Abstract
In Arabidopsis, genes encoding functional enzymes for the synthesis and degradation of trehalose have been detected recently. In this study we analyzed how trehalose affects the metabolism and development of Arabidopsis seedlings. Exogenously applied trehalose (25 mm) strongly reduced the elongation of the roots and, concomitantly, induced a strong accumulation of starch in the shoots, whereas the contents of soluble sugars were not increased. When Arabidopsis seedlings were grown on trehalose plus sucrose (Suc), root elongation was restored, but starch still accumulated to a much larger extent than during growth on Suc alone. The accumulation of starch in the shoots of trehalose-treated seedlings was accompanied by an increased activity of ADP-glucose pyrophosphorylase and an induction of the expression of the ADP-glucose pyrophosphorylase gene, ApL3. Even in the presence of 50 mm Suc, which itself also slightly induced ApL3, trehalose (5 mm) led to a further increase in ApL3 expression. These results suggest that trehalose interferes with carbon allocation to the sink tissues by inducing starch synthesis in the source tissues. Furthermore, trehalose induced the expression of the β-amylase gene, AT-β-Amy, in combination with Suc but not when trehalose was supplied alone, indicating that trehalose can modulate sugar-mediated gene expression.
The non-reducing disaccharide trehalose, α-d- glucopy-ranosyl-[1,1]-α-d-glucopyranoside, is a common sugar of bacteria, fungi, and invertebrate animals (Elbein, 1974). Trehalose has also been shown to occur in the “resurrection plants,” Selaginella lepidophylla and Myrothamnus flabellifolia, where it probably serves as a stress protectant during desiccation (Adams et al., 1990; Bianchi et al., 1993; Drennan et al., 1993; Müller et al., 1995a; Zentella et al., 1999). In other plants such as Arabidopsis, genes with homologies to the yeast genes encoding enzymes of trehalose synthesis (trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase) have been identified and shown to encode functional enzymes (Blázquez et al., 1998; Vogel et al., 1998; Goddijn and van Dun, 1999). These findings indicate that trehalose might generally be synthesized in plants (Goddijn and Smeekens, 1998; Goddijn and van Dun, 1999; Müller et al., 1999).
To make use of trehalose as a stress protectant, attempts have been made to increase trehalose contents in plants by overexpression of microbial genes encoding the enzymes for trehalose synthesis. It is interesting that transgenic plants expressing the Escherichia coli or Saccharomyces cerevisiae genes for trehalose synthesis not only exhibit increased drought tolerance (Holström et al., 1996; Romero et al., 1997; Pilon-Smits et al., 1998) but also show strong developmental alterations, such as stunted growth, lancet-shaped leaves, and disturbed root systems (Goddijn et al., 1997; Romero et al., 1997).
The mechanisms by which trehalose metabolism alters plant development are largely unknown. Trehalose itself could affect development by acting as signal molecule in carbohydrate metabolism. For example, trehalose induces enzymes of fructan synthesis in barley (Wagner et al., 1986; Müller et al., 2000) and Suc synthase activity in soybean (Müller et al., 1998). In general, sugars such as Suc and Glc act as signals in the regulation of gene expression (Koch, 1996). Whereas the expression of several source-specific genes is probably regulated by hexoses in a hexokinase-dependent signaling pathway (e.g. Jang and Sheen, 1994; Jang et al., 1997; Dai et al., 1999), the regulation of the expression of some other genes appears to be directly mediated by Suc without prior cleavage to hexoses (e.g. Yokoyama et al., 1994; Chiou and Bush, 1998; Rook et al., 1998). It is conceivable that trehalose, which is structurally similar to Suc, might act as an analog of Suc in sugar sensing. In addition to the trehalose that may be produced by the plants themselves, plants are also exposed to trehalose formed by microorganisms in mutualistic, as well as in pathogenic interactions. Trehalose formed by rhizobia during nodulation appears to have a strong effect on the carbohydrate contents in root nodules (Müller et al., 1995b). It is possible that trehalose-producing plant symbionts and/or pathogens can exploit the effects of trehalose to gain control over the sugar-sensing system of the plant. If this is the case, the trehalose-degrading enzyme trehalase, which is widespread among higher plants and is found in multiple tissues, may provide a safeguard against potentially deleterious effects of trehalose on carbohydrate allocation in plant-microbe interactions (Müller et al., 1995a; Aeschbacher et al., 1999).
In this study we analyzed which way trehalose alters plant metabolism and development and how it affects sugar-mediated gene expression. We show that in Arabidopsis seedlings, trehalose specifically induced the expression ApL3, which encodes a large subunit of ADP-Glc pyrophosphorylase (ADP-Glc-PPase), the first enzyme in starch biosynthesis (Preiss, 1982). In addition, the activity of ADP-Glc-PPase was increased and starch accumulated in source tissues, thereby leading to a reduced supply of carbon to the roots and developing leaves.
RESULTS
Effect of Trehalose on the Development of Arabidopsis
In previous experiments we have observed that trehalose is taken up by Arabidopsis seedlings and that it inhibits root elongation in a concentration-dependent manner (Aeschbacher et al., 2000). For the present study we analyzed the effects of trehalose at a concentration of 25 mm, a concentration we knew to reduce the growth of Arabidopsis seedlings without affecting the time of germination. Mannitol was used as an osmotic control, and Suc was used as a metabolizable carbon source known to affect gene expression. Since Arabidopsis contains trehalase activity, we also supplemented the growth media with validamycin A, which has been shown to inhibit the activity of trehalase from soybean (Müller et al., 1992; Müller et al., 1995b). During the growth of mature Arabidopsis plants on 10 mm trehalose, addition of validamycin A led to a 10-fold increase in the accumulation of trehalose in the rosette leaves (up to 10 mg g−1 dry weight; J. Müller, R. A. Aeschbacher, T. Boller, and A. Wiemken, unpublished data), indicating that validamycin A also inhibits the degradation of trehalose in Arabidopsis. Therefore, treatment with validamycin A allows distinguishing between the effects of trehalose as a carbon source and as a potential signal molecule.
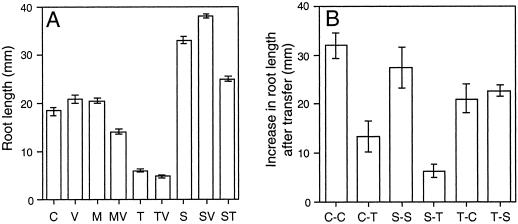
Growth on trehalose strongly inhibited root elongation, especially in the presence of validamycin A (Fig. 1A). In contrast, Suc increased root elongation and also restored root elongation in the presence of trehalose. This effect could also be observed by supplying Glc together with trehalose (data not shown), indicating that trehalose did not directly inhibit root growth but probably led to a starvation of the root tissue.
