Summary
Objectives
Summary evidence of influenza vaccine effectiveness (IVE) against hospitalized influenza is lacking. We conducted a meta-analysis of studies reporting IVE against laboratory-confirmed hospitalized influenza among adults.
Methods
We searched Pubmed (January 2009 to November 2016) for studies that used test-negative design (TND) to enrol patients hospitalized with influenza-associated conditions. Two independent authors selected relevant articles. We calculated pooled IVE against any and (sub)type specific influenza among all adults, and stratified by age group (18–64 and 65 years and above) using random-effects models.
Results
We identified 3411 publications and 30 met our inclusion criteria. Between 2010–11 and 2014–15, the pooled seasonal IVE was 41% (95%CI:34;48) for any influenza (51% (95%CI:44;58) among people aged 18–64y and 37% (95%CI:30;44) among ≥65 years). IVE was 48% (95%CI:37;59), 37% (95%CI:24;50) and 38% (95%CI:23;53) against influenza A(H1N1)pdm09, A(H3N2) and B, respectively. Among persons aged ≥65 year, IVE against A(H3N2) was 43% (95%CI:33;53) in seasons when circulating and vaccine strains were antigenically similar and 14% (95%CI: −3;30) when A(H3N2) variant viruses predominated.
Conclusions
Influenza vaccines provided moderate protection against influenza-associated hospitalizations among adults. They seemed to provide low protection among elderly in seasons where vaccine and circulating A(H3N2) strains were antigenically variant.
Keywords: Influenza, Vaccine effectiveness, Hospitalization, Adults, Systematic review, Meta-analysis
Background
Each year, seasonal influenza epidemics affect 20–30% of children and 5–10% of adults globally1 and that they cause three to five million severe (hospitalized) cases and 250,000 to 500,000 deaths worldwide.2 Pulmonary complications, as a direct consequence of influenza infection, after secondary bacterial infection or through the exacerbation of chronic conditions,3 and neuromuscular or cardiac complications4 may cause severe forms of influenza. Consequently, individuals at risk of developing severe influenza are those whose immune system is likely to sub-optimally respond to viral or secondary bacterial infection5 and patients who may suffer from an exacerbation of these conditions due to influenza infection.6,7 The mean annual incidence of influenza related hospitalizations among persons 65 years and older typically ranges between 136 and 309 episodes per 100,000 persons in the United States, and England8–11 and the case fatality among hospitalized patients is estimated to be 7%.12
Vaccination is the primary means of preventing influenza illnesses and reducing their burden. The World Health Organization (WHO) recommends annual vaccination to individuals at increased risk of severe influenza illness, including adults with chronic medical conditions and persons 65 years and older.1 Most middle and high income countries provide vaccination through routine immunization programs targeting these groups.13,14 While a goal of reaching 75% vaccination coverage among persons 65 years and older by 2010 was set during the 2003 World Health Assembly,15 few regions have reached this target. In Europe, vaccine uptake was below 50% in this group in 2014.16 Vaccine delivery in developed countries currently faces various challenges, including a decrease in populations’ trust in vaccine effectiveness.17,18
As recommendations to annually vaccinate high risk groups have been adopted internationally, conducting clinical trials to determine vaccine efficacy has become impossible for ethical reasons. To monitor the IVE, post-marketing (Phase IV) studies have been conducted using observational data. Such studies have historically built on existing outpatient-based sentinel surveillance networks, with a focus on the prevention of medically attended influenza like illnesses (ILI). More recently, a growing number of health authorities and research teams have set up hospital-based studies to measure IVE in preventing hospitalized influenza-associated outcomes.19–21 First developed to measure IVE against medically attended outcomes,22 the test-negative design (TND)23,24 has become increasingly popular for use in hospital based studies. In this approach, investigators enroll patients based on clinical criteria and measure the IVE derived from the relative difference between the odds of vaccination among patients testing positive and those testing negative for influenza viruses. Because influenza-associated hospitalization is a rare outcome, these studies have mostly reported IVE estimates with broad confidence intervals and limited conclusive evidence about the effectiveness of vaccines against influenza-associated hospitalization. Providing robust evidence of influenza vaccine effectiveness (IVE) in preventing severe influenza illness is important to inform current vaccination strategies. While there have been published reports of meta-analyses of studies reporting IVE against medically attended influenza25,26 or against hospitalized outcomes in high risk groups,27 there is a gap regarding meta-analyses of IVE focusing on severe outcomes associated with influenza viruses among adults. To provide precise estimates of IVE against laboratory-confirmed influenza-associated hospitalizations, we reviewed published results and summarized IVE estimates by adult age groups (18–64 years, ≥ 65 years of age), influenza subtype/lineage and influenza season.
Methods
We conducted a systematic review and meta-analysis of extracted IVE estimates.
Search strategy and selection criteria
Two review authors (M.R. & N.E.) used the following search terms on Pubmed: (“influenza” OR “flu”) AND (“vaccine” OR “vaccinat*”) AND (“hospital” OR “hospitali*” OR “patient” OR “inpatient”). They independently extracted, selected and reviewed articles.
A preliminary review of the literature showed very scarce data prior to 2009. To enable the computation of season-specific IVE meta-estimates, we restricted the search to studies measuring IVE from 2009 onwards. Studies published in English, French, Spanish or Portuguese were considered. The review was initially conducted on 02/06/2016 and was updated on 11/11/2016. The references of retrieved articles were also screened. Titles identified through the initial search were screened independently by two review authors (M.R. & N.E.). Abstracts of title based selected articles were reviewed and the full text of those considered relevant were retrieved and reviewed. Pandemic monovalent, and seasonal trivalent and quadrivalent influenza virus vaccines were considered.
In this meta-analysis, we included original analyses of IVE against hospitalized laboratory confirmed influenza among adults. After applying these criteria and classifying studies by study design, we observed that most published studies (39/50) used a TND approach. In order to reduce qualitative heterogeneity among studies included in this meta-analysis, we restricted studies to those using a TND. We included studies with any method of vaccination status ascertainment and used any laboratory techniques for case confirmation, including rapid diagnostic tests. We did not assess the risk of bias of the included studies since no risk-of-bias tools are suitable to TND studies.
Exclusion
We excluded duplicate reports, studies reporting secondary analyses of previously-published IVE data and interim reports superseded by a final report. We also excluded reports where IVE estimates were calculated using all ages (children and adults), unless their authors could provide us with adult-specific results. We excluded site-specific estimates for studies included in multicenter projects. We reported only season-specific IVE and excluded multiple-season pooled estimates. To ensure comparability between results, and due to the very limited number of TND studies providing such estimates, we excluded studies restricted to intensive care unit (ICU) admissions associated influenza.
We excluded estimates reporting IVE for the 2009–10 seasonal influenza vaccines containing the A/Brisbane/59/2007-like seasonal A(H1N1) virus against A(H1N1)pdm09 (A/California/7/2009-like viruses), because the seasonal influenza vaccine was not expected to provide protection against the pandemic virus.
Data collection
We used a structured electronic collection tool to screen and extract quantitative data from the studies reviewed and used a semi-formatted form to compile qualitative information. For each article, one review author extracted the information and another one checked the extracted data. Disagreements between the two authors were solved through discussion. We collected information about the country, influenza season, study population, age group, vaccine type, laboratory test used, data sources, clinical criteria to include patients in the study and maximum number of days between onset and specimen collection. For each age group and outcome [any influenza, A(H1N1)pdm09, A(H3N2) and B], we collected IVE estimates, their 95% confidence interval (95%CI) and the list of covariates used in the multivariable analysis. Similar to a previous review,25 for each study reporting IVE against A(H3N2), we retrieved the authors’ conclusion about the antigenic similarity between vaccine and circulating strains. When no conclusion was provided by the authors, we looked at the WHO recommendation for compositions of the influenza vaccine; if the A(H3N2) component was updated in the following season, we assumed that the vaccine component and circulating strains during the prior season were not antigenically optimally similar and we categorized them as “variant” in this review.
