Abstract
Ibrutinib is highly active in treating mantle cell lymphoma (MCL), an aggressive B-cell lymphoma. We pooled data from three ibrutinib studies to explore the impact of baseline patient characteristics on treatment response. Patients with relapsed/refractory MCL (n = 370) treated with ibrutinib had an objective response rate (ORR) of 66% (20% complete response; 46% partial response); median duration of response (DOR), progression-free survival (PFS) and overall survival (OS) were 18.6, 12.8 and 25.0 months, respectively. Univariate analyses showed patients with one versus >one prior line of therapy had longer OS. Multivariate analyses identified that one prior line of therapy affected PFS; Eastern Cooperative Oncology Group (ECOG) performance status, simplified MCL international prognostic index (sMIPI) score, bulky disease, and blastoid histology affected OS and PFS. Patients with blastoid versus non-blastoid histology had similar time to best response, but lower ORR, DOR, PFS and OS. OS and PFS were longer in patients with better sMIPI, patients with ECOG performance status 0–1, non-bulky disease and non-blastoid histology. Additionally, the proportion of patients with poor prognostic factors increased with increasing lines of therapy. Together, results suggest that patient outcomes following treatment failure with ibrutinib are related to the natural biological evolution of the disease.
Keywords: ibrutinib, mantle cell lymphoma, pooled analysis
Introduction
Mantle cell lymphoma (MCL) is a rare, clinically aggressive B-cell lymphoma that accounts for 6–8% of all non-Hodgkin lymphoma cases (Dreyling et al, 2014). It is a disease that predominantly affects older men (median age, 65 years), usually presents as late-stage disease and is associated with a poor prognosis (Dreyling et al, 2016). The median overall survival (OS) of patients with MCL is 4–5 years (Herrmann et al, 2009; Smith et al, 2015). The majority of patients respond to initial therapy but then relapse. Following progression, subsequent treatment is often ineffective and survival is short (Herrmann et al, 2009).
Ibrutinib, a first-in-class, once-daily, oral, covalent inhibitor of Bruton tyrosine kinase (BTK), is a member of the cytoplasmic tyrosine (Tec) kinase family that is important for signalling via B-cell receptors and other B-cell surface receptors (Khan, 2012). Ibrutinib binds in a potent and covalent manner to a cysteine residue (Cys-481) in the active site ATP-binding domain of BTK. This binding inhibits B-cell receptor signalling within the malignant B-cell, leading to downstream mitigation of cell growth, proliferation, survival, adhesion and migration (Buggy & Elias, 2012; Cinar et al, 2013; de Rooij et al, 2012; Herman et al, 2011; Honigberg et al, 2010; Ponader et al, 2012; Cheng et al, 2014). Based on the results of a phase II study (PCYC-1104) in patients with relapsed or refractory (R/R) MCL, ibrutinib was approved in the United States, the European Union, and elsewhere around the world for patients with MCL who have received at least one prior line of therapy (Wang et al, 2013).
Two additional studies have been reported with ibrutinib in patients with R/R MCL: the phase II MCL2001 (SPARK) (Wang et al, 2014) and phase III MCL3001 (RAY) (Dreyling et al, 2016) studies. In both of these studies, a high overall response rate (ORR) was seen, which appeared to be largely independent of traditional poor-risk prognostic factors associated with this disease. Recent reports (Cheah et al, 2015; Martin et al, 2016) suggest that outcomes following ibrutinib progression may be poor, but the key question that remains is whether this is due to a biological effect of ibrutinib or to the disease biology of the patient population itself. Interestingly, other studies (Dreyling et al, 2016; Rule et al, 2015) suggest that post-progression outcomes in ibrutinib-treated patients are less negatively affected, with significantly longer progression-free survival 2 (PFS2) with ibrutinib versus temsirolimus. This indicates that improvement with ibrutinib is preserved following post-progression treatments. Another major finding of this study is that within the ibrutinib-treated cohort, PFS is longer in patients treated with one versus more than one prior line of therapy (Fig S1) (Rule et al, 2015).
In this analysis, we pooled patient-level data from three single-agent ibrutinib studies, PCYC-1104, SPARK and RAY (ibrutinib-treated cohort), to further characterize the efficacy profile of single-agent ibrutinib in MCL and to assess the best potential place for its use in patients with R/R MCL. In particular, an improved understanding of the baseline factors influencing outcomes may provide guidance to help determine the optimal clinical use of ibrutinib.
