Abstract
Blastocystis is an anaerobic protist, commonly inhabiting the intestinal tract of both humans and other animals. Blastocystis is extremely diverse comprising 17 genetically distinct subtypes in mammals and birds. Pathogenicity of this enteric microbe is currently disputed and knowledge regarding its distribution, diversity and zoonotic potential is fragmentary. Most research has focused on Blastocystis from primates, while sampling from other animals remains limited. Herein, we investigated the prevalence and distribution of Blastocystis in animals held within a conservation park in South East England. A total of 118 samples were collected from 27 vertebrate species. The barcoding region of the small-subunit ribosomal RNA was used for molecular identification and subtyping. Forty one per cent of the species were sequence positive for Blastocystis indicating a high prevalence and wide distribution among the animals in the park. Six subtypes were identified, one of which is potentially novel. Moreover, the majority of animals were asymptomatic carriers, suggesting that Blastocystis is not pathogenic in animals. This study provides a thorough investigation of Blastocystis prevalence within a wildlife park in the UK and can be used as a platform for further investigations on the distribution of other eukaryotic gut microbes.
Key words: Blastocystis, genetic diversity, phylogeny, prevalence, subtype
Introduction
Blastocystis is a microbial eukaryote that inhabits the gastrointestinal tract of a variety of animals including humans, other primates, amphibians, reptiles and even insects (Abe, 2004; Stensvold et al. 2009; Parkar et al. 2010; Roberts et al. 2013; Yoshikawa et al. 2016). After fungi, Blastocystis is one of the most prevalent microbial eukaryotes in metazoans (Scanlan et al. 2014).
Until recently, Blastocystis was overlooked due to its small size and lack of distinct morphological features. Nonetheless, the advent of molecular methods has revealed an astounding genetic heterogeneity of Blastocystis. To date, 17 genetically diverse lineages have been reported in mammals and birds (subtypes; ST), based on the differences of the small subunit ribosomal RNA (SSU rRNA) (Stensvold and Clark, 2016). Blastocystis has wide host range, with the same subtype found in several animal genera. Emerging data, however, suggests that host specificity should be assessed based on lower than genus level taxonomy (Alfellani et al. 2013c). Of the 17 STs, only the first nine (ST1–ST9) and recently, ST12 have been found in humans (Ramirez et al. 2016; Stensvold and Clark, 2016). Blastocystis has been reported in wild animals, pets and domesticated animals and those that are housed in zoos (Parkar et al. 2010; Ruaux and Stang, 2014; Schar et al. 2014; Wang et al. 2014; Amenu et al. 2015; Figueroa, 2015; Puebla et al. 2017). Nonetheless, the comprehensive range of non-primate hosts of the various STs remains unclear, since only a limited number of studies focus on screening such animals (Abe et al. 2002; Lim et al. 2008; Perez Cordon et al. 2008; Parkar et al. 2010; Roberts et al. 2013).
The presence in various animals of Blastocystis isolates that belong to the same STs as those in humans has led to the speculation that the organism has zoonotic potential (Rajah Salim et al. 1999; Parkar et al. 2010; Ramirez et al. 2016). Nonetheless, this scenario has come under scrutiny in recent years, since cases where the direction of transmission has been established conclusively are absent. Moreover, most molecular investigations of Blastocystis isolates from domesticated animals and their keepers have not revealed any shared subtypes, though there are notable exceptions (Alfellani et al. 2013b; Wang et al. 2014). Due to this controversy, there is an urgent need for further investigations on the distribution of Blastocystis in animals in captivity, since prevalence data and molecular characterization of Blastocystis in such animals remain sparse.
Herein, we examine Blastocystis isolates from Wildwood Trust, a wildlife park in East Kent, UK. The park's collection consists mostly of UK native and previously native wildlife, meaning that the chance of the identified isolates being local is very high. The aim of this study was to investigate the prevalence, distribution, genetic diversity and host range of Blastocystis STs in animals at Wildwood Trust.
Materials and methods
Study site – source of specimens
A total of 118 faecal samples were collected from 27 different host species (Table 1) located at Wildwood Trust (Herne Bay, Kent, UK). Sampling covered a range of mammalian species, four bird species and one reptile species (Table 1).
Table 1.