Figure 1.
Inhibition of root elongation by trehalose. A, Arabidopsis seedlings were grown for 10 d on one-half-strength Murashige and Skoog medium (C) supplemented with 10 μm validamycin A (V), 25 mm mannitol (M), 25 mm trehalose (T), 25 mm Suc (S), or combinations thereof (MV, TV, SV, and ST). Data are means ± se of 94 to 140 plants from at least six different agar plates. B, The seedlings were transferred for an additional time of 6 d from one-half-strength Murashige and Skoog medium onto a fresh plate (C-C) or onto 25 mm trehalose (C-T), from 25 mm Suc onto a fresh plate (S-S) or onto 25 mm trehalose (S-T), and from 25 mm trehalose onto one-half-strength Murashige and Skoog medium (T-C) or onto 25 mm Suc (T-S). Data are means ± se of seven to nine plants.
To study if the effect of trehalose only applied to young seedlings that germinated on trehalose-con-taining medium we also transferred seedlings between the different media and measured the increase in root length (Fig. 1B). While the roots of plants transferred onto plates without sugar or with Suc continued growing, transfer onto trehalose-containingmedium led to an inhibition of root elongation, particularly when the plants had been growing on Suc before. On the other hand, plants transferred from trehalose-containing medium onto medium without sugar or onto Suc-containing medium recovered, and the roots started growing, showing that the effect of trehalose is reversible (Fig. 1B).
Effect of Trehalose on Photosynthetic Proteins
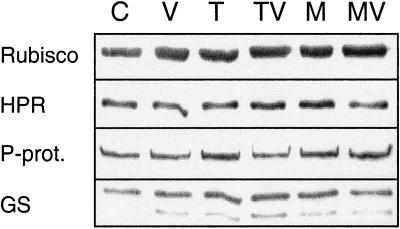
Since the restoration of root elongation by Suc or Glc indicates that growth on trehalose leads to a reduced supply of carbon to the roots, we studied whether trehalose impairs photosynthetic carbon assimilation by inhibiting the development of the photosynthetic apparatus. Seedlings grown in the presence of trehalose did not show any signs of chlorosis (data not shown). The amounts of the photosynthetic or photorespiratory proteins, Rubisco, NADH-dep-endent hydroxypyruvate reductase, P-protein of the Gly decarboxylase complex, or plastidic Gln synthetase, as determined by western blotting, were not affected by growth on trehalose (Fig. 2). This shows that growth on trehalose is unlikely to result in the down-regulation of photosynthetic gene expression.
Figure 2.
Western blots for photosynthetic and photorespiratory proteins in total shoots. Arabidopsis seedlings were grown for 14 d on one-half-strength Murashige and Skoog medium (C) supplemented with 10 μm validamycin A (V), 25 mm trehalose (T), 25 mm mannitol (M), or combinations thereof (TV, MV). Total protein (2.5 μg) was loaded per lane. The blots were probed with antibodies against Rubisco, NADH-dependent hydroxypyruvate reductase (HPR), P-protein of the Gly decarboxylase complex (P-prot.), and Gln synthetase (GS). The higher Mr band represents the plastidic isoform of Gln synthetase.
Effect of Trehalose on Carbohydrate Contents
If trehalose acted by inhibiting photosynthetic activity, one would expect a reduction in carbohydrate contents. In the shoots of seedlings grown on trehalose, however, the content of total non-structural carbohydrates was increased on a dry weight basis. This was due to a strong accumulation of starch, whereas Glc and Fru were not affected and the Suc content was reduced (Fig. 3).
Figure 3.
Contents of Suc, Glc, Fru, and starch in total shoots. Arabidopsis seedlings were grown for 10 d on one-half-strength Murashige and Skoog medium without (control, white columns) or with addition of 25 mm trehalose (black columns). Data are means ± se of seedlings harvested from three different agar plates.
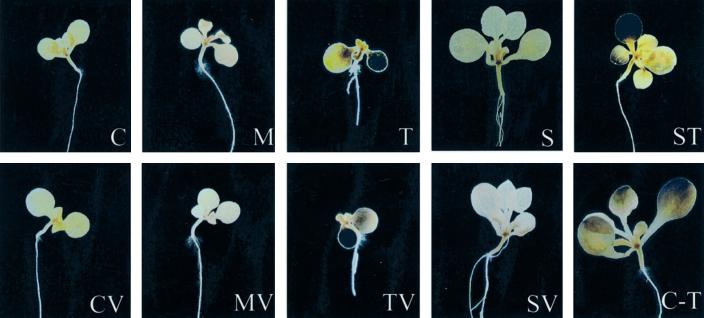
To analyze the distribution of starch we stained the starch in whole seedlings using KI/I2 (Fig. 4). Seedlings grown on medium without sugar or with addition of mannitol did not accumulate major amounts of starch. During growth on 25 mm trehalose, starch strongly accumulated in the cotyledons. One of the cotyledons of these plants was often smaller and more strongly stained than the other one. Starch also accumulated in young leaves that had typically developed poorly. To study if trehalose directly served as a carbon source for the formation of starch we inhibited the breakdown of trehalose by addition of validamycin A. Nevertheless, trehalose still induced starch synthesis. When the seedlings were grown in the dark, trehalose did, however, not induce starch formation (data not shown), indicating that the starch was synthesized from carbon fixed during photosynthesis. During growth on 25 mm Suc as a metabolizable carbon source, starch only accumulated in a small area in the upper part of the hypocotyl, but not in the cotyledons. It is interesting that in the presence of trehalose plus Suc, the plants grew well but still accumulated starch in the cotyledons and in the leaves (Fig. 4). The effect of trehalose on starch accumulation was, therefore, separable from the inhibition of growth that was induced by trehalose alone. Transfer of 10-d-old seedlings grown without an external carbon source onto trehalose-containing medium for an additional time of 6 d also led to increased starch contents in the leaves, showing again that Arabidopsis seedlings retain the capacity to react to trehalose even after initial growth without external sugar.
Figure 4.
