Abstract
ISG15 is an interferon-induced ubiquitin-like protein (Ubl) that has antiviral properties. The core El, E2 and E3 enzymes for conjugation of human ISG15 are Ube1L, UbcH8 and Herc5, all of which are induced at the transcriptional level by Type 1 interferon signaling. Several proteomics studies have, together, identified over 300 cellular proteins as ISG15 targets. These targets include a broad range of constitutively expressed proteins and approximately 15 interferon-induced proteins. This chapter provides an overview of the target identification process and the validation of these targets. We also discuss the limited number of examples where the biochemical effect of ISG15 conjugation on target proteins has been characterized.
INTRODUCTION
The innate immune response of mammalian cells is a first-line defense against viral and microbial infections.1 The system is nonspecific with respect to antigen recognition and is instead geared to recognize classes of molecules that are commonly produced by microbes or viruses, such as lipopolysaccharides, flagellin and double-stranded RNA. These molecules are referred to as PAMPs, for Pattern Associated Molecular Patterns. Pathogen Recognition Receptors (PRRs), such as Toll-like receptors, RIG-I and double-stranded RNA-activated protein kinase (PKR), directly recognize PAMPs and trigger signaling pathways that lead to the production of Type I interferons (IFNs).2 The two major forms of Type I IFNs are IFNα and β. IFNα is produced primarily by leukocytes, while IFNβ is produced by a wide range of cell types, including fibroblasts and epithelial cells. Type 1 IFNs are secreted by infected cells and bind to the interferon receptor (IFNAR) of surrounding cells, as well as the initially infected cell, resulting in the transcription of hundreds of interferon stimulated genes (ISGs). The expression of ISGs leads to the generation of a multi-faceted defense system that serves to limit viral or microbial infection.
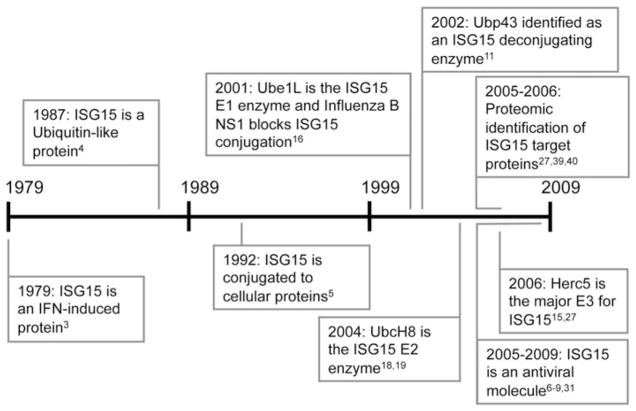
ISG15 is an ISG denoted by the approximate molecular mass of the protein, although the actual molecular mass of the mature form of the protein is 17.1 kD. It was first identified based on its rapid induction in Ehrlich ascites tumor cells after IFN stimulation.3 About a decade later. Art Haas and coworkers recognized ISG15 as what would turn out to be the first of many ubiquitin-like protein (Ubl) modifiers.4,5 As discussed further below, it would be nearly another two decades before ISG15 was shown to actually have antiviral activity against a range of viruses, including Influenza, Sindbis, Herpes, HIV and Ebola virus.6–9 There have so far been no reports demonstrating an antimicrobial function for ISG15. A timeline of discovery for ISG15, spanning 30 years from 1979 to 2009, is shown in Figure 1.
Figure 1.
Important discoveries in the ISG15 field. The timeline highlights the progress made since the discovery of ISG15 in 1979.