Animal samples collected from study hosts
| Host | Scientific name | Location | Sample number | PCR positive (clone number) |
|---|---|---|---|---|
| Carnivora | ||||
| Badger | Meles meles | Wildwood | 2 | – |
| European Brown Bear | Ursus arctos arctos | Wildwood | 2 | – |
| Lynx | Lynx lynx | Wildwood | 3 | – |
| Otter | Lutra lutra | Wildwood | 7 | – |
| Pine Marten | Martes martes | Wildwood | 2 | 1 [1] |
| Polecat | Mustela putorius | Wildwood | 1 | – |
| Red Fox | Vulpes vulpes | Wildwood | 3 | – |
| Scottish Wild Cat | Felis silvestris | Wildwood | 11 | – |
| Stoat | Mustela ermine | Wildwood | 3 | – |
| Anseriformes | ||||
| Barnacle Goose | Branta leucopsis | Wildwood | 1 | – |
| Pink Footed Goose | Anser brachyrhynchus | Wildwood | 1 | – |
| Artiodactyla | ||||
| Muntjac Deer | Muntiacus reevesi | Wildwood | 1 | 1 [1] |
| European Bison | Bison bonasus | Wildwood | 3 | 3 [11] |
| Eurasian Elk | Alces alces | Wildwood | 2 | 1 [4] |
| Pygmy Goat | Capra aegagrus hircus | Wildwood | 2 | 2 [3] |
| Red Deer | Cervus elaphus | Wildwood | 1 | 1 [8] |
| Reindeer | Rangifer tarandus | Wildwood | 1 | – |
| Soay Sheep | Ovis aries | Wildwood | 1 | 1 [1] |
| Wild Boar | Sus scrofa | Wildwood | 2 | 1 [1] |
| Squamata | ||||
| Four-lined Snake | Elaphe quatuorlineata | Wildwood | 1 | – |
| Eulipotyphla | ||||
| Hedgehog | Erinaceus europaeus | Wildwood | 1 | – |
| Water Shrew | Neomys fodiens | Wildwood | 6 | – |
| Passeriformes | ||||
| Raven | Corvus corax | Wildwood | 3 | – |
| Red Billed Chough | Pyrrhocorax pyrrhocorax | Wildwood | 1 | – |
| Rodentia | ||||
| Red Squirrel | Sciurus vulgaris | Wildwood | 3 | 2 [1] |
| Water Vole | Arvicola amphibious | Wildwood | 3 | 2 [12] |
| Water Vole | Arvicola amphibious | Bulphan | (5) 30a | 5 [17] |
| Water Vole | Arvicola amphibious | Tilbury | (3) 18a | 3 [9] |
| Diprotodontia Wallaby | Macropus rufogriseus | Wildwood | 3 | 2 [2] |
High sample number due to repetitive sampling from a small population. Numbers in parentheses denote number of water vole individuals.
Sample collection and storage
A licensed veterinarian visits the park on a monthly basis to monitor the animals’ health, during the week of sampling no animals exhibit diarrhoea. Faecal samples were collected between the months of July 2016 to January 2017. Wildwood Trust staff collected samples under the guidance of laboratory members; A minimum of one sample was collected per animal species, where possible (Table 1). In the cases where multiple animals of the same species were enclosed together, several samples were collected.
Once collected, samples were placed in sealed, sterile falcon tubes and stored at 4 °C until DNA extraction. The faecal samples were subdivided shortly after collection to be used for microscopy, culturing and DNA extraction. Heat-fixed slides were made from all samples collected within an hour of collection.
Culturing
Within half an hour of sampling, a small amount of faecal sample from randomly selected animals were separately inoculated in sterile falcon tubes containing the following media: two tubes containing modified LYSGM [16·07 mm potassium phosphate dibasic, 2·94 mm potassium phosphate monobasic, 128·34 mm sodium chloride, 2·5 g L−1 yeast extract, 0·5 g L−1 liver extract, 5% adult bovine (Sigma)/horse serum (Gibco); modified TYSGM-9, without mucin (Diamond, 1982), http://entamoeba.lshtm.ac.uk/xenic.htm], two tubes of TYM (22·2 g L−1 trypticase peptone, 11·1 g L−1 yeast extract, 16·23 mm maltose, 9·17 mm L-cysteine, 1·26 mm L-ascorbic acid, 5·1 mm potassium phosphate dibasic, 6·53 mm potassium phosphate monobasic) (Diamond, 1957, 1983) enriched with 5% fetal bovine serum (FBS; Sigma) and 2 tubes with 0·5% Liver Digest (LD) medium (0·5 g L−1 Oxoid liver extract). One tube of each medium type was incubated at 35 °C and the rest were left at room temperature. Samples were examined every 3 days under light microscope with neutral red staining (see below). Cultures positive for Blastocystis, were subcultured every 10 days.
Staining and microscopy
For the identification of live cells within cultures, a neutral red staining technique was employed (DeRenzis and Schechtman, 1973). Ninety-four μL of re-suspended cultured samples were mixed with equal volumes of freshly prepared 0·04% neutral red staining (Sigma, N2889) in 0·5 mL tubes and incubated for 10 min at the temperature in which samples were cultured. The samples were then centrifuged at 5000 g for 30 s. The supernatant was removed and the pellet was re-suspended in 20 µL of 1 × PBS (pH 7·2) by vortexing. Ten μL of the mixture was placed on a glass slide under a 22-mm square coverslip and individual cells were observed under 200× and 400× magnification.