Distribution of starch after growth on trehalose. Arabidopsis seedlings were grown for 10 d on one-half-strength Murashige and Skoog medium (C) supplemented with 10 μm validamycin A (V), 25 mm mannitol (M), 25 mm trehalose (T), 25 mm Suc (S), or combinations thereof (MV, TV, SV, and ST) and after transfer from one-half-strength Murashige and Skoog medium onto medium supplemented with 25 mm trehalose (C-T) for an additional time of 6 d. The seedlings were stained for starch with KI/I2 solution.
Effect of Trehalose on the Expression of AtSUC2
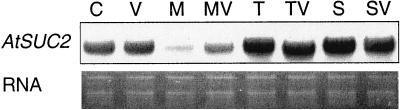
A possible reason for the accumulation of starch in the source tissues was that trehalose interfered with the export of assimilates in form of Suc to the growing sinks. We, therefore, determined if the expression of Suc transporters was altered in trehalose-treated seedlings. Whereas AtSUC1 is specifically expressed in the flowers of Arabidopsis plants (Stadler et al., 1999), AtSUC2 is probably involved in phloem loading in source tissues (Truernit and Sauer, 1995; Stadler et al., 1999). Correspondingly, expression of AtSUC1 was not observable in the shoots of seedlings by RNA-blot hybridization (data not shown), where-as AtSUC2 was strongly expressed. Compared with plants grown without external sugar, the expression of AtSUC2 was reduced by growth on 25 mm mannitol, which was used as an osmotic control (Fig. 5). In contrast, growth on 25 mm trehalose or on 25 mm Suc did not reduce the expression but even slightly induced it. Addition of validamycin A to these treatments did not affect the induction.
Figure 5.
RNA-blot analysis of the expression of the Suc transporter gene, AtSUC2, in total shoots. Arabidopsis seedlings were grown for 10 d on one-half-strength Murashige and Skoog medium (C) supplemented with 10 μm validamycin A (V), 25 mm mannitol (M), 25 mm trehalose (T), 25 mm Suc (S), or combinations thereof (MV, TV, and SV). The probe used was a 868-bp fragment corresponding to AtSUC2. The lower panel of the figure shows the ethidium bromide-stained RNA gel.
Effect of Trehalose on ADP-Glc-PPase
To study if trehalose directly enhanced starch synthesis we measured the activity of ADP-Glc-PPase. The activity of ADP-Glc-PPase was significantly increased after growth in the presence of 25 mm trehalose, independent of the addition of validamycin A (Fig. 6). Growth on Suc also led to a slight, but not statistically significant, increase in ADP-Glc-PPase activity. Mannitol had no effect on ADP-Glc-PPase activity.
Figure 6.
Activity of ADP-Glc-PPase in total shoots. Arabidopsis seedlings were grown for 10 d on one-half-strength Murashige and Skoog medium (C) supplemented with 10 μm validamycin A (V), 25 mm mannitol (M), 25 mm trehalose (T), 25 mm Suc (S), or combinations thereof (MV, TV, and SV). Data are means ± se of seedlings harvested from three to four different Petri dishes. Asterisks indicate a statistically significant difference from the control treatment (Fisher's protected lsd; P ≤ 0.05).
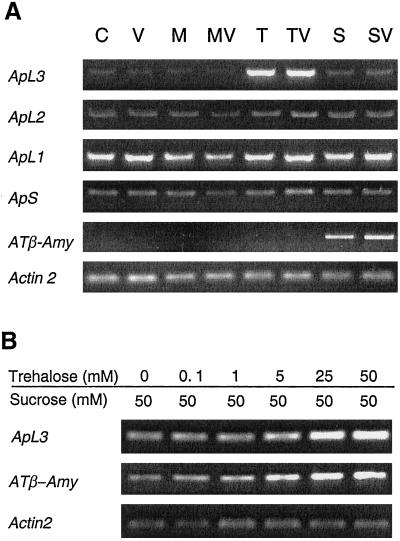
We analyzed whether this increase in ADP-Glc-PPase activity was due to changes in gene expression. The four known ADP-Glc-PPase genes from Arabidopsis, ApS, ApL1, ApL2, and ApL3, show high sequence homologies in the coding region (Villand et al., 1993). To circumvent the problem of a potential cross-hybridization that might occur in RNA-blot hybridization experiments, we performed reverse transcriptase (RT)-PCR experiments using specific primers for each gene. The primers were designed in a way that single gene-specific cDNA fragments with a diagnostic size were amplified (see “Materials and Methods”).
In the shoots of seedlings 25 mm trehalose led to an induction of ApL3 expresssion (Fig. 7A). Addition of validamycin A to the medium did not influence this effect. In the presence of 25 mm Suc the content of ApL3 mRNA was also slightly increased, but to a much lower extent compared with trehalose. Mannitol-treated seedlings did not show an increase in ApL3 mRNA content, which rules out a possible osmotic induction of ApL3. No substantial effect of 25 mm trehalose or 25 mm Suc on the contents of ApS1, ApL1, or ApL2 mRNAs could be detected by RT-PCR. Expression of the β-amylase gene, AT-β-Amy, was specifically induced by Suc but not by trehalose when either sugar was supplied alone. Amplification of the Arabidopsis actin gene, Act2, was similar in all samples, indicating that first strand cDNA synthesis was comparable in all samples.
Figure 7.
RT-PCR analysis of the expression of the ADP-Glc-PPase genes, ApS, ApL1, ApL2, and ApL3, and the β-amylase gene in total shoots. A, Arabidopsis seedlings were grown for 10 d on one-half-strength Murashige and Skoog medium (C) supplemented with 10 μm validamycin A (V), 25 mm mannitol (M), 25 mm trehalose (T), 25 mm Suc (S), or combinations thereof (MV, TV, and SV). B, The seedlings were grown for 10 d on one-half-strength Murashige and Skoog medium containing 50 mm Suc plus different concentrations of trehalose. ATβ-Amy, β-Amylase gene.
Since seedlings grown on trehalose alone developed poorly, we could not exclude that the altered morphology or the different developmental stage, rather than trehalose, was the cause of the altered expression of ApL3. Therefore, we analyzed the effect of different concentrations of trehalose in the presence of 50 mm Suc (Fig. 7B). Under these conditions Suc overrode the inhibitory effect of trehalose on growth, i.e. addition of trehalose did not visibly alter the morphology of the seedlings. In the presence of Suc, ApL3 mRNA contents were already increased by addition of 5 mm trehalose, and the expression was further stimulated with increasing concentrations of trehalose (Fig. 7B). It is interesting that in the presence of Suc, increasing concentrations of trehalose also increased the content of AT-β-Amy mRNA. This induction was not influenced by addition of the trehalase inhibitor validamycin A (data not shown).