Methods
Patients with R/R MCL enrolled in three separate studies, PCYC-1104, SPARK and RAY (ibrutinib arm only), received ibrutinib 560 mg orally once daily until progressive disease or unacceptable toxicity. Inclusion and exclusion criteria were similar in all three studies; however, SPARK required patients to have received prior rituximab and bortezomib, and RAY required patients to have received prior rituximab. For further details on eligibility criteria, please refer to the individual studies (PCYC-1104 (Wang et al, 2013); SPARK (Wang et al, 2014); RAY (Dreyling et al, 2016)). Blastoid histology was not centrally reviewed but was investigator-categorized at baseline. All patients provided written informed consent. Patient-level data from these studies were integrated for analyses. In addition to descriptive statistics for both efficacy and safety parameters, exploratory analyses were conducted using Kaplan-Meier estimates for efficacy endpoints PFS and OS. Hazard ratios (HR) and 95% confidence intervals (CIs) were calculated using both univariate and multivariate Cox regression model. Common prognostic factors that were deemed to have prognostic value (P < 0.1) in the univariate analysis and were collected in all three studies were included as covariates in a multivariate Cox model adjusted by study. The multivariate Cox regression adjusted by study included the following covariates: age, extra nodal disease, Eastern Cooperative Oncology Group (ECOG) performance status, simplified MCL international prognostic index (sMIPI) risk, prior lines of therapy, bulky disease, blastoid histology and bone marrow involvement. ORR was presented over time and by prior lines of therapy. Best overall response was summarized by patient baseline characteristics. Common treatment-emergent adverse events (AEs) (≥10% of patients) were summarized by preferred terms and toxicity grades.
Results
The baseline demographic and disease characteristics of the individual ibrutinib-treated populations (PCYC-1104, n = 111; SPARK, n = 120; RAY, n = 139) were generally comparable, although the PCYC-1104 population had a slightly higher proportion of patients with intermediate/high-risk MCL (86% vs. 76% in SPARK and 69% in RAY). Full baseline characteristics for patients in each trial and for the pooled population (n = 370) are shown in Table SI.
Trial outcomes were similar between PCYC-1104, SPARK and RAY, respectively: ORR was 68%, 63% and 72%; median PFS was 13.9, 10.5 and 14.6 months; median OS was 22.5 months, 25.4 months and not reached; and estimated OS at 18 months was 58%, 61% and 58%. Median duration of follow-up for PCYC-1104, SPARK and RAY was 15.5, 14.9 and 20 months, respectively.
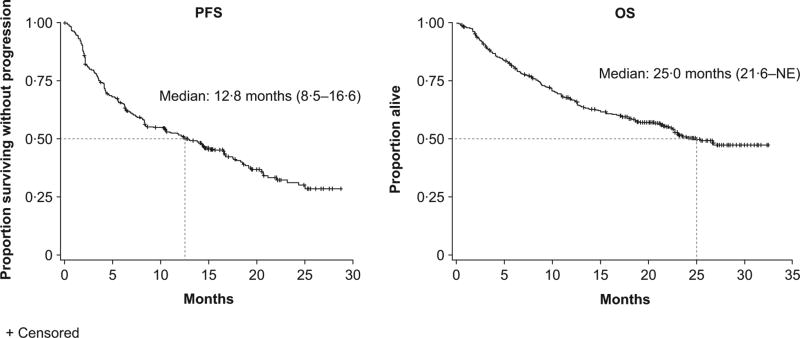
For the pooled population, the median age was 67.5 years, with patients having received a median of two prior lines of therapy. Seventy-six per cent of patients had intermediate/high-risk sMIPI and 49% had bulky disease (longest diameter ≥5 cm). The overall median treatment duration across the three studies was 11 months, with a median dose intensity of 98.4%, and the median duration of follow-up was 24–25 months. Thirty-five per cent of patients were still on therapy at 18 months. The median PFS of the overall MCL population was 12.8 months and median OS was 25.0 months (Fig 1). The median (range) time to first response and best response were 2.07 (0 53–13 73) and 2.14 (0.53–24.74) months, respectively.
Fig 1.
Progression-free survival (PFS) and overall survival (OS) for MCL pooled population.
NE, not evaluated
Overall, the most common sites of progression were the lymph nodes, with the mediastinum, external iliac and abdominal sites most commonly involved (37%, 35% and 32% of progressed patients, respectively). Extranodal sites that were commonly involved at progression were the liver, lung, and gastrointestinal tract (34%, 23% and 20% of progressed patients, respectively). In total, four (3.1%) patients showed central nervous system (CNS) involvement at progression in this poor risk patient cohort (e.g., high sMIPI, 32% of patients, high lactate dehydrogenase [LDH] 54% of patients).
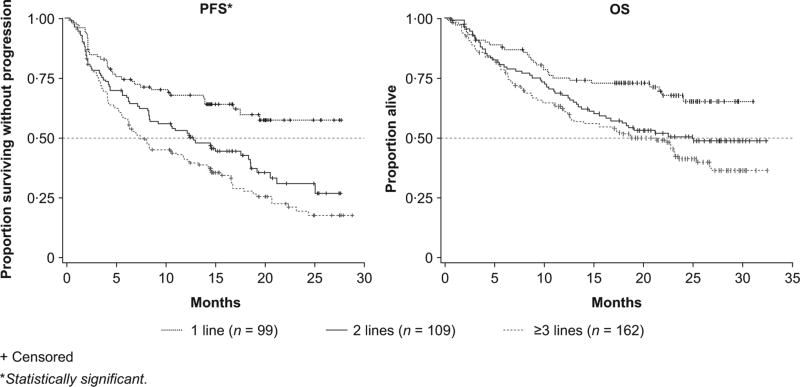
PFS and OS were markedly different in patients based on the number of previous lines of therapy. Patients who had received only one prior line of therapy had the longest PFS and OS (median not reached), and 2-year PFS and OS estimates were 57% and 68%, respectively (Fig 2).
Fig 2.
Progression-free survival (PFS) and overall survival (OS) by prior lines of therapy.