Data analyses
We defined IVE as 100% × (1 –ratio of odds of vaccination among influenza cases versus that among test-negative controls). We assessed heterogeneity among studies using the χ2-based Q test (Cochran’s Q) and I2 statistic28 and we pooled study specific data to calculate summary estimates. We computed meta-estimates using random-effect models, assuming IVE would not be fixed across study sites and seasons because of different levels of antigenic match between vaccine components and circulating strains. We used inverse variances that incorporated an estimate of the between-study variance to calculate the weights for the model.28,29 We computed pooled pandemic IVE for all adult ages against monovalent A(H1N1)pdm09 vaccines in 2009–10. We computed summary seasonal IVE by age group (all ages ≥ 18 years, 18–64 years and ≥ 65 years) against any influenza viruses, and separately for influenza A(H1N1)pdm09, A(H3N2) and B viruses, pooling estimates of the 2010–11 and subsequent seasons. We computed season specific summary estimates for all adult ages against any type of influenza virus, grouping each southern hemisphere season with the following northern hemisphere season. We calculated summary estimates of IVE against A(H3N2) by adult age group and antigenic similarity.
In sensitivity analyses, we computed summary estimates by age group and (sub)type of influenza viruses restricting our data to studies using a clearly stated set of clinical criteria [e.g., ILI or severe acute respiratory infection (SARI)] to enroll patients, and to studies using exclusively RT-PCR for laboratory testing.
When authors did not report age group specific IVE (18–64 years, ≥ 65 years) but did provide IVE estimates for smaller breakdowns of these age groups (for example 18–49 years and 50–64 years), we computed a study specific age group IVE meta-estimates and their 95%CI using fixed effects models.
We assessed the possibility of publication bias by plotting the log of studies’ variability (standard error of the OR) against the log of the size of the reported effect (OR).30 The symmetry of the resulting “funnel plots” was assessed both visually, and formally with the Egger’s test.31 We did all analyses with STATA version 14.2.
Role of the funding source
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
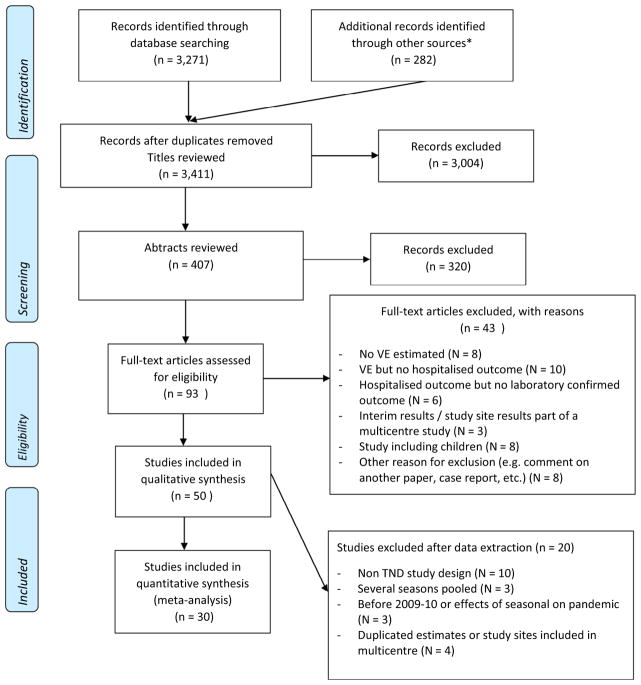
We identified 3411 unduplicated publications, of which we selected 407 for abstract review and further selected 93 for full-text review. We extracted data from 50 articles and included 30 of them in our IVE meta-analysis21,32–60 (Fig. 1, Table S1, Table S2). Nineteen studies were conducted in the Northern hemisphere and included studies covering seasons 2009–10 through 2015–16 (Table 1). In 22/30 articles, a clear set of clinical criteria was used to select patients to swab. In the remaining eight articles, the selection of patients to swab was left to the discretion of the clinician. A maximum allowed number of days between onset of clinical illness and swabbing to enroll patients was mentioned in 21/30 reports. All 27 studies reporting seasonal IVE presented estimates adjusted for age and presence of comorbidities and 13/27 further adjusted for calendar time. The three studies reporting pandemic IVE adjusted for calendar time and 2/3 further adjusted for age; none of them adjusted for comorbidities (Table S1).
Figure 1.
Flow chart for selection of studies.
* References of retrieved articles
Table 1.
Characteristics of the 30 studies included in this review reporting influenza vaccine effectiveness estimates against laboratory confirmed hospitalized influenza, 2008–2016a.
| Characteristics of selected published studies | N | |
|---|---|---|
| Number of unique studies | 30 | |
| Hemisphere | North | 19 |
| South | 11 | |
| By country income (World bank classification)b | Upper-middle-income economies | 2 |
| High income economies | 28 | |
| Continent | Europe | 11 |
| North America | 6 | |
| Oceania | 10 | |
| Asia | 3 | |
| Influenza season | 2009/10 | 3 |
| 2010/11 | 6 | |
| 2011/12 | 4 | |
| 2012/13 | 3 | |
| 2013/14 | 4 | |
| 2014/15 | 9 | |
| 2015/16 | 1 | |
| Vaccine type | Seasonal trivalent vaccine | 27 |
| Pandemic monovalent | 3 |
Southern hemisphere seasons were grouped with the following northern hemisphere season.
Overall, we compiled 116 IVE estimates, including 59 estimates against any influenza, 18 against influenza A(H1N1)pdm09, 28 against A(H3N2) and 11 against B viruses (Table S3).
Estimates against any type of influenza
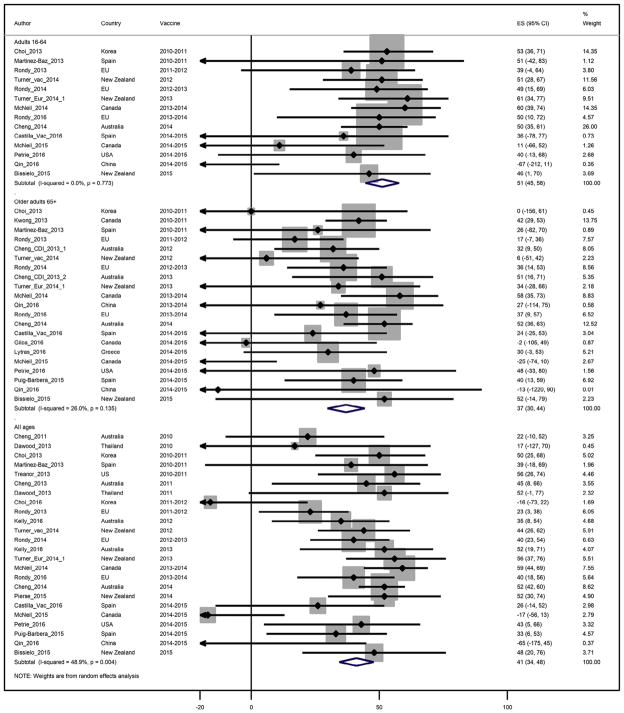
Twenty-four studies through six seasons reported seasonal IVE estimates against any type of influenza virus among adults of all ages, with IVE point estimates ranging from −65% to 59% (Fig. 2). Heterogeneity was moderate at I2 = 48%, and the pooled IVE estimate for all ages was 41% (95%CI: 34;48).
Figure 2.
Study specific and pooled seasonal influenza vaccine effectiveness against any influenza by age group.
For adults younger than 65 years of age, IVE point estimates ranged from −67% to 61%, I2 was 0%, and the pooled IVE estimate was 51% (95%CI: 44;58). For adults aged ≥65 years, IVE ranged from −25% to 58%, I2 was 26% and the pooled IVE estimate was statistically lower at 37% (95%CI: 30;44) (Table 2).
Table 2.
