Abstract
Fanconi Anemia (FA) is a cancer-prone inherited bone marrow failure and cancer susceptibility syndrome with at least 13 complementation groups (FANCA, -B, -C, -D1, -D2, -E,-F, -G, -I, -J, -L, -M, -N). Our laboratory has previously described several regulatory phosphorylation events for core complex member proteins FANCG and FANCA by phosphorylation. In this study we report a novel phosphorylation site S331 of FANCD2, the pivotal downstream player of the FA pathway. Phosphorylation of S331 is important for its DNA damage inducible monoubiquitylation, resistance to DNA crosslinkers and in vivo interaction with FANCD1/BRCA2. A phosphomimetic mutation at S331 restores all of these phenotypes to wild type. In vitro and in vivo experiments show phosphorylation of S331 is mediated by CHK1, the S phase checkpoint kinase implicated in the FA DNA repair pathway.
Keywords: phosphorylation, FANCD2, Genomic instability, BRCA2, Fanconi Anemia
Introduction
Fanconi anemia is a rare genetic disorder characterized by bone marrow failure, developmental abnormalities, and increased susceptibility to cancer(1). The cellular hallmark of Fanconi anemia is hypersensitivity and chromosomal breakage upon exposure to DNA interstrand cross-linkers such as mitomycin C (MMC), suggesting a defect in DNA damage response(2). So far, thirteen subtypes of FA have been identified and corresponding genes cloned, including three breast cancer related genes, FANCD1/BRCA2(3), FANCJ/BRIP1(4) and FANCN/PALB2(5). Eight of the FA proteins (A, B, C, E, F, G, L(6, 7), and M(8, 9)) associate to form a nuclear multi-protein “core” complex, which is necessary for monoubiquitylation of FANCD2 on lysine 561, a critical step for activation of the FA pathway in response to S phase progression or DNA damage(10).
Cell cycle checkpoint kinases play important roles in various DNA damage responses including the FA pathway. Ataxia telangiectasia and Rad3 related protein kinase (ATR), involved in the DNA damage response, interacts with the FA pathway and couples monoubiquitylation of FANCD2 to DNA damage(11). ATR has been reported to directly phosphorylate serine 717 and threonine 691 on FANCD2 to promote monoubiquitylation and resistance to DNA cross-linking agents(12). Furthermore, CHK1, a substrate of ATR, is also required for initiation of ICL induced S phase checkpoint(13) and monoubiquitylation of FANCD2(14). CHK1 directly phosphorylates the FANCE subunit of FA core complex on two conserved sites (threonine 346 and serine 374), which is functionally important though dispensable for FANCD2 monoubiquitylation(15)
In an attempt to further understand the extensive interactions between ATR-CHK1 and the FA pathway, we have identified a novel DNA damage-inducible FANCD2 phosphorylation site. Here we demonstrate that this DNA damage-inducible phosphorylation site on FANCD2, serine 331, is essential for normal cellular resistance to MMC and for DNA damage inducible monoubiquitylation of FANCD2. Furthermore, phosphorylation at serine 331 occurs independently of the FA core complex and is necessary for interaction of FANCD2 with BRCA2. A phospho-mimetic mutation at S331 restores all of these wild type functions to FANCD2. Phosphorylation at this site is dependent on CHK1, signifying the importance of the S phase checkpoint in the activation of FA pathway.
Materials and Methods
Cell Culture
Cells were maintained at 37°C in a CO2 incubator. HeLa cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). FA-D2 deficient PD20 cells and its derived cells were grown in DMEM plus 15% FBS. Lymphoblast cell lines BD180 (WT), HSC72 (FA-A), HSC536 (FA-C), EUFA143 (FA-G) and EUFA868 (FA-L), fibroblast cell line GM6914 (FA-A) and their cDNA complemented derivatives were grown as previously described (16, 17). EUFA868 cell lines were generously provided by Ruhikanta Meetei. For stable transduction, PD20 cells corrected with pMMP-derived wild type and mutant FANCD2 constructs were selected under 1 μg/ml puromycin for 1 week. 293GPG producer cells were grown as described previously(18).
Generation of Phosphospecific Antibody
Phosphospecific antibodies for human FANCD2 were generated by immunization of rabbits with keyhole limpet hemocyanin (KLH)-conjugated phospho-peptide KSKGRAS[P]SSGNQESS with serine 331 of FANCD2 in the middle (Proteintech group). Antibody was affinity purified using the phosphorylated peptide-conjugated gels and non-specificity was removed by absorption with unphosphorylated peptide-conjugated gels. Preparation of peptide-coupled affinity gel was carried out with AminoLink coupling gel (Pierce Group) according to the manufacturer's instruction.
Clonogenic Survival and Growth Inhibition Assays
Clonogenic survival assays were performed by plating 500 cells in log-growth phase on a 10 cm dish and allowing them to spread for 8 hours, at which point cells were treated with varying concentration of MMC in DMEM+15% FBS for 1 hour. Then cells were washed with PBS 2 times and allowed to grow in fresh media for 10-12 days into visible colonies. Colonies with more than 50 cells were stained with 0.1% crystal violet in methanol and counted. Growth inhibition by MMC was as previously described (17).
Immunoprecipitation, Immunoblotting, and Phosphatase Treatment
Cell pellets were lysed in buffer containing 350 mM NaCl, 50 mM Tris (pH 7.4), 0.5% NP-40, 5 mM EDTA, 2 mM sodium pyrophosphate, 1 mM β–glycerophosphate, plus proteinase inhibitors followed by high-speed centrifugation. To pull down Flag-tagged FANCD2, 30 μl of 50% anti-Flag M2 agarose (Sigma) slurry was added to 1 mg total cellular lysate and incubated in 4°C for 4 hours. Beads were then washed in extraction buffer once and TBS buffer 3 times. To pull down endogenous FANCD2, 1 mg total lysate was incubated with 2 μg FANCD2 antibody H-300 (Santa Cruz) on ice or one hour, followed by addition of 40 μl 40% slurry of protein A beads (Sigma). Immunoblotting in FA core complex mutant cell lines and immunoprecipitation of FANCD2 and BRCA2 were as previously described (16, 17, 19).
For dephosphorylation, beads with immunoprecipitated FANCD2 were dried by fine needle aspiration and then re-suspended in 30 μl λ-phosphatase reaction buffer with or without 400 U λ-phosphatase or withλ-phosphatase plus or minus phosphatase inhibitor (1 mM sodium pyrophosphate) and incubated at 30°C for 30 minutes.