DISCUSSION
Research on transgenic plants overexpressing microbial genes for trehalose synthesis has shown that increased trehalose synthesis can have strong effects on plant development (Goddijn et al., 1997; Romero et al., 1997). In Arabidopsis seedlings, exogenously supplied trehalose inhibits the development of roots and leaves (Aeschbacher et al., 2000; this study). We show here that supply of carbon in form of metabolizable sugars can override the growth inhibition caused by trehalose: The morphology of seedlings grown on trehalose plus Suc (Figs. 1 and 4) or Glc (data not shown) was comparable with plants grown on Suc alone. However, the seedlings still showed a specific effect of trehalose treatment, namely accumulation of starch in the cotyledons and in the leaves. This indicates that Suc did not simply inhibit the uptake of trehalose. In Arabidopsis seedlings grown in liquid culture, the starch content increased up to 6-fold within 24 h after addition of trehalose (data not shown), showing that the effect of trehalose was rapid and independent of developmental alterations. We also show that the effect of trehalose can be reversed by transfer onto medium without trehalose (Fig. 1). Furthermore, since growth on trehalose did not lead to increased hexose contents (Fig. 3) and since trehalose was also effective in the presence of validamycin A (Figs. 1, 4, 5, 6, and 7), we can rule out that the effects of trehalose were due to its cleavage to Glc.
Trehalose Alters the Shoot-Root Allocation of Carbon
Since the addition of external carbon sources restored root growth in the presence of trehalose, the effect of trehalose was probably resulting from a reduced supply of carbon to the roots. A reduced supply of carbon could be caused by a down-regulation of photosynthetic activity due to decreased amounts of photosynthetic enzymes. Such a negative effect of sugars on photosynthesis has been demonstrated to occur when other sugars (Suc and hexoses) accumulate in the leaves (Stitt et al., 1990). For example, feeding of Glc leads to a decline in NADH-dependent hydroxypyruvate reductase and Rubisco proteins in discs of tobacco leaves (Wingler et al., 1998) and in Arabidopsis seedlings (data not shown). As the contents of photosynthetic proteins were normal in trehalose-treated seedlings (Fig. 2), we exclude that trehalose had a negative effect on the development of the photosynthetic apparatus. The light-dependent increase in starch in the shoots (Fig. 3) further rules out that reduced photosynthesis was the cause of the growth alterations.
The accumulation of starch in the shoots of seedlings grown on trehalose (Figs. 3 and 4) could point at an impairment of the export of carbon in form of Suc to the sinks. Chiou and Bush (1998) have shown that feeding of Suc to leaves of sugar beet results in the down-regulation of the expression and of the activity of the Suc transporter. It was conceivable that trehalose, which is structurally similar to Suc, could have the same effect. We can, however, rule out that trehalose induced such a phenomenon in Arabidopsis; instead of leading to a down-regulation of the AtSUC2 gene, which encodes the Suc transporter probably responsible for phloem loading in source tissues (Truernit and Sauer, 1995; Stadler et al., 1999), trehalose even slightly induced the expression of AtSUC2 (Fig. 5). It is also unlikely that trehalose impairs phloem transport by inhibiting the activity of the Suc transporter. A decreased phloem transport would have resulted in the accumulation of soluble sugars (Kühn et al., 1999) that was, however, not the case (Fig. 3). The accumulation of starch was, therefore, probably not due to an interference of trehalose with the export of Suc to the roots.
Trehalose Induces Starch Biosynthesis
The accumulation of starch during growth on trehalose (Figs. 3 and 4) was paralleled by an increase in the activity of ADP-Glc-PPase (Fig. 6), the first enzyme in starch synthesis, and by an induction of ApL3 expression (Fig. 7). Thus trehalose appears to affect starch synthesis by directly inducing components of the starch biosynthetic pathway. In vivo, the activity of most plant ADP-Glc-PPases is regulated by activation by 3-phosphoglycerate and inhibition by Pi (Preiss, 1982). One might, therefore, argue that increased amounts of ADP-Glc-PPase protein, as reflected by higher in vitro activities, do not necessarily result in higher rates of starch synthesis. However, Neuhaus and Stitt (1990) have shown that the amount of ADP-Glc-PPase exerts appreciable control on starch formation in Arabidopsis leaves.
For Arabidopsis, one gene encoding a small subunit of ADP-Glc-PPase, ApS, and three genes encoding large subunits, ApL1, ApL2, and ApL3, are known (Villand et al., 1993). Mutant analysis has shown that, under normal conditions, ApL1 (= ADG2) is the large subunit protein that is mainly responsible for starch synthesis (Lin et al., 1988; Wang et al., 1997). However, in seedlings grown on trehalose, ApL3 appears to gain importance. A prerequisite for increased catalytic activity in the presence of trehalose is, of course, that the amount of the small subunit protein, ApS, is not limiting. It is not known in which cells the individual ADP-Glc-PPase large subunit proteins are expressed and how ADP-Glc-PPase complexes containing different large subunit proteins differ in their catalytic properties. Induction of genes encoding proteins of different localization or catalytic properties could be an important means of regulating starch synthesis in Arabidopsis.
A negative effect of excess ADP-Glc-PPase activity on plant development has also been reported by Stark et al. (1992). Similar to Arabidopsis plants grown in the presence of trehalose, transgenic potato plants expressing a bacterial ADP-Glc-PPase under control of the 35S promoter could only be maintained on Suc-containing medium. These results indicate that the inhibitory effect of trehalose on growth is a secondary effect caused by the increased ADP-Glc-PPase activity in the shoots and not a direct effect of trehalose on the roots. However, additional effects of trehalose on source metabolism, such as an inhibition of starch breakdown, an inhibition of the export of triose-phosphates out of the chloroplast, or an inhibition of Suc synthesis, cannot be ruled out.
