Clinics of North America
Fanconi anemia (FA) is an autosomal and X-linked recessive disorder characterized by bone marrow failure, acute myelogenous leukemia, solid tumors and developmental abnormalities. At the molecular level, cells derived from FA patients display hypersensitivity to DNA crosslinking agents, resulting in increased numbers of chromosomal abnormalities including translocations and radial chromosomes. This hypersensitivity made treating FA patients a challenge in the past as traditional treatments of their symptoms resulted in more harm than good. However, recent years have seen a dramatic improvement in FA patient treatment, resulting in a greater survival of children into adulthood. These improvements have been made despite the fact that a definitive cellular function for the proteins in the FA pathway has yet to be elucidated. Delineating the cellular functions of the FA pathway could help further improve the treatment options for FA patients and further reduce the probability of succumbing to the disease. This article will review the current clinical aspects of FA including presentation, diagnosis, and treatment followed by a review of the molecular aspects of FA as they are currently understood.
Clinical aspects of FA
In earlier times, children with FA had the inevitable outcome of death, as most FA patients present with aplastic anemia and little in the way of supportive care was available. In the first part of the 20th century, the advent of modern blood banking allowed the clinician to stem the immediacy of anemia and thrombocytopenia that resulted in death. As a result, the next major issue for these children became infection, even with the development of antibiotics. Neutropenic infections are generally not well tolerated and typically not curable with antibiotics alone, and many FA children succumbed to bacterial and fungal infections. Finally, even when a child could be supported through the huge problem of aplastic anemia, the looming issue of acute myelogenous leukemia (AML) nonetheless inevitably and inexorably presented itself. Thus it was the exceptionally rare patient who survived to adulthood1,2,3.
Recent years have revolutionized the care of the FA patient. While hematopoietic stem cell transplantation (SCT) has been performed on FA patients for almost 30 years, it is only in recent years that such approaches have been done more safely and successfully4. Even with the greater survival of children into adulthood as a result of SCT, the specter of potential of solid tumors such as squamous cell carcinomas of the head, neck, and genitourinary track remains as a serious problem5, 6, 7, 8.
Presentation
Even though a classic set of features generally characterize these patients, FA children typically present in the first decade of life upon recognition of aplastic anemia1, 2, 3. Nonetheless, classic features of FA consist of thumb and radial absence, malformation, or even less obvious features such as a deeper cleft between the first two digits. In much the same way as the facial features of children affected by Down’s syndrome allow easy recognition of their affliction, children with FA display a collection of subtle facial features which allow them to be easily recognizable as a group.
A less striking and less specific array of characteristics may be present as well and are summarized in Table 1. Even more interesting is the fact that a subset of FA patients has no discernible abnormalities at all, in a fraction estimated at up to one third. As a result, the index of suspicion of the clinician must be high in order to recognize the potential for the diagnosis of FA in the wake of aplastic anemia.
Table 1.
Physical abnormalities seen in FA patients.(132)
| Physical Abnormality | Percent of FA patients |
|---|---|
| Skin discolorations | 55% |
| Hand, Arm, and other skeletal abnormalities | 51% |
| Abnormal Reproductive Organs | 35% |
| Small Head or Eyes | 26% |
| Kidney Problems | 21% |
| Low Birth Weight | 11% |
| Heart Defects | 6% |
| Gastrointenstinal Problems (Bowel) | 5% |
Diagnosis
Once it is recognized that a patient has a production defect resulting in the occurrence of more than one cell line abnormally low, it is incumbent on the clinician to then proceed to an examination of the bone marrow. At the time of the bone marrow procedure, it is critical to not only perform aspiration but also a biopsy in order that 1) cellularity be assessed and 2) pathological examination for evidence of leukemia be undertaken. Aspirate samples are sent typically for flow cytometry to rule out further evidence of clonal cell populations and for examination of cell morphology.
The gold standard tests for FA quantify chromosomal breakage in cells exposed to crosslinking agents to which FA cells are hypersensitive. In this test lymphocytes from patients are stimulated and exposed or not to diepoxybutane (DEB) or mitomycin C (MMC). After inducing mitotic arrest, the cells are then dropped onto slides, and the chromosomes are scored for the number of induced breaks. The hallmark of FA is increased chromosome breakage in a statistically significant way. The chromosomal breakage assay is actually a clue to the basic biology of FA, as the cells themselves that are derived from FA patients display increased cell death when exposed to a whole range of chemical crosslinkers, including DEB, mitomycin C (MMC), and cisplatin3, 2, 4, 9.
On occasion, in spite of the strong suspicion of FA being present in a patient, the chromosome fragility test test can be negative. This phenomenon is associated with somatic reversion in which the hematopoietic lineages (in all or in part) have undergone mutation at a second site within the affected FA gene, resulting in restoration of at least partial function of that FA protein product10, 11, 12, 13. This phenomenon occurs probably due to a combination of increased genetic instability inherent in the phenotype of FA in combination with selective pressure of the relatively rapidly overturning hematopoietic compartment. Such a phenomenon also has implication for the potential for gene therapy approaches to FA, as will be discussed below. In the face of this possibility, if a negative DEB or MMC result has been obtained in the setting of strong suspicion of an FA diagnosis, then a skin biopsy should be obtained for culture and subsequent DEB testing.
AML
Even though 90% of FA patients first present with bone marrow failure, a certain percentage will nonetheless display AML as the first evidence of FA. These cases of AML are typically M1-M4 FAB subtype and display no characteristic cytogenetic or molecular abnormality althougth numerous translocations, deletions, and other aneuploidogenic changes can be found14, 15. The most ominous part of a diagnosis of AML is the fact that FA patients cannot be treated in a typical fashion as other AML patients, owing to their inability to tolerate standard doses of alkylating agents. One might expect that AMLs derived from FA patients would display greater sensitivity to chemotherapy and thus potentially be more curable, but the morbidity to the patient precludes an aggressive approach. In addition, analysis of cells derived from these AML cases reveal that they are heterogeneous in their cell culture response to agents that normally confer marked toxicity in the patient.
SCT
The decision to go forward with SCT in an FA patient is one to not be taken lightly. Under the best of circumstances in a patient unafflicted by FA, going forward electively with SCT presents risks and potential for morbidity. Total body irradiation and cyclophosphamide, which are typical parts of conditioning regimens, can result in long-term effects upon growth, cognition, and secondary malignancy. In addition, the potential of graft versus host disease can result in long term complications that may result in death. Most clinicians prefer to wait if they have the choice until children are out of the first decade of life, after which such effects are somewhat ameliorated.
It is an even more difficult decision for the FA patient because he or she is so susceptible to toxicity from the regimen. However, those caring for the FA patient must realize that an educated guess must be made as to when to transplant the patient so as to preempt the onset of leukemia, avoid the long term effects of blood product provision, and proceed when the patient is in good clinical shape and unaffected by serious infections, such as those from invasive organisms like aspergillus.
An assessment should be made as to the likelihood that proceeding to SCT will be necessary. Typically, once the diagnosis of FA has been made, an attempt to identify the complementation group should be made. It has become clear from the experience of FA clinicians that FA-D1 patients are at significant and early risk of progression to AML, often before the presentation of aplastic anemia16, 1, 17. In addition, other genotypes associated with early AML progression include the FA-C Ashkenazi Jewish mutation (deletion of exon 4) 18, 19. In general terms, it is thought that such a risk of early AML progression is coincident with a more severely displayed FA phenotype. Finally, a careful watch of patient’s cell counts and yearly bone marrow evaluation by the clinician is necessary in order to identify a decline in numbers that could result in the need for transfusion therapy. Chronic transfusions are generally associated with poorer outcome in the transplant setting, resulting in increased iron burden with the concomitant potential for organ damage, and signaling the potential for neutropenia that can result in infections that can delay or even proscribe the start of SCT20, 21, 22.
Because the clinical status of FA patients can change rapidly, it is prudent to be prepared for SCT by early identification of a donor well before the need to proceed. Provided the patient has a sibling, that sibling will have an approximately 20% chance of being a donor. Because of improved techniques in transplant and the potential for donors in the extended family if ethnically homogeneous, donors can often be found in parents and other family members. If no donor is found in the family then a search is initiated in the unrelated pool, including adults in the National Marrow Donor Registry (NMDR) and cord blood banks. At this time, the typical analysis of HLA loci involves molecular analysis at both alleles, resulting in report on 10 loci. Transplants can proceed with matches of as little as 8/10, especially in cord bloods. Several centers are conducting trials with haplo donors from parents23, 24, 25, 26.
Historically, the challenges of SCT in FA patients have been numerous. The issue of graft failure has been inherent with a prevalence of 10%. Avoiding this outcome necessitates the use of significant conditioning, which of course is associated with toxicity. In recent years the use of fludaribine has shrunk this number to less than 1%. As a result, efforts at reduction of conditioning have been steady and the use of TBI has been diminished down to doses of 400–600 cGy. In addition, the use of cyclophosphamide has also been decreased in recent years. With an allogeneic related transplant, the long-term survival is often greater than 80%27, 28, 29.
Matched unrelated transplants have posed a greater challenge with a greater incidence of graft versus host disease (GVHD). Toxicity associated GVHD has proved to occur with greater intensity in the FA patient, perhaps because of the greater degree of toxicity due to conditioning. Such toxicity is synergistic with the increased GVHD risk. With diminished toxicity has come greater GVHD control and subsequent increased survival for FA patients undergoing matched unrelated transplants30, 27, 31, 32.