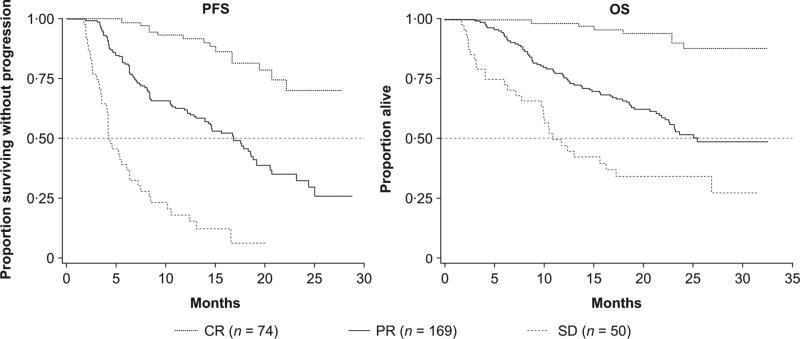
ORRs and complete response (CR) rates improved over time (Fig S2A). Overall, almost one-third of responders achieved a CR, with an overall CR rate of 20% and partial response (PR) rate of 46%. Response rates also differed based on the numbers of prior lines of treatment (Fig S2B), non-refractory status and low sMIPI risk scores. For prior lines of treatment, patients treated with ibrutinib at second line achieved the highest ORR and CR rate (73% and 27%, respectively) (Fig S2B). The depth of response markedly affected long-term outcomes; patients who achieved a CR exhibited the longest PFS and OS (Fig 3): median PFS and OS were not reached, with landmark rates of PFS and OS at 2 years of 79% and 92%, respectively.
Fig 3.
Progression-free survival (PFS) and overall survival (OS) by best response.
CR, complete response; PR, partial response; SD, stable disease.
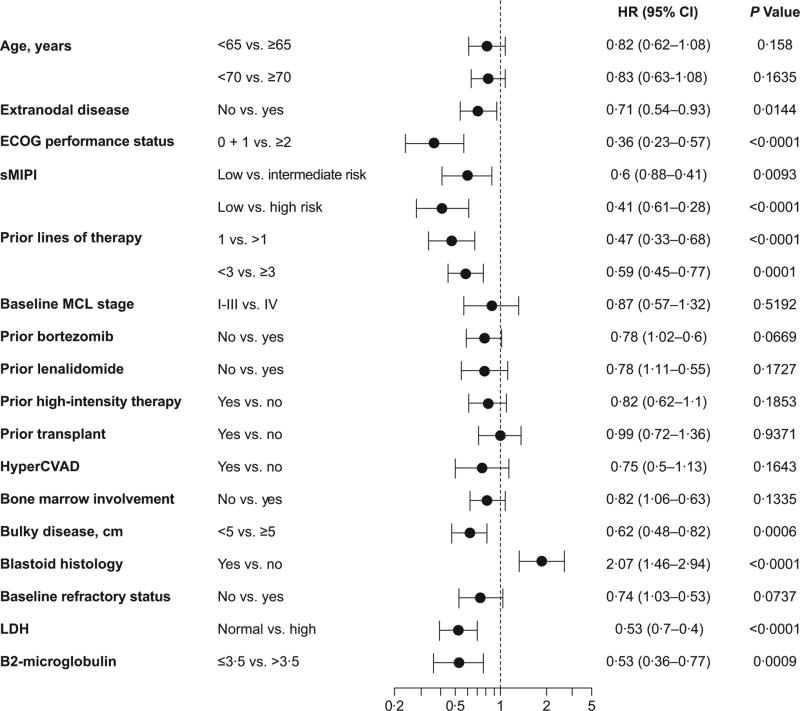
ORR (Table SII) was summarized by baseline characteristics. PFS and OS were explored in a univariate non-stratified Cox regression model by fitting baseline prognostic factors (Fig 4 and Fig S3). PFS and OS outcomes demonstrated similar trends regarding patient outcomes by baseline characteristics. Prior bortezomib did not appear to have any effect on PFS or OS. Trends toward a difference in outcomes were observed with regard to refractory status, disease stage and prior transplant; however, these did not reach statistical significance. Baseline characteristics, such as age, ECOG performance status, sMIPI risk, prior lines of therapy, bulky disease, bone marrow involvement, beta-2 microglobulin (B2M) levels, normal LDH and blastoid histology did show statistical significance (Fig 4 and Fig S3).
Fig 4.
Forest plot of progression-free survival for pooled MCL analysis.
CI, confidence interval; HyperCVAD, hyper-fractionated cyclophosphamide, vincristine, adriamycin and dexamethasone; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; LDH, lactate dehydrogenase; MCL, mantle cell lymphoma; sMIPI, simplified MCL international prognostic index.