Trehalose Modulates Sugar-Mediated Gene Expression
Suc and Glc have been shown to induce the expression of several ADP-Glc-PPase genes in plants (Müller-Röber et al., 1990; Krapp and Stitt, 1994; Krapp and Stitt, 1995; La Cognata et al., 1995; du Jardin et al., 1997). In Arabidopsis, the effect of Suc on the expression of the different ADP-Glc-PPase genes has been studied in detail (Sokolov et al., 1998). Feeding of 100 or 300 mm Suc to detached rosette leaves in the dark induced the expression of ApS, ApL2, and ApL3, whereas it decreased the expression of ApL1. Illumination had a similar effect, and feeding of Suc in the light further increased the expression of ApL3. In our experiments, in which we supplied a lower concentration of 25 mm Suc in the light, we only found a slight induction of ApL3 (Fig. 7). Compared with Suc, trehalose had a much stronger effect and not only induced the expression of ApL3 when supplied alone but also in the presence of 50 mm Suc even when its concentration was as low as 5 mm. This either shows that trehalose is a very potent activator of the Suc signaling pathway or that the pathways for Suc and trehalose signaling are, at least partially, different.
β-Amylase is also known to be induced by sugars (Caspar et al., 1989; Nakamura et al., 1991; Mita et al., 1995; Datta et al., 1999). Because of its extra-chloro-plastic localization, the role of β-amylase in plant metabolism is still obscure. Although Suc strongly induced the expression of the β-amylase gene, AT-β-AMY, we were unable to detect an induction by trehalose in our RT-PCR assays when trehalose was supplied alone. Trehalose, however, enhanced the effect of Suc on the expression of AT-β-AMY in a concentration-dependent manner. Given the absence of induction of AT-β-AMY expression by trehalose alone, it is possible that Suc and trehalose synergistically activate the expression of AT-β-AMY. Since trehalose alone inhibited the growth of the seedlings, it is also possible that the lack of an induction of AT-β-AMY was due to the altered morphology.
Glc contents increase strongly when Suc is fed to Arabidopsis leaves (Sokolov et al., 1998). Since Glc induces the expression of ApL3 (Sokolov et al., 1998) and of the β-amylase gene (Mita et al., 1995), the induction of these genes may not be due to Suc itself but mediated by Glc and involve hexokinase-dependent signaling. In contrast, the effects of trehalose are not caused by an accumulation of hexoses (Fig. 3) and are, therefore, probably not hexokinase-dependent.
We have shown that trehalose on its own is able to affect gene expression and that it also has the capacity to modulate sugar-regulated gene expression. Using trehalose as a tool for altering gene expression may, therefore, allow to gain a better understanding of sugar signaling pathways and their consequences for plant metabolism and development. In addition, elucidating the mechanisms of trehalose action may help to clarify the question whether or not pathogenic or symbiotic trehalose-producing microorganisms can make use of such mechanisms to affect the allocation of carbohydrates to their favor.
MATERIALS AND METHODS
Plant Material
Arabidopsis (WS-0) seeds were surface sterilized and germinated in vertically oriented Petri dishes on one-half-strength Murashige and Skoog medium solidified with purified agar (Oxoid, Basingstoke, Hampshire, UK) as previously described (Benfey et al., 1993). The plants were grown in a daily cycle of 18 h of light (80 μmol m−2 s−1) at 22°C and 6 h of darkness at 18°C. Ten days after germination, the root length was measured, and the shoots of the seedlings were harvested at midday for further analyses.
Analysis of Soluble Carbohydrates
Arabidopsis tissue was killed in hot (80°C) 80% (v/v) ethanol. After vacuum drying, soluble carbohydrates were extracted and derivatized as described in Müller et al. (1995b) and analyzed by capillary gas chromatography. The gas chromatograph (Carlo Erba Mega 3500, Brechbühler, Zurich) was equipped with a glass column (Capillary JW, 30 m × 0.323 mm, coated with DB-17, Brechbühler) and with a flame ionization detector (340°C). After the injection, the column was kept at 70°C for 2 min, then heated progressively with a rate of 25°C per min until 170°C was reached, and followed by a rate of 7°C per min to 300°C and 5 min at 300°C.
Quantification of Starch and Starch Staining
Starch was extracted from the insoluble pellets remaining from the extraction of soluble carbohydrates by incubation in 0.2 mL of 0.5 m NaOH at 60°C for 60 min. After addition of 0.2 mL of 0.5 m HCl, starch was digested overnight at 37°C by addition of 0.6 mL of 0.2 m sodium acetate buffer (pH 4.5) containing 1 unit of amyloglucosidase (Boehringer Mannheim, Mannheim, Germany). The reaction was stopped by boiling for 2 min, and Glc in the extracts was determined by HPLC (Dionex, Olten, Switzerland) on a PA-1 column and with pulsed-amperometric detection. For analysis of the distribution of starch, whole seedlings were taken at midday, destained in 95% (v/v) ethanol, stained in 43.4 mm KI/5.7 mm I2 and washed in water.
Western Analysis
Proteins were extracted by homogenizing the tissue in 200 mm Bicine (N-N′-bis[2-hydroxyethyl]glycine)-KOH, pH 9.0, 25 mm dithiothreitol and 1% (w/v) SDS. The extracts were boiled with equal volumes of solubilization buffer (62.5 mm Tris [tris(hydroxymethyl)aminomethane]-HCl, pH 6.8, 20% [v/v] glycerol, 2.5% [w/v] SDS, and 5% [v/v] 2-mercaptoethanol). After separation by SDS-PAGE on 10% (w/v) gels, the proteins were transferred onto PVDF membranes (Immobilon P, Millipore, Bedford, MA) and probed with antisera raised against Rubisco (provided by R.C. Leegood, University of Sheffield, UK), NADH-dependent hydroxypyruvate reductase (provided by P.J. Lea, Lancaster University, UK), P-protein of the Gly decarboxylase complex (provided by J. Lorang, Oregon State University, Corvallis), and Gln synthetase (provided by G. Ochs, Universität Mainz, Germany). A peroxidase-conjugated secondary antibody was used, and immuno-reactive bands were visualized with an enhanced chemiluminescence kit (Amersham Pharmacia, Little Chalfont, UK).