Like ubiquitin (Ub) and other Ubls, ISG15 is synthesized as a precursor protein that must be processed to reveal a C-terminal glycine residue. The identity of the processing enzyme is unknown, but Ubp43, an ISG15 deconjugating enzyme, is clearly not essential for processing as the Ubp43 knockout mouse still generates processed and conjugation-competent ISG15.10 There are also inconsistencies in the literature concerning the role of murine Ubp43 as a specific ISG15 deconjugating enzyme. It has been reported to have activity against both ubiquitin and ISG1511,12 and neurologic phenotypes of the Ubp43 knockout mouse were also seen in the Ubp43/ISG15 double knockout mouse,10,13 indicating that accumulation of ISG15 conjugates cannot account for the Ubp43−/− phenoytpes. The difficulty is identifying a specific deISGylating enzyme, if one indeed exists, is likely complicated by the fact that the last six residues of ISG15 are identical to those of ubiquitin (LRLRGG) and these residues must be recognized by the active site of deconjugating enzymes. Consistent with this, other presumed deubiquitinating enzymes have also been shown to have activity against ISG15 conjugates.14
Conjugation of human ISG15 to target proteins occurs via an enzymatic cascade consisting of a single E1 enzyme (Ube1L), E2 enzyme (UbcH8) and a single major E3 enzyme (Herc5). The genes encoding these proteins are all transcriptionally induced by IFNα/β15 although their induction is delayed relative to that of ISG15. Therefore, while free (unconjugated) ISG15 is rapidly induced by interferon stimulation,3 conjugation of ISG15 does not occur to an appreciable degree until approximately 18–24 hours after stimulation.5 It is not understood why this delay is built into the conjugation system, although it cannot be ruled out that that free ISG15 has a function independent of conjugation. Ube1L is the ISG15 E1 enzyme and this is a monomeric E1-like enzyme,16 unlike the heterodimeric E1 enzymes for Nedd8 and Sumo. The E2 enzyme for ISG15 is UbcH8/Ube2L6.17–19 While UbcH8 has been reported to be an E2 for ubiquitin,20–26 determination of kinetic constants of Ube1L and Ube1 (E1Ub) for UbcH8 and UbcH7 indicated that UbcH8 is unlikely to function as a ubiquitin E2 in vivo.17 The major E3 for ISG15 in human cells is Herc5, a HECT domain ligase with N-terminal RCC1 repeats.15,27 Herc5 depletion leads to a dramatic decrease in overall ISG15 conjugation activity, affecting conjugation to the vast majority of cellular target proteins.15 While additional proteins may be required for ISG15 conjugation, Ube1L, UbcH8 and Herc5 form the core IFN-induced components of the system, as their co-expression with ISG15 reconstitutes robust ISG15 conjugation in non-IFN-stimulated cells.15,27,28 ISGylation of target proteins has so far not been reconstituted in vitro. The difficulty appears to lay at the level of the E3 enzyme, as ISG15, Ube1L and UbcH8 are all biochemically active in vitro.17
Interestingly, human Herc5 does not have a direct equivalent in mice or rats.29 However, the human Herc5 and Herc6 genes are adjacent to each other on chromosome 4 and are clearly related to each other through a gene duplication event.15 Both genes are transcriptionally regulated by interferon signaling and the human Herc5 and Herc6 proteins are 50% identical with very similar domain organizations.29 Mouse Herc6 is interferon-induced and located at the corresponding genomic position as human Herc6. While it was proposed that Herc5 was the result of a gene duplication of Herc6 in the primate lineage,29 this is likely incorrect since other mammals (dogs, cow, sheep) have both the Herc5 and Herc6 genes in a similar arrangement as in the human genome, suggesting that Herc5 was lost in the evolution of the rodent lineage. Although there is no evidence that human Herc6 plays a significant role in ISG15 conjugation, it is conceivable that murine Herc6 plays the equivalent role to human Herc5 in conjugation of murine ISG15. To our knowledge, this possibility has not yet been experimentally tested.
Support for an antiviral function for ISG15 was first reported in 2001 when it was found that influenza B virus blocked the conjugation of ISG15 to cellular proteins.16,30 Since then, ISG15 has been reported to have antiviral effects against Influenza, Sindbis, Vaccinia (VACV), Herpes Simplex I, murine γ-Herpesvirus, HIV-1 and Ebola virus.6–9,31 Recent studies with mice lacking Ube1L highlight the importance of ISG15 conjugation, as Ube1L-null mice infected with either Sindbis or influenza B virus produced free ISG15, but no conjugates and were shown to have increased susceptibility to both viruses.32,33 In addition to Influenza B, other viruses have also evolved mechanisms for interfering with ISG15 conjugation: SARS coronavirus encodes a protease that can deconjugate ISG15 from target proteins,34 while vaccinia virus encodes a viral early protein, E3, which binds ISG15 and prevents ISGylation.35
Importantly, the precise biochemical function of ISG15 and the biochemical basis of its antiviral activities remain unknown. In order to understand the mechanism by which ISG15 conjugation contributes to antiviral responses, it is important to identify the proteins targeted by the ISG15 pathway. As described below, proteomic studies have identified over 300 cellular ISG15 targets. The functional consequences of ISGylation will also be discussed, although this has only been determined for a small number of target proteins.