Secondary effects of SCT predictably have greater consequences for FA patients presumably because of their underlying issues of growth delay, endocrine dysfunction, and risk of malignancy, all of which are associated with long term effects of undergoing SCT. A markedly increased risk of acquisition of squamous cell carcinoma is seen posttransplantation, out of proportion to that observed anyway in FA patients. These cases are associated with patients having significant toxicity during SCT and are only somewhat linked to HPV33, 7, 34.
The idea that FA cells are hypersensitive to endogenous and exogenous stimuli suggests that FA stem cells in the bone marrow are susceptible to a sort of “natural selection.” This is probably why somatic reversion is observed in some FA patients. As a result, it has been postulated that FA patients would be ideal for gene therapy clinical trials. Trials entailing the most common complementation group, FA-A, have been instituted using a lentiviral transduction system of HSC from FA patients, manipulated ex vivo. In vitro data suggest that HSCs can be transduced with subsequent colony forming assays suggesting increased growth and reconstitution. However, such trails have thus far been disappointing as lack of permanent transduction of progenitors has led to failure to establish long term hematopoiesis 35, 36, 37, 38.
Traditionally, androgens have proved to be an efficacious treatment in aplastic patients, FA patients included. Androgens generally stimulate more effective hematopoiesis, resulting in an increase in peripheral blood counts. However, the use of androgens has been marked by their difficulty in use in females, given the masculinizing side effects. In addition, their use has been associated with increased risk of liver adenomas39, 40.
Molecular Aspects of FA
At the molecular level, cells derived from FA patients display hypersensitivity to DNA cross-linking agents such as mitomycin C (MMC) and diepoxybutane (DEB). Treatment with these agents induces an abnormally prolonged cell cycle arrest in S phase and an accumulation of cells with 4N DNA41. As the result of this response, the FA pathway has been hypothesized to function in sensing DNA damage induced by these agents and in initiating its repair. This hypothesis has been supported by work elucidating the interactions of FA proteins with established DNA repair proteins. On the other hand, a significant body of work has pointed to a role for the FA pathway in cell signaling in response to stress stimuli and in apoptosis in the cytoplasm, affecting maintenance of the hematopoietic machinery. While the exact role of the FA pathway has yet to be discovered, what is known about the FA proteins and their interactions will be reviewed.
The FA pathway is composed of at least 13 genes42. Each of these genes, when biallelically mutated, causes FA. The encoded proteins (Table 2) can be subdivided within the FA pathway into three groups: proteins that make up the core complex, the FANCD2 and FANCI proteins which compose the ID complex, and three downstream effector proteins: FANCD1/BRCA2, FANCJ/BRIP1/BACH1, and FANCN/PALB2. Many of the FA proteins contain no recognizable motifs, which has made discovering their contributions to the FA pathway and the main function of the FA pathway more challenging. The following section will delineate what is known about each of the three FA protein subdivisions and how these groups interact to form an intact FA pathway.
Table 2.
The 13 FA genes and proteins they encode
| Complement-ation Group | Responsible Gene | Chromosome Location | Protein Molecular Weight (kDa) | Known Motifs | Necessary for FANCD2 mono-ubiquitylation |
|---|---|---|---|---|---|
| A | FANCA | 16q24.3 | 163 | 2 NLSs, 5 NESs | YES |
| B | FANCB | Xp22.31 | 95 | NLS | YES |
| C | FANCC | 9q22.3 | 63 | None | YES |
| D1 | FANCD1/BRCA2 | 13q12.13 | 380 | 8 BRC repeats, HD, 3 OBs, TD | NO |
| D2 | FANCD2 | 3p25.3 | 155, 162 | None | YES |
| E | FANCE | 6p21–22 | 60 | 2 NLSs | YES |
| F | FANCF | 11p15 | 42 | None | YES |
| G | FANCG/XRCC9 | 9p13 | 68 | 7 TPRs | YES |
| I | FANCI/KIAA1794 | 15q25–26 | 146 | None | YES |
| J | FANCJ/BRIP1/BACH1 | 17q22–24 | 130 | ATPase, 7 Helicase Specific Motifs | NO |
| L | FANCL/PHF9 | 2p16.1 | 43 | 3 WD40s, PHD | YES |
| M | FANCM | 14q21.3 | 250 | 7 Helicase Specific Motifs, degenerate endonuclease domain, ATPase | YES |
| N | FANCN/PALB2 | 16p12 | 130 | 2 WD40s | NO |
FA Core Complex Proteins
The nuclear “core” complex is composed of seven of the 13 FA proteins (FANCA, FANCB, FANCC, FANCE, FANCF, FANCG, FANCL and FANCM. This “core” complex is required for the monoubiquitylation of FANCD2 and FANCI42. While the reason for the necessity of an intact FA core complex for these modifications is not well understood, biallelic mutation or deletion of any one of the genes that encode these eight core proteins results in failure to monoubiquitylate FANCD2 and FANCI. The exact mechanism by which the core complex facilitates the monoubiquitylation of FANCD2 and FANCI has yet to be worked out, but a few core complex members contain motifs which allow us to speculate as to their functions within the complex if not the pathway.
Several of the core complex proteins including FANCA, FANCB, and FANCE contain nuclear localization signals (NLS) supporting lines of evidence showing that the core complex fully assembles in the nucleus43,44,45. FANCL/PHF9 has been proposed to be the catalytic E3 ubiquitin ligase subunit of the FA core complex required for the monoubiquitylation of FANCD246. FANCL contains three WD40 motifs which are required for interaction with other FA core complex proteins as well as a plant homeodomain (PHD) motif which when mutated impairs FANCD2 monoubiquitylation47. The FANCL protein has displayed autoubiquitylation activity in vitro46; however in vivo ubiquitin ligase activity has yet to be shown. FANCM has been proposed to act as a scaffolding protein for the FA core complex and is necessary for its localization to chromatin48. FANCM possesses 7 helicase specific motifs and a degenerate endonuclease domain42. While displaying considerable homology to the archeal Hef (helicase associated endonuclease for fork-structured DNA) protein, which has functional helicase and endonuclease domains and resolves stalled replication forks49, the FANCM protein has not displayed any of these activities thus far. However, the FANCM protein has been shown to display DNA-dependent ATPase activity and promotes the dissociation of DNA triplexes, acting as a DNA translocase50. The FANCM protein has been found to require interaction with the FA associated protein 24 (FAAP24) protein for functional integrity, as depletion of FAAP24 has been shown to disrupt the chromatin association of FANCM and to destabilize FANCM, leading to failure of the core complex to localize to chromatin48. Several of the core complex proteins including FANCA, FANCG, and FANCM are also regulated by phosphorylation and dephosphorylation throughout the cell cycle52,53,51,48. These modifications also seem to be necessary for intact FA pathway function.
While evidence has shown that an intact core complex is unequivocally necessary for activation of the FA pathway through regulation of the monoubiquitylation of FANCD2, it has been proposed that the core complex proteins may also perform other functions. Consistent with this hypothesis, the eight FA core complex proteins also segregate into several distinct subcomplexes amongst themselves. FANCB and FANCL directly interact and have been found to interact with FANCA through complex purification experiments. The interaction of these three proteins is disrupted in FA-G and FA-M cells but intact in FA-C, FA-E, and FA-F cells suggesting that two discrete subcomplexes exist, one composed of FANCA, FANCB, FANCG, FANCL, and FANCM, and another composed of FANCC, FANCE, and FANCF54. While the function of these subcomplexes remain elusive, the FANCA protein has been shown to directly interacts with a central portion of the BRCA1 protein in a DNA damage independent manner55, the FANCG protein has been shown to interact with XRCC3 and FANCD1/BRCA256, and the FANCM protein has been found to be capable of catalyzing branch migration of Holliday junctions and replication forks in vitro50. This suggests that the FANCA, FANCB, FANCG, FANCL, and FANCM subcomplex may participate in the cellular response to DNA cross-links both upstream of FANCD2 and FANCI monoubiquitylation in the core complex and downstream in the aforementioned subcomplex within the FA pathway. The interaction of the two subcomplexes seems to be mediated by FANCG, which interacts with both FANCA and FANCF57. Finally, FANCE seems to mediate the interaction of the core complex with FANCD2 as FANCE has been demonstrated to interact with FANCD2 both in vitro and in vivo58.
FANCD2-FANCI
Following treatment with DNA crosslinking agents 59 or during S phase of the cell cycle60, FANCD2 and FANCI become monoubiquitylated. These modifications result in the translocation of the two proteins to chromatin within cells where they colocalize with DNA repair proteins including the downstream effector FA proteins at sites of DNA damage61,62,63. As previously stated, an intact core complex is necessary for the monoubiquitylation of FANCD2 on lysine 56161 and FANCI on lysine 52362,63. FANCI is a relatively new member of the FA pathway, identified by a screen for phosphopeptides corresponding to consensus substrates of ATM/ATR64. While the role of the FANCI protein in the FA pathway has yet to be identified, FA-I cells display a lack of FANCD2 monoubiquitylation and a subsequent failure in FANCD2 chromatin associated foci formation while maintaining a normal intact core complex65. Interestingly, FANCI shares sequence homology to FANCD2 and associates with FANCD2 as the FANCI-FANCD2 (ID) complex, which translocates to chromatin following DNA damage62. Importantly, monoubiquitylation modifications on FANCD2 and FANCI are important for the preservation of monoubiquitylation on the other protein in the ID complex, respectively62. Phosphorylation is also a regulatory mechanism for FANCD2 and FANCI as phosphorylation of key residues on each protein is required for monoubiquitylation and focus formation of both66, 67. Many of the proteins which colocalize with FANCD2 and FANCI in discrete nuclear foci following treatment with DNA cross-linking agents are proteins which function in DNA damage sensing and repair. These interactions will be discussed more in depth later in this review.