Pooled seasonal vaccine effectiveness (VE) against influenza hospitalizations by type and subtype of influenza virus and by age group.
| Pooled VE (%) | 95%CI | Number of VE estimates | p-value for heterogeneity | I2 | |
|---|---|---|---|---|---|
| Any influenza | |||||
| All adults | 41 | 34;48 | 24 | 0,005 | 48 |
| Under 65 years | 51 | 44;58 | 14 | 0,762 | 0 |
| 65 years and above | 37 | 30;44 | 21 | 0,137 | 26 |
| A(H1N1)pdm09 | |||||
| All adults | 48 | 37;59 | 7 | 0,212 | 28 |
| Under 65 years | 55 | 34;76 | 3 | 0,948 | 0 |
| 65 years and above | 54 | 26;82 | 5 | 0,026 | 64 |
| A(H3N2) | |||||
| All adults | 37 | 24;50 | 9 | 0,021 | 56 |
| Under 65 years | 50 | 38;62 | 7 | 0,775 | 0 |
| 65 years and above | 33 | 21;45 | 11 | 0,137 | 33 |
| B | |||||
| All adults | 38 | 23;53 | 5 | 0,640 | 0 |
| Under 65 years | 45 | 8;81 | 2 | 0,907 | 0 |
| 65 years and above | 31 | 11;51 | 4 | 0,812 | 0 |
Pooled season-specific seasonal IVE estimates against any influenza viruses in all adults ranged between 31% in 2011–12 and 2014–15 and 53% in 2013–14. Summary monovalent pandemic IVE against influenza A(H1N1)pdm09 hospitalization in 2009–10 was 72% (95%CI: 22;100) (Table 3).
Table 3.
Pooled vaccine effectiveness (VE) against influenza A(H3N2) hospitalizations among all adults by antigenic similarity between circulating and vaccine strains.
| Age group | Pooled VEa (%) | 95%CI | Number of VE estimates | p-value for heterogeneity | I2 | |
|---|---|---|---|---|---|---|
| Similar | All | 52 | 39;66 | 3 | 0,387 | 0 |
| 16–64 years | 59 | 38;80 | 2 | 0,332 | 0 | |
| 65 years and above | 43 | 33;53 | 5 | 0,829 | 0 | |
| Variant | All | 29 | 13;44 | 6 | 0,082 | 49 |
| 16–64 years | 46 | 30;61 | 5 | 0,857 | 0 | |
| 65 years and above | 14 | −3;30 | 6 | 0,486 | 0 |
And 95% confidence interval in parentheses.
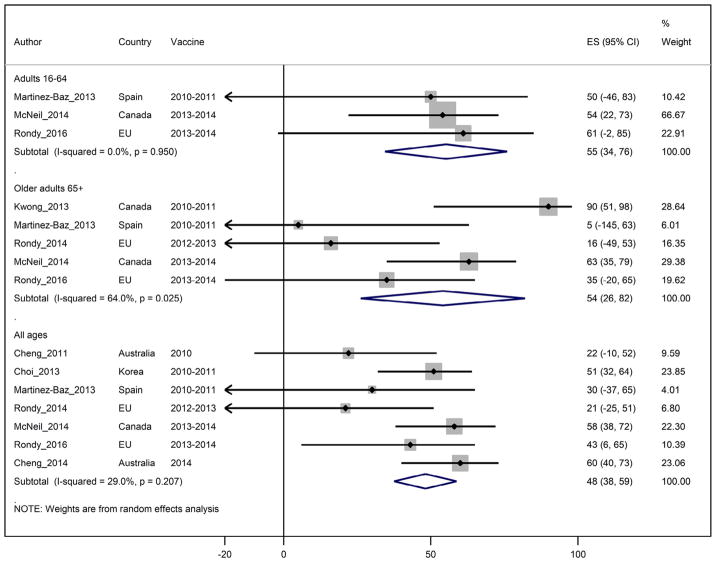
Seasonal vaccine effectiveness against influenza A(H1N1)pdm09 viruses
Seven TND studies through four seasons reported seasonal IVE against hospitalized A(H1N1)pdm09 among adults of all ages. The pooled IVE estimate was 48% (95%CI: 37;59) (Fig. 3). Heterogeneity was low at I2 = 28%. For adults <65 years of age, the summary IVE against influenza A(H1N1)pdm09 viruses was 55% (95%CI: 34;76) with I2 = 0%. For adults ≥ 65 years of age, summary IVE was 54% (95%CI: 26;82) with I2 = 64% (Table 2).
Figure 3.
Study specific and pooled seasonal influenza vaccine effectiveness against influenza A(H1N1)pdm09 by age group.
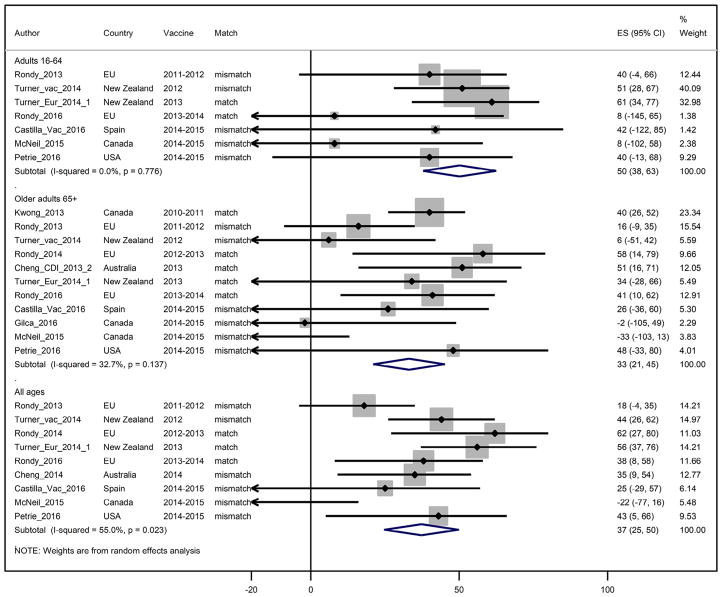
Seasonal vaccine effectiveness against influenza A(H3N2) viruses
Based on nine reported estimates through four seasons, the pooled IVE against A(H3N2) influenza viruses among adults of all ages was 37% (95%CI: 24;50) (Fig. 4). Heterogeneity was moderate at I2 = 56%. For adults <65 years of age, the summary IVE against influenza A(H3N2) viruses was 50% (95%CI: 38;62) with low heterogeneity (I2 = 0%) and for persons 65 years and older, summary IVE was 33% (95%CI: 21;45) with low heterogeneity between estimates (I2 = 33%) (Table 2).
Figure 4.
Study specific and pooled seasonal influenza vaccine effectiveness against influenza A(H3N2) by age group.
Information regarding antigenic similarity between vaccine and circulating strains was mentioned in all studies reporting IVE against A(H3N2) except one,46 for which we assumed similarity based on the fact that there had been no change in the A(H3N2) vaccine component in the subsequent season. When restricting to seasons with antigenically similar vaccine and circulating strains, pooled IVE against A(H3N2) was 52% (95%CI: 39;66) among all adults, 59% (95%CI: 38;80) among those aged <65 years and 43% (95%CI: 33; 53) among persons 65 years and older (Table 4). In seasons with reported A(H3N2) variant viruses, pooled IVE against A(H3N2) was 29% (95%CI: 13;44), 46% (95%CI: 30;61) and 14% (95%CI: −3;30) among all age adults, adults <65 years and persons 65 years and older. Of note, the pooled IVE among persons 65 years and older of 43% against A(H3N2) during seasons with similar vaccine and circulating strains was statistically higher than the IVE of 14% during seasons with variant A(H3N2) viruses (with 95% CI that did not overlap).
Table 4.