Densitometric Quantification
Image J was used for densitometric analysis*.
Mass spectrometry analysis
the Mass Spectrometry Core at the University of Virginia performed all mass spectrometry work. Briefly, FA-D2 deficient cells corrected with Flag-tagged wild type FANCD2 were treated with 0.5 μM MMC for 18 hours and 1 μM okadaic acid 20 minutes before cell lysate preparation. FANCD2 was then immunoprecipitated as described above and separated on SDS-PAGE gel. The silver stained bands (SilverQuest, Invitrogen) corresponding to FANCD2 were trypsin digested and subjected to liquid chromatography mass spectrometry (LC-MS) on an ion spray mass spectrometer. Spectra were analyzed and identified using the Sequest search algorithm as described previously(19).
Production of wild type and mutant FANCD2 constructs
Single point mutagenesis was carried out by employing PCR-ligation-PCR method(20). The mutated PCR products were then purified and used as templates to generate full length FANCD2 by PCR mediated ligation. Final PCR products were sequenced to verify accuracy before being sub-cloned into Flag- or Flag-EGFP pMMP vector.
The resulting pMMP constructs were transfected into 293GPG producer lines with LipofectAMINE (Amersham Biosciences), and viral supernatants were collected daily between 3 and 5 days after transfection. Retroviral supernatants were used for subsequent transduction(19).
In vitro CHK1 Kinase Assay
Peptide-based in vitro kinase assays were carried out as described previously(19). Briefly, FANCD2 peptides encompassing amino acids 324 to 338 (KSKGRASSSGNQESS) or with singly mutated peptide S331A (KSKGRASASGNQESS) were synthesized by Keck Facility center of Yale University. In vitro kinase reaction was carried out under room temperature in a volume of 25 μl with indicated amount of synthesized peptide (10 mM Hepes, pH 7.5, 10 mM MgCl2, 0.5 mM EGTA, 0.2 mM ATP, 10 μCi [gamma-32P]ATP (3000 Ci/mmol) 50 ng recombinant GST-CHK1, 0.1mM DTT). The reaction was stopped after 30 minutes by adding 45 μl ice-cold 10% trichloroacetic acid (TCA). After centrifugation at 10K rpm for 2 minutes, 30 μl supernatants were spotted onto Whatman P81 filter circles, which were then washed with cold 0.5% phosphoric acid and acetone. The filter circles were analyzed in a scintillation counter (Beckman).
Results
Serine 331 is a novel phosphorylation site
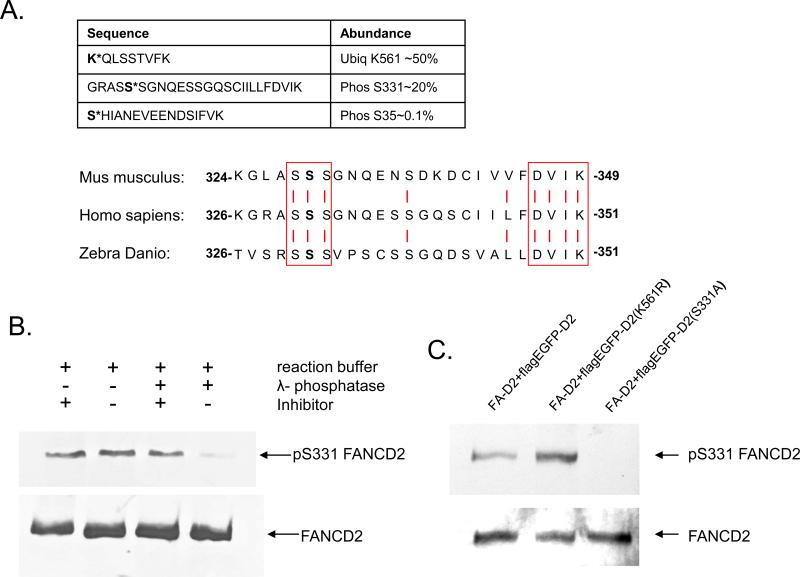
In order to examine the regulatory role of FANCD2 phosphorylation in the FA pathway, we investigated the phosphorylation of FANCD2 following genotoxic stress by mass spectrometry analysis as described above. The result revealed several modification sites (Fig. 1A), among which the previously unreported phosphorylation event at serine 331 had a relative abundance of 20%, second only to monoubiquitylation at lysine 561. In addition, sequence alignment of FANCD2 homologs from human, mouse and zebra fish showed that the three serine residues from 330 to 332 are highly conserved during evolution, suggesting functional importance (Fig. 1A).
Figure 1. Serine 331 is a novel phosphorylation site.
A). Mass spectroscopy analysis reveals a novel phosphorylation site of FANCD2 at serine 331. FA-D2 cells corrected with Flag-FANCD2 were treated with MMC, and Flag immunoprecipitated FANCD2 was subjected to mass spectrometry analysis. Phospho- or monoubiquitylated peptides are listed with relative abundance (modified peptide fragment/total peptide fragment). Serine 331 is a previously unreported phosphorylation site, which is highly conserved during evolution as shown by sequence alignment (vertical bar indicates identical residues)
B). Endogenous phosphorylation of serine 331 of FANCD2 is abolished by lambda phosphatase experiment. HeLa whole cell lysate was immunoprecipitated with anti-FANCD2 antiserum, and the immunoprecipitates were treated either with (lane 1) or without (lane 2) phosphatase inhibitor sodium pyrophosphate (Na4P2O7), or λ-phosphatase plus (lane 3) or minus (lane 4) the inhibitor sodium pyrophosphate as indicated. Dephosphorylation by lambda phosphatase abolishes phospho-specific signals detected by affinity purified anti-[P]S331 antibody(top panel), which is preserved by the phosphatase inhibitor sodium pyrophosphate. Immunoblotting against total FANCD2 (bottom panel) shows equal amount of FANCD2 present.
C) Phosphorylation of serine 331 is not dependent on monoubiquitylation 2 mg of total cell lysate made from FA-D2 deficient PD20 cells corrected with Flag-EGFP tagged wild type FANCD2, FANCD2(K561R) or FANCD2(S331A) were immunoprecipitated and blotted against phospho-serine331 specific antibody. The result (top panel) shows phosphorylation of serine 331 in K561R mutant as well as wild type FANCD2, but not in S331A mutant. Bottom panel shows total FANCD2 equal in all lanes.