The “Downstream” Proteins: FANCD1, FANCJ, and FANCN
The FANCD1 gene is identical to the familial breast/ovarian cancer susceptibility gene BRCA2 and as such, biallelic mutation of the FANCD1/BRCA2 gene results in the FA-D1 subtype of FA, while monoallelic mutation results in increased breast and ovarian cancer susceptibility68. The main contribution of the FANCD1 protein to the FA pathway is through its ability to recruit Rad51 into the DNA damage inducible nuclear foci, which FANCD2 translocates into following monoubiquitylation69. Rad51 is a recombinase, which, like its bacterial homologue RecA, binds ssDNA and promotes homologous recombination70. The FANCD1/BRCA2 protein consists of 8 BRC repeats which have been shown to bind RAD5171 as well as five C-terminal domains consisting of a helical domain (HD), three oligonucleotide/oligosaccharide-binding folds (OB), and a tower domain (TD)72. The OB domains participate in ssDNA binding while the TD domain participates in dsDNA binding, allowing the FANCD1/BRCA2 protein to nucleate RAD51 filament formation at ssDNA/dsDNA junctions to promote homologous recombination72. The protein DSS1 also interacts with FANCD1/BRCA2 and is necessary for the protein’s stability73.
The FANCJ gene is identical to the BRIP1 (BRCA1 interacting protein C-terminal helicase 1) and BACH1 (BRCA1 associated carboxyl terminal helicase 1) genes74,75,76. As the names of BRIP1 and BACH1 imply, the FANCJ/BRIP1/BACH1 protein directly binds to the BRCT domain of BRCA177. The BRCT domain is a phospho-protein binding domain, and phosphorylation of FANCJ/BRIP1/BACH1 on serine 990 is required for this interaction78. The FANCJ/BRIP1/BACH1 protein is a DNA-dependent ATPase and a 5′ to 3′ DNA helicase (DEAH helicase), which contains 7 helicase specific motifs77. FANCJ has been shown to be a structure specific helicase, dissociating guanine quadruplex DNA (G4 DNA) in vitro79.
FANCN/PALB2 (partner and localizer of BRCA2) is an interacting protein of FANCD1/BRCA2 upon which BRCA2 contributions to DNA double strand break repair (DSBR) and HR are at least partially reliant80. The only recognizable motifs in the FANCN/PALB2 protein are two WD40 repeat like motifs in its carboxyl terminus80. Cells deficient in FANCN/PALB2 show cellular phenotypes similar to those seen in cells deficient in FANCD1/BRCA2 such as lack of formation of Rad51 foci after ionizing radiation81. FANCN deficient cells also lack FANCD1/BRCA2 chromatin association, which is necessary for proper DSBR and HR80.
The FA pathway and DNA Repair
Cells derived from FA patients display hypersensitivity to DNA cross-linking agents such as MMC and DEB59. Treatment of cells derived from FA patients with DNA cross-linking drugs has been shown to induce an abnormally prolonged cell cycle arrest in S phase as well as an accumulation of cells containing 4N DNA41. The mechanism by which the inter- and intra-strand cross-links (ICLs) induced by these drugs are resolved in mammals in not well understood. While it is known that nucleotide excision repair (NER), homologous recombination (HR) and translesion synthesis (TLS) repair pathways play a role in repairing ICLs in bacteria and that NER, Rad6/Rad18 dependent postreplication repair, HR, and cell cycle checkpoint pathways play a role in repairing ICLs in yeast82, lack of a convenient and accurate approach to studying ICLs in mammals has delayed the field.
In 2005, Nojima, et.al., inferred that multiple pathways are utilized to resolve ICLs in chicken DT40 cells and deduced that the FA pathway is epistatic with TLS and HR mediated ICL repair83. While a definitive role for the FA proteins in the TLS or HR pathway has yet to be elucidated in vertebrates, many studies have linked the FA pathway to TLS and HR through interactions between FA proteins and proteins which participate in these DNA repair pathways. The following paragraphs will briefly describe these known interactions alluding to a role for FA proteins in TLS and HR mediated ICL repair.
The FA pathway and HR proteins
One of the most obvious connections between the FA and HR pathways is the interaction between FANCD1/BRCA2 and RAD51. RAD51 is the mammalian homolog of the bacterial RecA protein, which is a ssDNA binding protein necessary for catalyzing the strand invasion step of HR84. The interaction between BRCA2 and RAD51 was first discovered through a yeast two-hybrid screen85. The importance of this interaction to the FA pathway was not understood until the gene mutated in FA-D1 patients was discovered to be identical to BRCA268. Since then, it has been discovered that the internal BRC repeat motifs within the BRCA2 protein are required for this interaction with RAD5171 and that this interaction promotes RAD51 nucleoprotein filament formation which is a necessary early step in the HR pathway72. It has also more recently been discovered that the BRCA2 binding protein PALB2 is the protein mutated in the FA-N complementation group80. This discovery also then connects FANCN/PALB2 to the HR pathway as FANCN deficient cells lack FANCD1/BRCA2 chromatin association, which is necessary for proper HR80.
The familial breast cancer protein BRCA1 contributes to the HR pathway upstream of BRCA2 and plays a much broader role, participating in multiple cellular processes in response to DNA damage86. While previous studies provided evidence of indirect interactions between BRCA1 and FANCD1/BRCA287,85,88, it was not until 1998 that the two proteins were shown to co-immunoprecipitate and co-localize in nuclear foci during S phase of the cell cycle89, foci which were later shown to also contain FANCD259. Interestingly, through the use of yeast two-hybrid analysis, it was discovered that the amino-terminus of the FANCA protein directly interacts with a central section of the BRCA1 protein and this interactions is independent of DNA damage55. Finally, perhaps the strongest connection between BRCA1 and the FA pathway lies in the interaction between FANCJ/BRIP1/BACH1 and BRCA1. As its two alternate names suggest, the FANCJ protein was originally identified as a BRCA1 interacting protein as it binds directly to the BRCT domain of BRCA1 and promotes BRCA1s known roles in DNA repair77.
The BLM protein is a RecQ family helicase with an ATP-dependent 3′-5′ DNA helicase activity. This helicase appears to be highly DNA structure specific, showing in vivo activity on branched DNA structures such as Holliday junctions which can occur during the repair of stalled or collapsed replication forks by HR90. Interestingly, purified FANCD2 has also been shown to bind DNA with an increased propensity for dsDNA ends and Holliday junctions91. The interaction between the BLM protein and the FA pathway was first elucidated through the purification of a BLM-associated multiprotein complex composed of multiple FA proteins as well as known BLM-interacting proteins since called the BRAFT complex. Under high salt conditions, BLM and its associated proteins dissociate from the FA proteins leaving the FA core complex92. Colocalization in nuclear foci and co-immunoprecipitation of FANCD2 and BLM following DNA damage provide further evidence linking the FA pathway to the HR pathway through interaction with BLM93.
In response to forms of DNA damage such as DSBs, the mammalian histone H2A variant H2AX is incorporated into DNA at sites surrounding the damage and is phosphorylated at serine 139 (serine 136 in mice) to generate γH2AX. The γH2AX histone variant serves as a marker of DSBs and helps to initiate the accumulation of DNA damage sensing and repairing proteins such as NBS1 and BRCA1 to these sites of damage in order to activate DNA damage signaling pathways and ultimately DNA repair through HR or NHEJ94. A functional connection between γH2AX and the FA pathway was first discovered in H2AX−/− mouse embryonic fibroblasts (MEFs). While UV irradiation in wild type MEFs prompted the formation of FANCD2 nuclear foci, the formation of FANCD2 foci was abolished in H2AX−/− cells95. FANCD2 and H2AX have since been shown to co-immunoprecipitate, an interaction dependent on H2AX Consistent with the idea that phosphorylation. γH2AX is important for FANCD2 recruitment to nuclear foci and for DNA repair, H2AX−/− and phosphorylation defective H2AX mutant cells have been shown to be hypersensitive to MMC treatment. Interestingly, this sensitivity is not further exacerbated by siRNA-mediated knockdown of FANCD2, suggesting that the two proteins function epistatically in the cellular response to DNA damage induced by MMC treatment95.
The FA pathway and Translesion synthesis (TLS) proteins
In order to continue replicating through sites of DNA damage, which block replicative polymerases and lead to replication fork stalling, cells employ the use of TLS polymerases. Each TLS polymerase is specialized to replicate through a specific type of DNA lesion and thus keep the replication fork moving regardless of DNA damage96. The protein proliferating cell nuclear antigen (PCNA) plays an essential role in this switch from replicative to TLS polymerase. PCNA functions as a polymerase clamp, tethering a polymerase to DNA in need of replication in order to increase processivity97. An interaction between PCNA and FA proteins was first suggested by studies indicating that PCNA colocalizes with FANCD1/BRCA2 as well as BRCA1 and RAD51 in nuclear foci following treatment with ultraviolet irradiation and hydroxyurea (HU)89. It was later discovered that PCNA also colocalizes in foci containing FANCD2 in cells treated with HU98, verifying the observation that DNA damage induces an interaction between PCNA and the FA pathway.