DNA extraction, PCR, cloning and sequencing
DNA from feces and cultures were extracted using the Microbiome DNA Purification Kit Purelink (Fisher, UK), following the manufacturer's specifications and protocols. The extracted DNA was stored at −20 °C for long-term usage. To amplify the fragment of interest, polymerase chain reaction (PCR) was carried out using the extracted DNA. DNA extracted from an axenic Blastocystis NandII culture was used as positive control in every PCR application. The conditions of amplification were as follows: 2 µL of the extracted DNA was used for amplification of a Blastocystis sp SSUrRNA product. 10 µL 5× buffer (Promega), 1 mm MgCl2, 0·4 µm forward primer, 0·4 µm reverse primer, 0·2 mm dNTPs (Promega), 0·25 µL Taq polymerase, 30·75 µL HPLC grade water 2 µL DNA. The fragment was amplified in a total of 50 µL reaction, according to the standard conditions of for HiFI Taq polymerase (Promega). The broad specificity primers RD3 5′-GGGATCCTGATCCTTCCGCAGGTTCACCTAC-3′ and RD5 5′-GGAAGCTTATCTGGTTGATCCTGCCAGTA-3′ (Clark, 1997) were used for the first PCR. Cycling conditions were as follows: 95 °C 5 min, 35 cycles of denaturation at 95 °C for 30 s, annealing 55 °C for 30 s, extension at 72 °C for 1 min 40 s and final extension at 72 °C for 5 min.
A second nested PCR was performed using the forward RD5F 5′-ATCTGGTTGATCCTGCCAGT-3′ and reverse BhRDr 5′-GAGCTTTTTAACTGCAACAACG-3′ (Scicluna et al. 2006) primers giving a fragment at approximately 650 bp. This fragment is considered the barcoding region for Blastocystis identification. Concentration of reagents in each reaction and PCR conditions were the same as above. One μL from the PCR mentioned above was used as template.
Positive PCR reactions from the nested-PCR were gel-extracted using the Thermo Scientific GeneJET Gel Extraction Kit (following manufacturer's instructions) and subsequently cloned in the pGEM-T vector (Promega) using the manufacturer's protocol. Five to ten colonies from each transformation were selected for sub culturing and plasmid purification using the GeneJET Plasmid Miniprep Kit. Positive plasmids were screened by digestion with EcoRI restriction enzyme, to confirm presence of the fragment of interest. Positive plasmids were bidirectionally sequenced using T7 and SP6 universal primers by Eurofins, UK.
Genetic distance and phylogenetic analysis
The obtained sequences were trimmed to eliminate vector fragments and forward and reverse sequences of each sample were joined using Sequencher. Blast searches against GenBank using the sequences obtained were performed to exclude bacterial contamination. A dataset including all new sequences identified as Blastocystis along with sequences spanning the breadth of diversity of Blastocystis subtypes was build and aligned using MAFFT v.7 (Katoh and Toh, 2010). The alignment was further improved by visual check where necessary. Genetic distance was calculated using the Kimura2 parameter criterion. Gaps were considered as complete deletions. For this calculation, only the barcoding region of Blastocystis was used.
For the phylogenetic analysis, four additional outgroup taxa were included to the alignment and the entire sequence of SSUrRNA was used. The alignment contained a total of 90 taxa. Several sequences were represented only by their barcoding region in which case, the missing part of the sequence was considered as missing data. Following alignment with MAFFT, ambiguous positions were removed using trimAL (Capella-Gutierrez et al. 2009). After trimming the alignment contained 1163 sites. Phylogenetic trees were constructed by using maximum likelihood and Bayesian inference methods. Maximum likelihood trees were computed using the RAxML software (Stamatakis, 2006). For each dataset bootstrap support was evaluated from 1000 bootstrap replicates. Bayesian inference tree was computed using MrBayes (Ronquist and Huelsenbeck, 2003). Posterior probabilities were computed by running four chains with sampling occurring every 100th generation, whilst 25% of trees were discarded as burn-in. Trees were run for 1 500 000 generations at which point all parameters converged at 0·01.
Results
Culturing
Blastocystis grew in the tubes containing LYSGM and TYM + FBS at both 35 °C and room temperature. There was no Blastocystis growth in the 0·5% LD medium.