To confirm this finding, we raised a rabbit polyclonal antiserum against a synthesized 12-mer peptide flanking phospho-serine 331. Immunoblotting with our phosphospecific antibody detected a specific signal in PD20+Flag-FANCD2, which was abolished by lambda phosphatase treatment (Fig. 1B), demonstrating the specificity of this phospho-antiserum.
To test in vivo phosphorylation of FANCD2 at serine 331, stable FA-D2 deficient PD20 cells expressing Flag-EGFP tagged wild type FANCD2 or its derivative mutants K561R or S331A were made by infection with respective retroviral pMMP constructs. Cell lysate was prepared and subjected to Flag-affinity immunoprecipitation and subsequent immunoblotting using the affinity purified phospho-antiserum. Expression of phosphorylated serine 331 was detected in the PD20 cell line corrected with wild type FANCD2, but not mutant FANCD2 with substitution of serine 331 to alanine, confirming the specificity of the antiserum (Fig. 1C). This phosphorylation event is not dependent on monoubiquitylation, as evidenced by phospho-specific signal present in FANCD2 mutant K561R, suggesting that phosphorylation of serine 331 is upstream of activation of FANCD2.
Phosphorylation of serine 331 is essential for conferring resistance to MMC
The current paradigm of the FA pathway highlights the pivotal role of FANCD2 in the FA pathway: upon replication fork stalling by DNA damage, FANCD2 is activated by monoubiquitylation mediated by the FA core complex and deposited onto chromatin, where it is proposed to promote the bypass and repair of DNA lesions by translesion synthesis and homologous recombination repair (21),(22) . To address the functional significance of the phosphorylation of serine 331, we constructed stable PD20 cells transduced with wild type Flag-EGFP FANCD2, Flag-EFGP FANCD2 (S331A) or empty vector and compared their sensitivity to the DNA crosslinking agent MMC by clonogenic survival assay. Abolishing phosphorylation of serine 331 resulted in complete loss of resistance to MMC (Fig. 2A), since PD20 cells expressing the point mutation S331A behaved essentially like the uncorrected parental PD20 cell line.
Figure 2. Phosphorylation of serine 331 is essential for conferring resistance to MMC.
A). FANCD2 (S331A) fails to correct MMC sensitivity in FA-D2 cells. Resistance of FA-D2 deficient cell PD20+vector, PD20 + wild type FlagEGFP-FANCD2, or PD20 + FlagEGFP-D2 S331A cells against MMC were measured by clonogenic survivial assays. Points, means; bars,SE. n=3. B).
Disruption of phosphorylation at serine 331 abolishes inducible monoubiquitylation. FA-D2 deficient PD20 cells corrected with Flag-EGFP tagged wild FANCD2, FANCD2 (K561R) or FANCD2 (S331A) were treated with or without 0.5 μM MMC for 20 hours before analyzed by immunoblotting against total FANCD2.The result shows loss of monoubiquitylated FANCD2 (L-isoform) in the cases of S331A and K561R, compared with wild type FANCD2 (top panel). Immunoblot for Ku70 served as a loading control (bottom panel).
C). Hydroxyurea also induces monoubiquityation in a phosphoserine 331-dependent fashion. FA-D2 mutant cells transduced with either Flag-FANCD2 or Flag-FANCD2 (S331A) were treated with 1 mM hyroxyurea or 0.1 μM MMC for 24 hours. Immunoblotting revealed that hydroxyurea induced monoubiquitylation of FANCD2 only in wild type FANCD2 but not in FANCD2 (S331A).
D). FANCD2 (S331A) localizes to the nucleus. FA-D2 mutant cells expressing Flag-GFP-FANCD2 wild type or S331A mutant were plated and examined by direct fluorescent microscopy. The micrographs show that Flag-EGFP tagged FANCD2 (S331A) has similar nuclear cellular distribution as that of wild type FANCD2.
Monoubiquitylation of FANCD2 at lysine 561 has been shown to be a pivotal event in the activation of the FA pathway in response to stalling of DNA replication forks. Thus, hypersensitivity to MMC observed in phospho- deficient mutant S331A might be explained by an impairment of inducible monoubiquitylation. Indeed, immunoblotting revealed that expression of monoubiquitylated FANCD2 (L-isoform) in PD20 cells transduced with Flag-EGFP FANCD2 (S331A) failed to increase in response to MMC treatment (Fig. 2B), suggesting that phosphorylation of serine 331 is important for inducible monoubiquitylation of FANCD2 in response to DNA damage. This failure of induction of FANCD2 monoubiquitylation could also be seen in the same cells treated with hydroxyurea (Fig. 2C).
The deficiency of inducible monoubiquitylation of FANCD2 (S331A) is unlikely explained by sequestering FANCD2 away from its E3 ubiquitin ligase as a result of improper nuclear transportation or retention of FANCD2, since direct fluorescence microscopy showed that the cellular distribution of Flag-EGFP-FANCD2 (S331A) is similar to that of wild type FANCD2 (Fig. 2D): both localize to the nucleus, as reported previously(23).
Phospho-mimetic mutation S331D restores the monoubiquitylation of FANCD2 and resistance to MMC
It is conceivable that the dysfunctions of mutant FANCD2 (S331A) was caused by gross structural alterations introduced by this specific point substitution. To exclude this possibility, we constructed a stable PD20 cell line transduced with Flag-tagged phospho- mimetic mutant FANCD2 construct with serine 331 mutated to aspartic acid (S331D). Interestingly, immunoblotting showed inducible monoubiquitylation of the phosphomimetic mutant FANCD2 (S331D) in response to MMC (Fig. 3A).
Figure 3. Phospho-mimetic mutation S331D restores mono-ubiquitylation of FANCD2 and resistance to MMC.
A). Phospho-mimetic mutation S331D restores inducible monoubiquitylation. Whole cell lysates were prepared from FA-D2 cells expressing wild type or S331D mutant FANCD2 and treated with MMC. FANCD2 immunoblotting revealed that FANCD2 (S331D) was monoubiquitylated following MMC treatment as wild type FANCD2.
B) Phospho-mimetic mutant FANCD2 (S331D) confers resistance against MMC. Clonogenic survival assay was conducted in triplicate for FA-D2 deficient cell PD20+vector, PD20 + Flag tagged wild type FANCD2, or phosphomimetic S331D cell line. The phospho-mimetic mutant S331D conferred near full MMC resistance. Points, means; bars,SE. n=3.