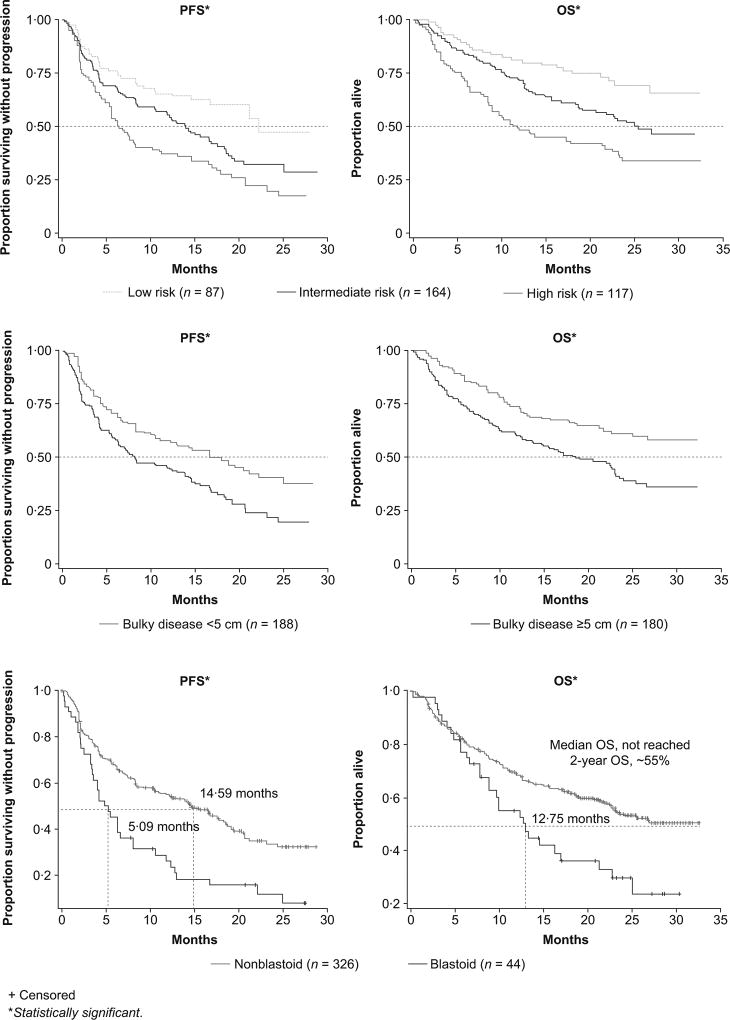
Better outcomes were associated with younger age, ECOG performance status 0–1, lower sMIPI score, fewer prior lines of therapy, normal LDH levels, lower B2M and non-bulky disease or non-blastoid histology (Fig 5 and Fig S4). Patients with blastoid and non-blastoid histology had an ORR of 50 0% and 67 8% and time to best response of 2.2 and 2.1 months, respectively; however, duration of response (DOR) (8.5 vs. 18.8 months), PFS (5.1 vs. 14.6 months) and OS (12.8 vs. not reached at 2 years) were significantly shorter in patients with blastoid histology.
Figure 5.
Progression-free survival (PFS) and overall survival (OS) by baseline factors: (A) simplified MCL international prognostic index (sMIPI: low vs. intermediate vs. high), (B) bulky disease, and (C) blastoid histology.
Additional exploratory analyses of PFS and OS were performed using a selected set of prognostic variables in a multivariate Cox regression model adjusted by study. The model revealed that ECOG performance status, sMIPI, bulky disease and blastoid histology significantly affect both PFS and OS, suggesting that each of these factors independently drives outcomes. As the multivariate analysis was only significant for PFS for one versus more than one prior line of therapy, these results suggest that ibrutinib, independent of baseline factors, significantly improves PFS in those patients who receive ibrutinib earlier versus later (Table I).
Table I.
Multivariate analysis of progression-free survival (PFS) and overall survival (OS).
| PFS HR (95% CI) |
P value | OS HR (95% CI) |
P value | |
|---|---|---|---|---|
| Age <65 years | 0.988 (0.722–1.352) | 0.9397 | 0.790 (0.549–1.137) | 0.2041 |
| No extranodal disease | 0.804 (0.604–1.070) | 0.1340 | 0.872 (0.633–1.201) | 0.4013 |
| ECOG performance status 0–1 | 0.584 (0.359–0.949) | 0.0299* | 0.454 (0.263–0.782) | 0.0045* |
| MIPI | ||||
|
| ||||
| High risk | 2.266 (1.431–3.589) | 0.0005* | 2.372 (1.401–4.016) | 0.0013* |
| Intermediate risk | 1.624 (1.081–2.440) | 0.0195* | 1.678 (1.046–2.692) | 0.0319* |
|
| ||||
| One prior line of therapy | 0.651 (0.448–0.946) | 0.0245* | 0.695 (0.459–1.055) | 0.0873 |
|
| ||||
| Non-bulky disease (<5 cm) | 0.703 (0.528–0.938) | 0.0164* | 0.608 (0.438–0.844) | 0.0029* |
| Non-blastoid histology | 0.442 (0.303–0.646) | <0.0001* | 0.397 (0.259–0.608) | <0.0001* |
|
| ||||
| No bone marrow involvement | 0.936 (0.709–1.236) | 0.6411 | 0.733 (0.537–1.002) | 0.0511 |
| Study PCYC-1104 (phase II) | 0.848 (0.589–1.223) | 0.3779 | 0.757 (0.499–1.150) | 0.1923 |
| Study MCL2001 (phase II) | 1.294 (0.914–1.831) | 0.1465 | 0.924 (0.628–1.358) | 0.6861 |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; MIPI, mantle cell lymphoma international prognostic index.
