Abstract
Hepatocellular carcinoma (HCC) is the third leading cause of cancer–related deaths worldwide, mainly because of its poor prognosis. A valid mechanism-based prognostic biomarker is urgently needed. γ-hydroxy-1,N2-propanodeoxyguanosine (γ-OHPdG) is an endogenously formed mutagenic DNA adduct derived from lipid peroxidation (LPO). We examined the relationship of γ-OHPdG with hepatocarcinogenesis in two animal models and its potential role as a prognostic biomarker for recurrence in HCC patients. Bioassays were conducted in the Xeroderma pigmentosum group A knockout mice (Xpa−/−), and the diethylnitrosamine (DEN)-injected mice, both prone to HCC development. γ-OHPdG levels in the livers of these animals were determined. The effects of antioxidant treatments on γ-OHPdG and hepatocarcinogenesis were examined. Using two independent sets of HCC specimens from patients, we examined the relationship between γ-OHPdG and survival or recurrence-free survival. γ-OHPdG levels in liver DNA showed an age-dependent increase and consistently correlated with HCC development in all three animal models. Theaphenon E treatment significantly decreased γ-OHPdG levels in the liver DNA of Xpa−/− mice, and remarkably reduced HCC incidence in these mice to 14% from 100% in the controls. It also effectively inhibited HCC development in the DEN-injected mice. Using clinical samples from two groups of patients, our study revealed that higher levels of γ-OHPdG are strongly associated with low survival (p < 0.0001) and low recurrence-free survival (p = 0.007), respectively. Conclusion: These results support γ-OHPdG as a mechanism-based biologically relevant biomarker for predicting the risk of HCC and its recurrence.
Keywords: Hepatocellular Carcinoma, DNA lesion, Biomarker, Antioxidant, Cancer Prevention
Genetic and epigenetic alterations in oncogenes and tumor-suppressor genes are crucial for carcinogenesis (1, 2). Somatic mutations may arise from DNA lesions that are not repaired. During lifetime, human genome will host a wide-spectrum of mutagenic DNA lesions, induced by chemical carcinogens, viruses, and reactive oxygen and nitrogen species. This is believed to be the case for human liver as it is a major detoxifying organ that is exposed to a large number of risk factors (3–5).
Primary liver cancer is the third most common cause of cancer-related death stems from the lack of suitable biomarker for early detection, inadequate understanding of the molecular features, and resistance to current chemotherapy (6). There are more than one million newly diagnosed liver cancer cases annually, which renders liver cancer a global health care problem (7). HCC accounts for approximately 85% of liver cancer and is an inflammation-associated cancer (8). It is known that chronic inflammation leads to oxidative/nitrosative stress and lipid peroxidation (LPO) (9–12). Upon oxidation, membrane polyunsaturated fatty acids generate highly reactive α,β-unsaturated aldehydes (enals) which can modify DNA bases, forming promutagenic cyclic DNA adducts (13–15).
Oxidative stress caused by chronic inflammation has emerged as a major player in liver carcinogenesis of different etiologies, including HCV/HBV, alcoholism, and obesity (16–18). LPO-derived endogenous cyclic DNA adducts in cancer initiation/promotion have been investigated as potential markers for various types of inflammatory cancer-prone diseases (e.g. chronic pancreatitis, Crohn`s disease, ulcerative colitis, alcohol related hepatitis, H. pylori infection) (19). Among the LPO-derived DNA adducts, γ-hydroxy-1,N2-propanodeoxyguanosine (γ-OHPdG) is one of the most abundant adducts, which has been ubiquitously detected in mammalian tissues (20). γ-OHPdG is mutagenic and induces predominantly G to T and G to A mutations (21, 22). One study showed that γ-OHPdG, derived from acrolein as a major constituent of cigarette smoke, occurs at the sites of p53 gene that coincide with its mutation hotspots in lung cancer of smokers (23). These results support γ-OHPdG’s role in human cancer by causing somatic mutations.
The link between hemochromatosis patients with iron overload and p53 mutation in HCC development and the correlations of inflammatory cells, LPO-derived protein adduct, and oxidized DNA bases as general oxidative markers, in liver tissues of HCV-associated HCC patients underlies the role of oxidative stress in liver carcinogenesis (24). Epidemiological studies have associated antioxidants in the reduced risks of certain types of cancer (25). However, conflicting results have been obtained in intervention trials (26, 27). A mechanism-based biomarker is crucial for identifying the population that will actually benefit from taking antioxidants.
To date, there has been no report of a specific LPO-derived DNA adduct as a predictive biomarker for HCC. In this work, we studied γ-OHPdG as a biomarker of hepatocarcinogenesis in animal models, involving the Xpa−/− transgenic mice, the diethylnitrosamine (DEN)-exposed mice, and the Long Evans Cinnamon (LEC) rats and its prevention by antioxidants. Furthermore, we demonstrated in two sets of liver specimens from HCC patients after surgical resection that γ-OHPdG is a highly reliable predictor of survival and HCC recurrence.
Experimental Procedures
Chemicals and Enzymes
All reagents purchased are analytical or HPLC grade.
Animals
The details of the study design are listed in the supplementary materials.