DETECTION OF ISG15 CONJUGATION
ISGylation of target proteins can be detected by two cell-based methods: either by treatment of interferon-responsive cells (e.g., A549, HeLa) with purified IFN-β, or by cotransfection of non-interferon-stimulated cells with four plasmids expressing the core ISG15 conjugation components (ISG15, Ube1L, UbcH8 and Herc5). In HeLa cells, conjugation of ISG15 can be detected approximately 18 hours after treatment with IFNβ, with maximal accumulation of conjugates by 48 hours posttreatment.5,16 For cotransfection experiments, HEK293T cells can be transfected with plasmids expressing Ube1L, UbcH8, Herc5 and tagged (3X-FLAG) or untagged ISG15. In both cases, extracts are typically prepared 24–48 hours after IFNβ treatment or transfection and total cellular proteins are separated by SDS-PAGE and transferred to nitrocellulose membrane. The membrane is then probed with anti-ISG15 antibody (Santa Cruz Antibodies) or with anti-FLAG antibody (Sigma) to detect conjugation of FLAG-ISG15. It is often useful to use a 12% acrylamide gel in order to observe the free (unconjugated) ISG15. Conjugates typically range in size from approximately 45 kD to very high molecular weight adducts that migrate near the top of the gel. Figure 2 shows a time course of ISG15 conjugation of FLAG-ISG15 following cotransfection of plasmids expressing 3XFLAG-ISG15, Ube1L, UbcH8 and Herc5. It should be noted that, at least in our hands, HEK293T respond poorly to IFNβ stimulation and little if any endogenous ISG15 conjugation can be detected in these cells.
Figure 2.
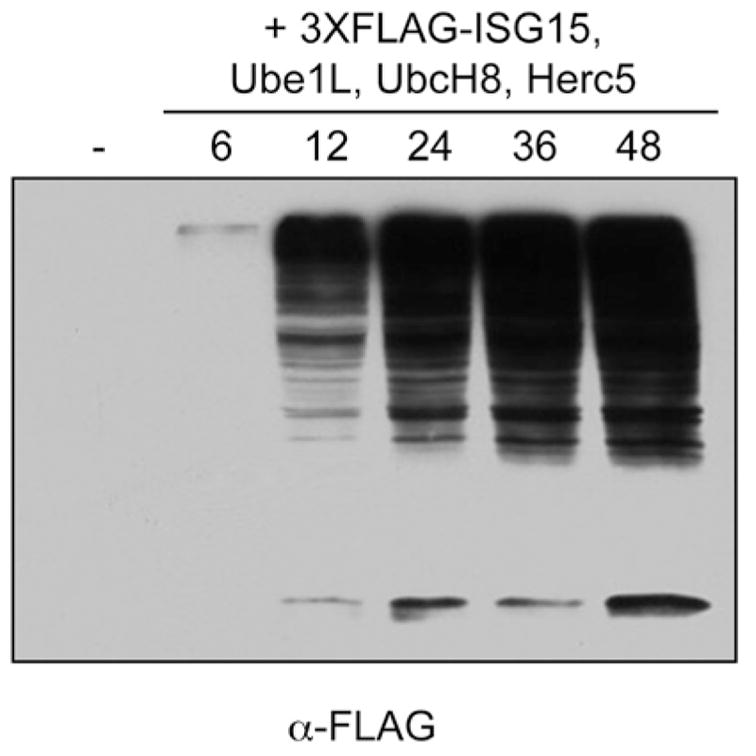
Timecourse of total ISG15 conjugation in non-interferon-treated cells. HEK293T cells were transfected with plasmids expressing Ube1L, UbcH8, Herc5 and 3X-FLAG-ISG15. Cell extracts were collected at the indicated time points and analyzed by immunoblotting with anti-FLAG antibody.
IDENTIFICATION OF ISG15 TARGETS
For ubiquitin, large-scale mass spectrometry-based identification of target in yeast proteins was first reported in 2003.36 This approach involved expression of an N-terminally tagged (6xHis) ubiquitin molecule, affinity purification of conjugates under denaturing conditions and multidimensional liquid chromatography coupled with tandem mass spectrometry to identify conjugated proteins. The basis of mass spectrometry identification of ubiquitin and Ubl modifiers and the criteria for considering a protein as target have been discussed elsewhere.37,38 Based on this general principle, three proteomics studies were performed to identify cellular proteins conjugated to ISG15.27,39,40 Zhao, et al, expressed a double-tagged ISG15 protein (6xHis and FLAG N-terminal tags), along with Ube1L and UbcH8, in HeLa cells and then treated the transfected cells with IFN-β. Extracts were prepared 24 hours after IFN treatment and conjugates were purified under denaturing conditions on Ni-NTA resin, followed by purification on anti-FLAG beads. Purification under denaturing conditions assured that conjugated proteins would be identified, as opposed to proteins associated with conjugated proteins. Isolated proteins were separated by SDS-PAGE and regions of the gel were subjected to trypsin digestion, peptides were separated and identified by liquid chromatography and mass spectrometry, as described.40 This study identified approximately 158 high-confidence targets. In a second study, Giannakopolous, et al, identified ISG15 conjugates in interferon-treated human cells (U937 cells) and in mouse Ubp43-null MEFs.39 Conjugates were immunopurified using antibody recognizing endogenously expressed ISG15. Mass spectrometry identified a total of 76 mouse and human proteins in the immunoprecipitates. Finally, Wong, et al, stably expressed FLAG-ISG15 in A549 cells and immunopurified conjugates after 48 hours of IFNβ treatment.27 174 ISG15 target proteins were identified in this study and together, these three studies identified slightly more than 300 unique proteins as potential ISG15 target proteins.