RT-PCR
Total RNA was extracted from shoots of 10-d-old Arabidopsis seedlings using the Rneasy kit (Qiagen, Basel). The RNA was reverse-transcribed using a reverse-transcription kit (Boehringer Mannheim) with both a random as well as an oligo(dT) primer in the reaction. PCR amplification was done using pairs of one forward and one reverse primer on this first strand cDNA. One microliter of the cDNA preparations was used per PCR reaction in a total volume of 30 μL. The cycle number was adapted for each gene analyzed to be able to compare expression levels at non-saturating conditions. For ApL1 (accession no. X73367), ApL2 (accession no. X73366), as well as ApL3 (X73364), 26 cycles were performed. For ApS1 (accession no. X73365) and Act2 (accession no. U41998) 22 cycles were sufficient. AT-β-AMY (accession no. S77076) was amplified with 30 cycles. The primers for the ADP-Glc-PPase genes map to regions where the four genes differ strongly, especially at their 3′ end. Diagnostic fragments, which, in addition, differed in size were, therefore, obtained for each ADP-Glc-PPase gene. Primers used for the amplification were designed to have similar annealing temperatures and to span at least one intron to be able to distinguish the amplified cDNA from any potential genomic DNA contaminants. Genes tested, primers used, and cDNA fragment sizes were the following: ADP-Glc-PPase ApS, primer o300 5′-GATGTAATGCTAGACTTACTAC-3′ and primer o301 5′-GTCAGTAACATCAGCATCAAG-3′ (278 bp); ADP-Glc-PPase ApL1, o302 5′-TCTATGTGAATGCTTATCTCTC-3′ and o303 5′-CTATGCTCAATCAAGCAGTTGG-3′ (237 bp); ADP-Glc-PPase ApL2, o304 5′-TTCTAAGGTCAAGTTATC- CTAC-3′ and o305 5′-TCCTGAAGCTCTACTCCAGAC-3′ (351 bp); ADP-Glc-PPase ApL3, o306 5′-ATGTTCAAGGATA- CATCTACAG-3′ and o307 5′-CTGAAGCTCAACACC- ATAGTCA-3′ (285 bp); actin ACT2, o176 5′-GGAAGGATCT- GTACGGTAAC-3′ and o177 5′-TGTGAACGATTCCTGGA- CCT-3′ (247 bp); β-amylase primer o168 5′-GAGTATCTC- TCAATCGGTGTTG-3′ and o169 5′-CTTTGGCTCCATAG- GTCTCT-3′ (830 bp). Amplifications were done under the following conditions: initial denaturation at 94°C for 2 min followed by the indicated number of cycles with the following steps: 45-s denaturation at 94°C, 45-s annealing at 50°C, and extension for 1 min 30 s at 72°C. A final extension for 8 min at 72°C was performed. Taq polymerase I (Pharmacia, Dübendorf, Switzerland) was used. Aliquots of each PCR reaction were analyzed by agarose gel electrophoresis using ethidium bromide to visualize amplified products under UV light. Single bands of the expected sizes were obtained.
RNA-Blot Hybridization
An 868-bp fragment from AtSUC2 (accession no. X75382) from Arabidopsis was amplified using the primer o279 5′-CGCTTCTCCTCATAGTCACTT-3′ and primer o277 5′-GAAAGAGAGCCAAACAACCAC-3′ from the plasmid pTF2035, encoding the SUC2 protein (Sauer and Stolz, 1994). Thirty-five cycles were performed using the PCR profile described above. One nanogram of this fragment was re-amplified using the same conditions for 20 cycles in the presence of 125 μm alkali labile Dig-11dUTP (Boehringer Mannheim). An aliquot of this fragment was run on an agarose gel and showed the typical increase in size indicative of incorporation of Dig-11dUTP. One-half of this fragment (approximately 200 ng) was denatured and used as a probe for the RNA-blot hybridization. Twenty micrograms of total RNA per sample was denatured by glyoxal and separated by electrophoresis on a 1.3% (w/v) agarose gel using the sodium-phosphate buffer system as described (Ausubel et al., 1992). A separate lane was cut off from the gel and stained with ethidium bromide to visualize the ribosomal RNA bands. The remainder of the gel was transferred to nylon membranes. To ensure loading of equal amounts of the RNA, 5 μg of each RNA sample was loaded on a separate gel that was electrophoresed in the presence of ethidium bromide. Hybridization and washings were done under high stringency conditions (45°C hybridization temperature; washings in 0.1× SSC, 0.2% [v/v] SDS at 72°C). Hybridization was done in DigEasyhyb buffer (Boehringer Mannheim). Detection of the signal for the digoxigenin probe was performed according to the instructions of the supplier.
Determination of ADP-Glc-PPase Activity
ADP-Glc-PPase was extracted as described by Neuhaus and Stitt (1990), but with addition of 10 mg mL−1 insoluble polyvinylpyrrolidone (Polyclar AT) and assayed according to Sowokinos (1976). Protein concentrations in the extracts were determined with the protein assay (Bio-Rad Laboratories, Hercules, CA) according to Bradford (1976).
ACKNOWLEDGMENTS
We are grateful to P.J. Lea, R.C. Leegood, J. Lorang, and G. Ochs for providing antisera and to N. Sauer for providing the plasmid pTF2035. We would also like to thank A.D. Meyer for critical reading of the manuscript and for scanning the slides and J. Oetiker for stimulating discussions.
Footnotes
This work was supported by the Swiss National Science Foundation (grant no. 3100–042535.94 to A.W. and grant no. 3100–040837.94 to T.B.).