The REV1 protein is a eukaryotic member of the Y family of DNA polymerases, which function as TLS polymerases, replicating through sites of DNA damage. The REV1 protein is an error-prone TLS polymerase which functions as a deoxycytidyl transferase, inserting cytidine nucleotides opposite any template strand nucleotide as well as abasic sites during TLS mediated replication99. Studies in the DT40 chicken cell line revealed that cells deficient in FANCC, REV1, or another TLS polymerase REV3 showed similar levels of hypersensitivity to cisplatin treatment as measured by cell survival percentage and number of chromosomal aberrations per metaphase, leading to the inference that the proteins function epistatically in response to DNA crosslinking treatment100. Further studies in FA-A, FA-G and FA-D2 patient derived cell lines show that the core complex proteins FANCA and FANCG are required for REV1 nuclear foci formation while FANCD2 is not. Interestingly, mutation of the BRCT domain of REV1, which is necessary for its interaction with PCNA, does not further impair assembly of REV1 into nuclear foci in FANCG deficient cells, indicating that FANCG may facilitate localization of REV1 in nuclear foci101.
The USP1 protein is a ubiquitin specific protease or deubiquitylating enzyme (DUB), capable of cleaving ubiquitin moieties off of proteins102. The USP1 protein was found to play a role in the FA pathway through a gene family RNAi library screen, which showed that inhibition of USP1 resulted in increased accumulation of ubiquitylated FANCD2. Further experiments showed that USP1 physically interacts with FANCD2 and that the two proteins colocalize in chromatin after DNA damage103. This interaction between USP1 and FANCD2 ties the FA pathway to the TLS pathway as USP1 has since been found to also be the DUB for PCNA. Similar to results seen with FANCD2, inhibition of USP1 resulted in increased accumulation of monoubiquitylated PCNA104. These results have been replicated in a USP1 null chicken DT40 cell line in which eradication of USP1 also resulted in elevated levels of monoubiquitylated FANCD2 and PCNA105.
The FA Pathway and Cell Signaling
The FA pathway also interacts with the DNA damage response through the DNA sensing and signaling proteins ataxia telangiectasia (AT) mutated (ATM) kinase, ATM and Rad3-related (ATR) kinase, and Chk1 kinase. Cells from patients with AT display radio-hypersensitivity, cell cycle checkpoint defects, and chromosomal instability. The ATM protein is a serine/threonine kinase and is a member of the phosphatidylinositol-3 kinase –related protein kinase (PIKK) family of kinases. The main function of ATM seems to be recognizing and responding to DNA double strand breaks (DSBs) by initiating a signaling cascade which results in activation of DNA repair factors and ultimately DSB repair106. Taniguchi et al have shown that FANCD2−/− patient derived cells display a defect in S phase checkpoint response after treatment with IR similar to that seen in AT cells. A direct interaction between the ATM signaling pathway and the FA pathway was made as ATM was shown to phosphorylate FANCD2 on serine 222 in normal cells in response to IR and this phosphorylation event was shown to be necessary for proper cellular response to DSBs. Interestingly, the FANCD2-S222A phosphorylation mutant was monoubiquitylated and translocated into discrete nuclear foci following treatment with MMC implying that phosphorylation on serine 222 is not necessary for FANCD2 monoubiquitylation108.
Patients with biallelic mutations of the ATR kinase develop Seckel syndrome, an extremely rare autosomal recessive disease characterized by microcephaly, growth retardation, and mental retardation. Like ATM, ATR is a serine/threonine kinase and is a member of the phosphatidylinositol-3 kinase –related protein kinase (PIKK) family of kinases. The two proteins also share an overlapping set of protein substrates, which influence DNA repair and cell cycle arrest. However, unlike ATM, which becomes activated as a result of DSBs, ATR becomes activated every S phase of the cell cycle in order to signal for repair of collapsed replication forks and to prevent early initiation of mitosis109. An interaction between ATR and the FA pathway was first hinted at by experiments showing that FANCD2 and ATR colocalized in nuclear foci following treatment with DNA cross-linking agents110,111. Further experiments showed that depletion of ATR resulted in inhibition of FANCD2 foci formation and the development of radial chromosomes mimicking those seen in FA patient derived cells. Interestingly, two major sites of ATR mediated phosphorylation on FANCD2 are T691 and S717, phosphorylation of which is required for FANCD2 monoubiquitylation and correction of MMC sensitivity in FA-D2 patient derived cells112.
ATR also interacts with other members of the FA pathway. Phosphorylation of FANCG on serine 7 by ATR is necessary for the interaction of FANCG with FANCD1/BRCA2, XRCC2, and FANCD2113. ATR has also been shown to phosphorylate FANCI on serines 730 and 1121 as well as threonine 95262. Finally, downregulation of FANCM or its associated protein FAAP24 dysregulates ATR meditated checkpoint signaling, further suggesting some interplay between ATR mediated checkpoint signaling and the FA pathway114.
Another of the many substrates of ATR is the effector kinase Chk1. Activation of Chk1 in S phase suspends DNA replication, stabilizes stalled replication forks, and prevents preemptive mitosis initiation115. In an examination of the effects of Chk1 on the FA pathway, two highly conserved Chk1 phosphorylation consensus sequences were discovered in the FANCE protein at threonine 346 and serine 374. In vitro and in vivo experiments confirmed these residues as Chk1 phosphorylation sites. While expression of the FANCE-T346A/S374A double mutant in FA-E cells resulted in a failure to correct MMC sensitivity, FANCD2 monoubiquitylation and foci formation after MMC treatment were left intact. Further experiments showed that FANCE phosphorylated on T346 colocalized with FANCD2 in discrete nuclear foci following UV irradiation, suggesting that phosphorylated FANCE plays a role in DNA damage repair outside of the canonical FA pathway116.
The FA pathway, Oxidative Stress, Cytokine Sensitivity
Teleologically, the involvement of very specific developmental abnormalities in FA patients implies that the FA proteins have the potential for other functions aside from those they perform in protecting the genome. Others have argued that the main function of the FA pathway is to regulate oxidative stress, as reactive oxygen species (ROS) have been documented to be involved in bone marrow failure117,118, cancer119, endocrinopathies120, abnormalities in skin pigmentation121, and malformations122. This explanation becomes even more plausible when considering the redox-related functions of some FA proteins. Specifically, FANCC has been shown to associate with NADPH cytochrome P450 reductase and glutathione S-transferase, two proteins with redox functions123,124. Microarray studies comparing mRNA expression levels found that nuclear factor-1 (NF-1), heat shock protein 70 (HSP70), and cyclooxygenase 2 (COX-2) were consistently overexpressed in FANCC deficient cells as compared to their corrected counterparts125. The FANCG protein has been shown to interact with cytochrome P450 2E1 (CYP2E1), which has been shown to be involved in metabolism of xenobiotics such as MMC126. Finally, both FANCA and FANCG have been shown to be redox-sensitive proteins which multimerize following H2O2 treatment, lending plausibility to the hypothesis that the FA pathway may function in oxidative stress management in cells127.
Several lines of evidence have shown that excessive apoptosis and consequent malfunction of the hematopoietic stem cell compartment lead to progressive bone marrow failure in FA patients. The FANCC protein functions independently of the FA core complex to suppress apoptosis in hematopoietic cells in response to environmental cues, which induce expression or secretion of certain cytokines128. FA patients exhibit altered expression levels of some growth factors and cytokines, including unusually high levels of intracellular TNF-α, a cytokine capable of initiating the apoptotic pathway. In fact, hematopoietic stem cells with inactivating mutations in the FANCC gene are hypersensitive to cytokines such as IFNγ and TNF-α128. However, neoplastic stem cell clones, which are resistant to these cytokines frequently evolve in FA patients and result in leukemia. This phenomenon was more closely examined by Li, et.al., through the use of a murine Fancc−/− model. Recapitulating what likely happens in FA patients, exposure of murine Fancc−/− stem cells to TNF-α results in inhibition of growth in the short term but promotes evolution of clones resistant to TNF-α when treated for longer periods of time. These long term treated TNF-α resistant outgrowth cells, when transplanted into wild type mice, result in acute myelogenous leukemia, mimicking their human counterparts. Importantly, expression of FANCC cDNA in the fancc−/− stem cells prevented the formation of leukemic clonal outgrowths, implying that the FA pathway and the FANCC protein are necessary for an intact cellular response to TNF-α129.
The growth inhibition seen in murine fancc−/− stem cells after short-term exposure to TNF-α was further explored by the Pang group and found to correlate with accumulation of reactive oxygen species (ROS). Deletion of the TNF-α receptor in fancc−/− mice resulted in a reduction in the amount of ROS produced as well as reduced levels of hematopoietic senescence. Cells from TNF-α treated fancc−/− mice also showed increased levels of chromosomal aberrations and decreased levels of repair of DNA damage caused by ROS, indicating that FANCC may also play a role in the cellular response to oxidative DNA damage129.
The FANCC protein has also been found to interact with and be necessary for the proper localization of the STAT1 transcription factor following stimulation with IFNγ. Loss of functional FANCC results in reduced levels of STAT1 activation and impaired Th1 differentiation, possibly leading to a slight immunological defect in FA patients130. Stimulation with IFNγ has also been found to activate the RNA dependent protein kinase PKR, which has in fact been found to be constitutively activated in FANCC deficient cells. Activated PKR phosphorylates the translation initiation factor eIF2 to arrest protein synthesis. The FANCC protein has been found to interact with the molecular chaperone protein Hsp70 which suppresses PKR activation. The two proteins acting together are able to inactivate PKR and prevent apoptosis caused by IFNγ stimulation131. From these lines of evidence it can be inferred that the FANCC protein is necessary for the cell to properly respond to IFNγ stimulation, which is necessary for proper immunological differentiation and apoptosis avoidance.