Pooled vaccine effectiveness (VE) against influenza hospitalizations among adults by season.
| Vaccine type | Pooled VEa (%) | 95%CI | Number of VE estimates | p-value for heterogeneity | |
|---|---|---|---|---|---|
| Any influenza | |||||
| 2009–10 | pandemic | 72 | 22;100 | 3 | 0,286 |
| 2010–11 | seasonal | 43 | 34;52 | 6 | 0,613 |
| 2011–12 | seasonal | 31 | 12;49 | 5 | 0,143 |
| 2012–13 | seasonal | 39 | 29;48 | 4 | 0,824 |
| 2013–14 | seasonal | 53 | 45;61 | 6 | 0,704 |
| 2014–15 | seasonal | 31 | 15;47 | 9 | 0,003 |
And 95% confidence interval in parentheses.
Seasonal vaccine effectiveness against influenza B viruses
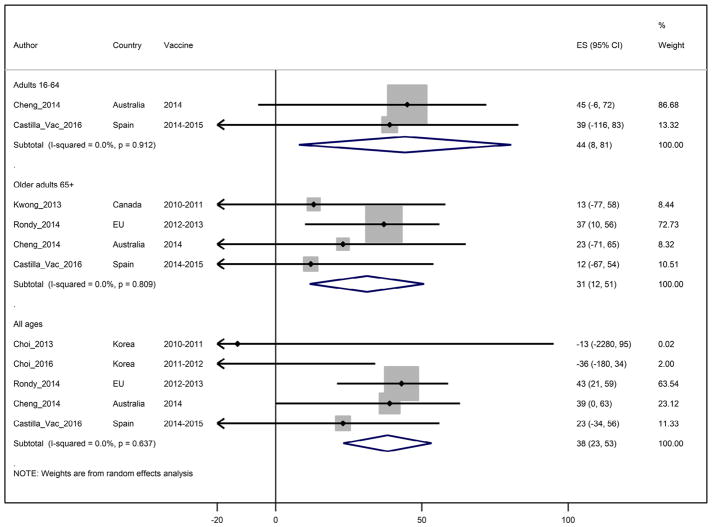
Based on five reported estimates through four seasons, with I2 = 0% heterogeneity, the pooled IVE estimate against influenza B viruses among adults of all ages was 38% (95%CI: 23;53) (Fig. 5). For adults aged <65 years, the summary IVE against influenza B was 45% (95%CI: 8;81; I2 = 0%) and for persons 65 years and older, summary IVE was 31% (95%CI: 11;51; I2 = 0%) (Table 2).
Figure 5.
Study specific and pooled seasonal influenza vaccine effectiveness against influenza B by age group.
Sensitivity analysis
Sensitivity analyses, whereby we excluded data from studies not using clear clinical criteria for patients’ inclusion or those not exclusively using RT-PCR for laboratory testing, resulted in similar summary estimates (Table S4, Table S5). Of note, the gap in IVE against any influenza hospitalization between adults aged <65 years (52%, 95%CI: 44; 59) and adults aged ≥65 years was wider (32%, 95%CI: 21;43) when limited to studies using clear clinical criteria.
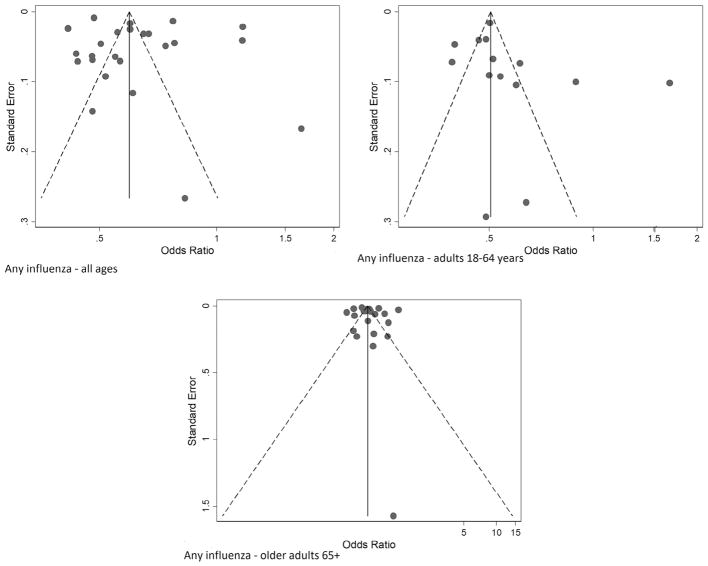
Publication bias assessment
The funnel plots for IVE against any influenza were symmetrical around a single peak (Fig. 6). There was no statistically significant difference between the results in small and large studies (Egger’s test, p = 0.475, p = 0.252 and p = 0.606 among adults of all ages, 18–64 years and 65 years and older respectively). Similar results were obtained for (sub)types specific estimates (data not shown).
Figure 6.
Funnel plots of effect size of individual studies included in the meta-analysis of influenza vaccine effectiveness against any influenza among adults all ages, 18–64 years and 65 years and older. Points correspond to OR from individual studies, diagonal lines show the expected 95% confidence intervals around the summary estimate. Odds ratios are plotted on a logarithmic scale.
Discussion
Our meta-analysis estimated at 41% (95%CI: 34;48) the overall seasonal IVE against hospitalizations associated with laboratory confirmed influenza virus infections among adults, with (sub)type IVE of 48% (95%CI :37;59) against influenza A(H1N1)pdm09, 37% (95%CI: 24;50) against influenza A(H3N2) and 38% (95%CI: 23;53) against influenza B viruses. Monovalent pandemic vaccine yielded to the highest pooled IVE at 72% (95%CI: 22;100). Our results suggested that IVE was significantly higher among adults aged less than 65 years compared to those aged 65 years or older (51% vs. 37%, respectively). In seasons with antigenic dissimilarity between A(H3N2) vaccine and circulating strains, IVE against hospitalized influenza A(H3N2) was close to null among elderly at 14% (95%CI: −3;30).
Our estimates were in line with the recently published meta-estimates of IVE against medically attended influenza illnesses.25 Compared to influenza illnesses in outpatient settings, we found slightly lower IVE estimates against influenza A(H1N1)pdm09 and B virus hospitalizations. In contrast, our IVE point estimates against A(H3N2) virus hospitalizations were a few percentage points higher than the findings from outpatient settings.25 These comparisons are also in line with a recent meta-analysis comparing outpatient and inpatient based IVE estimates within the same season and population, which concluded that IVE for outpatient and inpatient influenza were consistent most of the time.61
Although prior reviews have noted lower influenza vaccine immunogenicity among older adults62 and lower IVE point estimates among persons 65 years and older compared to adults aged <65 year,25 this is the first review to document with sufficient precision that IVE against influenza hospitalization is significantly lower for the elderly. This gap in vaccine protection was especially notable against A(H3N2) hospitalizations.
Our results suggest that IVE against A(H3N2) was particularly low in seasons predominated by variant A(H3N2) viruses. Lower IVE point estimates during seasons predominated by variant A(H3N2) viruses were noted for all adults, but the difference was only statistically significant among persons 65 years and older (43% vs. 14% in antigenically similar vs. variant seasons). The reasons why a poorly matched A(H3N2) vaccine component would provide less protection to older adults is unclear, but may include a narrower and more specific immune response to influenza vaccines62–64 and possibly age-cohort specific differences in A(H3N2) virus exposure history.65
Our meta-analysis of published IVE against hospitalizations associated with influenza virus infections presented several limitations. Firstly, we solely searched the Pubmed database to identify relevant studies, which captures the journals that influenza TND studies are published in.Comparison of databases suggests Pubmed offers optimal frequency and timely updates.66 Furthermore, using funnel plots and the Egger’s test, we observed no evidence of publication biases.30,31 The limited number of observations made the computation of subtype specific estimates by season difficult. While our overall estimates are useful evidence for public health decision makers, they do not reflect inter-seasonal variability of IVE. Suboptimal IVE may be due to mismatch between WHO-recommended and circulating strains but also to manufacturing processes, as described for the A(H3N2) vaccine component (e.g.,67). We were not able to collect and compute influenza B lineage-specific IVE, though primary care based published studies suggest the existence of influenza B cross-lineage protection.68,69
We observed low to moderate heterogeneity (I2 ranging between 0 and 64%) across IVE estimates included in the various meta-estimates. However, the small number of estimates and the large study-specific confidence intervals may hinder proper quantitative assessment of heterogeneity between studies.70 Following Greenland’s recommendations on the validation of meta-analysis approaches,71 we compared our results with values obtained using a fixed-model approach and found very small differences in IVE point estimates (data not shown).