Quantification of γ-OHPdG
The LC/MS/MS method was described previously (28).
Patients and Database
Paraffin-embedded liver biopsy or HCC specimens, from patients who had liver biopsies or curative resection of HCC as part of standard medical care, were obtained from Georgetown University Medical Center. Informed consent was obtained from all patients under an approved IRB protocol (# 1992-048). A secure database with proper safeguards was constructed for the management of patient data. An Institutional Review Board approved the ethical, legal, and social implications of the project.
Whole Exome Sequencing
Genomic DNA extraction, library preparation, and whole exome sequencing was performed by Otogenetics (Norcross, GA, USA). The genomic mouse DNA samples were constructed into libraries and hybridized to Agilent SureSelect Mouse All Exon 51 Mb probes (Santa Clara, CA, USA). Paired end sequencing was executed on the Illumina HiSeq 2500 (San Diego, CA, USA) and raw data files were pushed through the DNAnexus standard exome analysis pipeline to complete alignment, quality control, coverage analysis, and variant calling.
Immunohistochemical Staining
Immunohistochemical detection of γ-OHPdG in healthy and cancerous tissues was performed by using tissue microarrays (HLiv-HCC060CD-01 and HLiv-HCC180Sur-02, US Biomax, Rockville, MD), PFA-fixed and paraffin-embedded blocks of tumor tissue from cases operated at Georgetown University. All patients gave informed consent, and the study was authorized by the respective Hospital Ethics Committees. Immunoscores were calculated by adding intensity and region of staining: 0, 1, 2, 3 correspond to no, weak, moderate, and high intensity staining; 0, 1, 2, 3 correspond to no, focal, regional, and diffuse region staining.
Statistical Analysis
The differences in adduct levels were compared using Student’s t-test. Fisher’s exact test was used in analyzing categorical data. Kaplan-Meier method was used to analyze the survival data and Log-rank test was used to compare the survival between different groups. Z-test was employed to calculate the p-values of tumor incidences. P-values < 0.05 were considered statistically significant. The SAS software (SAS Inc, Cary, NC) version 9.3 was used for statistical analysis.
Results
Increased levels of γ-OHPdG is associated with hepatocarcinogenesis in Xpa−/− mice
The mutagenicity of γ-OHPdG has been demonstrated using in vitro assays (29), but the evidence of its role in carcinogenesis in vivo is elusive. We first examined the association between γ-OHPdG and liver tumorigenesis in Xpa−/− mice deficient in nucleotide excision repair (NER) (30). γ-OHPdG is the only endogenous DNA adduct known to be repaired by NER (31). Xpa−/− C57/B6 mice are a model for skin carcinogenesis by ultraviolet-B irradiation. Intriguingly, these mice also develop spontaneous liver tumors (32). We proposed that the cumulated γ-OHPdG levels, due to the lack of its repair, contribute to hepatocarcinogenesis. To further enhance the spontaneous HCC, we back-crossed Xpa−/− C57/B6 mice with C3H/HeNCrl mice which have a high rate of spontaneous liver cancer (fig. S1). Compared to WT controls, Xpa−/− mice not only developed a higher incidence of liver tumors, but also showed significantly larger tumor sizes and increased multiplicity (fig. 1A and B). γ-OHPdG levels in liver DNA were quantified by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The data showed that it increased age-dependently in Xpa−/− mice (p < 0.05, fig. 1C). Mouse gender had no influence on the γ-OHPdG levels (fig. S3). We were able to see a statistically significant difference in the hepatic γ-OHPdG levels and the liver cancer development between Xpa−/− and WT mice (fig. 1). Unlike Xpa−/− mice, LEC rats are inflicted with increased LPO because of abnormal copper accumulation, mimicking that of human Wilson’s disease (33). As a result, LEC rats develop acute hepatitis, followed by chronic hepatitis, and eventually HCC. We found that γ-OHPdG levels in the livers of LEC rats were significantly higher than that of the WT Long Evans rats (fig. S4). These results provide evidence that the elevated liver γ-OHPdG levels are associated with an increased risk of HCC.
Fig.1.
Bioassay of WT and Xpa−/− mice. (A) Representative livers of WT and Xpa−/− mice at 72 weeks after feeding control diet. (B) Tumor multiplicity, maximum tumor diameter and incidences. (C) γ-OHPdG levels detected by LC-MS/MS in livers of mice at 8-week, 16-week and 32-week time points. n = 6–7 for each group, error bars represent SD values. *p < 0.05. n = 12–16 for 72-week tumor bioassay.