Concluding Remarks
Our knowledge about the pathway and the disease seems to grow exponentially with each passing year, as two of the FA proteins (FANCI and FANCN) were discovered and characterized in 2007. The body of work has delineated a pathway with three distinct subdivisions of proteins, but many questions remain to be answered. Some questions involve the function of individual FA proteins: Is FANCL the E3 ubiquitin ligase for FANCD2 and FANCI? Is the helicase activity of FANCM important to the FA pathway or does FANCM solely serve as a DNA translocase? Does the FANCJ helicase interact directly with the FA core complex or downstream partners? Several questions involve the mechanisms of the FA pathway proteins complexes: What are the functions of the subcomplexes composed by core complex proteins? What is the function of the BRAFT complex? What is the function of the FANCD2 and FANCI proteins within nuclear foci? How are the downstream effector FA proteins recruited to these nuclear foci? Other outstanding questions are much broader and involve the pathway itself: Is the FA pathway truly a DNA damage response and repair pathway, an oxidative stress response pathway, or a general stress response pathway for stem cells? Have we even discovered all of the proteins within the pathway? While the field has come a long way within the past few years, there is still much to learn. Elucidation of the intricacies of the FA pathway will ultimately allow for more individualized and efficacious treatment of FA patients and may provide insights into other cancer susceptibility diseases.
Figure 1. The FA pathway proteins.

The FA pathway is composed of at least 13 genes. Each of these genes, when biallelically mutated, causes FA. The encoded proteins can be subdivided within the FA pathway into three groups: proteins that make up the core complex, the FANCD2 and FANCI proteins which compose the ID complex, and three downstream effector proteins: FANCD1/BRCA2, FANCJ/BRIP1/BACH1, and FANCN/PALB2. Following treatment with DNA crosslinking agents or during S phase of the cell cycle, FANCD2 and FANCI become monoubiquitylated. An intact core complex is required for these modifications which result in the translocation of the two proteins to chromatin within cells. Within chromatin, FANCD2 and FANCI colocalize with DNA repair proteins including the downstream effector FA proteins at sites of DNA damage in nuclear foci. FA proteins are in blue.
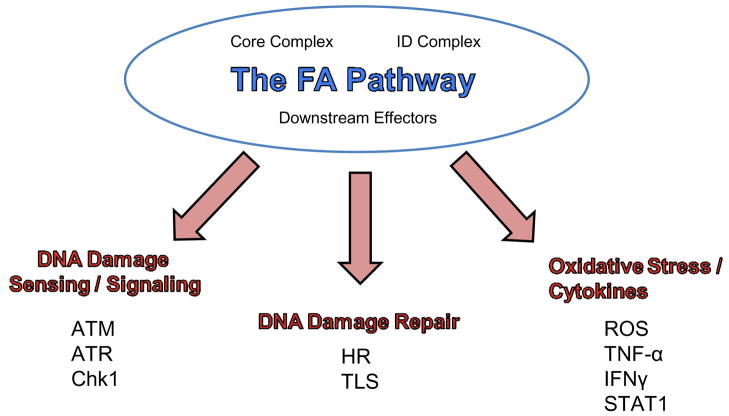
Figure 2. FA pathway functions.
While an exact mechanism for the FA pathway has yet to be elucidated, it seems to function in sensing DNA damage induced by DNA crosslinking agents such as MMC and DEB likely plays some role in initiating DNA repair. A significant body of work has also pointed to a role for the FA pathway in cell signaling in response to stress stimuli and in apoptosis in the cytoplasm, affecting maintenance of the hematopoietic machinery so it is likely that at least some of these proteins are multifunctional.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bagby GC, Alter BP. Fanconi Anemia. Semin Hematol. 2006;43(3):147–156. doi: 10.1053/j.seminhematol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 2.D’Andrea AD, Dahl N, Guinan EC, et al. Marrow Failure. Hematology Am Soc Hematol Educ Program. 2002:58–72. doi: 10.1182/asheducation-2002.1.58. [DOI] [PubMed] [Google Scholar]
- 3.Tischkowitz M, Dokal I. Fanconi anaemia and leukemia- clinical and molecular aspects. Br J Haematol. 2004;126(2):176–191. doi: 10.1111/j.1365-2141.2004.05023.x. [DOI] [PubMed] [Google Scholar]
- 4.Alter BP. Fanconi’s anemia, transplantation, and cancer. Pediatr Transplant. 2005;9(Suppl 7):81–86. doi: 10.1111/j.1399-3046.2005.00440.x. [DOI] [PubMed] [Google Scholar]
- 5.Lustig JP, Lugassy G, Neder A, et al. Head and neck carcinoma in Fanconi’s anaemia--report of a case and review of the literature. Eur J Cancer B Oral Oncol. 1995;31B(1):68–72. doi: 10.1016/0964-1955(94)00044-5. [DOI] [PubMed] [Google Scholar]
- 6.Lowy Dr, Gillison ML. A new link between Fanconi anemia and human papillomavirus-associated malignancies. J Natl Cancer Inst. 2003;95(22):1648–1650. doi: 10.1093/jnci/djg125. [DOI] [PubMed] [Google Scholar]
- 7.Masserot C, Peffault de Latour R, Rocha V, et al. Head and neck squamous cell carcinoma in 13 patients with Fanconi anemia after hematopoietic stem cell transplantation. Cancer. 2008 doi: 10.1002/cncr.23954. [DOI] [PubMed] [Google Scholar]
- 8.Socie G, Scieux C, Gluckman E, et al. Squamous cell carcinomas after allogeneic bone marrow transplantation for aplastic anemia: further evidence of a multistep process. Transplantation. 1998;66(5):667–670. doi: 10.1097/00007890-199809150-00023. [DOI] [PubMed] [Google Scholar]
- 9.Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2003 doi: 10.1002/0471142905.hg0807s37. Chapter 8: Unit8.7. [DOI] [PubMed] [Google Scholar]
- 10.Hirschhorn R. In vivo reversion to normal of inherited mutations in humans. J Med Genet. 2003;40(10):721–728. doi: 10.1136/jmg.40.10.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregory JJ, Jr, Wagner JE, Verlander PE, et al. Somatic mosaicism in Fanconi anemia: evidence of genotypic reversion in lymphohematopoietic stem cells. Proc Natl Acad Sci. 2001;98(5):2532–2537. doi: 10.1073/pnas.051609898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soulier J, Leblanc T, Larghero J, et al. Detection of somatic mosaicism and classification of Fanconi anemia patients by analysis of the FA/BRCA pathway. Blood. 2005;105(3):1329–1336. doi: 10.1182/blood-2004-05-1852. [DOI] [PubMed] [Google Scholar]
- 13.Lo Ten Foe JR, Kwee ML, Rooimans MA, et al. Somatic mosaicism in Fanconi anemia: molecular basis and clinical significance. Eur J Hum Genet. 1997;5(3):137–148. [PubMed] [Google Scholar]
- 14.Xie Y, de Winter JP, Waisfisz Q, et al. Aberrant Fanconi anemia protein profiles in acute myeloid leukemia cells. Br J Haematol. 2000;111(4):1057–1064. [PubMed] [Google Scholar]
- 15.Velez-Ruelas MA, Martinez-Jaramillo G, Arana-Trejo RM, et al. Hematopoietic changes during progression from Fanconi anemia into acute myeloid leukemia: case report and brief review of the literature. Hematology. 2006;11(5):331–334. doi: 10.1080/10245330500397703. [DOI] [PubMed] [Google Scholar]
- 16.Alter BP, Rosenberg PS, Brody LC, et al. Clinical and molecular features associated with biallelic mutations in FANCD1/BRCA2. J Med Genet. 2007;44(1):1–9. doi: 10.1136/jmg.2006.043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer S, Fergusson WD, Whetton AD, et al. Amplification and translocation of 3q26 with overexpression of EVI1 in Fanconi anemia-derived childhood acute myeloid leukemia with biallelic FANCD1/BRCA2 disruption. Genes Chromosomes Cancer. 2007;46(4):359–372. doi: 10.1002/gcc.20417. [DOI] [PubMed] [Google Scholar]
- 18.Gillio AP, Verlander PC, Batish SD, et al. Phenotypic consequences of mutations in the Fanconi anemia FAC gene: an International Fanconi Anemia Registry study. Blood. 1997;90(1):105–110. [PubMed] [Google Scholar]
- 19.Auerbach AD. Fanconi anemia: genetic testing in Ashkenazi Jews. Genet Test. 1997;1(1):27–33. doi: 10.1089/gte.1997.1.27. [DOI] [PubMed] [Google Scholar]
- 20.Farzin A, Davies SM, Smith FO, et al. Matched sibling donor haematopoietic stem cell transplantation in Fanconi anemia: an update of the Cincinnati Children’s experience. Br J Haematol. 2007;136(4):633–640. doi: 10.1111/j.1365-2141.2006.06460.x. [DOI] [PubMed] [Google Scholar]
- 21.Pasquini R, Carreras J, Pasquini MC, et al. HLA-matched sibling hematopoietic stem cell transplantation for Fanconi anemia: comparison of irradiation and nonirradiation containing conditioning regimens. Biol Blood Marrow Transplant. 2008;14(10):1141–1147. doi: 10.1016/j.bbmt.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gluckman E, Wagner JE. Hematopoietic stem cell transplantation in childhood inherited bone marrow failure syndromes. Bone Marrow Transplant. 2008;41(2):127–132. doi: 10.1038/sj.bmt.1705960. [DOI] [PubMed] [Google Scholar]