Excluding IVE estimates focused only on intensive care unit (ICU) outcomes, and including only TND based studies in our estimates, we tried to limit potential qualitative heterogeneity across study methods. However, we did not apply restrictions to other methodological features, such as symptom eligibility criteria, vaccination status ascertainment, laboratory tests and specimen collection procedures, inclusion criteria based on the number of days between illness onset and specimen collection. A systematic review of TND IVE studies72 concluded that the most common variation in their practices was the analytical approach. Similarly, we noted considerable variability in the variables used to adjust IVE estimates across the studies in this review; however, all studies adjusted for age and presence of comorbidities, which are the most consistently included covariates in IVE TND studies.72 We believe that differences in other adjustment variables reflect local settings’ specificities. Indeed, variations in viruses’ circulation and access to vaccines across study sites are likely to lead to different confounding factors when measuring IVE.73
In 8/30 articles, patients’ inclusion was based on the physicians’ diagnosis rather than on a clear set of signs and symptoms. Such an inclusion approach could have led to a selection bias if the decision to include/exclude a patient was based on his/her vaccination status. One study in France comparing ad-hoc and systematic sampling of ILI patients by general practitioners showed a higher propensity of the physicians to select influenza positive cases and vaccinated patients.74 Although clinician testing has not been shown consistently to be associated with vaccination status,75 such a bias, if present in the hospital based studies would lead to underestimating the IVE. However, we found similar results when we restricted our analysis to studies using clearly defined sets of clinical criteria.
To reduce qualitative heterogeneity between studies included in the meta-analysis, we restricted our analyses to articles reporting results from TND studies. Other study designs may be used to measure IVE against laboratory confirmed hospitalized influenza. Cohort studies are scarce as they usually rely on vaccine registries to allow defining cohorts of vaccinated and unvaccinated individuals and require a systematic swabbing of SARI patients in all hospitals covering the source population.76 In the screening method,77–80 the odds of vaccination among cases are compared with the odds of vaccination in a reference population (based on administrative data). However, it is usually difficult to obtain vaccine coverage stratified on all potential confounders, which may bias IVE estimates. Consequently, WHO recommends against its use to measure IVE.73 In case control studies, controls must have experienced the same exposure of interest (here, influenza vaccination) as the population giving rise to the cases. The source population of hospitalized influenza cases may be defined as those at increased risk of SARI. In this context, non-influenza SARI patients may represent an appropriate group of controls and the TND a suitable study design to measure IVE. A recent modeling-based article suggested that measuring IVE against hospitalized influenza among inpatients was subject to biases if recruited test negative controls were included in the study because patients with exacerbation of underlying cardiopulmonary (CP) disease would be over-sampled.81 Such a bias would lead to recruiting a higher proportion of patients with CP in the study compared to the source population giving rise to hospitalized cases. If the population with CP were more likely to be vaccinated than the source population, such a bias would result in an overrepresentation of vaccinated patients in the control group and, ultimately, an overestimation of the IVE. In our meta-analysis, the presence of underlying conditions was controlled for in all studies reporting seasonal IVE. Furthermore, published observational studies conducted in Navarra (Spain) reported similar IVE estimates against influenza hospitalizations using cohort and TND designs.76
Our review could not examine the possible role of prior vaccination history in modifying current season IVE against severe outcomes, which has been suggested by an increasing number of publications.82,83 Repeat influenza vaccination over multiple years has been associated with decreased clinical IVE against influenza A(H3N2) and B viruses associated medical visits.84 Given that documenting current year influenza vaccination status is especially challenging in hospital settings,32,33 it is not surprising that the effect of prior vaccination on IVE was reported in very few articles.36,41,58 Nonetheless, research that considers the possible modification of current season IVE by prior vaccination history among hospitalized patients is needed, especially when consecutive identical vaccine components are followed by an antigenically distinct circulating strain. This can result in a blunting of IVE as described by Smith et al.85 and observed in 2014–15.86,87
Due to the limited number of TND studies reporting very severe outcomes,45,52,88 we could not compute pooled IVE against ICU admission associated with laboratory confirmed influenza. Castilla et al.88 reported a higher IVE against ICU compared to hospitalized influenza and concluded that vaccination lowered the severity of hospitalized cases of influenza. For the same reason of paucity of published data, we could not explore the effects of more potent vaccines. Adjuvanted vaccines may induce a more rapid and broader immune response89 and an observational study suggested a reduction by 25% of the risk of hospitalization for influenza or pneumonia with adjuvanted versus non-adjuvanted trivalent inactivated vaccines.90 Increasing the size and the number of studies using ICU admissions and deaths associated with laboratory confirmed influenza as outcomes as well as more potent influenza vaccines would be useful to further guide influenza vaccination policies.
Conclusion
In conclusion, our review of the published literature suggests that among vaccinated individuals influenza vaccines may prevent nearly half of the laboratory confirmed hospitalizations associated with influenza viruses. We observed lower IVE among persons 65 years and older compared to adults aged 18–64 years. We also noted poor performance of the seasonal influenza vaccines against influenza A(H3N2) viruses among the elderly in seasons characterized by a mismatch between vaccine and circulating strains. Real-time monitoring of antigenic drift during influenza A(H3N2) epidemics may facilitate the early implementation of alternative prevention measures, such as prophylactic use of antivirals, among the elderly.
Despite the lower effectiveness of influenza vaccines compared to other vaccines of the expanded programs on immunization, seasonal vaccination remains the best and safest public health measure to reduce morbidity and mortality due to influenza. Improving communication about IVE against severe influenza could increase influenza vaccine uptake and sustain investments in the vaccines. Larger studies providing insight into the effectiveness of different vaccine types (e.g., adjuvanted/unadjuvanted, high-dose/standard dose) in preventing severe influenza illness over various seasons could better target vaccination strategies, especially among high risk populations. Developing more immunogenic vaccines should however remain a public health priority.
Supplementary Material
Acknowledgments
Funding: None.
We thank authors of the following included studies for responding to queries and providing additional data: John Treanor, David Shay, Jessie Chung, Brendan Flannery, Jesus Castilla Catalan, Itziar Casado Buesa, Iván Martínez Baz. We would like to thank Marta Valenciano and Esther Kissling for their methodological input.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jinf.2017.09.010.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributors
MR and NEO designed the study. MR and NEO screened and abstracted publications. MR and SS analysed data. MR, NEO, and MT interpreted the results. MR wrote the manuscript, with editorial contributions from NEO, AM, AL, MT and SS. All authors reviewed the manuscript for accuracy and scientific content.
Conflict of interest
All authors declare no competing interests.
References
- 1.World Health Organization. Influenza vaccines. WHO position paper. Wkly Epidemiol Rec. 2005;80(33):279–87. [Google Scholar]
- 2.WHO. [Accessed 27 December 2012];WHO | influenza (seasonal) [Internet] 2012 Available from: http://www.who.int/mediacentre/factsheets/fs211/en/index.html.
