 3. Hotho D. M., de Bruijne J., Spaan M., Treitel M. A., Boonstra A., de Knegt R. J., Janssen H. L., and Reesink H. W. (2013) Sustained virologic response after therapy with the HCV protease inhibitor narlaprevir in combination with peginterferon and ribavirin is durable through long-term follow-up. J. Viral Hepat. 20, e78–e81 10.1111/jvh.12012 [DOI] [PubMed] [Google Scholar]
- 4. Petersen J., Thompson A. J., and Levrero M. (2016) Aiming for cure in HBV and HDV infection. J. Hepatol. 65, 835–848 10.1016/j.jhep.2016.05.043 [DOI] [PubMed] [Google Scholar]
- 5. Schneider W. M., Chevillotte M. D., and Rice C. M. (2014) Interferon-stimulated genes: a complex web of host defenses. Annu. Rev. Immunol. 32, 513–545 10.1146/annurev-immunol-032713-120231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee H. M., Nguyen D. T., and Lu L. F. (2014) Progress and challenge of microRNA research in immunity. Front. Genet. 5, 178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sedger L. M. (2013) microRNA control of interferons and interferon induced anti-viral activity. Mol. Immunol. 56, 781–793 10.1016/j.molimm.2013.07.009 [DOI] [PubMed] [Google Scholar]
- 8. Turner M., Galloway A., and Vigorito E. (2014) Noncoding RNA and its associated proteins as regulatory elements of the immune system. Nat. Immunol. 15, 484–491 10.1038/ni.2887 [DOI] [PubMed] [Google Scholar]
- 9. Yates L. A., Norbury C. J., and Gilbert R. J. (2013) The long and short of microRNA. Cell 153, 516–519 10.1016/j.cell.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 10. Yang C. H., Li K., Pfeffer S. R., and Pfeffer L. M. (2015) The type I IFN-induced miRNA, miR-21. Pharmaceuticals 8, 836–847 10.3390/ph8040836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Connell R. M., Taganov K. D., Boldin M. P., Cheng G., and Baltimore D. (2007) MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. U.S.A. 104, 1604–1609 10.1073/pnas.0610731104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Su C., Hou Z., Zhang C., Tian Z., and Zhang J. (2011) Ectopic expression of microRNA-155 enhances innate antiviral immunity against HBV infection in human hepatoma cells. Virol. J. 8, 354 10.1186/1743-422X-8-354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Y., Yang L., Wang H., Zhang G., and Sun X. (2016) Respiratory syncytial virus non-structural protein 1 facilitates virus replication through miR-29a-mediated inhibition of interferon-α receptor. Biochem. Biophys. Res. Commun. 478, 1436–1441 10.1016/j.bbrc.2016.08.142 [DOI] [PubMed] [Google Scholar]
- 14. Schmitt M. J., Philippidou D., Reinsbach S. E., Margue C., Wienecke-Baldacchino A., Nashan D., Behrmann I., and Kreis S. (2012) Interferon-γ-induced activation of signal transducer and activator of transcription 1 (STAT1) up-regulates the tumor suppressing microRNA-29 family in melanoma cells. Cell Commun. Signal. 10, 41 10.1186/1478-811X-10-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zheng Q., Hou J., Zhou Y., Yang Y., and Cao X. (2016) Type I IFN-inducible downregulation of microRNA-27a feedback inhibits antiviral innate response by upregulating Siglec1/TRIM27. J. Immunol. 196, 1317–1326 10.4049/jimmunol.1502134 [DOI] [PubMed] [Google Scholar]
- 16. O'Connell R. M., Rao D. S., Chaudhuri A. A., and Baltimore D. (2010) Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 10, 111–122 10.1038/nri2708 [DOI] [PubMed] [Google Scholar]
- 17. Reinsbach S., Nazarov P. V., Philippidou D., Schmitt M., Wienecke-Baldacchino A., Muller A., Vallar L., Behrmann I., and Kreis S. (2012) Dynamic regulation of microRNA expression following interferon-γ-induced gene transcription. RNA Biol. 9, 978–989 10.4161/rna.20494 [DOI] [PubMed] [Google Scholar]