GC>TA is the dominant somatic mutation in HCC of Xpa−/− mice
Studies have shown that γ-OHPdG causes predominantly GC>TA and GC>AT mutations (21, 22). We reasoned that the increased γ-OHPdG in the livers of Xpa−/− mice can lead to a somatic mutation pattern in which GC>TA and GC>AT mutations are the most frequent alterations. We obtained the mutation frequencies through comparing liver tumor nodules versus adjacent normal liver tissues from two Xpa−/− mice using whole exome next generation sequencing. Whole exome sequencing produced a mean yield of 23.8 million reads or 2.5 gigabases of data per sample with 94.1% > Q30. The samples were sequenced to a mean coverage of 29X, and 99.4% of the reads were mapped to the target regions. We found 60 and 100 variants in the two liver nodules (fig 2A), with GC>TA mutation as the dominant alteration accounting for 92% and 86% mutations, respectively (fig. 2B). While examining the Sorting Intolerant from Tolerant prediction scores, we also noted that more than 35% of the variants in both samples were predicted as deleterious mutations (fig 2C). The high GC>TA mutation frequency in the Xpa−/− mouse liver tumors implies that γ-OHPdG may play a role in the mutagenesis of HCC development. Different from other solid tumors in which CG>TA transitions are the highest variations in the mutation spectrum, with the exception of lung cancer, variants in human HCC also showed an over-representation of GC>TA transversion (1). We identified a number of mutant genes within mouse liver nodules that were reported in human HCC, including ABCA1, CSMD1, LAMA2, TRRAP, and TRANK1 (1), suggesting this model is relevant to human liver carcinogenesis (table S1).
Fig.2.
Profiles of mutations found in liver tumors from two Xpa−/− mice. (A) Distribution of somatic mutations in each tumor sample. (B) Frequency of indels and nucleotide substitutions in each tumor sample. (C) Percentage of tolerated and deleterious mutations (Sorting Intolerant from Tolerant data).
Antioxidants suppress liver γ-OHPdG levels and HCC development in Xpa−/− mice
To further assess the role of γ-OHPdG in HCC development, we next studied if suppression of γ-OHPdG formation in the mouse liver would inhibit hepatocarcinogenesis. Xpa−/− mice yield 100% liver cancer incidence at the end of the 72-week bioassay. Three antioxidants (Theaphenon E, α-lipoic acid, and α-tocopherol) were used (34). To determine whether these antioxidants suppress the liver γ-OHPdG levels, Xpa−/− mice were fed diets containing antioxidants: α-lipoic acid (2 g/kg), Theaphenon E (20 g/kg) and α-tocopherol(1.8 g/kg) (28, 35–37) (details of bioassay are in SI). We observed a significant decrease of γ-OHPdG in the livers of mice fed the antioxidant diets with different potencies: Theaphenon E > α-lipoic acid > α-tocopherol (fig. 3). No significant changes of γ-OHPdG levels were found in the lungs, a non-target organ. We also examined the ratio of reduced to oxidized glutathione (GSH/GSSG), an indicator of oxidative stress. The increases in the ratio of GSH/GSSG in the liver tissues from the mice fed different antioxidants for 32 weeks are consistent with the decreases of γ-OHPdG (fig. 3C). We reported previously in LEC rats that Theaphenon E, but not α-tocopherol, suppresses γ-OHPdG formation (28).
Fig.3.
A 32-week bioassay of Xpa−/− mice with and without the treatment of antioxidants. (A) A diagram of experimental protocol. (B) γ-OHPdG levels in the livers of Xpa−/− mouse detected by LC-MS/MS after feeding various antioxidant diets (Theaphenon E (20 g/kg), α-lipoic acid (2 g/kg), and α-tocopherol (1.8 g/kg)) and control diet. (C) GSH/GSSG ratio changes in the livers of mice fed different diets for 32 weeks. *p < 0.05, **p < 0.01, ***p < 0.001. (D)(E)(F) γ-OHPdG levels detected by LC-MS/MS in livers and lungs from mice after feeding different antioxidants diets for 8, 16, and 32 weeks., error bars represent SD values.
Having demonstrated that antioxidants can suppress γ-OHPdG formation, we examined the relationships of γ-OHPdG with hepatocarcinogenesis in a 72-week life-time tumor bioassay in Xpa−/− mice. Mice randomized into four groups were fed with diets containing antioxidants throughout the bioassay period (28, 35–37). Theaphenon E showed a strong inhibition with a remarkable reduction of tumor incidence to 14% from 100% in mice on control diet, and a significant decrease in tumor numbers and size (fig. 4B, C, and D). α-Lipoic acid also decreased HCC incidence, but less effectively, whereas α-tocopherol had no significant effect. The potency of antioxidants to inhibit HCC showed a close correlation with their effects on γ-OHPdG levels. The protective effects of antioxidants against HCC development were similar in both male and female mice (fig. S5), consisting with their suppressing effects on γ-OHPdG in both genders (fig. S3). We compared the γ-OHPdG adduct levels in non-tumorous livers from control or Theaphenon E group at 72 weeks (fig. S6). Consistent with that at earlier time points, a significant reduction was observed in the Theaphenon E group. Although a significant loss in body weight gains was observed in the Theaphenon E group (fig. S6A), probably related to thermogenesis and fat oxidation caused by green tea extract (38), no food consumption difference was noted and mice in this group were leaner and healthy without any overt adversary effects (fig. S6B).
Fig.4.
A 72-week tumor bioassay of Xpa−/− mice. (A) Representative livers of mice kept on control or different antioxidant diets (Theaphenon E, α-lipoic acid, and α-tocopherol) from week 4 to week 72. (B) Tumor multiplicity. (C) Tumor size. (D) Tumor incidence. n = 17–18 for each group. Error bars represent SD values. *p < 0.05, **p < 0.01, ***p < 0.001, ns = non-significant.