C) Growth inhibition by MMC confirms clonogenic survival assay. Cells (106) were plated into 75 cm2 flasks in growth medium containing MMC, grown until near-confluence was reached in control cultures, trypsinized and cell numbers determined by hemocytometer. The response of the phospho-mimetic FANCD2(S331D) cell line is indistinguishable from wild type, while the FANCD2(S331A) cell line is considerably much more MMC-hypersensitive. Points, means; bars,SE. n=3
We further tested whether the phospho-mimetic mutation S331D also restored resistance to DNA crosslinkers in FA-D2 deficient PD20 cells. In a clonogenic survival assay, FANCD2 (S331D) conferred near wild type resistance against MMC (Fig. 3B). Complementation of MMC hypersensitivity by FANCD2 (S331D) was confirmed in a growth inhibition assay (Fig. 3C). Here the response of PD20+FANCD2 (S331D) was indistinguishable from the wild type corrected cell line.
Serine 331 phosphorylation is inducible in response to DNA damage
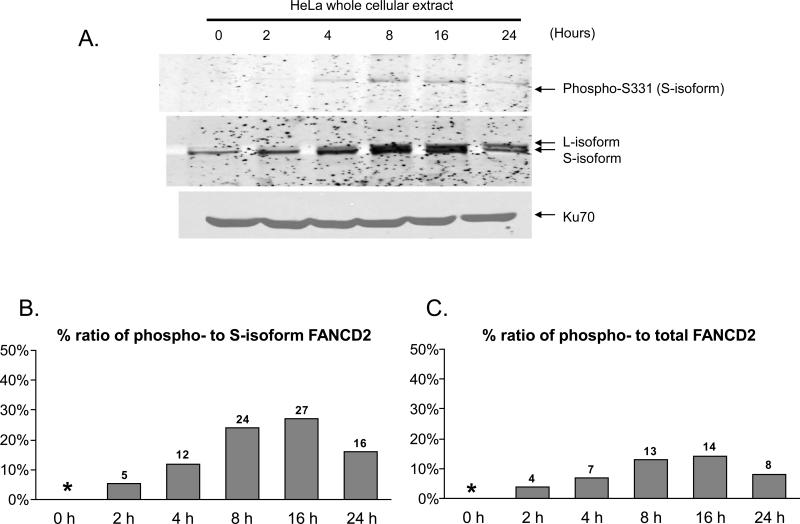
Previously phosphorylation of FANCD2 on T691 and S717 was shown to increase dynamically following DNA damage (12). Thus we tested kinetics of phosphorylation of FANCD2 at serine 331to determine whether this phosphorylation event is constitutive or temporally regulated. HeLa cells were treated with 1 μM MMC for different amounts of time before cellular extracts were prepared and analyzed by SDS-PAGE. Immunoblotting against phosphoserine 331 specific antiserum showed that expression of phosphorylated FANCD2 rises substantially by 8 hours (Fig. 4A). Interestingly, total levels of FANCD2 rose concomitantly with phosphorylation, suggesting that phosphorylation may be a part of protein level regulation. Densitometric quantification showed that not only the expression level of FANCD2 but the relative abundance of phosphorylated FANCD2 increased over time as well (Fig. 4B). Surprisingly, phosphorylation of serine 331 only appeared in the S-isoform but not in L-isoform, suggesting coordinated dephosphorylation at serine 331 upon addition of the ubiquitin moiety.
Figure 4. Serine 331 phosphorylation is inducible in response to DNA damage.
A) Temporal pattern of phosphorylation at serine 331 is similar to that of inducible monoubiquitylation. HeLa cells were treated with 0.5 μM MMC for the indicated time interval followed by immunoblotting. Expression of phospho-FANCD2 was increased by 8 hours following MMC treatment. The temporal pattern of inducible phosphorylation is similar to that of monoubiquitylation of FANCD2. Furthermore, the phospho-FANCD2 comigrated with unmonoubiquitylated FANCD2 (S-isoform). Ku70 immunoblot served as a loading control.
B) Percentage of ratio of the phospho-specific signals to corresponding total FANCD2 signals. To quantify the amount of phosphorylated FANCD2 relative to total FANCD2 following DNA damage, the immunoblot images above were analyzed further by ImageJ to calculate the ratio of the intensity of phospho-specific signal to corresponding intensity of S-isoform. The results confirmed that not only the total level but the proportion of phospho-FANCD2 as well were increased and peaked around 8~16 hours following MMC treatment. * indicates data not applicable.
Phospho-serine 331 FANCD2 is expressed in FA core complex mutant cell lines
In order to establish whether phosphorylation of FANCD2 at serine 331 is also dependent on the integrity of FANC core complex like monoubiquitylation, we analyzed expression of phospho-S331 FANCD2 in a number of human FA cell lines (Fig. 5A). Immunoblotting with the S331 phospho-specific FANCD2 antibody showed that similar levels of protein were detectable in FA-A, FA-C, FA-G, and FA-L cell lines as well as their cDNA complemented counterparts. Phospho-Ser331 FANCD2 was also present in PD20-3-15 and PD20-K651R. These results indicate that neither an intact core complex nor monoubiquitylation of FANCD2 is required for efficient phosphorylation at Ser331.
Figure 5. Phosphorylation of serine 331 is independent of the FA core complex and required for FANCD2/BRCA2 interaction.
A) Phospho-serine 331 FANCD2 is expressed in FA core complex mutant cell lines. FA-D2 deficient PD20 cell and its derived cells expressing Flag tagged FANCD2 S331A(PD20-S331A), FANCD2 561R(PD20-K561R) or wild type FANCD2 (PD20-315) , lymphoblast cell lines BD180 (WT), HSC72 (FA-A), HSC536 (FA-C), EUFA143 (FA-G) and EUFA868 (FA-L), fibroblast cell line GM6914 (FA-A) and their respective cDNA complemented derivatives were analyzed by immunoblotting. Expression of phospho-FANCD2 was present in each of the uncomplemented core complex mutant cell lines and wild type cells, except PD20 and PD20-S331A.
B) FANCD1/BRCA2 is co-immunoprecipitated with FANCD2. Cell lysates were immunoprecipitated with antibody to FANCD2 and probed either with an anti-BRCA2 C-terminal antibody (amino acids 3245–3418) to determine FANCD2-BRCA2 interaction (upper panel) or with the same FANCD2 antibody used for immunoprecipitation (lower panel). BRCA2 co-precipitated with FANCD2 in cell lines expressing wild type (PD20-3-15, PD20+FANCD2) and FANCD2 (K561R) proteins. (PD20-3-15 cells are derived from complementing FA-D2 deficient PD20 cells with human chromosome 3p). Co-precipitation was absent in FA-D2 deficient PD20 cells and expression of FANCD2 (S331A) failed to restore this interaction. In contrast, in PD20 cells expressing the phospho-mimetic mutation S331D, interaction between FANCD2 and BRCA2 was restored. The input lane represents 1/50 of the protein extract used for immunoprecipitation in the cell lines indicated.