Screening of faecal samples
A total of 118 samples from 27 species were examined of which 71 clones were sequence positive belonging to 11 species (41%) (Table 1). Nonetheless, there was a notable difference in the presence of Blastocystis across hosts. With the exception of a single case, all sequence positive samples came from non-carnivorous animals. This was despite repeated sampling and sequencing attempts (Table 1). Specifically, 7/8 (87·5%) of artiodactyls, 2/2 (100%) of rodents and 1/9 (11%) of carnivores were sequence positive for Blastocystis. No sequence positive samples were found in birds, snakes and insectivores (Table 1).
Subtype identification and distribution in various hosts
Among the 71 Blastocystis-positive samples, six STs were detected (Table 2, Fig. 1): ST1, ST4, ST5, ST10, ST14 and a potentially new subtype. Subtypes 4 and 10 colonized the most species (seven and six respectively) followed by ST14 (three), ST1 (two), ST5 (one) and a novel subtype (one). We provide the first molecular data and subtyipng of Blastocystis from elk, water voles, pine martens and red squirrels. The Eurasian elk (artiodactyl) were the hosts harbouring the widest range of subtypes, followed by pygmy goat (artiodactyl) and water vole (rodent). Most notably, four subtypes were found in the elk (ST4, ST10, ST14, novel), while goat and water vole harboured three (ST1, ST10 and ST14 in goat and ST1, ST4 and ST10 in water vole). The hosting of multiple subtypes within elk is of no doubt, as there is just a single elk in the park. The same cannot be verified for the goats and voles as the park houses several of them. Nonetheless, only two faecal samples were collected, which means that there are at least two subtypes present in a single goat. The three subtypes in water vole were identified only in the captive population of which three were sampled. The presence of all subtypes can be confirmed here, due to cloning being used rather than PCR purification of a single product.
Table 2.
Subtype results from sequencing positive samples
| Blastocystis ST | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Host | Name | Location | PCR positive samples | Sequence positive clones | ST1 | ST4 | ST5 | ST10 | ST14 | ST? |
| European Bison | Bison bonasus | Wildwood | 3 | 11 | – | – | – | 11/11 | – | – |
| Eurasian Elk | Alces alces | Wildwood | 1 | 4 | – | 1/4 | – | 1/4 | 1/4 | 1/4 |
| Muntjac Deer | Muntiacus reevesi | Wildwood | 1 | 1 | – | – | – | – | 1/1 | – |
| Pine Marten | Martes martes | Wildwood | 1 | 1 | – | 1/1 | – | – | – | – |
| Pygmy Goat | Capra aegagrus hircus | Wildwood | 2 | 3 | 1/3 | – | – | 1/3 | 1/3 | – |
| Red Deer | Cervus elaphus | Wildwood | 1 | 8 | – | 2/8 | – | 6/8 | – | – |
| Red Squirrel | Sciurus vulgaris | Wildwood | 2 | 1 | – | 1/1 | – | – | – | – |
| Soay Sheep | Ovis aries | Wildwood | 1 | 1 | – | – | – | – | 1/1 | – |
| Wallaby | Macropus rufogriseus | Wildwood | 2 | 2 | – | – | – | 2/2 | – | – |
| Water Vole | Arvicola amphibius | Wildwood | 2 | 12 | 1/12 | 10/12 | – | 1/12 | – | – |
| Water Vole PP | Arvicola amphibius | Bulphan | 5 | 17 | – | 17/17 | – | – | – | – |
| Water Vole TB | Arvicola amphibius | Tilbury | 3 | 9 | – | 9/9 | – | – | – | – |