γ-OHPdG levels correlate with DEN-induced hepatocarcinogenesis in mice
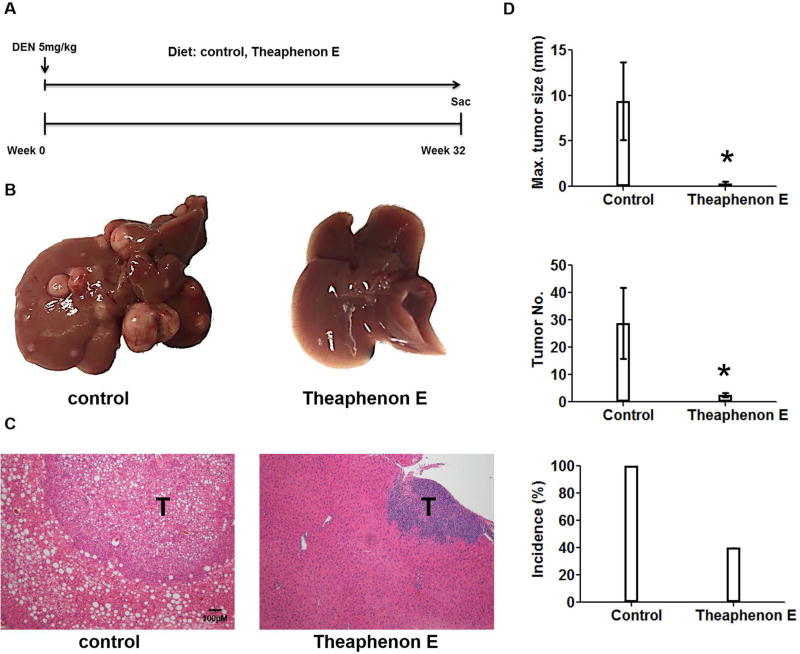
The relationship of γ-OHPdG in HCC was further examined in C57/B6 mice involving a single injection of procarcinogen DEN. Mice develop poorly differentiated HCC nodules within 32 weeks after DEN exposure (39). A time-dependent development of steatosis was observed in the DEN-injected mice fed the control diet. Malignant liver nodules were observed after eight months (fig. 5). To determine whether γ-OHPdG levels are associated with hepatocarcinogenesis, we measured γ-OHPdG in the livers obtained at four time intervals, shortly before and, 8, 16, and 24 weeks after DEN injection, by LC-MS/MS. Similar to Xpa−/− mice, an age-dependent increase of γ-OHPdG was seen in the livers of these mice, but not in the lungs (a non-target tissue) (fig. 5B). These results are consistent with previous reports that DEN causes oxidative stress and induces LPO (40). Similar to the results in Xpa−/− mice, Theaphenon E showed a remarkable suppression of HCC formation in these mice by decreasing tumor size from 10 mm to < 1 mm, tumor multiplicity from 30 to 3 nodules per mouse, and incidence from 100% to 40% (fig. 6).
Fig.5.
A 32-week bioassay of DEN-treated C56B/6 mice. (A) H&E immunohistochemistry staining of livers from mice of different age. (B) γ-OHPdG levels detected by LC-MS/MS in mouse livers and lungs. (C) Steatosis was evaluated and quantified using this scoring system: no steatosis = 0, minimum steatosis = 1, mild steatosis = 2, moderate steatosis = 3, severe steatosis = 4. *p < 0.05.
Fig.6.
A 32-week tumor bioassay of DEN-treated C56B/6 mice fed diets with and without Theaphenon E. (A) A diagram of experimental protocol. 14-day old C56BL/6 male pups were given a single i.p. injection of DEN (5mg/kg). Recipients were sacrificed 8 months later for liver tumor analysis. (B) Representative livers of mice under control diet and Theaphenon E diet. (C) Representative immunohistochemistry staining of H&E for mouse liver. (D) Maxiumum tumor diameter, tumor multiplicity, and incidences. n = 5 for each group, error bars represent SD values. * p < 0.05.