The identification of ISG15 target proteins was highly anticipated, as it was hoped that this would provide valuable insight into the biologic function of ISG15. This, for example, was the case for identification of targets of Sumo, where target identification revealed a very high percentage of targets to be nuclear proteins involved in regulation of DNA- and RNA-related processes.41,42 Unfortunately, the identification of ISG15 target proteins was not particularly revealing. The targets were largely abundant constitutively expressed proteins, with the exception of approximately 15 targets that were themselves interferon-induced proteins. The interferon-induced targets included some of the better-characterized antiviral ISGs, including PKR, p56, MxA and RIG-I.43,44 PKR and RIG-I, as mentioned above, are pathogen recognition receptors. p56 is a translational inhibitor and MxA is an endoplasmic reticulum-associated GTPase. The constitutively expressed proteins included both cytoplasmic and nuclear proteins involved in a diverse array of cellular functions, including cytoskeletal organization, stress responses, translation, transcription, RNA splicing and general metabolism. Therefore, a clear prediction of the biologic function for ISG15 could not be inferred from this diverse set of target proteins. Finally, it should be noted that a common primary sequence motif has not been identified within this set of target proteins that might serve as a recognition signal for ISGylation and the basis for substrate recognition by the ISGylation machinery remains unknown.
VALIDATION OF ISG15 TARGETS
Validation of ISG15 targets can be performed in several different ways depending on the availability of an antibody to the target, the expression level of the endogenous target protein and the degree to which the target protein is modified with ISG15. In the simplest case, IFNβ can be used to induce the ISG15 conjugation system for 24–48 hours, followed by immunoblotting for the endogenous target protein. The presence of ISG15 conjugates is then seen as the appearance of modified forms of the protein corresponding to the 15 kD Ubl. Multiple conjugates at 15 kD intervals are seen among on some targets. There is no evidence that these represent poly-ISG15 chains and mass spectrometry based mapping of modification sites on individual target proteins strongly suggests that these represent single ISG15 moieties at multiple lysine residues of the target protein (unpublished results). The pattern of modified bands may appear more complicated due to the fact that mono-ISGylated proteins modified at different sites of a target protein may result in slightly different migration on SDS-PAGE gels. In any case, these modified forms can be confirmed to be ISG15 conjugates by siRNA knockdown of one or more of the ISG15 conjugation enzymes. Importantly, only a small number of target proteins have been validated by direct western blotting of IFN-treated cells and these have primarily been those that are IFN-induced and highly expressed, such as p56 and MxA.38 This may primarily be due to the fact that ISGylation is an inefficient process. Typically, the most robust conjugation observed will be on the order of 10–20% of the total pool of the target and in most cases it is much lower. Alternatively, immunoprecipitation of a target protein, followed by immunoblotting for ISG15 (IP-westerns), may serve to enrich for the target protein and result in more sensitive detection of conjugates. ISG15 conjugates can also be concentrated by immunoprecipitating total conjugates and then immunoblotting for an individual target protein.
The single most successful way to validate ISGylation of targets has been to transiently express the target protein along with the conjugation components (ISG15, Ube1L, UbcH8, with or without Herc5) in non-IFN-treated cells. Prior to the identification of Herc5 as the major ISG15 E3, modification of targets was in some cases validated by co-expression of the target with ISG15, Ube1L and UbcH8.39,40 In retrospect, this combination of components was likely sufficient for modification of some proteins because of a basal level of expression of Herc5.15 That is, while conjugation can be detected in HEK293T or HeLa cells by co-expression of ISG15, Ube1L and UbcH8, an siRNA against Herc5 dramatically decreases the level of conjugates, while co-expression of Herc5 boosts the level of conjugation.15 Figure 3 shows the modification of four epitope-tagged target proteins (V5-Ran, FLAG-p56, V5-moesin and TAP-Hsc70) in the presence of a triple (ISG15, Ube1L, UbcH8) or quadruple (ISG15, Ube1L, UbcH8, Herc5) combination of the conjugation components. This demonstrates that modification can in some cases be detected with only basal levels of Herc5 expression, but that it in all cases it is strongly enhanced by co-expression of Herc5.