| Wild Boar | Sus scrofa | Wildwood | 1 | 1 | – | – | 1/1 | – | – | – |
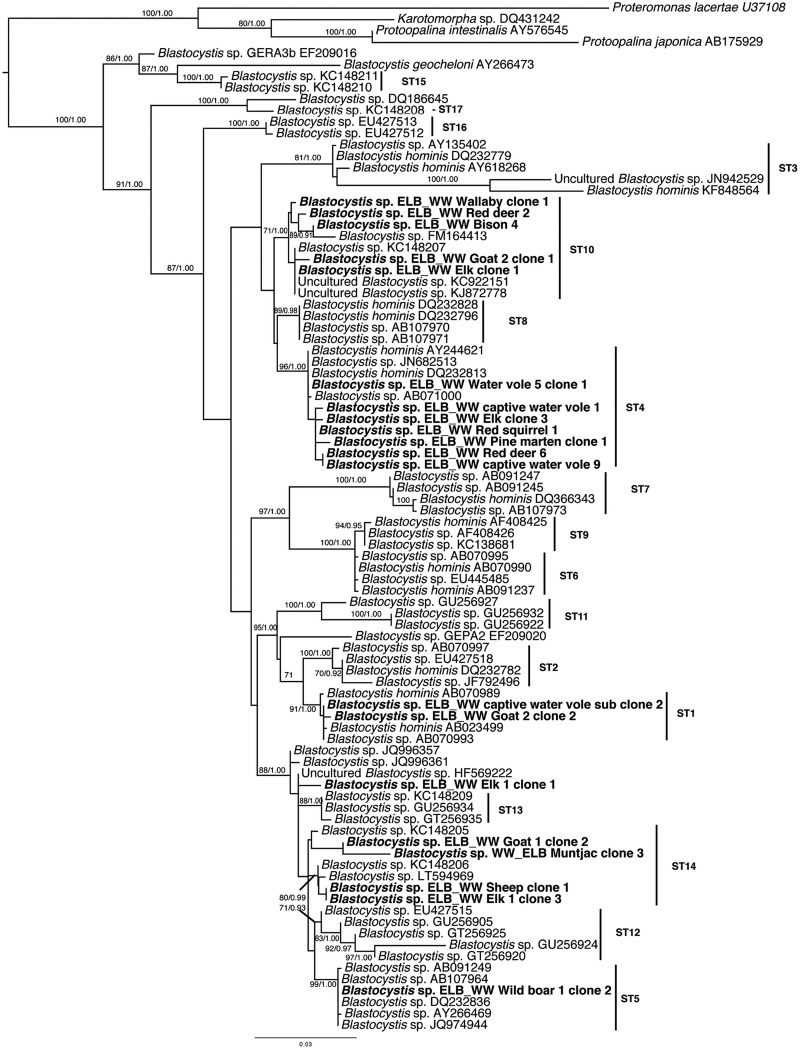
Fig. 1.
Maximum likelihood phylogenetic tree inferred from 90 SSUrRNA sequences and 1163 sites. Newly generated sequences are shown in bold. Numerical values on the branches indicate bootstrap percentages and posterior probabilities in this order. Only bootstrap support values greater than 70 are shown. The accession numbers of all newly generated sequences are presented in online Supplementary Table S1.
Several samples were collected from two rodent species; the red squirrel and water vole. Subtype 4 was commonly detected in both species, while the range of subtypes previously reported within rodents can be expanded to include ST1. Several colonies were also screened from wallabies, diprotodontid marsupials. All samples from wallabies harboured ST10, which had not been reported previously from these marsupials.
Phylogenetic analysis
Though 71 clones were sequenced, only 20 of them were used in the phylogenetic analyses. In the cases where clusters contained identical clones, only a few representative sequences were kept. In total, the new sequences were subtyped as follows: ST4 (n = 41); ST10 (n = 22); ST14 (n = 4); ST1 (n = 2); ST5 (n = 1) novel ST (n = 1). All Blastocystis sequences formed a strongly supported cluster (100BS/1·00BI). Most newly sequenced isolates grouped within clades formed by previously published subtypes (Fig. 1). The most basal sequences belonged to Blastocystis isolates from reptiles and cockroaches along with those from ST15, ST16 and ST17 in agreement with previous studies (Alfellani et al. 2013c; Yoshikawa et al. 2016). Subtype 3 sequences grouped together and sister to a clustered formed by ST10, ST8 and ST4. Subtypes 7, 9 and 6 clustered together, while ST11, ST2 and ST1 formed a separate clade. Subtypes 12 and 5 also grouped together. Subtypes 13 and 14 were not well resolved even when a subtree was constructed (data not shown). The ELB_WW Elk 1 clone 1 did not fall within any of the 17 STs and its position remains unresolved.
Discussion
Approximately 61 animals from 27 species were examined. Forty one per cent of all animals were sequence positive for Blastocystis. In select cases, we attempted to establish cultures of Blastocystis. The organism has been previously cultured in a wide range of media including egg slant medium with Locke's solution, Iscove's modified Dulbecco's medium, Robinson's medium and Jones’ medium (Clark and Diamond, 2002; Tan, 2008). The latter is a widely used formulation ideal for short term culturing of multiple subtypes (i.e. a few days). Blastocystis isolates originating from endothermic hosts are customarily cultured at 35 °C. Reported here was cultivation of Blastocystis from a water vole (Arvicola amphibius) in TYM medium enriched with FBS. The culture had been maintained in the laboratory for at least 11 months. Although the origin of the isolate is an endothermic animal, it grew over abundantly at room temperature. This indicates that some isolates of Blastocystis can grow at lower temperatures given certain types of media. Whether all isolates of Blastocystis or only some can grow in TYM + FBS at room temperature needs further study.