- 3.Murata Y, Walsh EE, Falsey AR. Pulmonary complications of interpandemic influenza A in hospitalized adults. J Infect Dis. 2007;195(7):1029–37. doi: 10.1086/512160. [DOI] [PubMed] [Google Scholar]
- 4.Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008;121(4):258–64. doi: 10.1016/j.amjmed.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuiken T, Riteau B, Fouchier RAM, Rimmelzwaan GF. Pathogenesis of influenza virus infections: the good, the bad and the ugly. Curr Opin Virol. 2012;2(3):276–86. doi: 10.1016/j.coviro.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Madjid M, Miller CC, Zarubaev VV, Marinich IG, Kiselev OI, Lobzin YV, et al. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34,892 subjects. Eur Heart J. 2007;28(10):1205–10. doi: 10.1093/eurheartj/ehm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57(RR–7):1–60. [PubMed] [Google Scholar]
- 8.Müller-Pebody B, Crowcroft NS, Zambon MC, Edmunds WJ. Modelling hospital admissions for lower respiratory tract infections in the elderly in England. Epidemiol Infect. 2006;134(6):1150–7. doi: 10.1017/S0950268806006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitman RJ, Melegaro A, Gelb D, Siddiqui MR, Gay NJ, Edmunds WJ. Assessing the burden of influenza and other respiratory infections in England and Wales. J Infect. 2007;54(6):530–8. doi: 10.1016/j.jinf.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng P-Y, Steiner C, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis. 2012;54(10):1427–36. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Preventio. [Accessed 17 May 2017];FluView: laboratory-confirmed influenza hospitalizations – preliminary cumulative rates as of May 06, 2017 [Internet] Available from: https://gis.cdc.gov/GRASP/Fluview/FluHospRates.html.
- 12.Molinari N-AM, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 13.Mereckiene J. VENICE technical report [Internet] Stockholm, Sweden: ECDC; 2016. [Accessed 28 June 2017]. Seasonal influenza vaccination and antiviral use in Europe Overview of vaccination recommendations and coverage rates in the EU Member States for the 2013–14 and 2014–15 influenza seasons. Available from: http://venice.cineca.org/Seasonal-influenza-vaccination-antiviral-use-europe.pdf. [Google Scholar]
- 14.Ropero-Álvarez AM, El Omeiri N, Kurtis HJ, Danovaro-Holliday MC, Ruiz-Matus C. Influenza vaccination in the Americas: progress and challenges after the 2009 A(H1N1) influenza pandemic. Hum Vaccin Immunother. 2016;12(8):2206–14. doi: 10.1080/21645515.2016.1157240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. [Accessed 25 March 2017];Prevention and control of influenza pandemics and annual epidemics, Resolution of the 56th World Health Assembly, WHA 56.19, 2003. [Internet] Available from: http://apps.who.int/gb/archive/pdf_files/WHA56/ea56r19.pdf.
- 16.OECD/EU. Health at a glance: Europe 2016: state of health in the EU cycle. Paris: OECD Publishing; 2016. Influenza vaccination for older people. [Google Scholar]
- 17.Brewer NT, Hallman WK. Subjective and objective risk as predictors of influenza vaccination during the vaccine shortage of 2004–2005. Clin Infect Dis. 2006;43(11):1379–86. doi: 10.1086/508466. [DOI] [PubMed] [Google Scholar]
- 18.Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy: an overview. Hum Vaccin Immunother. 2013;9(8):1763–73. doi: 10.4161/hv.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly PM, Kotsimbos T, Reynolds A, Wood-Baker R, Hancox B, Brown SGA, et al. FluCAN 2009: initial results from sentinel surveillance for adult influenza and pneumonia in eight Australian hospitals. Med J Aust. 2011;194(4):169–74. doi: 10.5694/j.1326-5377.2011.tb03764.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang QS, Turner N, Baker MG, Williamson DA, Wong C, Webby R, et al. Southern Hemisphere Influenza and Vaccine Effectiveness Research and Surveillance. Influenza Other Respir Viruses. 2015;9(4):179–90. doi: 10.1111/irv.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puig-Barberà J, Arnedo-Pena A, Pardo-Serrano F, Tirado-Balaguer MD, Pérez-Vilar S, Silvestre-Silvestre E, et al. Effectiveness of seasonal 2008–2009, 2009–2010 and pandemic vaccines, to prevent influenza hospitalizations during the autumn 2009 influenza pandemic wave in Castellón, Spain. A test-negative, hospital-based, case-control study. Vaccine. 2010;28(47):7460–7. doi: 10.1016/j.vaccine.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 22.Effectiveness of vaccine against medical consultation due to laboratory-confirmed influenza: results from a sentinel physician pilot project in British Columbia, 2004–2005. Can Commun Dis Rep. 2005;31(18):181–91. [PubMed] [Google Scholar]
- 23.Rodriguez L, Kirkwood B. Case-control designs in the study of common diseases: updates on the demise of the rare disease assumption and the choice of sampling scheme for controls. Int J Epidemiol. 1990;19(1):205–13. doi: 10.1093/ije/19.1.205. [DOI] [PubMed] [Google Scholar]
- 24.Valenciano M, Kissling E, Ciancio BC, Moren A. Study designs for timely estimation of influenza vaccine effectiveness using European sentinel practitioner networks. Vaccine. 2010;28(46):7381–8. doi: 10.1016/j.vaccine.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16:942–51. doi: 10.1016/S1473-3099(16)00129-8. [DOI] [PubMed] [Google Scholar]
- 26.Darvishian M, van den Heuvel ER, Bissielo A, Castilla J, Cohen C, Englund H, et al. Effectiveness of seasonal influenza vaccination in community-dwelling elderly people: an individual participant data meta-analysis of test-negative design case-control studies. Lancet Respir Med. 2017;5(3):200–11. doi: 10.1016/S2213-2600(17)30043-7. [DOI] [PubMed] [Google Scholar]
- 27.Restivo V, Costantino C, Bono S, Maniglia M, Marchese V, Ventura G, et al. Influenza vaccine effectiveness among high-risk groups: a systematic literature review and meta-analysis of case-control and cohort studies. Hum Vaccin Immunother. 2017:1–12. doi: 10.1080/21645515.2017.1321722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101–5. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner N, Pierse N, Bissielo A, Huang QS, Baker MG, Widdowson M-A, et al. The effectiveness of seasonal trivalent inactivated influenza vaccine in preventing laboratory confirmed influenza hospitalisations in Auckland, New Zealand in 2012. Vaccine. 2014;32(29):3687–93. doi: 10.1016/j.vaccine.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner N, Pierse N, Bissielo A, Huang Q, Radke S, Baker M, et al. Effectiveness of seasonal trivalent inactivated influenza vaccine in preventing influenza hospitalisations and primary care visits in Auckland, New Zealand, in 2013. Euro Surveill. 2014;19(34) pii: 20884. [PMC free article] [PubMed] [Google Scholar]
- 34.Treanor JJ, Talbot HK, Ohmit SE, Coleman LA, Thompson MG, Cheng P-Y, et al. Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis. 2012;55(7):951–9. doi: 10.1093/cid/cis574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rondy M, Puig-Barbera J, Launay O, Duval X, Castilla J, Guevara M, et al. 2011–12 seasonal influenza vaccines effectiveness against confirmed A(H3N2) influenza hospitalisation: pooled analysis from a European network of hospitals. A pilot study. PLoS ONE. 2013;8(4):e59681. doi: 10.1371/journal.pone.0059681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rondy M, Launay O, Puig-Barberà J, Gefenaite G, Castilla J, de Gaetano Donati K, et al. 2012/13 influenza vaccine effectiveness against hospitalised influenza A(H1N1)pdm09, A(H3N2) and B: estimates from a European network of hospitals. Euro Surveill. 2015;20(2) doi: 10.2807/1560-7917.es2015.20.2.21011. [DOI] [PubMed] [Google Scholar]