Figure 3.
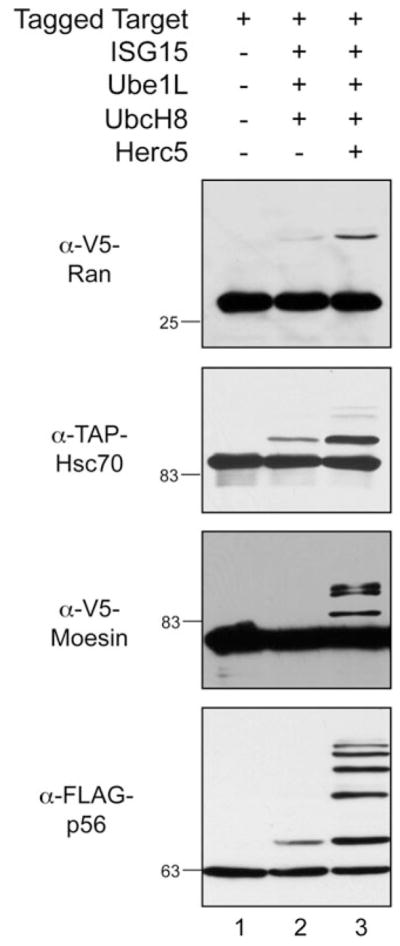
ISG15 conjugation to exogenously expressed targets is enhanced by the co-expression of Herc5. HEK293T cells were transfected with a plasmid expressing an epitope-tagged target protein either alone (lane 1), or combined with Ube1L, UbcH8 and ISG15 (lane 2), or combined with Ube1L, UbcH8, ISG15 and Herc5 (lane 3). Cell extracts were prepared 48 hours posttransfection and analyzed by immunoblotting using the indicated antibodies.
THE EFFECTS OF ISGYLATION ON TARGET PROTEIN FUNCTION
As described above, there are a number of cell-based approaches can be used to generate ISGylated proteins and validate modification of individual proteins. However, it is important to note that an in vitro ISGylation system has not yet been established. Without such a system, it is not possible to easily generate purified ISGylated target proteins for biochemical analyses. This is a key reason why only modest progress has been made on understanding the biochemical function of ISG15 conjugation. Nevertheless, a small number of studies have examined modification of different target cellular target proteins and described biochemical consequences of modification. The four targets discussed here are Ubc13, filamin B, PP2Cβ, and 4EHP.
Ubc13 is a ubiquitin E2 enzyme that functions in the generation of K63-linked polyubiquitin chains. Ubc13 was identified as a cellular target of ISGylation in one of the three proteomic studies discussed above.40 Two subsequent reports showed that Ubc13 is ISGylated at lysine residue K92, which lies near the active-site cysteine residue (C87) of the protein. Both studies showed that the ISGylated form of Ubc13 was defective for ubiquitin thioester formation.45,46 Given the proximity of C87 to K92 in the structure, the inhibition of thioester formation is likely to be simply a result of steric occlusion of C87 by the conjugated ISG15 molecule. The downstream effects of this inhibition have been suggested to result in prevention of NFkB activation,47 as K63-linked polyubiquitination catalyzed by the Ubc13/Mms2 complex is a critical component of the signaling pathway that leads to NFkB activation. NFkB activation, in turn, is critical for transcriptional activation of genes involved in the innate immune response, including IFNβ. An unresolved problem with this proposed mechanism is that only a small fraction of Ubc13 is ISGylated and it is not clear how this would lead to a significant inhibition of overall Ubc13 activity.
Filamin B is one of three related actin binding proteins that are critical for crosslinking of cortical actin filaments48 and it was identified in one of the three large-scale ISG15 proteomics projects discussed above.40 At early time points after interferon stimulation, filamin B tethers RAC1 and a MAP kinase module, which promotes activation of JNK and JNK-mediated apopotosis. The ISGylation of filamin B was shown to lead to release of RAC1, MEKK1 and MKK4 from the scaffold, preventing JNK activation.49 A model was proposed whereby ISGylation of filamin B, at relatively late time points after interferon stimulation, leads to the inactivation of JNK.