- 18. Cheng M., Si Y., Niu Y., Liu X., Li X., Zhao J., Jin Q., and Yang W. (2013) High-throughput profiling of α interferon- and interleukin-28B-regulated microRNAs and identification of let-7s with anti-hepatitis C virus activity by targeting IGF2BP1. J. Virol. 87, 9707–9718 10.1128/JVI.00802-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berezikov E., Chung W. J., Willis J., Cuppen E., and Lai E. C. (2007) Mammalian mirtron genes. Mol. Cell 28, 328–336 10.1016/j.molcel.2007.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Havens M. A., Reich A. A., Duelli D. M., and Hastings M. L. (2012) Biogenesis of mammalian microRNAs by a non-canonical processing pathway. Nucleic Acids Res. 40, 4626–4640 10.1093/nar/gks026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang P., Li Y., Hong W., Zhen J., Ren J., Li Z., and Xu A. (2012) The changes of microRNA expression profiles and tyrosinase related proteins in MITF knocked down melanocytes. Mol. Biosyst. 8, 2924–2931 10.1039/c2mb25228g [DOI] [PubMed] [Google Scholar]
- 22. Sármay G., Angyal A., Kertész A., Maus M., and Medgyesi D. (2006) The multiple function of Grb2 associated binder (Gab) adaptor/scaffolding protein in immune cell signaling. Immunol. Lett. 104, 76–82 10.1016/j.imlet.2005.11.017 [DOI] [PubMed] [Google Scholar]
- 23. Zheng Y., An H., Yao M., Hou J., Yu Y., Feng G., and Cao X. (2010) Scaffolding adaptor protein Gab1 is required for TLR3/4- and RIG-I-mediated production of proinflammatory cytokines and type I IFN in macrophages. J. Immunol. 184, 6447–6456 10.4049/jimmunol.0901750 [DOI] [PubMed] [Google Scholar]
- 24. Wolf I., Jenkins B. J., Liu Y., Seiffert M., Custodio J. M., Young P., and Rohrschneider L. R. (2002) Gab3, a new DOS/Gab family member, facilitates macrophage differentiation. Mol. Cell. Biol. 22, 231–244 10.1128/MCB.22.1.231-244.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaughan T. Y., Verma S., and Bunting K. D. (2011) Grb2-associated binding (Gab) proteins in hematopoietic and immune cell biology. Am. J. Blood Res. 1, 130–134 [PMC free article] [PubMed] [Google Scholar]
- 26. de Weerd N. A., Samarajiwa S. A., and Hertzog P. J. (2007) Type I interferon receptors: biochemistry and biological functions. J. Biol. Chem. 282, 20053–20057 10.1074/jbc.R700006200 [DOI] [PubMed] [Google Scholar]
- 27. Rusinova I., Forster S., Yu S., Kannan A., Masse M., Cumming H., Chapman R., and Hertzog P. J. (2013) Interferome v2.0: an updated database of annotated interferon-regulated genes. Nucleic Acids Res. 41, D1040–D1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carpenter S., Aiello D., Atianand M. K., Ricci E. P., Gandhi P., Hall L. L., Byron M., Monks B., Henry-Bezy M., Lawrence J. B., O'Neill L. A., Moore M. J., Caffrey D. R., and Fitzgerald K. A. (2013) A long noncoding RNA mediates both activation and repression of immune response genes. Science 341, 789–792 10.1126/science.1240925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mehta A., and Baltimore D. (2016) MicroRNAs as regulatory elements in immune system logic. Nat. Rev. Immunol. 16, 279–294 10.1038/nri.2016.40 [DOI] [PubMed] [Google Scholar]
- 30. Forster S. C., Tate M. D., and Hertzog P. J. (2015) MicroRNA as type I interferon-regulated transcripts and modulators of the innate immune response. Front. Immunol. 6, 334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Na Y. J., and Kim J. H. (2013) Understanding cooperativity of microRNAs via microRNA association networks. BMC Genomics 14, S17 10.1186/1471-2164-14-S5-S17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu X., Wang T., Wakita T., and Yang W. (2010) Systematic identification of microRNA and messenger RNA profiles in hepatitis C virus-infected human hepatoma cells. Virology 398, 57–67 10.1016/j.virol.2009.11.036 [DOI] [PubMed] [Google Scholar]
- 33. Cheng M., Si Y., Yang Y., Liu X., Gong Q., Zhao J., Niu Y., Li X., Jin Q., and Yang W. (2012) Recombinant human interleukin 28B: anti-HCV potency, receptor usage and restricted cell-type responsiveness. J. Antimicrob. Chemother. 67, 1080–1087 10.1093/jac/dks015 [DOI] [PubMed] [Google Scholar]
- 34. Dweep H., and Gretz N. (2015) miRWalk2.0: a comprehensive atlas of microRNA-target interactions. Nat. Methods 12, 697 10.1038/nmeth.3485 [DOI] [PubMed] [Google Scholar]
- 35. Ferdousi M., Habibi-Rezaei M., Balalaie S., Ramezanpour S., Sabouni F., Poursasan N., Sabokdast M., and Moosavi-Movahedi A. A. (2016) Toxicity of serum albumin on microglia upon seeding effect of amyloid peptide. J. Biochem. 160, 325–332 10.1093/jb/mvw042 [DOI] [PubMed] [Google Scholar]