- 37.Rondy M, Castilla J, Launay O, Costanzo S, Ezpeleta C, Galtier F, et al. Moderate influenza vaccine effectiveness against hospitalisation with A(H3N2) and A(H1N1) influenza in 2013–14: results from the InNHOVE network. Hum Vaccin Immunother. 2016;12(5):1217–24. doi: 10.1080/21645515.2015.1126013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin Y, Zhang Y, Wu P, Feng S, Zheng J, Yang P, et al. Influenza vaccine effectiveness in preventing hospitalization among Beijing residents in China, 2013–15. Vaccine. 2016;34(20):2329–33. doi: 10.1016/j.vaccine.2016.03.068. [DOI] [PubMed] [Google Scholar]
- 39.Puig-Barbera J, Mira-Iglesias A, Tortajada-Girbes M, Lopez-Labrador FX, Belenguer-Varea A, Carballido-Fernandez M, et al. Effectiveness of influenza vaccination programme in preventing hospital admissions, Valencia, 2014/15 early results. Euro Surveill. 2015;20(8) doi: 10.2807/1560-7917.es2015.20.8.21044. [DOI] [PubMed] [Google Scholar]
- 40.Pierse N, Kelly H, Thompson MG, Bissielo A, Radke S, Huang QS, et al. Influenza vaccine effectiveness for hospital and community patients using control groups with and without non-influenza respiratory viruses detected, Auckland, New Zealand 2014. Vaccine. 2016;34(4):503–9. doi: 10.1016/j.vaccine.2015.11.073. [DOI] [PubMed] [Google Scholar]
- 41.Petrie JG, Ohmit SE, Cheng CK, Martin ET, Malosh RE, Lauring AS, et al. Influenza vaccine effectiveness against antigenically drifted influenza higher than expected in hospitalized adults: 2014–2015. Clin Infect Dis. 2016;63(8):1017–25. doi: 10.1093/cid/ciw432. [DOI] [PubMed] [Google Scholar]
- 42.McNeil S, Shinde V, Andrew M, Hatchette T, Leblanc J, Ambrose A, et al. Interim estimates of 2013/14 influenza clinical severity and vaccine effectiveness in the prevention of laboratory-confirmed influenza-related hospitalisation, Canada, February 2014. Euro Surveill. 2014;19(9) doi: 10.2807/1560-7917.es2014.19.9.20729. [DOI] [PubMed] [Google Scholar]
- 43.McNeil SA, Andrew MK, Ye L, Haguinet F, Hatchette TF, ElSherif M, et al. Interim estimates of 2014/15 influenza vaccine effectiveness in preventing laboratory-confirmed influenza-related hospitalisation from the Serious Outcomes Surveillance Network of the Canadian Immunization Research Network, January 2015. Euro Surveill. 2015;20(5):21024. doi: 10.2807/1560-7917.es2015.20.5.21024. [DOI] [PubMed] [Google Scholar]
- 44.Martínez-Baz I, Martínez-Artola V, Reina G, Guevara M, Cenoz MG, Morán J, et al. Effectiveness of the trivalent influenza vaccine in Navarre, Spain, 2010–2011: a population-based test-negative case-control study. BMC Public Health. 2013;13:191. doi: 10.1186/1471-2458-13-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lytras T, Kossyvakis A, Melidou A, Andreopoulou A, Exindari M, Gioula G, et al. Influenza vaccine effectiveness in preventing hospitalizations with laboratory-confirmed influenza in Greece during the 2014–2015 season: a test-negative study. J Med Virol. 2016;88(11):1896–904. doi: 10.1002/jmv.24551. [DOI] [PubMed] [Google Scholar]
- 46.Kwong JC, Campitelli MA, Gubbay JB, Peci A, Winter A-L, Olsha R, et al. Vaccine effectiveness against laboratory-confirmed influenza hospitalizations among elderly adults during the 2010–2011 season. Clin Infect Dis. 2013;57(6):820–7. doi: 10.1093/cid/cit404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelly HA, Lane C, Cheng AC. Influenza vaccine effectiveness in general practice and in hospital patients in Victoria, 2011–2013. Med J Aust. 2016;204(2):76, e1–4. doi: 10.5694/mja15.01017. [DOI] [PubMed] [Google Scholar]
- 48.Hellenbrand W, Jorgensen P, Schweiger B, Falkenhorst G, Nachtnebel M, Greutélaers B, et al. Prospective hospital-based case-control study to assess the effectiveness of pandemic influenza A(H1N1)pdm09 vaccination and risk factors for hospitalization in 2009–2010 using matched hospital and test-negative controls. BMC Infect Dis. 2012;12:127. doi: 10.1186/1471-2334-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilca R, Skowronski DM, Douville-Fradet M, Amini R, Boulianne N, Rouleau I, et al. Mid-season estimates of influenza vaccine effectiveness against influenza A(H3N2) hospitalization in the elderly in Quebec, Canada, January 2015. PLoS ONE. 2015;10(7):e0132195. doi: 10.1371/journal.pone.0132195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dawood FS, Prapasiri P, Areerat P, Ruayajin A, Chittaganpitch M, Muangchana C, et al. Effectiveness of the 2010 and 2011 Southern Hemisphere trivalent inactivated influenza vaccines against hospitalization with influenza-associated acute respiratory infection among Thai adults aged = 50 years. Influenza Other Respir Viruses. 2014;8(4):463–8. doi: 10.1111/irv.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi WS, Noh JY, Seo YB, Baek JH, Lee J, Song JY, et al. Case-control study of the effectiveness of the 2010–2011 seasonal influenza vaccine for prevention of laboratory-confirmed influenza virus infection in the Korean adult population. Clin Vaccine Immunol. 2013;20(6):877–81. doi: 10.1128/CVI.00009-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi WS, Noh JY, Baek JH, Seo YB, Lee J, Song JY, et al. Suboptimal effectiveness of the 2011–2012 seasonal influenza vaccine in adult Korean populations. PLoS ONE. 2015;10(3):e0098716. doi: 10.1371/journal.pone.0098716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng AC, Kotsimbos T, Kelly PM FluCAN Investigators. Influenza vaccine effectiveness against hospitalisation with influenza in adults in Australia in 2014. Vaccine. 2015;33(51):7352–6. doi: 10.1016/j.vaccine.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 54.Cheng AC, Kotsimbos T, Kelly HA, Irving LB, Bowler SD, Brown SGA, et al. Effectiveness of H1N1/09 monovalent and trivalent influenza vaccines against hospitalization with laboratory-confirmed H1N1/09 influenza in Australia: a test-negative case control study. Vaccine. 2011;29(43):7320–5. doi: 10.1016/j.vaccine.2011.07.087. [DOI] [PubMed] [Google Scholar]
- 55.Cheng AC, Holmes M, Irving LB, Brown SGA, Waterer GW, Korman TM, et al. Influenza vaccine effectiveness against hospitalisation with confirmed influenza in the 2010–11 seasons: a test-negative observational study. PLoS ONE. 2013;8(7):e68760. doi: 10.1371/journal.pone.0068760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng AC, Dwyer DE, Holmes M, Irving LB, Brown SG, Waterer GW, et al. Influenza epidemiology, vaccine coverage and vaccine effectiveness in sentinel Australian hospitals in 2013: the Influenza Complications Alert Network. Commun Dis Intell Q Rep. 2014;38(2):E143–9. [PubMed] [Google Scholar]
- 57.Cheng AC, Brown S, Waterer G, Holmes M, Senenayake S, Friedman ND, et al. Influenza epidemiology, vaccine coverage and vaccine effectiveness in sentinel Australian hospitals in 2012: the Influenza Complications Alert Network (FluCAN) Commun Dis Intell Q Rep. 2013;37(3):E246–52. [PubMed] [Google Scholar]
- 58.Castilla J, Navascués A, Fernández-Alonso M, Reina G, Pozo F, Casado I, et al. Effectiveness of subunit influenza vaccination in the 2014–2015 season and residual effect of split vaccination in previous seasons. Vaccine. 2016;34(11):1350–7. doi: 10.1016/j.vaccine.2016.01.054. [DOI] [PubMed] [Google Scholar]
- 59.Bissielo A, Pierse N, Huang QS, Thompson MG, Kelly H, Mishin VP, et al. Effectiveness of seasonal influenza vaccine in preventing influenza primary care visits and hospitalisation in Auckland, New Zealand in 2015: interim estimates. Euro Surveill. 2016;21(1) doi: 10.2807/1560-7917.ES.2016.21.1.30101. [DOI] [PubMed] [Google Scholar]