γ-OHPdG as a prognostic biomarker for HCC recurrence in patients
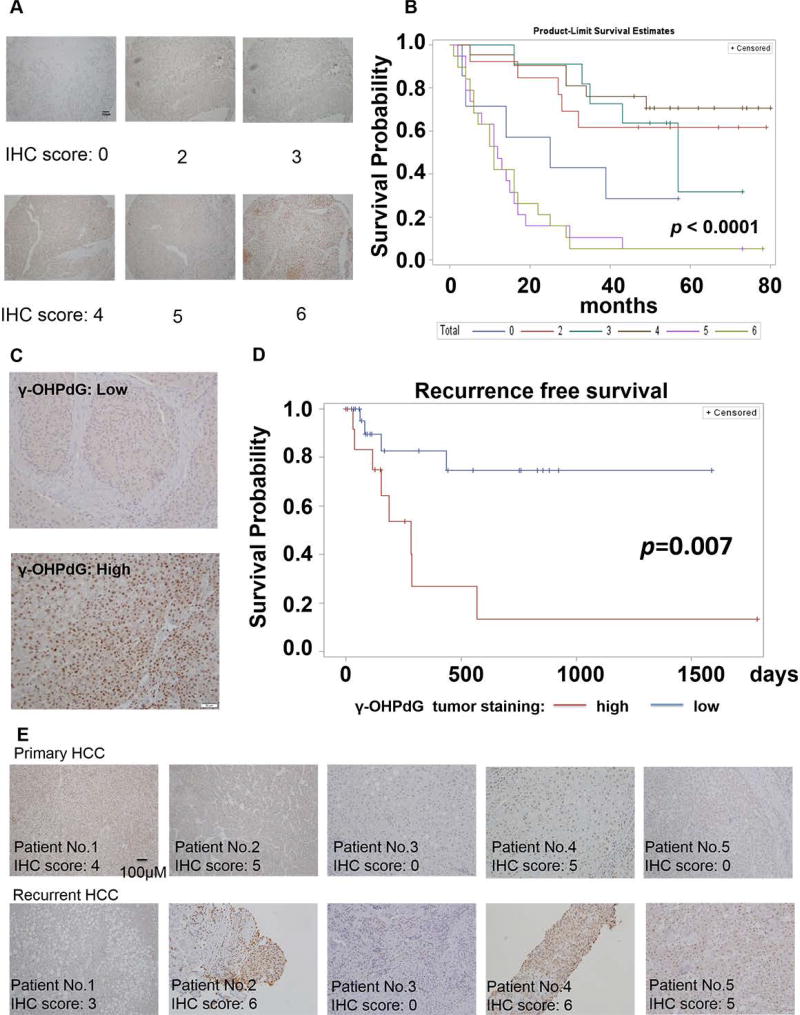
The results described above demonstrated that γ-OHPdG is closely associated with liver carcinogenesis in the animal models. To determine whether γ-OHPdG serves as a prognostic biomarker for the survival of HCC patients, we studied liver samples from a set of ninety HCC patients who underwent surgery without adjuvant therapy between August 2006 and November 2009. These patients were followed up for four to seven years. We found that the high γ-OHPdG levels in the liver cancerous tissues were strongly correlated (p < 0.0001) with poorer survival in these patients (fig. 7A and B). We then recruited 45 patients and examined the relationship of γ-OHPdG in the liver cancerous tissues from these patients after surgical resection with their recurrence-free survival. We did not observe a correlation between cancerous tissues and adjacent normal tissue in the levels of γ-OHPdG (fig S8). Patients with low γ-OHPdG tumors (IHC score ≤ 3) experienced a significantly prolonged HCC recurrence-free survival compared to patients with high γ-OHPdG tumors (IHC sore > 3) (p = 0.007, fig. 7C and D). After two years of follow-up from the date of curative surgical resection, the probability of no cancer recurrence in patients with low γ-OHPdG tumors is 75%, while the probability of no cancer recurrence in patients with high γ-OHPdG tumors is only 13% (p = 0.0002, table S2). We also compared the levels of γ-OHPdG in the recurrent tumors to that in the primary HCC, and found that 80% of them showed similar levels of γ-OHPdG compared to their primary HCC tumors (fig. 7E). The data from the clinical studies strongly support γ-OHPdG as a biomarker for predicting the risk of human HCC recurrence.
Fig. 7.
γ-OHPdG as a biomarker of survival and recurrence-free survival in HCC patients. (A) Representative images of γ-OHPdG staining in human liver tissue microarray samples contains 90 HCC patients: semi-quantitative scores were calculated by adding intensity and distribution of staining: 0, 1, 2, 3 correspond to negative, weak, moderate, and intense staining and 0, 1, 2, 3 correspond to negative, focal, regional, and diffuse distribution. (B) Kaplan-Meier Curve for γ-OHPdG staining demonstrated decreased survival in patients with high γ-OHPdG tumor (p < 0.0001). (C) Representative γ-OHPdG staining of liver samples from patients at Georgetown University clinical trial. (D) Kaplan-Meier Curve for γ-OHPdG staining using median cut-off point (high > 3, Low ≤ 3) showing decreased recurrence-free survival in patients with high γ-OHPdG (red line) in tumors compared to patients with low γ-OHPdG in tumors (p = 0.007). (E) Comparisons of immunostaining score of γ-OHPdG between primary tumor and recurrent tumor in HCC patients.
Discussion
Liver cancer is a type of cancer that evolves over the course of decades, and is extremely difficult to treat once diagnosed. Therefore, developing a mechanism-based biologically relevant biomarker is extremely important for liver cancer intervention. The role of γ-OHPdG, an endogenous promutagnic DNA lesions preferentially formed at the p53 mutation hotspots in human cancer (23), has not been investigated. In vitro studies showed that it is repaired by NER pathways, and it causes GC>TA and GC>AT mutation (21, 22). In this work, we provided the evidence in vivo that γ-OHPdG is repaired by NER in animals as its levels are significantly higher in Xpa−/− mice as compared to the WT. We also found that γ-OHPdG levels increase age-dependently in Xpa−/− mice, indicating that the endogenous lesions accumulate over time. A similar trend was observed in LEC rats and the DEN-injected mice. DEN-induced ROS accumulation, which is known to induce HCC development, may play a role in the increase of γ-OHPdG in the DEN-injected mice (41). Such increase of γ-OHPdG was not observed in the lungs of Xpa−/− mice, suggesting a tissue-specific accumulation in the liver, possibly reflecting its relatively high lipid content in the liver and inhibition of NER by acrolein (42).