PP2Cβ is a protein phosphatase identified in two ISG15 proteomics studies.40,50 PP2Cβ dephosphorylates the TAK1 and IKK kinases, leading to inhibition of NFkB signaling and it was shown that ISGylation of PP2Cβ inhibited its activity. Finally, in a case where ISGylation activates, rather than inactivates, a target protein, the 4EHP mRNA cap binding protein was reported to have increased cap binding activity following ISGylation.51 The biochemical basis of this enhanced binding is unknown.
CONCLUSION
A clearer picture has been to emerge of the enzymes required for ISGylation, the identity of the cellular targets of ISGylation and the spectrum of viruses sensitive to the antiviral effects of ISGylation. Some of the major gaps in our understanding concern (1) the basis for enzyme-substrate recognition in the conjugation process (that is, how a single major E3 enzyme target over 300 cellular proteins?), (2) how the ISGylation of the known targets are directly related to antiviral activities and (3) the precise biochemical effect of ISG15 on target proteins. The establishment of an in vitro ISGylation system will be important for addressing all of these problems, as will a better understanding of the precise steps that ISG15 inhibits in the course of the life cycles of relevant viruses.
Acknowledgments
We thank members of the Huibregtse lab for helpful comments and discussions. This work was supported by a grant to J. M. H. from the National Cancer Institute, National Institutes of Health (CA72943).
References
- 1.Seth RB, Sun L, Chen ZJ. Antiviral innate immunity pathways. Cell Res. 2006;16:141–7. doi: 10.1038/sj.cr.7310019. [DOI] [PubMed] [Google Scholar]
- 2.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–90. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 3.Farrell PJ, Broeze RJ, Lengyel P. Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumour cells. Nature. 1979;279:523–5. doi: 10.1038/279523a0. [DOI] [PubMed] [Google Scholar]
- 4.Haas AL, Ahrens P, Bright PM, et al. Interferon induces a 15-kilodalton protein exhibiting marked homology to ubiquitin. J Biol Chem. 1987;262:11315–23. [PubMed] [Google Scholar]
- 5.Loeb KR, Haas AL. The interferon-inducible 15-kDa ubiquitin homolog conjugates to intracellular proteins. J Biol Chem. 1992;267:7806–13. [PubMed] [Google Scholar]
- 6.Lenschow DJ, Giannakopoulos NV, Gunn LJ, et al. Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo. J Virol. 2005;79:13974–83. doi: 10.1128/JVI.79.22.13974-13983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okumura A, Pitha PM, Harty RN. ISG15 inhibits Ebola VP40 VLP budding in an L-domain-dependent manner by blocking Nedd4 ligase activity. Proc Natl Acad Sci USA. 2008;105:3974–9. doi: 10.1073/pnas.0710629105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okumura A, Lu G, Pitha-Rowe I, et al. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc Natl Acad Sci USA. 2006;103:1440–5. doi: 10.1073/pnas.0510518103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsiang TY, Zhao C, Krug RM. Interferon-induced ISG15 conjugation inhibits influenza A virus gene expression and replication in human cells. J Virol. 2009;83:5971–7. doi: 10.1128/JVI.01667-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritchie KJ, Malakhov MP, Hetherington CJ, et al. Dysregulation of protein modification by ISG15 results in brain cell injury. Genes Dev. 2002;16:2207–12. doi: 10.1101/gad.1010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malakhov MP, Malakhova OA, Kim KI, et al. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J Biol Chem. 2002;277:9976–81. doi: 10.1074/jbc.M109078200. [DOI] [PubMed] [Google Scholar]
- 12.Liu LQ, Ilaria R, Jr, Kingsley PD, et al. A novel ubiquitin-specific protease, UBP43, cloned from leukemia fusion protein AML1-ETO-expressing mice, functions in hematopoietic cell differentiation. Mol Cell Biol. 1999;19:3029–38. doi: 10.1128/mcb.19.4.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knobeloch KP, Utermohlen O, Kisser A, et al. Reexamination of the role of ubiquitin-like modifier ISG15 in the phenotype of UBP43-deficient mice. Mol Cell Biol. 2005;25:11030–4. doi: 10.1128/MCB.25.24.11030-11034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catic A, Fiebiger E, Korbel GA, et al. Screen for ISG15-crossreactive deubiquitinases. PLoS ONE. 2007;2:e679. doi: 10.1371/journal.pone.0000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dastur A, Beaudenon S, Kelley M, et al. Herc5, an interferon-induced HECT E3 enzyme, is required for conjugation of ISG15 in human cells. J Biol Chem. 2006;281:4334–8. doi: 10.1074/jbc.M512830200. [DOI] [PubMed] [Google Scholar]