Significant.
Treatment-emergent AEs were reported in 364 (98.4%) patients in the MCL pooled population. Grade ≥3 AEs were reported in 265 (71.6%) patients. The most frequently reported AEs (any grade) were diarrhoea (n = 146, 39.5%), fatigue (n = 129, 34.9%), cough (n = 81, 21.9%), nausea (n = 80, 21.6%), peripheral oedema and thrombocytopenia (both n = 74, 20.0%) (Table SIII). Other AEs of clinical interest occurred in a minority of patients, including grade ≥3 atrial fibrillation in 17 (4.6%) patients, and grade ≥3 major bleeding in 18 (4.9%) patients. Rash occurred in 57 (15.4%) patients. The incidence of other malignancies was 5.7% in the overall population, the majority of which (67%) were non-melanoma skin cancers.
Discussion
Outcomes are poor for patients with MCL who relapse after initial therapy. Until recently, limited treatment options have been available. New targeted therapies have been added to our armamentarium (e.g., bortezomib, lenalidomide and temsirolimus) in the last decade. These agents have activity, but the majority of patients do not respond well and responses are often short-lived. Ibrutinib was licensed by the US Food and Drug Administration in February 2013, based on phase II data in a single-arm study (PCYC-1104) (Wang et al, 2013). The results observed with ibrutinib showed ORR and CR rates higher than what had previously been seen with any other single agent (Fisher et al, 2006; Hess et al, 2009; Trneny et al, 2016). These results were confirmed in two subsequent studies (Dreyling et al, 2016; Wang et al, 2014). The phase III RAY study randomized patients to ibrutinib or temsirolimus and demonstrated a significant improvement in both ORR and PFS in favour of ibrutinib.
The pooled analysis confirms that response rates are consistently high across all the different subgroups examined, but PFS and OS are dependent on baseline characteristics. Both univariate and multivariate Cox regression models show similar and consistent results; together, these analyses indicate that blastoid histology, sMIPI, bulky disease and ECOG performance status remain prognostic in terms of PFS and OS with ibrutinib.
The pooled analysis allows us to look specifically at patients with blastoid histology (n = 44). The ORR was 50% in patients with blastoid histology (n = 44); however, both PFS and OS were shorter than in patients with non-blastoid histology. This is consistent with outcomes observed with other treatments in blastoid MCL (Bhatt et al, 2016). In the pooled analysis, 50% of patients with blastoid histology achieved an objective response and best response within 2 to 3 months, with a median DOR of 8 months, suggesting that consolidation of a response with bone marrow transplant should optimally be performed within 5 to 6 months of starting ibrutinib therapy.
Results from exploratory analyses of ibrutinib in chronic lymphocytic lymphoma suggest that PFS and OS outcomes may improve when ibrutinib is used earlier in treatment (Brown et al, 2014; Burger et al, 2015). A similar exploratory analysis was conducted in the ibrutinib phase III MCL study (Rule et al, 2015), which suggested the same improvement in outcomes (Fig S1). The pooled analysis confirms this finding with patients shown to have significantly longer PFS and OS when ibrutinib was used after initial relapse versus later in treatment. These results were further validated in the multivariate analysis when identifying line of therapy as an independent factor for PFS.
In the phase III RAY study, analysis of PFS2 (defined as time to progressive disease after next line of therapy, death or start of second subsequent therapy) (Dreyling et al, 2016) showed that post-progression outcome is poor, independent of prior ibrutinib exposure. In combination with those findings, the current results indicate that disease characteristics and line of therapy independently impact outcomes with ibrutinib. These findings suggest that the dismal outcomes reported after ibrutinib treatment (Cheah et al, 2015; Martin et al, 2016) are attributable mostly to adverse disease characteristics. This is further supported by the analysis showing that the proportion of patients with poor prognostic factors increases with increasing lines of therapy (Fig S5).
One important finding from this analysis is that the DOR observed with ibrutinib is proportionate to the depth of that response. In patients who achieved a CR with ibrutinib, 70% were progression-free and 90% were alive at 2 years. These data would support the concept of combination regimens involving ibrutinib in an attempt to maximize the CR rate. In R/R MCL, Maddocks et al (2015) previously reported a 94% ORR and 76% CR rate with ibrutinib in combination with bendamustine/ rituximab. Prior studies on bendamustine/ rituximab (without ibrutinib) demonstrated an ORR/CR of 97%/31% (Flinn et al, 2014) and 82%/40% (Czuczman et al, 2015). Wang et al (2016) reported an 88% ORR and 44% CR rate with ibrutinib plus rituximab. It is unknown whether the PFS and OS rates of the patients who achieved CR are similar to those observed in the current pooled analysis. However, this will probably be further addressed in the upcoming SHINE phase III study (NCT01776840), in which ibrutinib or placebo are being combined with bendamustine and rituximab in treatment-naïve patients aged ≥65 years who are not eligible for transplant.