C) FANCD2 is co-immunoprecipitated with FANCD1/BRCA2. The reciprocal immunoprecipiatation with anti-BRCA2 antibody was performed using the same cells as in (B) and subsequently immunoblotted with either FANCD2 antibody (upper panel) or BRCA2 antibody (lower panel). Blotting with FANCD2 antibody yielded two adjacent FANCD2 protein bands in PD20-3-15, PD20+FANCD2 and PD20+FANCD2 (S331D) cell lines, indicating that both isoforms of FANCD2 interact with BRCA2. No interaction was detected in PD20 cells. Neither isoform was co-precipitated in PD20+FANCD2 (S331A) cells, indicating that prior phosphorylation of FANCD2 at serine 331 is a requirement for interaction with BRCA2 regardless of monoubiquitylation status. BRCA2 is expressed in all cell lines (lower panel). The input lane represents 1/50 of the protein extract used for immunoprecipitation in the cell lines indicated.
Phosphorylation of FANCD2 at serine 331 is required for its in vivo interaction with BRCA2
We and others have previously demonstrated that FANCD1/BRCA2 and FANCD2 can be co-immunoprecipitated in mammalian cells (24). BRCA2 has been shown to have a role in homologous recombination repair, mediated via direct interaction with RAD51(25, 26). It was previously reported that FANCD2 binds to a highly conserved C terminal site of BRCA2 using yeast 2 hybrid analysis(24, 27). Interestingly, a region encompassing serine 331 (amino acid residues 248-359) is required for association between FANCD2 and BRCA2(24). In addition, we more recently demonstrated that the in vivo interaction of FANCD2 and BRCA2 takes place in core complex mutants such as FA-A and FA-C (16), as does the expression of phospho-Ser331 FANCD2 (Fig. 5A). These observations prompted us to investigate the impact of phosphorylation at serine 331 on FANCD2 interaction with BRCA2. Immunoprecipitation-immunoblotting experiments showed that interaction of FANCD2 with BRCA2 was abolished in FANCD2 deficient PD20 cells expressing mutant FANCD2-S331A (Fig. 5B). Analogous to monoubiquitylation and survival assays, expression of the phospho-mimetic FANCD2 mutation S331D restored the physical interaction between FANCD2 and BRCA2. When immunoprecipitating with anti-BRCA2 antibody (Fig 5C), we confirmed that both isoforms of FANCD2 interact in PD20 cells expressing wild type FANCD2 (16). These interactions are also present in PD20+FANCD2(S331D), while both interactions are absent in PD20+FANCD2(S331A). The absence of interaction between both isoforms of FANCD2 in PD20+FANCD2(S331A) suggests that prior phosphorylation of Ser331 is required for interaction with BRCA2, irrespective of monoubiquitylation status. This further demonstrates that phosphorylation at serine 331 is required for the functional interplay between BRCA2 and FANCD2.
Phosphorylation at serine 331 is mediated by CHK1
The sequence flanking serine 331(K-S-K-G-R-A-S-S-S) is highly conserved amongst mammalian species and is a partial fit for phosphorylation motif of CHK1(Arg-X-X-Ser)(28), an ATR substrate which has been implicated in regulating cell cycle checkpoint in the FA pathway –mediated genomic stress response (13, 29). Furthermore, CHK1 also directly regulates the FA pathway by phosphorylation of FA member protein FANCE(15). Thus we examined whether phosphorylation of serine 331 might be regulated by CHK1.
In order to test this idea, we synthesized 2 peptides of 15 amino acid residues around serine 331 either wild type or with the S331A substitution. In vitro CHK1 kinase experiments were performed to measure 1) if CHK1 could phosphorylate the peptide and 2) the substrate dependent nature of the phosphorylation. Marked specific CHK1 phosphorylation occurred on the wild type peptide as compared with S331A peptide (Fig. 6A).
Figure 6. Phosphorylation at serine 331 is mediated by CHK1.
A). Recombinant GST-CHK1 specifically phosphorylates wild type peptide encompassing serine 331. In vitro CHK1 kinase assay was conducted with indicated concentrations of either wild type or S331A peptides.Radioactivity of blank controls (reaction systems without peptide substrate) was subtracted from counts to calculate the mole amount of phosphate groupincorporated by peptides . Only wild type FANCD2 peptide was significantly phosphorylated. Points, means; bars,SE. n=3
B). CHK1 specifically phosphorylates peptide substrates as compared to CHK2. In vitro CHK1 or CHK2 kinase assay was conducted with wild type FANCD2 peptide substrates at a concentration of 0.2 mM or 30 μM CHKtide substrates as the positive control. The kinase activity of CHK1 with wild type FANCD2 peptide substrate was adjusted for differing enzymatic potency between CHK1 and CHK2 by multiplying the ratio of CHKtide phosphorylated by CHK2 to that by CHK1.. The result indicates that CHK1 displays roughly 8 fold more relative activity than CHK2 at the FANCD2 peptide. Columns, means; bars, SE.n=3
C). In vivo inhibition of CHK1 eliminates phosphorylation of FANCD2 at serine 331. HeLa whole cell extracts made from cells undergoing the indicated treatment (16 hours) were separated by SDS-PAGE and immunoblotted against phospho-serine 331 FANCD2, FANCA, and phospho-serine 1449 FANCA. Lane 1 to 4 from left to right are 1): Hela cells without treatment; 2): Hela cells treated with 1 μM MMC; 3): Hela treated with 1 uM MMC plus 5 μM CHK1 inhibitor, SB-218078; 4): HeLa cells treated with 1 uM MMC plus 1 mM CHK1 inhibitor. CHK1 inhibitor effectively inhibited inducible expression of phospho-FANCD2 while phospho-FANCA remains intact.
CHK1 and CHK2 share some substrates such as CDC25C(30, 31) but have different roles in DNA repair control and cell cycle arrest : ATR and ATM phosphorylate CHK1 and CHK2, respectively, to activate the checkpoint as well as DNA damage responses(32, 33). To determine the FANCD2 peptide substrate preference between CHK1 and CHK2, the kinase activity of CHK1 and CHK2 was first adjusted for differing enzyme potency by normalizing the ratio of each respective kinase activity on a control substrate, CHKtide (Millipore). In accordance with the fact that ATR and not ATM is required for resistance to MMC, the result showed that CHK1 phosphorylated the peptide substrate specifically compared to CHK2, further supporting that CHK1 targets FANCD2 at serine 331(Fig. 6B).