- 16.Yuan W, Krug RM. Influenza B virus NS1 protein inhibits conjugation of the interferon (IFN)-induced ubiquitin-like ISG15 protein. EMBO J. 2001;20:362–71. doi: 10.1093/emboj/20.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durfee LA, Kelley ML, Huibregtse JM. The basis for selective E1-E2 interactions in the ISG15 conjugation system. J Biol Chem. 2008;283:23895–902. doi: 10.1074/jbc.M804069200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KI, Giannakopoulos NV, Virgin HW, et al. Interferon-Inducible Ubiquitin E2, Ubc8, Is a Conjugating Enzyme for Protein ISGylation. Mol Cell Biol. 2004;24:9592–600. doi: 10.1128/MCB.24.21.9592-9600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao C, Beaudenon SL, Kelley ML, et al. The UbcH8 ubiquitin E2 enzyme is also the E2 enzyme for ISG15, an IFN-alpha/beta-induced ubiquitin-like protein. Proc Natl Acad Sci USA. 2004;101:7578–82. doi: 10.1073/pnas.0402528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chin LS, Vavalle JP, Li L. Staring, a novel E3 ubiquitin-protein ligase that targets syntaxin 1 for degradation. J Biol Chem. 2002;277:35071–9. doi: 10.1074/jbc.M203300200. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Kao WH, Howley PM. Physical interaction between specific E2 and Hect E3 enzymes determines functional cooperativity. J Biol Chem. 1997;272:13548–54. doi: 10.1074/jbc.272.21.13548. [DOI] [PubMed] [Google Scholar]
- 22.Moynihan TP, Ardley HC, Nuber U, et al. The ubiquitin-conjugating enzymes UbcH7 and UbcH8 interact with RING finger/IBR motif-containing domains of HHARI and H7-AP1. J Biol Chem. 1999;274:30963–8. doi: 10.1074/jbc.274.43.30963. [DOI] [PubMed] [Google Scholar]
- 23.Niwa J, Ishigaki S, Doyu M, et al. A novel centrosomal ring-finger protein, dorfin, mediates ubiquitin ligase activity. Biochem Biophys Res Commun. 2001;281:706–13. doi: 10.1006/bbrc.2001.4414. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka K, Suzuki T, Chiba T, et al. Parkin is linked to the ubiquitin pathway. J Mol Med. 2001;79:482–94. doi: 10.1007/s001090100242. [DOI] [PubMed] [Google Scholar]
- 25.Urano T, Saito T, Tsukui T, et al. Efp targets 14-3-3 sigma for proteolysis and promotes breast tumour growth. Nature. 2002;417:871–5. doi: 10.1038/nature00826. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Gao J, Chung KK, et al. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci USA. 2000;97:13354–9. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong JJ, Pung YF, Sze NS, et al. HERC5 is an IFN-induced HECT-type E3 protein ligase that mediates type I IFN-induced ISGylation of protein targets. Proc Natl Acad Sci USA. 2006;103:10735–40. doi: 10.1073/pnas.0600397103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeuchi T, Inoue S, Yokosawa H. Identification and Herc5-mediated ISGylation of novel target proteins. Biochem Biophys Res Commun. 2006;348:473–7. doi: 10.1016/j.bbrc.2006.07.076. [DOI] [PubMed] [Google Scholar]
- 29.Hochrainer K, Mayer H, Baranyi U, et al. The human HERC family of ubiquitin ligases: novel members, genomic organization, expression profiling and evolutionary aspects. Genomics. 2005;85:153–64. doi: 10.1016/j.ygeno.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Chang YG, Yan XZ, Xie YY, et al. Different roles for two ubiquitin-like domains of ISG15 in protein modification. J Biol Chem. 2008;283:13370–7. doi: 10.1074/jbc.M800162200. [DOI] [PubMed] [Google Scholar]