The potential importance of γ-OHPdG in HCC is also supported by the observations that there is an overwhelming representation of GC>TA mutation in Xpa−/− mouse liver tumors. Except for lung cancer, human HCC is the only solid cancer show a relatively high rate of GC>TA mutation (1). The mutation signature in Xpa−/− mice coincides with the signature of mutational process of human HCC with a prevalence at 12.1% (2), linking DNA lesions on G:C pair with HCC. When comparing the mutations in human HCC, we found a number of genes overlapped, for example, up to 121 mutations per nodule are detected with a high frequency mutations on G:C pair (1). This was explained primarily by the methylated cytosine (1). It is known that methylation at CpG sites enhances γ-OHPdG formation at these sites; moreover, CpG methylation greatly increases γ-OHPdG-induced GC>AT and GC>TA mutation frequency (43). Although other DNA lesions associated with oxidative stress may also induce GC>TA, such as 8-oxo-7,8-dihydro-2'-deoxyguanosine, data from these studies together implicate γ-OHPdG as a significant lesion that causes the high frequency mutations on CpG sites found in human HCC.
A cohort study in Ohsaki, Japan showed that the consumption of green tea is associated with a reduced risk of human liver cancer incidence (44). Other possible explanations for tumor inhibitory effects of theaphenon E come from the evidence showing tea polyphenols, especially epigallocatechin gallate, can alter DNA methylation patterns of genes and nuclear factor erythroid 2-related factor 2 (Nrf2) pathway (45). Tea polyphenols may possess multiple functional roles, however, we focused in this study on their effects to block the formation of an endogenous DNA adduct as a critical mechanism. Theaphenon E, a green tea extract, is a formulation containing a well-defined composition of polyphenols, mostly epigallocatechin gallate, the most abundant and potent anti-oxidative catechin in green tea (Table S5). α-Lipoic acid is an organosulfur antioxidant. Unlike α-lipoic acid and Theaphenon E, Α-tocopherol is a lipid-soluble antioxidant. In fact, α-tocopherol has been associated with an increased risk of prostate cancer in healthy men (27). In contrast, the α-Tocopherol, β-Carotene trial showed α-tocopherol reduces prostate cancer incidence of heavy smokers. These conflicting clinical outcomes are plausibly due to the lack of valid biomarkers and may highlight the importance to identify the high-risk population suitable for intervention trials.
Oxidative stress has emerged as a crucial factor in HCC development under various pathological conditions (46, 47). Moreover, it has been shown to be involved in migration, invasion, and metastasis of HCC (48). Serum quantification of derivatives of reactive oxygen metabolites levels, a simple method for measuring hydrogen peroxide, is found to predict the risk of HCC recurrence (49). These findings highlight the potential of developing oxidative stress-related biomarkers as prognostic tools for HCC and its recurrence.
Our studies demonstrated that the LPO-derived γ-OHPdG is closely associated with liver carcinogenesis; it serves as a mechanistically-relevant biomarker for HCC. Its levels in HCC, but not in normal adjacent liver, are highly reliable in predicting survival and recurrence-free survival in HCC patients (fig. S9). More importantly, γ-OHPdG is independent of other known prognostic biomarkers such as microvascular invasion and tumor differentiation (fig. S10) (50). The clinical applications of γ-OHPdG as a prognostic biomarker for HCC in guiding future intervention trials warrant studies.
Supplementary Material
Acknowledgments
Financial Support
This work was supported by the NCI grant: RO1-CA-134892 to F-L Chung and Carlucci Family Research Award in Cancer Prevent and Early Detection to Y. Fu.
We thank Drs. Bin Gao and Mingjiang Xu from National Institute on Alcohol Abuse and Alcoholism (NIAAA), Moon-Shong Tang from NYU School of Medicine, Xiaolin Wu from NCI for the discussions. We thank Dr. Yukihiko Hara for the generosity of providing Theaphenon E. We thank Maria Cruz, Carlos Benitez, Sanchita Sarangi, Zhuoli Xuan, Yuzana Khine Zaw, Umar Khan for the animal bioassay, DNA sample preparations.
Abbreviations
- HCC
hepatocellular carcinoma
- γ-OHPdG
γ-hydroxy-1,N2-propanodeoxyguanosine
- LPO
lipid peroxidation
- Xpa−/−
Xeroderma pigmentosum knockout
- DEN
diethylnitrosamine
- LEC
Long-Evans Cinnamon
- NER
nucleotide excision repair
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- GSH/GSSG
ratio of reduced to oxidized glutathione.