- 23.Brown JA, Boussiotis VA. Umbilical cord blood transplantation: basic biology and clinical challenges to immune reconstitution. Clin Immunol. 2008;127(3):286–297. doi: 10.1016/j.clim.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burt RK, Loh Y, Pearce W, et al. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA. 2008;299(8):925–936. doi: 10.1001/jama.299.8.925. [DOI] [PubMed] [Google Scholar]
- 25.Craddock CF. Full-intensity and reduced-intensity allogeneic stem cell transplantation in AML. Bone Marrow Transplant. 2008;41(5):415–423. doi: 10.1038/sj.bmt.1705975. [DOI] [PubMed] [Google Scholar]
- 26.Brunstein CG, Baker KS, Wagner JE. Umbilical cord blood transplantation for myeloid malignancies. Curr Opin Hematol. 2007;14(2):162–9. doi: 10.1097/MOH.0b013e32802f7da4. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhury S, Auerbach AD, Kernan NA, et al. Fludarabine-based cytoreductive regimen and T-cell-depleted grafts from alternative donors for the treatment of high-risk patients with Fanconi anaemia. Br J Haematol. 2008;140(6):644–655. doi: 10.1111/j.1365-2141.2007.06975.x. [DOI] [PubMed] [Google Scholar]
- 28.Gluckman E, Rocha V, Ionescu I, et al. Results of unrelated cord blood transplant in fanconi anemia patients: risk factor analysis for engraftment and survival. Biol Blood Marrow Transplant. 2007;13(9):1073–1082. doi: 10.1016/j.bbmt.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Bitan M, Or R, Shapira MY, et al. Fludarabine-based reduced intensity conditioning for stem cell transplantation of Fanconi anemia patients from fully matched related and unrelated donors. Biol Blood Marrow Transplant. 2006;12(7):712–718. doi: 10.1016/j.bbmt.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Huck K, Hanenberg H, Nurnberger W, et al. Favourable long-term outcome after matched sibling transplantation for Fanconi-anemia (FA) and in vivo T-cell depletion. Klin Padiatr. 2008;220(3):147–152. doi: 10.1055/s-2008-1065326. [DOI] [PubMed] [Google Scholar]
- 31.Balci YI, Akdemir Y, Gumruk F, et al. CD-34 selected hematopoetic stem cell transplantation from HLA identical family members for fanconi anemia. Pediatr Blood Cancer. 2008;50(5):1065–1067. doi: 10.1002/pbc.21424. [DOI] [PubMed] [Google Scholar]
- 32.Ayas M, Al-Jefri A, Al-Seraihi A, et al. Allogeneic stem cell transplantation in Fanconi anemia patients presenting with myelodysplasia and/or clonal abnormality: update on the Saudi experience. Bone Marrow Transplant. 2008;41(3):261–265. doi: 10.1038/sj.bmt.1705903. [DOI] [PubMed] [Google Scholar]
- 33.Millen FJ, Rainey MG, Hows JM, et al. Oral squamous cell carcinoma after allogeneic bone marrow transplantation for Fanconi anaemia. Br J Haematol. 1997;99(2):410–414. doi: 10.1046/j.1365-2141.1997.3683184.x. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg PS, Alter BP, Socie G, et al. Secular trends in outcomes for Fanconi anemia patients who receive transplants: implications for future studies. Biol Blood Marrow Transplant. 2005;11(9):672–679. doi: 10.1016/j.bbmt.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Croop JM. Gene therapy for fanconi anemia. Curr Hematol Rep. 2003;2(4):335–340. [PubMed] [Google Scholar]
- 36.Yamada K, Ramezani A, Hawley RG, et al. Phenotype correction of Fanconi anemia group A hematopoietic stem cells using lentiviral vector. Mol Ther. 2003;8(4):600–610. doi: 10.1016/s1525-0016(03)00223-5. [DOI] [PubMed] [Google Scholar]
- 37.Kelly PF, Radtke S, von Kalle C, et al. Stem cell collection and gene transfer in Fanconi anemia. Mol Ther. 2007;15(1):211–219. doi: 10.1038/sj.mt.6300033. [DOI] [PubMed] [Google Scholar]
- 38.Si Y, Ciccone S, Yang FC, et al. Continuous in vivo infusion of interferon-gamma (IFN-gamma) enhances engraftment of syngeneic wild-type cells in Fanca−/− and Fancg−/− mice. Blood. 2006;108(13):4283–4287. doi: 10.1182/blood-2006-03-007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozenne V, Paradis V, Vullierme MP, et al. Liver tumours in patients with Fanconi anaemia: a report of three cases. Eur J Gastroenterol Hepatol. 2008;20(10):1036–1039. doi: 10.1097/MEG.0b013e3282f824e9. [DOI] [PubMed] [Google Scholar]
- 40.Velazquez I, Alter BP. Androgens and liver tumors: Fanconi’s anemia and non-Fanconi’s conditions. Am J Hematol. 2004;77(3):257–267. doi: 10.1002/ajh.20183. [DOI] [PubMed] [Google Scholar]
- 41.Akkari YM, Bateman RL, Reifsteck CA, et al. The 4N cell cycle delay in Fanconi anemia reflects growth arrest in late S phase. Mol Genet Metab. 2001;74(4):403–412. doi: 10.1006/mgme.2001.3259. [DOI] [PubMed] [Google Scholar]
- 42.Meetei AR, Medhurst AL, Ling C, et al. A human ortholog of archaeal DNA repair protein Hef is defective in Fanconi anemia complementation group M. Nat Genet. 2005;37(9):958–963. doi: 10.1038/ng1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lightfoot J, Alon N, Bosnoyan-Collins L, et al. Characterization of regions functional in the nuclear localization of the Fanconi anemia group A protein. Human Mol Genet. 1999;8(6):1007–1015. doi: 10.1093/hmg/8.6.1007. [DOI] [PubMed] [Google Scholar]
- 44.Meetei AR, Levitus M, Xue Y, et al. X-linked inheritance of Fanconi anemia complementation group B. Nat Genet. 2004;36(11):1219–1224. doi: 10.1038/ng1458. [DOI] [PubMed] [Google Scholar]
- 45.de Winter JP, Leveille F, van Berkel CGM, et al. Isolation of a cDNA Representing the Fanconi Anemia Complementation Group E Gene. Am J Hum Genet. 2000;67(5):1306–1308. doi: 10.1016/s0002-9297(07)62959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meetei AR, de Winter JP, Medhurst AL, et al. A novel ubiquitin ligase is deficient in Fanconi anemia. Nat Genet. 2003;35(2):165–170. doi: 10.1038/ng1241. [DOI] [PubMed] [Google Scholar]
- 47.Gurtan AM, Stuckert P, D’Andrea AD. The WD40 Repeats of FANCL Are Required for Fanconi Anemia Core Complex Assembly. J Biol Chem. 2006;281(16):10806–10905. doi: 10.1074/jbc.M511411200. [DOI] [PubMed] [Google Scholar]
- 48.Kim JM, Kee Y, Gurtan A, et al. Cell cycle dependent chromatin loading of the Fanconi anemia core complex by FANCM/FAAP24. Blood. 2008;111(10):5215–5222. doi: 10.1182/blood-2007-09-113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komori K, Fujikane R, Shinagawa H, et al. Novel endonuclease in Archaea cleaving DNA with various branched structure. Genes Genet Syst. 2002;77(4):227–241. doi: 10.1266/ggs.77.227. [DOI] [PubMed] [Google Scholar]
- 50.Gari K, Decaillet C, Stasiak AZ, et al. The Fanconi Anemia Protein FANCM Can Promote Branch Migration of Holliday Junctions and Replication Forks. Mol Cell. 2008;29(1):141–148. doi: 10.1016/j.molcel.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 51.Yamashita T, Kupfer GM, Naf D, et al. The Fanconi anemia pathway requires FAA phosphorylation and FAA/FAC nuclear accumulation. PNAS. 1998;95(22):13085–13090. doi: 10.1073/pnas.95.22.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiao F, Mi J, Wilson JB, et al. Phosphorylation of Fanconi Anemia (FA) Complementation Group G Protein, FANCG, at Serine 7 Is Important for Function of the FA Pathway. J Biol Chem. 2004;279(44):46035–46045. doi: 10.1074/jbc.M408323200. [DOI] [PubMed] [Google Scholar]
- 53.Mi J, Qiao F, Wilson JB, et al. FANCG is Phosphorylated at Serines 383 and 387 during Mitosis. Mol Cell Biol. 2004;24(19):8576–8585. doi: 10.1128/MCB.24.19.8576-8585.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Medhurst AL, El Houari L, Steltenpool J, et al. Evidence for subcomplexes in the Fanconi anemia pathway. Blood. 2006;108(6):2072–2080. doi: 10.1182/blood-2005-11-008151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Folias A, Matkovic M, Bruun D, et al. BRCA1 interacts directly with the Fanconi anemia protein FANCA. Hum Mol Genet. 2002;11(21):2591–2597. doi: 10.1093/hmg/11.21.2591. [DOI] [PubMed] [Google Scholar]
- 56.Hussain S, Witt E, Huber PAJ, et al. Direct interaction of the Fanconi anemia protein FANCG with BRCA2/FANCD1. Hum Mol Genet. 2003;12(19):2503–2510. doi: 10.1093/hmg/ddg266. [DOI] [PubMed] [Google Scholar]
- 57.Gordon SM, Buchwald M. Fanconi anemia protein complex: mapping protein interactions in the yeast 2- and 3- hybrid systems. Blood. 2003;102(1):136–141. doi: 10.1182/blood-2002-11-3517. [DOI] [PubMed] [Google Scholar]