Most of the animals that we examined harboured a single subtype of Blastocystis. Nonetheless, some animals carried more than one subtype. Mixed colonization was confirmed, because we employed cloning and screened multiple colonies from each sample, while previous studies only used direct sequencing from PCR products (Stensvold et al. 2012; Roberts et al. 2013; Alfellani et al. 2013b, c; Stensvold, 2013). Using this strategy, it was found that elk (Alces alces) harboured four subtypes, with this being the first time Blastocystis has been reported in this mammal. In cases where multiple subtypes are found within a single host, it is important to exclude contamination from other sources. The park has a single elk, which is housed in an isolated enclosure on its own. Moreover, the faecal sample was collected at the moment of defecation precluding contamination from small, non-resident animals. More than one subtype was also detected in pygmy goats (ST = 3), red deer (ST = 2) and water voles (ST = 3). Unlike in the case of the elk, we cannot definitively conclude that the detected subtypes in goats originated from a single individual per se, since enclosures housed multiple animals of the same species. While colonization with multiple subtypes is rare in humans, not much information is available for other animal species (Meloni et al. 2012). In light of our findings, it is tempting to speculate that the microbiota of at least some animals includes Blastocystis subtypes. Sampling from more animals and use of methodologies similar to ours will shed further light as to whether presence of multiple subtypes is the norm within these and other animals.
Water voles also constitute an interesting case. There are two, temporary populations of water vole being held within the park, together with permanent residents. These two groupings of water vole are temporarily brought in to captivity as part of a licensed, development mitigation programme and are subsequently to be introduced back into their natural environment locations; two separate sites in Essex, UK. This study can report that ‘wild’ water vole harboured ST4 only, whereas those in permanent captivity also harboured ST1. Wild water voles were sampled multiple times, while captive ones provided only a limited number of samples. Despite considerable effort (PCR, cloning and screening of clones) we were unable to detect ST1 in wild water voles. It is tempting to speculate that the ‘captive’ water vole acquired ST1 after their introduction in the park and that this is one of the many microbiota-related alterations associated with life in captivity (Waite and Taylor, 2014; Kohl et al. 2017). However, since captive voles originated from two additional locations other than Essex, this hypothesis needs further testing involving surveys of all populations of origin.
ST10 and ST4 were the most widely distributed subtypes, each isolated from five species. As previously described, artiodactyls carried mostly ST10 (Alfellani et al. 2013c). It has been speculated that rodents are reservoirs of ST4 for human infection, though not all rodent species carry this specific ST (Alfellani et al. 2013b). Subtypes 3 and 17 were also found in rodents in previous investigations (Stensvold et al. 2009; Alfellani et al. 2013a). Herein, this study detected ST4 in all Blastocystis positive samples of rodents. Nonetheless, other subtypes were also found in the screened rodents: ST10 in red squirrels and ST1, ST5 and ST10 in water voles. Therefore, the study has been able to expand the number of subtypes recorded in rodents by identifying ST1 and ST10. It was also possible to expand the range of subtypes identified in goats to include ST14, along with the previously identified ST10, ST1, ST3, ST6 and ST7 (Alfellani et al. 2013b). The study also detected ST14 in four hosts, all of which belong to the artiodactyls.
To determine the monophyly and relationships among STs, phylogenetic analyses were performed. Traditionally, sampling of Blastocystis had focused on primates, especially humans. As a result, STs that were present in non-primates were reported infrequently and the clades of these STs remained sparsely populated. For instance, the resolution of the ST13 and ST14 has been problematic. Previously, Alfellani et al. (2013c) speculated that ST14 should be split into two subtypes, but refrained from doing so pending further sampling. The current study has shown that, when our isolates were added to the tree, ST14 splits into two distinct clades, with our samples populating both of these clades, hence, supporting the idea that it should be considered as two STs. Moreover, one isolate from elk grouped independently of all other STs, suggesting that this might be a novel ST. Genetic divergence analysis of the barcode region indicated that the genetic distance between our isolate and all other STs is over 5%, with the exception of ST13, with which it differed by 4·4%. The recommended threshold to define a new sequence is 5% divergence (Clark et al. 2013). Nonetheless, the full sequence and further samples are needed to confirm this finding since this is an individual partial sequence.
In summary, we present here a comprehensive study of Blastocystis prevalence focusing exclusively on non-primate animals in a zoo setting in the UK. Presented here has been the presence of six subtypes, with one potentially being novel. Through the use of cloning, it has been possible to conclusively record the presence of multiple STs within an individual animal. The sequences generated from this study have populated STs that were considered rare and for which not many sequences exist. Collectively, these highlight the need for sampling from a wide range of hosts.
Acknowledgements
This research was supported by BBSRC research grant (BB/M009971/1) to Dr Anastasios D. Tsaousis. Dr Eleni Gentekaki was supported by the Mae Fah Luang University research grant (601202). We thank members of the Dr Tsaousis laboratory (2016/2017), Prof. Martin Michaelis (University of Kent) and the keepers of the Wildwood Trust for assisting with sample collection and accommodating us during our visitations.