In order to confirm the importance of CHK1 for phosphorylation of FANCD2 on serine 331 in vivo, we measured intensity of phosphorylation of FANCD2 at this site in HeLa cells in the presence of a CHK1 inhibitor, SB-218078(34) following MMC treatment. Treatment with CHK1 inhibitor SB-218078 completely blocked the phosphorylation of serine 331 in FANCD2, while phosphorylation at serine 1449 of FANCA was preserved, which we have shown to be an ATR phosphorylatable site (Fig. 6C) (17). Taken together, these data suggest that CHK1 mediates the activation of the FA pathway by direct phosphorylation of serine 331 on FANCD2.
Discussion
Several groups, ours included, have shown that phosphorylation of FA proteins such as FANCA, FANCD2, and FANCG plays an important functional role in regulating the FA DNA repair pathway(16, 19, 35, 36). In this study, we have identified a novel phosphorylation site at serine 331 on FANCD2, the pivotal player downstream of the FA core complex.
This phosphorylation event is essential for activation of the FA pathway since single point substitution S331A diminishes the inducible monoubiquitylation of FANCD2 and fails to complement the MMC hypersensitivity of FA-D2 deficient PD20 cells. Our time course indicates that phosphorylation at serine 331 increases by 4 hours following drug treatment, just preceding the expression of monoubiquitylated FANCD2, further supporting the notion that phosphorylation of serine 331 is independent of, but necessary for inducible FANCD2 monoubiquitylation. Contrary to monoubiquitylation at lysine 561, the phosphorylation of FANCD2 at serine 331 does not require an intact FA core complex.
FANCD2 has been shown to directly interact with BRCA2, which is involved in homologous recombination via direct binding with RAD51(37). We recently demonstrated that the two proteins are components of a protein complex that also contains FANCG and the RAD51 paralog XRCC3 (16, 38). Serine 331 lies in the domain essential for FANCD2-BRCA2 interaction as evidenced in yeast 2 hybrid system,(24) and we now show that phosphorylation of serine 331 is required for their in vivo interaction. The effect on inducible monoubiquitylation and binding with BRCA2 is significant and specific since phospho-mimetic mutation (S331D) complements both phenotypes. Thus, our findings corroborate earlier studies on FANCD2 interacting partners by providing a mechanistic link between FANCD2 and BRCA2. We previously demonstrated that phosphorylation of FANCG at serine 7 is also required for interaction between FANCD2 and BRCA2 (16), and it will be of great interest to determine how these two phosphorylation events are coordinated mechanistically. At present, it is not precisely understood how the interaction of FANCD2 with BRCA2 modulates homologous recombination repair in response to DNA damage. Given that CHK1-phosphorylation of both RAD51 and BRCA2 regulates the binding of RAD51 to BRCA2 and the ensuing recruitment of RAD51 to sites of DNA damage (39), it is tempting to speculate that CHK-1 mediated phosphorylation of FANCD2 at Ser331 may also impact on this process. BRCA2 has been thought of as a downstream effector in a linear FA pathway, since monoubiquitylation of FANCD2 is independent of BRCA2. Given that phosphorylation precedes monoubiquitylation at lysine 331 in timecourse experiments, co-immunoprecipitation of FANCD2 and BRCA2 suggests that BRCA2 may act in concert with FANCD2 in the FA pathway, at least in part. That phosphorylation of Ser331 is simultaneously required for FANCD2-BRCA2 interaction and promotion of FANCD2 monoubiquitylation, implicates a complexity of response that is inconsistent with a simple linear pathway. Interestingly, the coprecipitation of FANCD2 by BRCA2 reveals both the short and long isoforms of FANCD2 in a phosphoserine 331 dependent fashion, even though phosphorylation was detectable only on the short form. This observation may be due to a regulated dephosphorylation event at the time of monoubiquitylation. Alternatively, our phosphospecific antibody may simply be unable to detect the serine 331 phosphorylated species of FANCD2 when in the monoubiquitylated state.
Cell cycle checkpoint machinery plays a significant role in genomic stress responses by allowing various DNA repair pathways enough time to repair DNA lesions. Previous studies show that ATR is the DNA damage sensor involved in the FA pathway: ATR acts upstream of the FA pathway in the ICL-induced intra-S phase checkpoint (13), and silencing of ATR by siRNA knocks down monoubiquitylated FANCD2 expression and sensitizes cells to DNA crosslinkers, probably mediated by direct phosphorylation of FANCD2 at S717 and T691(12). In this study we further extend the interactions between cellular checkpoint machinery and DNA repair pathways by establishing the links between phosphorylation of FANCD2 at serine 331 and CHK1, the substrate of ATR. This is consistent with the finding that loss of CHK1 impedes FANCD2 monoubiquitylation and resistance to MMC(14). Furthermore, CHK1 is also required for FANCD2-independent FA functions mediated by phosphorylation of FANCE at T346 and S374(15). While it may be impossible to rule out a role for CHK2 phosphorylation at serine 331 as we have not examined FANCD2 S331 phosphorylation after ionizing radiation, these published reports and our in vitro data suggest an exclusive role for CHK1.
The mechanism by which CHK1-dependent phosphorylation of serine 331 promotes FANCD2 monoubiquitylation may be mediated by regulating the interactions of FANCD2 with its binding partners. There are several possible mechanisms. First, phosphorylation of FANCD2 by CHK1 may enhance its affinity to the FANCL subunit of the FA core complex, the putative ubiquityl E3 ligase of FANCD2(6). Second, the phosphorylation event at serine 331 may facilitate monoubiquitylation by blocking access to the deubiquitylation enzyme USP1(40). Third, it is recently reported that CHK1 and its binding partner claspin are required for DNA –damage induced ubiquitylation of proliferating cell nuclear antigen (PCNA) by RAD18 (41). Since PCNA shares the same deubiquitylation enzyme USP1 with FANCD2 (42, 43) and PCNA and FANCD2 have been shown to colocalize in foci (24), it is possible that they share the same regulation through CHK1 as well. Given the general proximity of these key enzymes, it is certainly a possibility that phosphorylation regulates monoubiquitylation of FANCD2 in a coordinated way, allowing a dephosphorylation of FANCD2 at the same time, consistent with our data. Regardless of the mechanisms that promote FANCD2 monoubiquitylation and the interaction of FANCD2 with BRCA2, our study highlights the regulation of the FA/BRCA pathway by checkpoint kinases via phosphorylation.