- 31.Lenschow DJ, Lai C, Frias-Staheli N, et al. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes and Sindbis viruses. Proc Natl Acad Sci USA. 2007;104:1371–6. doi: 10.1073/pnas.0607038104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai C, Struckhoff JJ, Schneider J, et al. Mice lacking the ISG15 E1 enzyme UbE1L demonstrate increased susceptibility to both mouse-adapted and nonmouse-adapted influenza B virus infection. J Virol. 2009;83:1147–51. doi: 10.1128/JVI.00105-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giannakopoulos NV, Arutyunova E, Lai C, et al. ISG15 Arg151 and the ISG15-conjugating enzyme UbE1L are important for innate immune control of Sindbis virus. J Virol. 2009;83:1602–10. doi: 10.1128/JVI.01590-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindner HA, Lytvyn V, Qi H, et al. Selectivity in ISG15 and ubiquitin recognition by the SARS coronavirus papain-like protease. Arch Biochem Biophys. 2007;466:8–14. doi: 10.1016/j.abb.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerra S, Caceres A, Knobeloch KP, et al. Vaccinia virus E3 protein prevents the antiviral action of ISG15. PLoS Pathog. 2008;4:e1000096. doi: 10.1371/journal.ppat.1000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng J, Schwartz D, Elias JE, et al. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21:921–6. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 37.Kirkpatrick DS, Denison C, Gygi SP. Weighing in on ubiquitin: the expanding role of mass-spectrometry-based proteomics. Nat Cell Biol. 2005;7:750–7. doi: 10.1038/ncb0805-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denison C, Kirkpatrick DS, Gygi SP. Proteomic insights into ubiquitin and ubiquitin-like proteins. Curr Opin Chem Biol. 2005;9:69–75. doi: 10.1016/j.cbpa.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Giannakopoulos NV, Luo JK, Papov V, et al. Proteomic identification of proteins conjugated to ISG15 in mouse and human cells. Biochem Biophys Res Commun. 2005;336:496–506. doi: 10.1016/j.bbrc.2005.08.132. [DOI] [PubMed] [Google Scholar]
- 40.Zhao C, Denison C, Huibregtse JM, et al. Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc Natl Acad Sci USA. 2005;102:10200–5. doi: 10.1073/pnas.0504754102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panse VG, Hardeland U, Werner T, et al. A proteome-wide approach identifies sumoylated substrate proteins in yeast. J Biol Chem. 2004;279:41346–51. doi: 10.1074/jbc.M407950200. [DOI] [PubMed] [Google Scholar]
- 42.Denison C, Rudner AD, Gerber SA, et al. A proteomic strategy for gaining insights into protein sumoylation in yeast. Mol Cell Proteomics. 2005;4:246–54. doi: 10.1074/mcp.M400154-MCP200. [DOI] [PubMed] [Google Scholar]
- 43.Sen GC, Sarkar SN. The interferon-stimulated genes: targets of direct signaling by interferons, double-stranded RNA and viruses. Curr Top Microbiol Immunol. 2007;316:233–50. doi: 10.1007/978-3-540-71329-6_12. [DOI] [PubMed] [Google Scholar]
- 44.Sen GC. Novel functions of interferon-induced proteins. Semin Cancer Biol. 2000;10:93–101. doi: 10.1006/scbi.2000.0312. [DOI] [PubMed] [Google Scholar]
- 45.Zou W, Papov V, Malakhova O, et al. ISG15 modification of ubiquitin E2 Ubc13 disrupts its ability to form thioester bond with ubiquitin. Biochem Biophys Res Commun. 2005;336:61–8. doi: 10.1016/j.bbrc.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 46.Takeuchi T, Yokosawa H. ISG15 modification of Ubc13 suppresses its ubiquitin-conjugating activity. Biochem Biophys Res Commun. 2005;336:9–13. doi: 10.1016/j.bbrc.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 47.Minakawa M, Sone T, Takeuchi T, et al. Regulation of the nuclear factor (NF)-kappaB pathway by ISGylation. Biol Pharm Bull. 2008;31:2223–7. doi: 10.1248/bpb.31.2223. [DOI] [PubMed] [Google Scholar]
- 48.Stossel TP, Condeelis J, Cooley L, et al. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2:138–45. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- 49.Jeon YJ, Choi JS, Lee JY, et al. Filamin B serves as a molecular scaffold for type I interferon-induced c-Jun NH2-terminal kinase signaling pathway. Mol Biol Cell. 2008;19:5116–30. doi: 10.1091/mbc.E08-06-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeuchi T, Kobayashi T, Tamura S, et al. Negative regulation of protein phosphatase 2Cbeta by ISG15 conjugation. FEBS Lett. 2006;580:4521–6. doi: 10.1016/j.febslet.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 51.Okumura F, Zou W, Zhang DE. ISG15 modification of the eIF4E cognate 4EHP enhances cap structure-binding activity of 4EHP. Genes Dev. 2007;21:255–60. doi: 10.1101/gad.1521607. [DOI] [PMC free article] [PubMed] [Google Scholar]