Conflicts of interest
The authors declare no conflict of interest.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0031182017002347.
click here to view supplementary material
References
- Abe N (2004) Molecular and phylogenetic analysis of Blastocystis isolates from various hosts. Veterinary Parasitology 120, 235–242. [DOI] [PubMed] [Google Scholar]
- Abe N, Nagoshi M, Takami K, Sawano Y and Yoshikawa H (2002) A survey of Blastocystis sp. in livestock, pets, and zoo animals in Japan. Veterinary Parasitology 106, 203–212. [DOI] [PubMed] [Google Scholar]
- Alfellani MA, Jacob AS, Perea NO, Krecek RC, Taner-Mulla D, Verweij JJ, Levecke B, Tannich E, Clark CG and Stensvold CR (2013a) Diversity and distribution of Blastocystis sp. subtypes in non-human primates. Parasitology 140, 966–971. [DOI] [PubMed] [Google Scholar]
- Alfellani MA, Stensvold CR, Vidal-Lapiedra A, Onuoha ES, Fagbenro-Beyioku AF and Clark CG (2013b) Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta Tropica 126, 11–18. [DOI] [PubMed] [Google Scholar]
- Alfellani MA, Taner-Mulla D, Jacob AS, Imeede CA, Yoshikawa H, Stensvold CR and Clark CG (2013c) Genetic diversity of Blastocystis in livestock and zoo animals. Protist 164, 497–509. [DOI] [PubMed] [Google Scholar]
- Amenu K, Tesfaye D, Tilahun G and Mekibib B (2015) Gastrointestinal parasites of vervet monkeys around Lake Hawassa recreational sites, southern Ethiopia. Comparative Clinical Pathology 24, 1491–1496. [Google Scholar]
- Capella-Gutierrez S, Silla-Martinez JM and Gabaldon T (2009) Trimal: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics (Oxford, England) 25, 1972–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CG (1997) Extensive genetic diversity in Blastocystis hominis. Molecular and Biochemical Parasitology 87, 79–83. [DOI] [PubMed] [Google Scholar]
- Clark CG and Diamond LS (2002) Methods for cultivation of luminal parasitic protists of clinical importance. Clinical Microbiology Reviews 15, 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CG, van der Giezen M, Alfellani MA and Stensvold CR (2013) Recent developments in Blastocystis research. Advances in Parasitology 82, 1–32. [DOI] [PubMed] [Google Scholar]
- DeRenzis FA and Schechtman A (1973) Staining by neutral red and trypan blue in sequence for assaying vital and nonvital cultured cells. Stain Technology 48, 135–136. [DOI] [PubMed] [Google Scholar]
- Diamond LS (1957) The establishment of various trichomonads of animals and man in axenic cultures. The Journal of Parasitology 43, 488–490. [PubMed] [Google Scholar]
- Diamond LS (1982) A new liquid medium for xenic cultivation of Entamoeba histolytica and other lumen-dwelling protozoa. The Journal of Parasitology 68, 958–959. [PubMed] [Google Scholar]
- Diamond LS (1983) Lumen dwelling protozoa: Entamoeba, trichomonads, and Giardia In Jensen JB (ed.). In Vitro Cultivation of Protozoan Parasites, Boca Raton, FL: CRC Press, pp. 67–109. [Google Scholar]
- Figueroa J (2015) New records of parasites in free-ranging Andean bears from Peru. Ursus 26, 21–27. [Google Scholar]
- Katoh K and Toh H (2010) Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics (Oxford, England) 26, 1899–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl KD, Brun A, Magallanes M, Brinkerhoff J, Laspiur A, Acosta JC, Caviedes-Vidal E and Bordenstein SR (2017) Gut microbial ecology of lizards: insights into diversity in the wild, effects of captivity, variation across gut regions and transmission. Molecular Ecology 26, 1175–1189. [DOI] [PubMed] [Google Scholar]
- Lim YA, Ahmad RA and Smith HV (2008) Current status and future trends in Cryptosporidium and Giardia epidemiology in Malaysia. Journal of Water and Health 6, 239–254. [DOI] [PubMed] [Google Scholar]
- Meloni D, Poirier P, Mantini C, Noel C, Gantois N, Wawrzyniak I, Delbac F, Chabe M, Delhaes L, Dei-Cas E, Fiori PL, El Alaoui H and Viscogliosi E (2012) Mixed human intra- and inter-subtype infections with the parasite Blastocystis sp. Parasitology International 61, 719–722. [DOI] [PubMed] [Google Scholar]
- Parkar U, Traub RJ, Vitali S, Elliot A, Levecke B, Robertson I, Geurden T, Steele J, Drake B and Thompson RC (2010) Molecular characterization of Blastocystis isolates from zoo animals and their animal-keepers. Veterinary Parasitology 169, 8–17. [DOI] [PubMed] [Google Scholar]