Footnotes
Drug Names
DEN, diethylnitrosamine (Sigma-Aldrich, St. Louis, MO, USA)
References
- 1.Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, Calderaro J, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694–698. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks PJ, Theruvathu JA. DNA adducts from acetaldehyde: implications for alcohol-related carcinogenesis. Alcohol. 2005;35:187–193. doi: 10.1016/j.alcohol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Gupta RC. Nonrandom binding of the carcinogen N-hydroxy-2-acetylaminofluorene to repetitive sequences of rat liver DNA in vivo. Proc Natl Acad Sci U S A. 1984;81:6943–6947. doi: 10.1073/pnas.81.22.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Millonig G, Nair J, Patsenker E, Stickel F, Mueller S, Bartsch H, et al. Ethanol-induced cytochrome P4502E1 causes carcinogenic etheno-DNA lesions in alcoholic liver disease. Hepatology. 2009;50:453–461. doi: 10.1002/hep.22978. [DOI] [PubMed] [Google Scholar]
- 6.Marquardt JU, Andersen JB, Thorgeirsson SS. Functional and genetic deconstruction of the cellular origin in liver cancer. Nat Rev Cancer. 2015;15:653–667. doi: 10.1038/nrc4017. [DOI] [PubMed] [Google Scholar]
- 7.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 9.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 10.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 11.Dedon PC, Plastaras JP, Rouzer CA, Marnett LJ. Indirect mutagenesis by oxidative DNA damage: Formation of the pyrimidopurinone adduct of deoxyguanosine by base propenal. Proc Natl Acad Sci U S A. 1998;95:11113–11116. doi: 10.1073/pnas.95.19.11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liebler DC. Protein Damage by Reactive Electrophiles: Targets and Consequences. Chem Res Toxicol. 2007;21:117–128. doi: 10.1021/tx700235t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: A review of published adduct types and levels in humans. Free Radic Biol Med. 2007;43:1109–1120. doi: 10.1016/j.freeradbiomed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Blair IA. DNA Adducts with Lipid Peroxidation Products. J Biol Chem. 2008;283:15545–15549. doi: 10.1074/jbc.R700051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7:e330–341. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Ivanov AV, Bartosch B, Smirnova OA, Isaguliants MG, Kochetkov SN. HCV and oxidative stress in the liver. Viruses. 2013;5:439–469. doi: 10.3390/v5020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 19.Bartsch H, Nair J. Oxidative stress and lipid peroxidation-derived DNA-lesions in inflammation driven carcinogenesis. Cancer Detect Prev. 2004;28:385–391. doi: 10.1016/j.cdp.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Nath RG, Chung FL. Detection of exocyclic 1,N2-propanodeoxyguanosine adducts as common DNA lesions in rodents and humans. Proc Natl Acad Sci U S A. 1994;91:7491–7495. doi: 10.1073/pnas.91.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang IY, Chan G, Miller H, Huang Y, Torres MC, Johnson F, Moriya M. Mutagenesis by acrolein-derived propanodeoxyguanosine adducts in human cells. Biochemistry. 2002;41:13826–13832. doi: 10.1021/bi0264723. [DOI] [PubMed] [Google Scholar]
- 22.Minko IG, Washington MT, Kanuri M, Prakash L, Prakash S, Lloyd RS. Translesion synthesis past acrolein-derived DNA adduct, gamma -hydroxypropanodeoxyguanosine, by yeast and human DNA polymerase eta. J Biol Chem. 2003;278:784–790. doi: 10.1074/jbc.M207774200. [DOI] [PubMed] [Google Scholar]
- 23.Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci U S A. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain SP, Raja K, Amstad PA, Sawyer M, Trudel LJ, Wogan GN, Hofseth LJ, et al. Increased p53 mutation load in nontumorous human liver of Wilson disease and hemochromatosis: Oxyradical overload diseases. Proc Natl Acad Sci U S A. 2000;97:12770–12775. doi: 10.1073/pnas.220416097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004;134:3479S–3485S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 26.Lee JE, Chan AT. Fruit, vegetables, and folate: cultivating the evidence for cancer prevention. Gastroenterology. 2011;141:16–20. doi: 10.1053/j.gastro.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu Y, Nath RG, Dyba M, Cruz IM, Pondicherry SR, Fernandez A, Schultz CL, et al. In vivo detection of a novel endogenous etheno-DNA adduct derived from arachidonic acid and the effects of antioxidants on its formation. Free Radic Biol Med. 2014;73:12–20. doi: 10.1016/j.freeradbiomed.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HW, Wang HT, Weng MW, Hu Y, Chen WS, Chou D, Liu Y, et al. Acrolein- and 4-Aminobiphenyl-DNA adducts in human bladder mucosa and tumor tissue and their mutagenicity in human urothelial cells. Oncotarget. 2014;5:3526–3540. doi: 10.18632/oncotarget.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi Y, Nakatsuru Y, Zhang S, Shimizu Y, Kume H, Tanaka K, Ide F, et al. Enhanced spontaneous and aflatoxin-induced liver tumorigenesis in xeroderma pigmentosum group A gene-deficient mice. Carcinogenesis. 2002;23:627–633. doi: 10.1093/carcin/23.4.627. [DOI] [PubMed] [Google Scholar]