- 58.Pace P, Johnson M, Tan WM, et al. FANCE: the link between Fanconi anemia complex assembly and activity. EMBO. 2002;21(13):3414–3423. doi: 10.1093/emboj/cdf355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Higuera I, Taniguchi T, Ganesan S, et al. Interaction of the Fanconi Anemia Proteins and BRCA1 in a Common Pathway. Mol Cell. 2001;7:249–262. doi: 10.1016/s1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 60.Taniguchi T, Garcia-Higuera I, Andreassen PR, et al. S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51. Blood. 2002;100:2414–2420. doi: 10.1182/blood-2002-01-0278. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, Andreassen PR, D’Andrea AD. Functional interaction of monoubiquitinated FANCD2 and BRCA2/FANCD1 in chromatin. Mol Cell Biol. 2004;24(13):5850–5862. doi: 10.1128/MCB.24.13.5850-5862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smogorzewska A, Matsuoka S, Vinciguerra P, et al. Identification of the FANCI Protein, a Monoubiquitinated FANCD2 Paralog Required for DNA Repair. Cell. 2007;129(2):289–301. doi: 10.1016/j.cell.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sims AE, Spiteri E, Sims RJ, 3rd, et al. FANCI is a second monoubiquitinated member of the Fanconi anemia pathway. Nat Struct Mol Biol. 2007;14(6):564–567. doi: 10.1038/nsmb1252. [DOI] [PubMed] [Google Scholar]
- 64.Dorsman JC, Levitus M, Rockx D, et al. Identification of the Fanconi anemia complementation group I gene, FANCI. Cell Oncol. 2007;29(3):211–218. doi: 10.1155/2007/151968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levitus M, Rooimans MA, Steltenpool J, et al. Heterogeneity in Fanconi anemia: evidence for 2 new genetic subtypes. Blood. 2004;103(7):2498–2503. doi: 10.1182/blood-2003-08-2915. [DOI] [PubMed] [Google Scholar]
- 66.Taniguchi T, Garcia-Higuera I, Xu B, et al. Convergence of the Fanconi anemia and Ataxia telangiectasia signaling pathways. Cell. 2002;109(4):459–472. doi: 10.1016/s0092-8674(02)00747-x. [DOI] [PubMed] [Google Scholar]
- 67.Ishiai M, Kitao H, Smogorzewska A, et al. FANCI phosphorylation functions as a molecular switch to turn on the Fanconi anemia pathway. Nat Struct Mol Biol. 2008;15(11):1138–1146. doi: 10.1038/nsmb.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Howlett NG, Taniguchi T, Olson S, et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;297(5581):606–609. doi: 10.1126/science.1073834. [DOI] [PubMed] [Google Scholar]
- 69.Godthelp BC, Wiegant WW, Waisfisz Q, et al. Inducibility of nuclear Rad51 foci after DNA damage distinguishes all Fanconi anemia complementation groups from D1/BRCA2. Mut Research. 2006;594(1–2):39–48. doi: 10.1016/j.mrfmmm.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 70.Shinohara A, Ogawa H, Ogawa T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992;69(3):457–470. doi: 10.1016/0092-8674(92)90447-k. [DOI] [PubMed] [Google Scholar]
- 71.Wong AKC, Pero R, Ormonde PA, et al. RAD51 interacts with the evolutionarily conserved BRC motifs in the human breast cancer susceptibility gene brca2. J Biol Chem. 1997;272(51):31941–31944. doi: 10.1074/jbc.272.51.31941. [DOI] [PubMed] [Google Scholar]
- 72.Yang H, Jeffrey PD, Miller J, et al. BRCA2 function in DNA binding and recombination from a BRCA2-DSS1-ssDNA structure. Science. 2002;297(5588):1837–1848. doi: 10.1126/science.297.5588.1837. [DOI] [PubMed] [Google Scholar]
- 73.Li J, Zou C, Bai Y, et al. DSS1 is required for the stability of BRCA2. Oncogene. 2006;25(8):1186–1194. doi: 10.1038/sj.onc.1209153. [DOI] [PubMed] [Google Scholar]
- 74.Levitus M, Waisfisz Q, Godthelp BC, et al. The DNA helicase BRIP1 is defective in Fanconi anemia complementation group. J Nat Genet. 2005;37(9):934–935. doi: 10.1038/ng1625. [DOI] [PubMed] [Google Scholar]
- 75.Levran O, Attwooll C, Henry RT, et al. The BRCA1-interacting helicase BRIP1 is deficient in Fanconi anemia. Nat Genet. 2005;37(9):931–933. doi: 10.1038/ng1624. [DOI] [PubMed] [Google Scholar]
- 76.Litman R, Peng M, Jin Z, et al. BACH1 is critical for homologous recombination and appears to be the Fanconi anemia gene product FANCJ. Cancer Cell. 2005;8(3):255–265. doi: 10.1016/j.ccr.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Cantor SB, Bell DW, Ganesan S, et al. BACH1, a Novel Helicase-like Protein, Interacts Directly with BRCA1 and Contributes to Its DNA Repair Function. Cell. 2001;105(1):149–160. doi: 10.1016/s0092-8674(01)00304-x. [DOI] [PubMed] [Google Scholar]
- 78.Yu X, Chini CCS, He M, et al. The BRCT Domain is a Phospho-protein Binding Domain. Science. 2003;302(5645):639–642. doi: 10.1126/science.1088753. [DOI] [PubMed] [Google Scholar]
- 79.Wu Y, Shin-ya K, Brosh RM., Jr FANCJ helicase defective in Fanconi anemia and breast cancer unwinds G-quadruplex DNA to defend genomic instability. Mol Cell Biol. 2008;28(12):4116–4128. doi: 10.1128/MCB.02210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xia B, Dorsman JC, Ameziane N, et al. Fanconi anemia is associated with a defect in the BRCA2 partner PALB2. Nat Genet. 2007;39(2):159–161. doi: 10.1038/ng1942. [DOI] [PubMed] [Google Scholar]
- 81.Reid S, Schindler D, Hanenberg H, et al. Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat Genet. 2007;39(2):162–164. doi: 10.1038/ng1947. [DOI] [PubMed] [Google Scholar]
- 82.Dronkert ML, Kanaar R. Repair of DNA interstrand cross-links. Mutat Res. 2001;486(4):217–247. doi: 10.1016/s0921-8777(01)00092-1. [DOI] [PubMed] [Google Scholar]
- 83.Nojima K, Hochegger H, Saberi A, et al. Multiple repair pathways mediate tolerance to chemotherapeutic cross-linking agents in vertebrate cells. Cancer Res. 2005;65(24):11704–11711. doi: 10.1158/0008-5472.CAN-05-1214. [DOI] [PubMed] [Google Scholar]
- 84.Shin DS, Chahwan C, Huffman JL, et al. Structure and function of the double strand break repair machinery. DNA Repair (Amst) 2004;4(2):91–98. doi: 10.1016/j.dnarep.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 85.Sharan SK, Morimatsu M, Albrecht U, et al. Embryonic lethality and radiation hypersensitivity mediated by Rad51in mice lacking Brca2. Nature. 1997;386(6627):804–810. doi: 10.1038/386804a0. [DOI] [PubMed] [Google Scholar]
- 86.Scully R, Xie A, Nagaraju G. Molecular functions of BRCA1 in the DNA damage response. Cancer Biol Ther. 2004;3(6):521–527. doi: 10.4161/cbt.3.6.842. [DOI] [PubMed] [Google Scholar]
- 87.Rajan JV, Wang ST, Marquis ST, et al. BRCA2 is coordinately regulated with BRCA1 during proliferation and differentiation in mammary epithelial cells. Proc Natl Acad Sci. 1996;93(23):13078–13083. doi: 10.1073/pnas.93.23.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scully R, Chen J, Plug A, et al. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88(2):265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- 89.Chen J, Silver DP, Walpita D, et al. Stable interaction between the products of the BRCA1 and BRCA2 tumor suppressor genes in mitotic and meiotic cells. Mol Cell. 1998;2(3):317–328. doi: 10.1016/s1097-2765(00)80276-2. [DOI] [PubMed] [Google Scholar]
- 90.Seki M, Tada S, Enomoto T. Function of recQ family helicase in genome stability. Subcell Biochem. 2006;40:49–73. doi: 10.1007/978-1-4020-4896-8_5. [DOI] [PubMed] [Google Scholar]
- 91.Park WH, Margossian S, Horwitz AA, et al. Direct DNA binding activity of the Fanconi anemia D2 protein. J Biol Chem. 2005;280(25):23593–23958. doi: 10.1074/jbc.M503730200. [DOI] [PubMed] [Google Scholar]
- 92.Meetei AR, Sechi S, Wallisch M, et al. A multiprotein nuclear complex connects Fanconi anemia and Bloom syndrome. Mol Cell Biol. 2003;23(10):3417–3426. doi: 10.1128/MCB.23.10.3417-3426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pichierri P, Franchitto A, Rosselli F. BLM and the FANC proteins collaborate in a common pathway in response to stalled replication forks. EMBO. 2004;23(15):3154–3163. doi: 10.1038/sj.emboj.7600277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kinner A, Wu W, Staudt C, et al. Gamma-H2AX in recognition and signaling of DNA double strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36(17):5678–5694. doi: 10.1093/nar/gkn550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bogliolo M, Lyakhovich A, Callen E, et al. Histone H2AX and Fanconi anemia FANCD2 function in the same pathway to maintain chromosome stability. EMBO. 2007;26(5):1340–1351. doi: 10.1038/sj.emboj.7601574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCulloch SD, Kunkel TA. The fidelity of DNA synthesis by eukaryotic replicative and translesion synthesis polymerases. Cell Res. 2008;18(1):148–161. doi: 10.1038/cr.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andersen PL, Xu F, Xiao W. Eukaryotic DNA damage tolerance and translesion synthesis through covalent modifications of PCNA. Cell Res. 2008;18(1):162–173. doi: 10.1038/cr.2007.114. [DOI] [PubMed] [Google Scholar]