- Perez Cordon G, Hitos Prados A, Romero D, Sanchez Moreno M, Pontes A, Osuna A and Rosales MJ (2008) Intestinal parasitism in the animals of the zoological garden ‘Pena Escrita’ (Almunecar, Spain). Veterinary Parasitology 156, 302–309. [DOI] [PubMed] [Google Scholar]
- Puebla LEJ, Núñez FA, Rivero L, Hernández YR, Millán IA and Müller N (2017) Prevalence of intestinal parasites and molecular characterization of Giardia duodenalis from dogs in La Habana, Cuba. Veterinary Parasitology: Regional Studies and Reports 8, 107–112. [DOI] [PubMed] [Google Scholar]
- Rajah Salim H, Suresh Kumar G, Vellayan S, Mak JW, Khairul Anuar A, Init I, Vennila GD, Saminathan R and Ramakrishnan K (1999) Blastocystis in animal handlers. Parasitology Research 85, 1032–1033. [DOI] [PubMed] [Google Scholar]
- Ramirez JD, Sanchez A, Hernandez C, Florez C, Bernal MC, Giraldo JC, Reyes P, Lopez MC, Garcia L, Cooper PJ, Vicuna Y, Mongi F and Casero RD (2016) Geographic distribution of human Blastocystis subtypes in South America. Infection, Genetics and Evolution: Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases 41, 32–35. [DOI] [PubMed] [Google Scholar]
- Roberts T, Stark D, Harkness J and Ellis J (2013) Subtype distribution of Blastocystis isolates from a variety of animals from New South Wales, Australia. Veterinary Parasitology 196, 85–89. [DOI] [PubMed] [Google Scholar]
- Ronquist F and Huelsenbeck JP (2003) Mrbayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics (Oxford, England) 19, 1572–1574. [DOI] [PubMed] [Google Scholar]
- Ruaux CG and Stang BV (2014) Prevalence of Blastocystis in shelter-resident and client-owned companion animals in the US Pacific Northwest. PLoS ONE 9, e107496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan PD, Stensvold CR, Rajilic-Stojanovic M, Heilig HG, De Vos WM, O'Toole PW and Cotter PD (2014) The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiology Ecology 90, 326–330. [DOI] [PubMed] [Google Scholar]
- Schar F, Inpankaew T, Traub RJ, Khieu V, Dalsgaard A, Chimnoi W, Chhoun C, Sok D, Marti H, Muth S and Odermatt P (2014) The prevalence and diversity of intestinal parasitic infections in humans and domestic animals in a rural Cambodian village. Parasitology International 63, 597–603. [DOI] [PubMed] [Google Scholar]
- Scicluna SM, Tawari B and Clark CG (2006) DNA barcoding of Blastocystis. Protist 157, 77–85. [DOI] [PubMed] [Google Scholar]
- Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics (Oxford, England) 22, 2688–2690. [DOI] [PubMed] [Google Scholar]
- Stensvold CR (2013) Comparison of sequencing (barcode region) and sequence-tagged-site PCR for Blastocystis subtyping. Journal of Clinical Microbiology 51, 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensvold CR and Clark CG (2016) Current status of Blastocystis: a personal view. Parasitology International 65, 763–771. [DOI] [PubMed] [Google Scholar]
- Stensvold CR, Alfellani MA, Norskov-Lauritsen S, Prip K, Victory EL, Maddox C, Nielsen HV and Clark CG (2009) Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a new subtype. International Journal for Parasitology 39, 473–479. [DOI] [PubMed] [Google Scholar]
- Stensvold CR, Alfellani M and Clark CG (2012) Levels of genetic diversity vary dramatically between Blastocystis subtypes. Infection, Genetics and Evolution: Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases 12, 263–273. [DOI] [PubMed] [Google Scholar]
- Tan KS (2008) New insights on classification, identification, and clinical relevance of Blastocystis spp. Clinical Microbiology Reviews 21, 639–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite DW and Taylor MW (2014) Characterizing the avian gut microbiota: membership, driving influences, and potential function. Frontiers in Microbiology 5, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Owen H, Traub RJ, Cuttell L, Inpankaew T and Bielefeldt-Ohmann H (2014) Molecular epidemiology of Blastocystis in pigs and their in-contact humans in Southeast Queensland, Australia, and Cambodia. Veterinary Parasitology 203, 264–269. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Koyama Y, Tsuchiya E and Takami K (2016) Blastocystis phylogeny among various isolates from humans to insects. Parasitology International 65, 750–759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0031182017002347.
click here to view supplementary material