LITERATURE CITED
- Adams RP, Kendall E, Kartha KK. Comparison of free sugars in growing and desiccated plants of Selaginella lepidophylla. Biochem Syst Ecol. 1990;18:107–110. [Google Scholar]
- Aeschbacher RA, Müller J, Boller T, Wiemken A. Purification of the trehalase GMTRE1 from soybean nodules and cloning of its cDNA: GMTRE1 is expressed at a low level in multiple tissues. Plant Physiol. 1999;119:489–495. doi: 10.1104/pp.119.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aeschbacher RA, Wingler A, Fritzius T, Brodmann D, Boller T, Wiemken A. Trehalose metabolism affects development of Arabidopsis by regulating the sugar sensing mechanism. 9th Swiss Plant Cell and Molecular Biology Conference March 8–10, 2000, Villar sur Ollon, Switzerland. 2000. pp. 51–53. [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Short Protocols in Molecular Biology. New York: Greene Publishing Associates/John Wiley & Sons; 1992. [Google Scholar]
- Benfey PN, Linstead PJ, Roberts K, Schiefelbein JW, Hauser MT, Aeschbacher RA. Root development in Arabidopsis: four mutants with dramatically altered root morphogenesis. Development. 1993;119:57–70. doi: 10.1242/dev.119.Supplement.57. [DOI] [PubMed] [Google Scholar]
- Bianchi G, Gamba A, Limiroli R, Pozzi N, Elster R, Salamini F, Bartels D. The unusual sugar composition in leaves of the resurrection plant Myrothamnus flabellifolia. Physiol Plant. 1993;87:223–226. [Google Scholar]
- Blázquez MA, Santos E, Flores C-L, Martínez-Zapater JM, Salinas J, Gancedo C. Isolation and molecular characterization of the Arabidopsis TPS1 gene, encoding trehalose-6-phosphate synthase. Plant J. 1998;13:685–689. doi: 10.1046/j.1365-313x.1998.00063.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caspar T, Lin T-P, Monroe J, Bernard W, Spilatro S, Preiss J, Sommerville C. Altered regulation of β-amylase activity in mutants of Arabidopsis with lesions in starch metabolism. Proc Natl Acad Sci USA. 1989;86:5830–5833. doi: 10.1073/pnas.86.15.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou T-J, Bush DR. Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA. 1998;95:4784–4788. doi: 10.1073/pnas.95.8.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai N, Schaffer A, Petreikov M, Shahak Y, Giller Y, Ratner K, Levine A, Granot D. Overexpression of Arabidopsis hexokinase in tomato plants inhibits growth, reduces photosynthesis, and induces rapid senescence. Plant Cell. 1999;11:1253–1266. doi: 10.1105/tpc.11.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta R, Vally KJM, Sharma R. Sugar mimics the light-mediated β-amylase induction and distribution in maize and pearl millet leaves. J Plant Physiol. 1999;154:665–672. [Google Scholar]
- Drennan PM, Smith MT, Goldsworthy D, van Staden J. The occurrence of trehalose in the leaves of the desiccation-tolerant angiosperm Myrothamnus flabellifolius Welw. J Plant Physiol. 1993;142:493–496. [Google Scholar]
- du Jardin P, Harvengt L, Kirsch F, Le V-Q, Nguyen-Quoc B, Yelle S. Sink-cell-specific activity of a potato ADP-glucose pyrophosphorylase B-subunit promoter in transgenic potato and tomato plants. Planta. 1997;203:133–139. [Google Scholar]
- Elbein AD. The metabolism of α,α-trehalose. Adv Carbohydr Chem. 1974;30:227–256. doi: 10.1016/s0065-2318(08)60266-8. [DOI] [PubMed] [Google Scholar]
- Goddijn O, Smeekens S. Sensing trehalose biosynthesis in plants. Plant J. 1998;14:143–146. doi: 10.1046/j.1365-313x.1998.00140.x. [DOI] [PubMed] [Google Scholar]
- Goddijn OJM, van Dun K. Trehalose metabolism in plants. Trends Plant Sci. 1999;4:315–319. doi: 10.1016/s1360-1385(99)01446-6. [DOI] [PubMed] [Google Scholar]
- Goddijn OJM, Verwoerd TC, Voogd E, Krutwagen RWHH, de Graaf PTHM, Poels J, van Dun K, Ponstein AS, Damm B, Pen J. Inhibition of trehalase activity enhances trehalose accumulation in transgenic plants. Plant Physiol. 1997;113:181–190. doi: 10.1104/pp.113.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holström K-O, Mäntylä E, Welin B, Mandal A, Palva ET, Tunnela OE, Londesborough J. Drought tolerance in tobacco. Science. 1996;397:683–684. [Google Scholar]
- Jang J-C, León P, Zhou L, Sheen J. Hexokinase as a sugar sensor in higher plants. Plant Cell. 1997;9:5–19. doi: 10.1105/tpc.9.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J-C, Sheen J. Sugar sensing in higher plants. Plant Cell. 1994;6:1665–1679. doi: 10.1105/tpc.6.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Krapp A, Stitt M. Influence of high carbohydrate content on the activity of plastidic and cytosolic isoenzyme pairs in photosynthetic tissues. Plant Cell Environ. 1994;17:861–866. [Google Scholar]
- Krapp A, Stitt M. An evaluation of direct and indirect mechanisms for the “sink-regulation” of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta. 1995;195:313–323. [Google Scholar]
- Kühn C, Barker L, Bürkle L, Frommer WB. Update on sucrose transport in higher plants. J Exp Bot. 1999;50:935–953. [Google Scholar]
- La Cognata U, Willmitzer L, Müller-Röber B. Molecular cloning and characterization of novel isoforms of potato ADP-glucose pyrophosphorylase. Mol Gen Genet. 1995;246:538–548. doi: 10.1007/BF00298960. [DOI] [PubMed] [Google Scholar]
- Lin T-P, Caspar T, Sommerville CR, Preiss J. A starch deficient mutant of Arabidopsis thaliana with low ADP glucose pyrophosphorylase activity lacks one of the two subunits of the enzyme. Plant Physiol. 1988;88:1175–1181. doi: 10.1104/pp.88.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita S, Suzuki-Fujii K, Nakamura K. Sugar-inducible expression of a gene for β-amylase in Arabidopsis thaliana. Plant Physiol. 1995;107:895–904. doi: 10.1104/pp.107.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Aeschbacher RA, Sprenger N, Boller T, Wiemken A. Disaccharide-mediated regulation of sucrose:fructan-6-fructosyltransferase, a key enzyme of fructan synthesis in barley leaves. Plant Physiol. 2000;123:265–273. doi: 10.1104/pp.123.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Boller T, Wiemken A. Trehalose and trehalase in plants: recent developments. Plant Sci. 1995a;112:1–9. [Google Scholar]
- Müller J, Boller T, Wiemken A. Effects of validamycin A, a potent trehalase inhibitor, and phytohormones on trehalose metabolism in roots and nodules of soybean and cowpea. Planta. 1995b;197:362–368. [Google Scholar]