- 31.Bohr VA. DNA repair fine structure and its relations to genomic instability. Carcinogenesis. 1995;16:2885–2892. doi: 10.1093/carcin/16.12.2885. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa T, Zhang SS, Qin X, Takahashi Y, Oda H, Nakatsuru Y, Ide F. DNA repair and cancer: lessons from mutant mouse models. Cancer Sci. 2004;95:112–117. doi: 10.1111/j.1349-7006.2004.tb03190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masuda R, Yoshida MC, Sasaki M, Dempo K, Mori M. High Susceptibility to Hepatocellular Carcinoma Development in LEC Rats with Hereditary Hepatitis. Cancer Sci. 1988;79:828–835. doi: 10.1111/j.1349-7006.1988.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasegawa R, Chujo T, Sai-Kato K, Umemura T, Tanimura A, Kurokawa Y. Preventive effects of green tea against liver oxidative DNA damage and hepatotoxicity in rats treated with 2-nitropropane. Food Chem Toxicol. 1995;33:961–970. doi: 10.1016/0278-6915(95)00064-9. [DOI] [PubMed] [Google Scholar]
- 35.Ho YS, Lai CS, Liu HI, Ho SY, Tai C, Pan MH, Wang YJ. Dihydrolipoic acid inhibits skin tumor promotion through anti-inflammation and anti-oxidation. Biochem Pharmacol. 2007;73:1786–1795. doi: 10.1016/j.bcp.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Yan Y, Wang Y, Tan Q, Haray Y, Yun TK, Lubet RA, You M. Efficacy of polyphenon E, red ginseng, and rapamycin on benzo(a)pyrene-induced lung tumorigenesis in A/J mice. Neoplasia. 2006;8:52–58. doi: 10.1593/neo.05652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pratico D, Tangirala RK, Rader DJ, Rokach J, FitzGerald GA. Vitamin E suppresses isoprostane generation in vivo and reduces atherosclerosis in ApoE-deficient mice. Nat Med. 1998;4:1189–1192. doi: 10.1038/2685. [DOI] [PubMed] [Google Scholar]
- 38.Westerterp-Plantenga MS. Green tea catechins, caffeine and body-weight regulation. Physiol Behav. 2010;100:42–46. doi: 10.1016/j.physbeh.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Verna L, Whysner J, Williams GM. N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther. 1996;71:57–81. doi: 10.1016/0163-7258(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 40.Thirunavukkarasu C, Sakthisekaran D. Effect of selenium on N-nitrosodiethylamine-induced multistage hepatocarcinogenesis with reference to lipid peroxidation and enzymic antioxidants. Cell Biochem Funct. 2001;19:27–35. doi: 10.1002/cbf.895. [DOI] [PubMed] [Google Scholar]
- 41.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 42.Wang HT, Hu Y, Tong D, Huang J, Gu L, Wu XR, Chung FL, et al. Effect of carcinogenic acrolein on DNA repair and mutagenic susceptibility. J Biol Chem. 2012;287:12379–12386. doi: 10.1074/jbc.M111.329623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H-T, Weng M-w, Chen W-c, Yobin M, Pan J, Chung F-L, Wu X-R, et al. Effect of CpG methylation at different sequence context on acrolein- and BPDE-DNA binding and mutagenesis. Carcinogenesis. 2013;34:220–227. doi: 10.1093/carcin/bgs323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ui A, Kuriyama S, Kakizaki M, Sone T, Nakaya N, Ohmori-Matsuda K, Hozawa A, et al. Green tea consumption and the risk of liver cancer in Japan: the Ohsaki Cohort study. Cancer Causes Control. 2009;20:1939–1945. doi: 10.1007/s10552-009-9388-x. [DOI] [PubMed] [Google Scholar]
- 45.Li W, Guo Y, Zhang C, Wu R, Yang AY, Gaspar J, Kong AN. Dietary Phytochemicals and Cancer Chemoprevention: A Perspective on Oxidative Stress, Inflammation, and Epigenetics. Chem Res Toxicol. 2016;29:2071–2095. doi: 10.1021/acs.chemrestox.6b00413. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki Y. Does oxidative stress participate in the development of hepatocellular carcinoma? J Gastroenterol. 2006;41:1135–1148. doi: 10.1007/s00535-006-1982-z. [DOI] [PubMed] [Google Scholar]
- 47.Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253–257. doi: 10.1038/nature16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung JS, Park S, Park SH, Park ER, Cha PH, Kim BY, Chung YM, et al. Overexpression of Romo1 promotes production of reactive oxygen species and invasiveness of hepatic tumor cells. Gastroenterology. 2012;143:1084–1094. e1087. doi: 10.1053/j.gastro.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki Y, Imai K, Takai K, Hanai T, Hayashi H, Naiki T, Nishigaki Y, et al. Hepatocellular carcinoma patients with increased oxidative stress levels are prone to recurrence after curative treatment: a prospective case series study using the d-ROM test. J Cancer Res Clin Oncol. 2013;139:845–852. doi: 10.1007/s00432-013-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jonas S, Bechstein WO, Steinmuller T, Herrmann M, Radke C, Berg T, Settmacher U, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.