FANCI, the paralog of FANCD2, associates with FANCD2 to form so-termed ID complex(44). Both FANCD2 and FANCI undergo interdependent monoubiquitylation and re-localization to nuclear foci following DNA damage or replication fork stress(45). Recently it was reported that mono-ubiquitylation of FANCI is dependent on phosphorylation at six conserved and clustered Ser/Thr-Gln motifs and phospho-mimetic mutations induce persistent monoubiquitylation and foci formation(46). We are interested to investigate whether FANCI association is disrupted in phospho-mutant FANCD2(S331A) as well.
Footnotes
ImageJ software (internet). Washington DC: National institutes of Health, 2009. Available from http://rsbweb.nih.gov/ij/download.html.
References
- 1.Taniguchi T, D'Andrea AD. Molecular pathogenesis of Fanconi anemia: recent progress. Blood. 2006;107(11):4223–33. doi: 10.1182/blood-2005-10-4240. [DOI] [PubMed] [Google Scholar]
- 2.D'Andrea AD, Grompe M. The Fanconi anaemia/BRCA pathway. Nat Rev Cancer. 2003;3(1):23–34. doi: 10.1038/nrc970. [DOI] [PubMed] [Google Scholar]
- 3.Kitao H, Yamamoto K, Matsushita N, Ohzeki M, Ishiai M, Takata M. Functional interplay between BRCA2/FancD1 and FancC in DNA repair. The Journal of biological chemistry. 2006;281(30):21312–20. doi: 10.1074/jbc.M603290200. [DOI] [PubMed] [Google Scholar]
- 4.Levitus M, Waisfisz Q, Godthelp BC, et al. The DNA helicase BRIP1 is defective in Fanconi anemia complementation group. J. Nature genetics. 2005;37(9):934–5. doi: 10.1038/ng1625. [DOI] [PubMed] [Google Scholar]
- 5.Wang W. Emergence of a DNA-damage response network consisting of Fanconi anaemia and BRCA proteins. Nature reviews. 2007;8(10):735–48. doi: 10.1038/nrg2159. [DOI] [PubMed] [Google Scholar]
- 6.Meetei AR, Yan Z, Wang W. FANCL replaces BRCA1 as the likely ubiquitin ligase responsible for FANCD2 monoubiquitination. Cell cycle (Georgetown, Tex. 2004;3(2):179–81. [PubMed] [Google Scholar]
- 7.Meetei AR, de Winter JP, Medhurst AL, et al. A novel ubiquitin ligase is deficient in Fanconi anemia. Nature genetics. 2003;35(2):165–70. doi: 10.1038/ng1241. [DOI] [PubMed] [Google Scholar]
- 8.Xue Y, Li Y, Guo R, Ling C, Wang W. FANCM of the Fanconi anemia core complex is required for both monoubiquitination and DNA repair. Human molecular genetics. 2008;17(11):1641–52. doi: 10.1093/hmg/ddn054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gari K, Decaillet C, Stasiak AZ, Stasiak A, Constantinou A. The Fanconi anemia protein FANCM can promote branch migration of Holliday junctions and replication forks. Molecular cell. 2008;29(1):141–8. doi: 10.1016/j.molcel.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Jacquemont C, Taniguchi T. The Fanconi anemia pathway and ubiquitin. BMC biochemistry. 2007;8(Suppl 1):S10. doi: 10.1186/1471-2091-8-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andreassen PR, D'Andrea AD, Taniguchi T. ATR couples FANCD2 monoubiquitination to the DNA-damage response. Genes & development. 2004;18(16):1958–63. doi: 10.1101/gad.1196104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho GP, Margossian S, Taniguchi T, D'Andrea AD. Phosphorylation of FANCD2 on two novel sites is required for mitomycin C resistance. Molecular and cellular biology. 2006;26(18):7005–15. doi: 10.1128/MCB.02018-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pichierri P, Rosselli F. The DNA crosslink-induced S-phase checkpoint depends on ATR-CHK1 and ATR-NBS1-FANCD2 pathways. The EMBO journal. 2004;23(5):1178–87. doi: 10.1038/sj.emboj.7600113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guervilly JH, Mace-Aime G, Rosselli F. Loss of CHK1 function impedes DNA damage-induced FANCD2 monoubiquitination but normalizes the abnormal G2 arrest in Fanconi anemia. Human molecular genetics. 2008;17(5):679–89. doi: 10.1093/hmg/ddm340. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Kennedy RD, Ray K, Stuckert P, Ellenberger T, D'Andrea AD. Chk1-mediated phosphorylation of FANCE is required for the Fanconi anemia/BRCA pathway. Molecular and cellular biology. 2007;27(8):3098–108. doi: 10.1128/MCB.02357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson JB, Yamamoto K, Marriott AS, et al. FANCG promotes formation of a newly identified protein complex containing BRCA2, FANCD2 and XRCC3. Oncogene. 2008;27(26):3641–52. doi: 10.1038/sj.onc.1211034. [DOI] [PubMed] [Google Scholar]
- 17.Collins NB, Wilson JB, Bush T, et al. ATR-dependent phosphorylation of FANCA on serine 1449 after DNA damage is important for FA pathway function. Blood. 2009;113(10):2181–90. doi: 10.1182/blood-2008-05-154294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiao F, Moss A, Kupfer GM. Fanconi anemia proteins localize to chromatin and the nuclear matrix in a DNA damage- and cell cycle-regulated manner. The Journal of biological chemistry. 2001;276(26):23391–6. doi: 10.1074/jbc.M101855200. [DOI] [PubMed] [Google Scholar]
- 19.Mi J, Qiao F, Wilson JB, et al. FANCG is phosphorylated at serines 383 and 387 during mitosis. Molecular and cellular biology. 2004;24(19):8576–85. doi: 10.1128/MCB.24.19.8576-8585.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali SA, Steinkasserer A. PCR-ligation-PCR mutagenesis: a protocol for creating gene fusions and mutations. BioTechniques. 1995;18(5):746–50. [PubMed] [Google Scholar]
- 21.Thompson LH, Hinz JM, Yamada NA, Jones NJ. How Fanconi anemia proteins promote the four Rs: replication, recombination, repair, and recovery. Environmental and molecular mutagenesis. 2005;45(2-3):128–42. doi: 10.1002/em.20109. [DOI] [PubMed] [Google Scholar]