- 98.Hussain S, Wilson JB, Medhurst AL, et al. Direct interaction of FANCD2 with BRCA2 in DNA damage response pathways. Hum Mol Genet. 2004;13(12):1241–1248. doi: 10.1093/hmg/ddh135. [DOI] [PubMed] [Google Scholar]
- 99.Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu Rev Biochem. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- 100.Niedzwiedz W, Mosedale G, Johnson M, et al. The Fanconi anemia gene FANCC promotes homologous recombination and error prone DNA repair. Mol Cell. 2004;15:607–620. doi: 10.1016/j.molcel.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 101.Mirchandani KD, McCaffrey RM, D’Andrea AD. The Fanconi anemia core complex is required for efficient point mutagenesis and Rev1 foci assembly. DNA Repair. 2008;7:902–911. doi: 10.1016/j.dnarep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fujiwara T, Saito A, Suzuki M, et al. Identification and chromosomal assignment of USP1, a novel gene encoding a human ubiquitin-specific protease. Genomics. 1998;54(1):155–158. doi: 10.1006/geno.1998.5554. [DOI] [PubMed] [Google Scholar]
- 103.Nijman SM, Huang TT, Dirac AM, et al. The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Mol Cell. 2005;17(3):331–339. doi: 10.1016/j.molcel.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 104.Huang TT, Nijman SM, Mirchandani KD, et al. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat Cell Biol. 2006;8(4):339–347. doi: 10.1038/ncb1378. [DOI] [PubMed] [Google Scholar]
- 105.Oestergaard VH, Langevin F, Kuiken HJ, et al. Deubiquitination of FANCD2 is required for DNA crosslink repair. Mol Cell. 2007;28(5):798–809. doi: 10.1016/j.molcel.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signaling and cancer. Mol Cell Biol. 2008;9:759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- 107.Djuzenova C, Flentje M, Plowman PN. Radiation response in vitro of fibroblasts from a Fanconi anemia patient with marked clinical radiosensitivity. Strahlentherapie und Onkologie. 2004;180(12):789–797. doi: 10.1007/s00066-004-1250-1. [DOI] [PubMed] [Google Scholar]
- 108.Taniguchi T, Garcia-Higuera I, Xu B, et al. Convergence of the Fanconi anemia and Ataxia-telangiectasia signaling pathways. Cell. 2002;109:459–472. doi: 10.1016/s0092-8674(02)00747-x. [DOI] [PubMed] [Google Scholar]
- 109.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Mol Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pichierri P, Rosselli F. The DNA crosslink induced S-phase checkpoint depends on ATR-CHK1 and ATR-NBS1-FANCD2 pathways. EMBO. 2004;23:1178–1187. doi: 10.1038/sj.emboj.7600113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Andreassen PR, D’Andrea AD, Taniguchi T. ATR couples FANCD2 monoubiquitination to the DNA damage response. Genes Dev. 2004;18:1958–1963. doi: 10.1101/gad.1196104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ho GP, Margossian S, Taniguchi T, et al. Phosphorylation of FANCD2 on two novel sites is required for mitomycin C resistance. Mol Cell Biol. 2006;26(18):7005–7015. doi: 10.1128/MCB.02018-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wilson JB, Yamamoto K, Marriott AS, et al. FANCG promotes formation of a newly identified protein complex containing BRCA2, FANCD2 and XRCC3. Oncogene. 2008;27:3641–3652. doi: 10.1038/sj.onc.1211034. [DOI] [PubMed] [Google Scholar]
- 114.Collis SJ, Ciccia A, Deans AJ, et al. FANCM and FAAP24 function in ATR mediated checkpoint signaling independently of the Fanconi anemia core complex. Mol Cell. 32:313–324. doi: 10.1016/j.molcel.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 115.Enders GH. Expanded roles for Chk1 in genome maintenance. J Biol Chem. 2008;283(26):17749–17752. doi: 10.1074/jbc.R800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang X, Kennedy RD, Ray K, et al. Chk1 mediated phosphorylation of FANCE is required for the Fanconi anemia/BRCA pathway. Mol Cell Biol. 2007;27(8):3098–3108. doi: 10.1128/MCB.02357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gordon-Smith EC, Rutherford TR. Fanconi anemia- constitutional, familial aplastic anemia. Bailliere’s Clin Haematol. 1989;2:139–152. doi: 10.1016/s0950-3536(89)80011-3. [DOI] [PubMed] [Google Scholar]
- 118.Bornman L, Baladi S, Richard MJ, et al. Differential regulation and expression of stress proteins and ferritin in human monocytes. J Cell Physiol. 1999;178:1–8. doi: 10.1002/(SICI)1097-4652(199901)178:1<1::AID-JCP1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 119.Kovacic P, Jacintho JD. Mechanisms of carcinogenesis: focus on oxidative stress and electron transfer. Curr Med Chem. 2001;8:773–796. doi: 10.2174/0929867013373084. [DOI] [PubMed] [Google Scholar]
- 120.Evans LM, Davies JS, Anderson RA, et al. The effect of GH replacement therapy on endothelial function and oxidative stress in adult growth hormone deficiency. Eur J Endocrinol. 2000;142:254–262. doi: 10.1530/eje.0.1420254. [DOI] [PubMed] [Google Scholar]
- 121.Memoli S, Napolitanto A, D’Ischia M, et al. Diffusible melanin-related metabolites are potent inhibitors of lipid peroxidation. Biochim Biophys Acta. 1997;1346:61–68. doi: 10.1016/s0005-2760(97)00018-0. [DOI] [PubMed] [Google Scholar]
- 122.Wells PG, Kim PM, Laposa RR, et al. Oxidative damage in chemical teratogenesis. Mutat Res. 1997;396:65–78. doi: 10.1016/s0027-5107(97)00175-9. [DOI] [PubMed] [Google Scholar]
- 123.Kruyt FA, Hoshino T, Liu JM, et al. Abnormal microsomal detoxification implicated in Fanconi anemia group C by interaction of the FAC protein with NADPH cytochrome P450 reductase. Blood. 1998;92:3050–3056. [PubMed] [Google Scholar]
- 124.Cumming RC, Lightfoot J, Beard K, et al. Fanconi anemia fropu C protein prevents apoptosis in hematopoietic cells through redox regulation of GSTP1. Nat Med. 2001;7:814–820. doi: 10.1038/89937. [DOI] [PubMed] [Google Scholar]
- 125.Zanier R, Briot D, Villard JA, et al. Fanconi anemia C gene product regulates the expression of genes involved in differentiation and inflammation. Oncogene. 2004;23:5004–5013. doi: 10.1038/sj.onc.1207677. [DOI] [PubMed] [Google Scholar]
- 126.Futaki M, Igarashi T, Watanabe S, et al. The FANCG Fanconi anemia protein interacts with CYP2E1: possible role in protection against oxidative DNA damage. Carcinogenesis. 2002;23:67–72. doi: 10.1093/carcin/23.1.67. [DOI] [PubMed] [Google Scholar]
- 127.Park S, Ciccone SLM, Beck BD, et al. Oxidative stress/damage induces multimerization and interaction of Fanconi anemia proteins. J Biol Chem. 2004;279(29):30053–30059. doi: 10.1074/jbc.M403527200. [DOI] [PubMed] [Google Scholar]
- 128.Fagerlie S, Lensch MW, Pang Q, et al. The Fanconi anemia group C gene product: Signaling functions in hematopoietic cells. Exp Hematol. 2001;29:1371–1381. doi: 10.1016/s0301-472x(01)00755-x. [DOI] [PubMed] [Google Scholar]
- 129.Li J, Sejas DP, Zhang X, et al. TNF-α induces leukemic clonal evolution ex vivo in Fanconi anemia group C murine stem cells. J Clin Invest. 2007;117(11):3283–3295. doi: 10.1172/JCI31772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pang Q, Fagerlie S, Christianson TA, et al. The Fanconi anemia protein FANCC binds to and facilitates the activation of STAT1 by gamma interferon and hematopoietic growth factors. Mol Cell Biol. 2000;20(13):4724–4735. doi: 10.1128/mcb.20.13.4724-4735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pang Q, Keeble W, Christianson TA, et al. FANCC interacts with Hsp70 to protect hematopoietic cells from IFNγ/TNF-α mediated cytotoxicity. EMBO. 2001;20(16):4478–4489. doi: 10.1093/emboj/20.16.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alter B. Diagnostic Evaluation of FA. Fanconi Anemia Standards for Clinical Care. In: Owen J, Frohnmayer L, Eiler ME, editors. The Fanconi Anemia Research Fund. 2. 2003. pp. 3–17. [Google Scholar]