- Müller J, Boller T, Wiemken A. Trehalose affects sucrose synthase and invertase activities in soybean (Glycine max [L.] Merr.) roots. J Plant Physiol. 1998;153:255–257. [Google Scholar]
- Müller J, Staehelin C, Mellor RB, Boller T, Wiemken A. Partial purification and characterization of trehalase from soybean nodules. J Plant Physiol. 1992;140:8–13. [Google Scholar]
- Müller J, Wiemken A, Aeschbacher RA. Trehalose metabolism in sugar sensing and plant development. Plant Sci. 1999;147:37–47. [Google Scholar]
- Müller-Röber BT, Koβmann J, Hannah LC, Willmitzer L, Sonnewald U. One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Mol Gen Genet. 1990;224:136–146. doi: 10.1007/BF00259460. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Ohto M, Yoshida N, Nakamura K. Sucrose-induced accumulation of β-amylase occurs concomitant with the accumulation of starch and sporamin in leaf-petiole cuttings of sweet potato. Plant Physiol. 1991;96:902–909. doi: 10.1104/pp.96.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus HE, Stitt M. Control analysis of photosynthate partitioning: impact of reduced activity of ADP-glucose pyrophosphorylase or plastid phosphoglucomutase on the fluxes to starch and sucrose in Arabidopsis thaliana (L.) Heynh. Planta. 1990;182:445–454. doi: 10.1007/BF02411398. [DOI] [PubMed] [Google Scholar]
- Pilon-Smits EAH, Terry N, Sears T, Kim H, Zayed A, Hwang S, van Dun K, Voogd E, Verwoerd TC, Krutwagen RWHH, Goddijn OJM. Trehalose-producing transgenic tobacco plants show improved growth performance under drought stress. J Plant Physiol. 1998;152:525–532. [Google Scholar]
- Preiss J. Regulation of the biosynthesis and degradation of starch. Annu Rev Plant Physiol. 1982;33:431–454. [Google Scholar]
- Romero C, Bellés JM, Vayá JL, Serrano R, Culiáñez-Macià FA. Expression of the yeast trehalose-6-phosphate synthase gene in transgenic tobacco plants: pleiotropic phenotypes include drought tolerance. Planta. 1997;201:293–297. doi: 10.1007/s004250050069. [DOI] [PubMed] [Google Scholar]
- Rook F, Gerrits N, Kortstee A, van Kampen M, Borrias M, Weisbeek P, Smeekens S. Sucrose-specific signalling represses translation of the Arabidopsis ATB2 bZIP transcription factor gene. Plant J. 1998;15:253–263. doi: 10.1046/j.1365-313x.1998.00205.x. [DOI] [PubMed] [Google Scholar]
- Sauer N, Stolz J. SUC1 and SUC2: two sucrose transporters from Arabidopsis thaliana. Expression and characterization in baker's yeast and identification of the histidine-tagged protein. Plant J. 1994;6:67–77. doi: 10.1046/j.1365-313x.1994.6010067.x. [DOI] [PubMed] [Google Scholar]
- Sokolov LN, Déjardin A, Kleczkowski LA. Sugars and light/dark exposure trigger differential regulation of ADP-glucose pyrophosphorylase genes in Arabidopsis thaliana (thale cress) Biochem J. 1998;336:681–687. doi: 10.1042/bj3360681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos JR. Pyrophosphorylase in Solanum tuberosum: I. Changes in ADP-glucose pyrophosphorylase activities associated with starch biosynthesis during tuberization, maturation, and storage of potatoes. Plant Physiol. 1976;57:63–68. doi: 10.1104/pp.57.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Truernit E, Gahrtz M, Sauer N. The AtSUC1 sucrose carrier may represent the osmotic driving force for anther dehiscence and pollen tube growth in Arabidopsis. Plant J. 1999;19:269–278. doi: 10.1046/j.1365-313x.1999.00527.x. [DOI] [PubMed] [Google Scholar]
- Stark DM, Timmermann KP, Barry GF, Preiss J, Kishore GM. Regulation of the amount of starch in plant tissues by ADP glucose pyrophosphorylase. Science. 1992;258:287–292. doi: 10.1126/science.258.5080.287. [DOI] [PubMed] [Google Scholar]
- Stitt M, von Schaewen A, Willmitzer L. “Sink” regulation of photosynthetic metabolism in transgenic tobacco plants expressing yeast invertase in their cell wall involves a decrease of the Calvin-cycle enzymes and an increase of glycolytic enzymes. Planta. 1990;183:40–50. doi: 10.1007/BF00197565. [DOI] [PubMed] [Google Scholar]
- Truernit E, Sauer N. The promoter of the Arabidopsis thaliana SUC2 sucrose-H+ symporter gene directs expression of β-glucuronidase to the phloem: evidence for phloem loading and unloading by SUC2. Planta. 1995;196:564–570. doi: 10.1007/BF00203657. [DOI] [PubMed] [Google Scholar]
- Villand P, Olsen O-A, Kleczkowski L-A. Molecular characterization of multiple cDNA clones for ADP-glucose pyrophosphorylase from Arabidopsis thaliana. Plant Mol Biol. 1993;23:1279–1284. doi: 10.1007/BF00042361. [DOI] [PubMed] [Google Scholar]
- Vogel G, Aeschbacher RA, Müller J, Boller T, Wiemken A. Trehalose-6-phosphate phosphatases from Arabidopsis thaliana: identification by functional complementation of the yeast tps2 mutant. Plant J. 1998;13:673–683. doi: 10.1046/j.1365-313x.1998.00064.x. [DOI] [PubMed] [Google Scholar]
- Wagner W, Wiemken A, Matile P. Regulation of fructan metabolism in leaves of barley (Hordeum vulgare L. cv Gerbel) Plant Physiol. 1986;81:444–447. doi: 10.1104/pp.81.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-M, Chu B, Lue W-L, Yu T-S, Eimert K, Chen J. adg2-1 represents a missense mutation in the ADPG pyrophosphorylase large subunit gene of Arabidopsis thaliana. Plant J. 1997;11:1121–1126. doi: 10.1046/j.1365-313x.1997.11051121.x. [DOI] [PubMed] [Google Scholar]
- Wingler A, von Schaewen A, Leegood RC, Lea PJ, Quick WP. Regulation of leaf senescence by cytokinin, sugars, and light: effects on NADH-dependent hydroxypyruvate reductase. Plant Physiol. 1998;116:329–335. [Google Scholar]
- Yokoyama R, Hirose T, Fujii N, Aspuria ET, Kato A, Uchimyia H. The rolC promoter of Agrobacterium rhizogenes Ri plasmid is activated by sucrose in transgenic tobacco plants. Mol Gen Genet. 1994;244:15–22. doi: 10.1007/BF00280182. [DOI] [PubMed] [Google Scholar]
- Zentella R, Mascorro-Gallardo JO, Van Dijck P, Folch-Mallol J, Bonini B, Van Vaeck C, Gaxiola R, Covarrubias AA, Nieto-Sotelo J, Thevelein JM, Iturriaga G. A Selaginella lepidophylla trehalose-6-phosphate synthase complements growth and stress-tolerance defects in a yeast tps1 mutant. Plant Physiol. 1999;119:1473–1482. doi: 10.1104/pp.119.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]