- 22.Wang W. A major switch for the Fanconi anemia DNA damage-response pathway. Nature structural & molecular biology. 2008;15(11):1128–30. doi: 10.1038/nsmb1108-1128. [DOI] [PubMed] [Google Scholar]
- 23.Marte B. A FANCy double life. Nature cell biology. 2002;4(6):E151. doi: 10.1038/ncb0602-e151. [DOI] [PubMed] [Google Scholar]
- 24.Hussain S, Wilson JB, Medhurst AL, et al. Direct interaction of FANCD2 with BRCA2 in DNA damage response pathways. Human molecular genetics. 2004;13(12):1241–8. doi: 10.1093/hmg/ddh135. [DOI] [PubMed] [Google Scholar]
- 25.Davies OR, Pellegrini L. Interaction with the BRCA2 C terminus protects RAD51-DNA filaments from disassembly by BRC repeats. Nature structural & molecular biology. 2007;14(6):475–83. doi: 10.1038/nsmb1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esashi F, Galkin VE, Yu X, Egelman EH, West SC. Stabilization of RAD51 nucleoprotein filaments by the C-terminal region of BRCA2. Nature structural & molecular biology. 2007;14(6):468–74. doi: 10.1038/nsmb1245. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Andreassen PR, D'Andrea AD. Functional interaction of monoubiquitinated FANCD2 and BRCA2/FANCD1 in chromatin. Mol Cell Biol. 2004;24(13):5850–62. doi: 10.1128/MCB.24.13.5850-5862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim MA, Kim HJ, Brown AL, et al. Identification of novel substrates for human checkpoint kinase Chk1 and Chk2 through genome-wide screening using a consensus Chk phosphorylation motif. Experimental & molecular medicine. 2007;39(2):205–12. doi: 10.1038/emm.2007.23. [DOI] [PubMed] [Google Scholar]
- 29.Pichierri P, Rosselli F. Fanconi anemia proteins and the s phase checkpoint. Cell cycle (Georgetown, Tex. 2004;3(6):698–700. [PubMed] [Google Scholar]
- 30.Wang Y, Wang H. CHK1 kinase activity assay. Methods in molecular biology (Clifton, NJ. 2004;281:143–51. doi: 10.1385/1-59259-811-0:143. [DOI] [PubMed] [Google Scholar]
- 31.O'Neill T, Giarratani L, Chen P, et al. Determination of substrate motifs for human Chk1 and hCds1/Chk2 by the oriented peptide library approach. The Journal of biological chemistry. 2002;277(18):16102–15. doi: 10.1074/jbc.M111705200. [DOI] [PubMed] [Google Scholar]
- 32.Wakabayashi M, Ishii C, Inoue H, Tanaka S. Genetic analysis of CHK1 and CHK2 homologues revealed a unique cross talk between ATM and ATR pathways in Neurospora crassa. DNA repair. 2008;7(12):1951–61. doi: 10.1016/j.dnarep.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene. 2007;26(56):7773–9. doi: 10.1038/sj.onc.1210881. [DOI] [PubMed] [Google Scholar]
- 34.Jackson JR, Gilmartin A, Imburgia C, Winkler JD, Marshall LA, Roshak A. An indolocarbazole inhibitor of human checkpoint kinase (Chk1) abrogates cell cycle arrest caused by DNA damage. Cancer Res. 2000;60(3):566–72. [PubMed] [Google Scholar]
- 35.Thomashevski A, High AA, Drozd M, et al. The Fanconi anemia core complex forms four complexes of different sizes in different subcellular compartments. The Journal of biological chemistry. 2004;279(25):26201–9. doi: 10.1074/jbc.M400091200. [DOI] [PubMed] [Google Scholar]
- 36.Qiao F, Mi J, Wilson JB, et al. Phosphorylation of fanconi anemia (FA) complementation group G protein, FANCG, at serine 7 is important for function of the FA pathway. The Journal of biological chemistry. 2004;279(44):46035–45. doi: 10.1074/jbc.M408323200. [DOI] [PubMed] [Google Scholar]
- 37.Goodsell DS. The molecular perspective: RAD51 and BRCA2. Stem Cells. 2005;23(9):1434–5. doi: 10.1634/stemcells.FCM5. [DOI] [PubMed] [Google Scholar]
- 38.Hussain S, Wilson JB, Blom E, et al. Tetratricopeptide-motif-mediated interaction of FANCG with recombination proteins XRCC3 and BRCA2. DNA repair. 2006;5(5):629–40. doi: 10.1016/j.dnarep.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Bahassi EM, Ovesen JL, Riesenberg AL, Bernstein WZ, Hasty PE, Stambrook PJ. The checkpoint kinases Chk1 and Chk2 regulate the functional associations between hBRCA2 and Rad51 in response to DNA damage. Oncogene. 2008;27(28):3977–85. doi: 10.1038/onc.2008.17. [DOI] [PubMed] [Google Scholar]
- 40.Nijman SM, Huang TT, Dirac AM, et al. The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Molecular cell. 2005;17(3):331–9. doi: 10.1016/j.molcel.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Yang XH, Shiotani B, Classon M, Zou L. Chk1 and Claspin potentiate PCNA ubiquitination. Genes & development. 2008;22(9):1147–52. doi: 10.1101/gad.1632808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedberg EC. Reversible monoubiquitination of PCNA: A novel slant on regulating translesion DNA synthesis. Molecular cell. 2006;22(2):150–2. doi: 10.1016/j.molcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Huang TT, Nijman SM, Mirchandani KD, et al. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nature cell biology. 2006;8(4):339–47. doi: 10.1038/ncb1378. [DOI] [PubMed] [Google Scholar]
- 44.Smogorzewska A, Matsuoka S, Vinciguerra P, et al. Identification of the FANCI protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell. 2007;129(2):289–301. doi: 10.1016/j.cell.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sims AE, Spiteri E, Sims RJ, 3rd, et al. FANCI is a second monoubiquitinated member of the Fanconi anemia pathway. Nature structural & molecular biology. 2007;14(6):564–7. doi: 10.1038/nsmb1252. [DOI] [PubMed] [Google Scholar]
- 46.Ishiai M, Kitao H, Smogorzewska A, et al. FANCI phosphorylation functions as a molecular switch to turn on the Fanconi anemia pathway. Nature structural & molecular biology. 2008;15(11):1138–46. doi: 10.1038/nsmb.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]