Clinical reports have recently shown that ibrutinib can penetrate into the CNS, with clinical responses reported in patients with Bing-Neel syndrome (Cabannes-Hamy et al, 2016) and CNS responses reported in MCL patients (Bernard et al, 2015; Choquet et al, 2016; Grommes et al, 2016). Most patients in this pooled analysis had at least one risk factor for CNS involvement. In these patients, blastoid histology, the presence of B-symptoms, increased serum LDH, ECOG performance status ≥2 and a high sMIPI score are considered risk factors for CNS involvement during the course of the disease (Cheah et al, 2013); however, among patients who progressed in our pooled analysis, 3.1% showed involvement of the CNS. The role of ibrutinib in the treatment of patients with CNS lymphoma is being further explored in clinical studies.
While not the primary focus of this pooled analysis, it is important to comment that in MCL and other B-cell malignancies, the overall safety of ibrutinib indicates a favourable risk-benefit profile. No new safety signals were observed in any of these three studies, and all of the findings were consistent with the known safety profile of ibrutinib in clinical settings.
These results support the use of ibrutinib earlier in the treatment algorithm, with significant improvements in both PFS and OS when used at first relapse rather than later. It also seems that the poor prognostic factors that increase in later lines of therapy not only impact traditional chemotherapy but also ibrutinib outcomes. However, ibrutinib also appears to overcome some of the common poor risk factors, such as refractory status and disease stage, and may even provide an important option for patients with blastoid histology, allowing them to bridge to therapies that may lead to better long-term outcomes. Overall, ibrutinib represents a significant advance in the treatment of this challenging lymphoma and further on-going studies will help define the optimal position in therapy as well as the best combination partner for ibrutinib.
Supplementary Material
Acknowledgments
The authors would like to thank Michelle Olsher, PhD for writing assistance (PAREXEL, Hackensack, NJ, USA), funded by Janssen Global Services, LLC. We also thank the patients who participated in this trial, their families, and the investigators and coordinators at each of the clinical sites. The investigators are listed in the supplemental material.
Grant: NIH/NCI P30 CA16672
Funding
This study was funded by Janssen Research & Development. Funders were involved in the study design, data collection, data analysis and interpretation, and provided writing support. Lead investigators (SR, MW) had full access to the data and analyses for compilation of this report.
SR reports grants and personal fees from Janssen, during the conduct of the study; personal fees from Roche, personal fees from Pharmacyclics, personal fees from Celgene, outside the submitted work. MD reports grants and personal fees from Janssen, outside the submitted work. GH reports grants and personal fees from Roche, grants and personal fees from Pfizer, grants from CTI, personal fees from Janssen, grants and personal fees from Celgene, outside the submitted work. RA reports personal fees from Janssen, other from Janssen, non-financial support from Janssen, personal fees from Bristol Myers Squibb, personal fees from Celgene, outside the submitted work. BK reports grants from Pharmacyclics, during the conduct of the study; personal fees from Gilead, personal fees from Infinity, personal fees from Pharmacyclics, outside the submitted work. NC, FC and DB are employees of Pharmacyclics. BL, SY, JG and MW are employees of Janssen Research & Development and hold stock in Johnson & Johnson outside the submitted work. JV is an employee of Janssen Biologics and holds stock in Johnson & Johnson outside the submitted work.
Footnotes
Authorship Contributions:
SR, MD, AG, GH, RA, BK, FC, JG, JV, MW and MW were responsible for study conception and design, provision of study materials or patients, collection and assembly of data, data analysis and interpretation, manuscript writing and manuscript approval. NC was responsible for collection and assembly of data, data analysis and interpretation and manuscript approval. BL and DB were responsible for data analysis and interpretation, manuscript writing and manuscript approval. SY was responsible for data analysis and interpretation and manuscript approval.
Conflict of Interest Disclosures
AG and MW declare no competing interests.
References
- Bernard S, Goldwirt L, Amorim S, Brice P, Briere J, de KE, Mourah S, Sauvageon H, Thieblemont C. Activity of ibrutinib in mantle cell lymphoma patients with central nervous system relapse. Blood. 2015;126:1695–1698. doi: 10.1182/blood-2015-05-647834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt VR, Loberiza FR, Jr, Smith LM, Armitage JO, Greiner TC, Bast M, Lunning MA, Bierman PJ, Vose JM, Bociek RG. Clinicopathologic features, management and outcomes of blastoid variant of mantle cell lymphoma: a Nebraska Lymphoma Study Group Experience. Leukemia & lymphoma. 2016;57:1327–1334. doi: 10.3109/10428194.2015.1094801. [DOI] [PubMed] [Google Scholar]