- 60.Andrews N, Waight P, Yung C-F, Miller E. Age-specific effectiveness of an oil-in-water adjuvanted pandemic (H1N1) 2009 vaccine against confirmed infection in high risk groups in England. J Infect Dis. 2011;203(1):32–9. doi: 10.1093/infdis/jiq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feng S, Cowling BJ, Sullivan SG. Influenza vaccine effectiveness by test-negative design – Comparison of inpatient and outpatient settings. Vaccine. 2016;34(14):1672–9. doi: 10.1016/j.vaccine.2016.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention (CDC) Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices–United States, 2013–2014. MMWR Recomm Rep. 2013;62(RR–07):1–43. [PubMed] [Google Scholar]
- 63.Camilloni B, Basileo M, Valente S, Nunzi E, Iorio AM. Immunogenicity of intramuscular MF59-adjuvanted and intradermal administered influenza enhanced vaccines in subjects aged over 60: a literature review. Hum Vaccin Immunother. 2015;11(3):553–63. doi: 10.1080/21645515.2015.1011562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marra F, Young F, Richardson K, Marra CA. A meta-analysis of intradermal versus intramuscular influenza vaccines: immunogenicity and adverse events. Influenza Other Respir Viruses. 2013;7(4):584–603. doi: 10.1111/irv.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mosterín Höpping A, McElhaney J, Fonville JM, Powers DC, Beyer WEP, Smith DJ. The confounded effects of age and exposure history in response to influenza vaccination. Vaccine. 2016;34(4):540–6. doi: 10.1016/j.vaccine.2015.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB J. 2008;22(2):338–42. doi: 10.1096/fj.07-9492LSF. [DOI] [PubMed] [Google Scholar]
- 67.Skowronski DM, Janjua NZ, De Serres G, Sabaiduc S, Eshaghi A, Dickinson JA, et al. 2012–13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS ONE. 2014;9(3):e92153. doi: 10.1371/journal.pone.0092153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skowronski DM, Janjua NZ, Sabaiduc S, De Serres G, Winter A-L, Gubbay JB, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011–2012 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis. 2014;210(1):126–37. doi: 10.1093/infdis/jiu048. [DOI] [PubMed] [Google Scholar]
- 69.Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, et al. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. 2013;11:153. doi: 10.1186/1741-7015-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol. 2008;8:79. doi: 10.1186/1471-2288-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greenland S. Invited commentary: a critical look at some popular meta-analytic methods. Am J Epidemiol. 1994;140(3):290–6. doi: 10.1093/oxfordjournals.aje.a117248. [DOI] [PubMed] [Google Scholar]
- 72.Sullivan SG, Feng S, Cowling BJ. Potential of the test-negative design for measuring influenza vaccine effectiveness: a systematic review. Expert Rev Vaccines. 2014;13(12):1571–91. doi: 10.1586/14760584.2014.966695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.World Health Organization. Evaluation of influenza vaccine effectiveness: a guide to the design and interpretation of observational studies. Geneva: World Health Organization; 2017. [Google Scholar]
- 74.Cohen JM, Kissling E, Daviaud I, van der Werf S, Lina B, Mosnier A. “Systematic” versus “ad hoc” selection of patients to swab, France 2009–2014: do influenza vaccine effectiveness estimates change? [Internet]. Poster presentation presented at: Options IX for the control of influenza; Chicago, USA. 2016. [Accessed 13 March 2017]. Available from: http://2016.isirv.org/sites/default/files/docs/2016/isirv-16-fp-web.pdf. [Google Scholar]
- 75.Ferdinands JM, Belongia EA, Nwasike C, Shay DK. Influenza vaccination status is not associated with influenza testing among children: implications for observational studies of vaccine effectiveness. Vaccine. 2011;29(10):1935–40. doi: 10.1016/j.vaccine.2010.12.098. [DOI] [PubMed] [Google Scholar]
- 76.Castilla J, Martínez-Artola V, Salcedo E, Martínez-Baz I, Cenoz MG, Guevara M, et al. Vaccine effectiveness in preventing influenza hospitalizations in Navarre, Spain, 2010–2011: cohort and case-control study. Vaccine. 2012;30(2):195–200. doi: 10.1016/j.vaccine.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 77.Widgren K, Magnusson M, Hagstam P, Widerström M, Örtqvist Å, Einemo IM, et al. Prevailing effectiveness of the 2009 influenza A(H1N1)pdm09 vaccine during the 2010/11 season in Sweden. Euro Surveill. 2013;18(15):20447. [PubMed] [Google Scholar]
- 78.Thomas HL, Andrews N, Green HK, Boddington NL, Zhao H, Reynolds A, et al. Estimating vaccine effectiveness against severe influenza in England and Scotland 2011/2012: applying the screening method to data from intensive care surveillance systems. Epidemiol Infect. 2013;142:126–33. doi: 10.1017/S0950268813000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bonmarin I, Belchior E, Le Strat Y, Levy-Bruhl D. First estimates of influenza vaccine effectiveness among severe influenza cases, France, 2011/12. Euro Surveill. 2012;17(18) doi: 10.2807/ese.17.18.20163-en. [DOI] [PubMed] [Google Scholar]
- 80.Remschmidt C, Rieck T, Bödeker B, Wichmann O. Application of the screening method to monitor influenza vaccine effectiveness among the elderly in Germany. BMC Infect Dis. 2015;15:137. doi: 10.1186/s12879-015-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Foppa IM, Ferdinands JM, Chaves SS, Haber MJ, Reynolds SB, Flannery B, et al. The case test-negative design for studies of the effectiveness of influenza vaccine in inpatient settings. Int J Epidemiol. 2016;45(6):2052–9. doi: 10.1093/ije/dyw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohmit SE, Petrie JG, Malosh RE, Cowling BJ, Thompson MG, Shay DK, et al. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis. 2013;56(10):1363–9. doi: 10.1093/cid/cit060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thompson MG, Naleway A, Fry AM, Ball S, Spencer SM, Reynolds S, et al. Effects of Repeated Annual Inactivated Influenza Vaccination among Healthcare Personnel on Serum Hemagglutinin Inhibition Antibody Response to A/Perth/16/2009 (H3N2)-like virus during 2010–11. Vaccine. 2016;34(7):981–8. doi: 10.1016/j.vaccine.2015.10.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McLean HQ, Thompson MG, Sundaram ME, Meece JK, McClure DL, Friedrich TC, et al. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis. 2014;59(10):1375–85. doi: 10.1093/cid/ciu680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci USA. 1999;96(24):14001–6. doi: 10.1073/pnas.96.24.14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Skowronski DM, Chambers C, Sabaiduc S, De Serres G, Winter A-L, Dickinson JA, et al. A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin Infect Dis. 2016;63(1):21–32. doi: 10.1093/cid/ciw176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Skowronski DM, Chambers C, De Serres G, Sabaiduc S, Winter A-L, Dickinson JA, et al. Serial vaccination and the antigenic distance hypothesis: effects on influenza vaccine effectiveness during A(H3N2) epidemics in Canada, 2010–11 to 2014–15. J Infect Dis. 2017 doi: 10.1093/infdis/jix074. http://dx.doi.org/10.1093/infdis/jix074. [DOI] [PMC free article] [PubMed]
- 88.Castilla J, Godoy P, Domínguez A, Martínez-Baz I, Astray J, Martín V, et al. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis. 2013;57(2):167–75. doi: 10.1093/cid/cit194. [DOI] [PubMed] [Google Scholar]
- 89.O’Hagan D, Tsai T, Reed S. Emulsion-based adjuvants for improved influenza vaccines. In: Del Giudice G, Rappuoli R, editors. Influenza vaccines for the future. Springer; 2011. pp. 327–57. [Google Scholar]
- 90.Mannino S, Villa M, Apolone G, Weiss NS, Groth N, Aquino I, et al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy. Am J Epidemiol. 2012;176(6):527–33. doi: 10.1093/aje/kws313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.