- Brown J, Hillman P, Obrien S. Updated efficacy including genetic and clinical subgroup analysis and overall safety in the phase 3 RESONATE™ trial of ibrutinib versus ofatumumab in previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma. American Society of Hematology. 2014 2014 Dec 6–9; [Google Scholar]
- Buggy JJ, Elias L. Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. International Reviews of Immunology. 2012;31:119–132. doi: 10.3109/08830185.2012.664797. [DOI] [PubMed] [Google Scholar]
- Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, Bairey O, Hillmen P, Bartlett NL, Li J, Simpson D, Grosicki S, Devereux S, McCarthy H, Coutre S, Quach H, Gaidano G, Maslyak Z, Stevens DA, Janssens A, Offner F, Mayer J, O'Dwyer M, Hellmann A, Schuh A, Siddiqi T, Polliack A, Tam CS, Suri D, Cheng M, Clow F, Styles L, James DF, Kipps TJ. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. New England Journal of Medicine. 2015;373:2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabannes-Hamy A, Lemal R, Goldwirt L, Poulain S, Amorim S, Perignon R, Berger J, Brice P, de KE, Bay JO, Sauvageon H, Beldjord K, Mourah S, Tournilhac O, Thieblemont C. Efficacy of ibrutinib in the treatment of Bing-Neel syndrome. American Journal of Hematology. 2016;91:E17–E19. doi: 10.1002/ajh.24279. [DOI] [PubMed] [Google Scholar]
- Cheah CY, George A, Gine E, Chiappella A, Kluin-Nelemans HC, Jurczak W, Krawczyk K, Mocikova H, Klener P, Salek D, Walewski J, Szymczyk M, Smolej L, Auer RL, Ritchie DS, Arcaini L, Williams ME, Dreyling M, Seymour JF. Central nervous system involvement in mantle cell lymphoma: clinical features, prognostic factors and outcomes from the European Mantle Cell Lymphoma Network. Annals of Oncology. 2013;24:2119–2123. doi: 10.1093/annonc/mdt139. [DOI] [PubMed] [Google Scholar]
- Cheah CY, Chihara D, Romaguera JE, Fowler NH, Seymour JF, Hagemeister FB, Champlin RE, Wang ML. Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Annals of Oncology. 2015;26:1175–1179. doi: 10.1093/annonc/mdv111. [DOI] [PubMed] [Google Scholar]
- Cheng S, Ma J, Guo A, Lu P, Leonard JP, Coleman M, Liu M, Buggy JJ, Furman RR, Wang YL. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia. 2014;28:649–657. doi: 10.1038/leu.2013.358. [DOI] [PubMed] [Google Scholar]
- Choquet S, Houillier C, Bijou F, Houot R, Boyle E, Gressin R, Nicolas-Virelizier E, Barrie M, Molucon-Chabrot C, Blonski M, El Yamani A, LeLez M-L, Clavert A, Coisy S, de la Bretonnière ME, Touitou V, Cassoux N, Boussetta S, Broussais F, Gelas-Dore B, Barzic N, Ghesquieres H, Hoang-Xuan K, Soussain C. Ibrutinib monotherapy in relapse or refractory primary CNS lymphoma (PCNSL) and primary vitreo-retinal lymphoma (PVRL). Result of the interim analysis of the iLOC phase II study from the Lysa and the French LOC network. Blood. 2016;128:784. doi: 10.1016/j.ejca.2019.05.024. [DOI] [PubMed] [Google Scholar]
- Cinar M, Hamedani F, Mo Z, Cinar B, Amin HM, Alkan S. Bruton tyrosine kinase is commonly overexpressed in mantle cell lymphoma and its attenuation by Ibrutinib induces apoptosis. Leuk Res. 2013;37:1271–1277. doi: 10.1016/j.leukres.2013.07.028. [DOI] [PubMed] [Google Scholar]
- Czuczman MS, Kahanic S, Forero A, Davis G, Munteanu M, Van Den Neste E, Offner F, Bron D, Quick D, Fowler N. Results of a phase II study of bendamustine and ofatumumab in untreated indolent B cell non-Hodgkin's lymphoma. Annals of Hematology. 2015;94:633–641. doi: 10.1007/s00277-014-2269-8. [DOI] [PubMed] [Google Scholar]
- de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, Pals ST, Spaargaren M. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- Dreyling M, Geisler C, Hermine O, Kluin-Nelemans HC, Le GS, Rule S, Shpilberg O, Walewski J, Ladetto M. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2014;25(Suppl 3):iii83–iii92. doi: 10.1093/annonc/mdu264. [DOI] [PubMed] [Google Scholar]
- Dreyling M, Jurczak W, Jerkeman M, Silva RS, Rusconi C, Trneny M, Offner F, Caballero D, Joao C, Witzens-Harig M, Hess G, Bence-Bruckler I, Cho SG, Bothos J, Goldberg JD, Enny C, Traina S, Balasubramanian S, Bandyopadhyay N, Sun S, Vermeulen J, Rizo A, Rule S. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387:770–778. doi: 10.1016/S0140-6736(15)00667-4. [DOI] [PubMed] [Google Scholar]
- Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de VS, Epner E, Krishnan A, Leonard JP, Lonial S, Stadtmauer EA, O'Connor OA, Shi H, Boral AL, Goy A. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. Journal of Clinical Oncology. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- Flinn IW, van der Jagt R, Kahl BS, Wood P, Hawkins TE, Macdonald D, Hertzberg M, Kwan YL, Simpson D, Craig M, Kolibaba K, Issa S, Clementi R, Hallman DM, Munteanu M, Chen L, Burke JM. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123:2944–2952. doi: 10.1182/blood-2013-11-531327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grommes C, Kaley TJ, Nolan C, Omura AMP, Wolfe J, Mellinghoff IK, DeAngelis LM. Phase I study of single agent ibrutinib in recurrent/refractory primary (PCNSL) and secondary CNS lymphoma. American Association of Clinical Oncology; June 2–6, 2016. Journal of Clinical Oncology. 2016;34 Abstract 2046. [Google Scholar]
- Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, Flynn J, Jones J, Blum KA, Buggy JJ, Hamdy A, Johnson AJ, Byrd JC. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann A, Hoster E, Zwingers T, Brittinger G, Engelhard M, Meusers P, Reiser M, Forstpointner R, Metzner B, Peter N, Wormann B, Trumper L, Pfreundschuh M, Einsele H, Hiddemann W, Unterhalt M, Dreyling M. Improvement of overall survival in advanced stage mantle cell lymphoma. Journal of Clinical Oncology. 2009;27:511–518. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- Hess G, Herbrecht R, Romaguera J, Verhoef G, Crump M, Gisselbrecht C, Laurell A, Offner F, Strahs A, Berkenblit A, Hanushevsky O, Clancy J, Hewes B, Moore L, Coiffier B. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. Journal of Clinical Oncology. 2009;27:3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, Buggy JJ. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan WN. Colonel Bruton's kinase defined the molecular basis of X-linked agammaglobulinemia, the first primary immunodeficiency. Journal of Immunology. 2012;188:2933–2935. doi: 10.4049/jimmunol.1200490. [DOI] [PubMed] [Google Scholar]
- Maddocks K, Christian B, Jaglowski S, Flynn J, Jones JA, Porcu P, Wei L, Jenkins C, Lozanski G, Byrd JC, Blum KA. A phase 1/1b study of rituximab, bendamustine, and ibrutinib in patients with untreated and relapsed/refractory non-Hodgkin lymphoma. Blood. 2015;125:242–248. doi: 10.1182/blood-2014-08-597914. [DOI] [PubMed] [Google Scholar]
- Martin P, Maddocks K, Leonard JP, Ruan J, Goy A, Wagner-Johnston N, Rule S, Advani R, Iberri D, Phillips T, Spurgeon S, Kozin E, Noto K, Chen Z, Jurczak W, Auer R, Chmielowska E, Stilgenbauer S, Bloehdorn J, Portell C, Williams ME, Dreyling M, Barr PM, Chen-Kiang S, DiLiberto M, Furman RR, Blum KA. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood. 2016;127:1559–1563. doi: 10.1182/blood-2015-10-673145. [DOI] [PubMed] [Google Scholar]
- Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, Keating MJ, O'Brien S, Chiorazzi N, Burger JA. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule S, Jurczak W, Jerkeman M, Santucci R, Rusconi C, Trneny M, Offner F, Caballero D, Joao C, Witzens-Harig M, Hess G, Bence-Bruckler I, Cho S-G, Balasubramanian S, Bandyopadhyay N, Sun S, Goldberg J, Bothos J, Traina S, Enny C, Rizo A, Vermeulen J, Dreyling MH. Ibrutinib versus temsirolimus: results from a phase 3, international, randomized, open-label, multicenter study in patients with previously treated mantle-cell lymphoma. Blood. 2015;126:469. [Google Scholar]
- Smith A, Crouch S, Lax S, Li J, Painter D, Howell D, Patmore R, Jack A, Roman E. Lymphoma incidence, survival and prevalence 2004-2014: sub-type analyses from the UK's Haematological Malignancy Research Network. British Journal of Cancer. 2015;112:1575–1584. doi: 10.1038/bjc.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trneny M, Lamy T, Walewski J, Belada D, Mayer J, Radford J, Jurczak W, Morschhauser F, Alexeeva J, Rule S, Afanasyev B, Kaplanov K, Thyss A, Kuzmin A, Voloshin S, Kuliczkowski K, Giza A, Milpied N, Stelitano C, Marks R, Trumper L, Biyukov T, Patturajan M, Bravo ML, Arcaini L. Lenalidomide versus investigator's choice in relapsed or refractory mantle cell lymphoma (MCL-002; SPRINT): a phase 2, randomised, multicentre trial. Lancet Oncology. 2016;17:319–331. doi: 10.1016/S1470-2045(15)00559-8. [DOI] [PubMed] [Google Scholar]
- Wang M, Goy A, Martin P, Ramchandren R, Alexeeva J, Popat R, Avivi I, Advani R, Le Gouill S, Horowitz N, Yuan Z, Kranenburg B, Rizo A, Zhuang SH, Deraedt W, Rule S. Efficacy and safety of single-agent ibrutinib in patients with mantle cell lymphoma who progressed after bortezomid therapy. Blood. 2014;124(4471) [Google Scholar]
- Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, Barrientos JC, Chmielowska E, Radford J, Stilgenbauer S, Dreyling M, Jedrzejczak WW, Johnson P, Spurgeon SE, Li L, Zhang L, Newberry K, Ou Z, Cheng N, Fang B, McGreivy J, Clow F, Buggy JJ, Chang BY, Beaupre DM, Kunkel LA, Blum KA. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. New England Journal of Medicine. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ML, Lee H, Chuang H, Wagner-Bartak N, Hagemeister F, Westin J, Fayad L, Samaniego F, Turturro F, Oki Y, Chen W, Badillo M, Nomie K, DeLa RM, Zhao D, Lam L, Addison A, Zhang H, Young KH, Li S, Santos D, Medeiros LJ, Champlin R, Romaguera J, Zhang L. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncology. 2016;17:48–56. doi: 10.1016/S1470-2045(15)00438-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.