Abstract
Objective
Although cross-sectional studies find altered cognition in youth with type 1 diabetes mellitus (T1DM), few longitudinal studies have examined the trajectories of their cognitive performance over time. The aims of this study were to explore longitudinal change in cognitive function in youth with T1DM as compared to non-diabetic sibling controls, and how glycemic control and age of onset influence cognitive performance over time.
Methods
We assessed crystallized intelligence, visual-spatial ability, delayed memory, and processing speed at three time points using the same cognitive tasks in youth with T1DM and sibling controls. Hierarchical linear modeling examined relationships between diabetes, hyperglycemia (HbA1c values), age of onset, and cognition over 5.5 years.
Results
Youth with diabetes performed worse than controls on visual-spatial ability and memory tasks over time, and did not improve as much in processing speed. Greater hyperglycemia was associated with lower crystallized intelligence and slower processing speed but better memory across all time points. There was a stronger negative relationship between hyperglycemia and visual-spatial ability for youth with earlier compared to later onset diabetes. Importantly, within-person decreases in hyperglycemia between time points were associated with improved visual-spatial ability and faster processing speed.
Conclusions
On average, differences in cognitive function between youth with T1DM and non-diabetic relatives are maintained or increase during childhood and adolescence. Hyperglycemia and age of onset can have negative effects on the developmental trajectories of cognitive processes in youth with T1DM. However, treatments that lower hyperglycemia may lead to improved cognitive function in youth with T1DM.
Keywords: Diabetes Mellitus, Type 1, Pediatrics, Hyperglycemia, Cognition, Memory
In cross-sectional studies, youth with type 1 diabetes mellitus (T1DM) perform worse than non-diabetic peers on tests of cognitive function across multiple domains (1–3). Cross-sectional research also suggests that greater hyperglycemia or severe hypoglycemia exposure and early age of diabetes onset are associated with lower intelligence, visual-spatial ability, and memory performance and slower processing speed (4–15). However, few truly longitudinal studies have directly statistically compared change in cognitive function over time in children and adolescents with T1DM relative to non-diabetic peers (16–19), limiting causal interpretations. Thus, little is currently known regarding whether differences in cognitive function between diabetic and non-diabetic youth are stable over time, increase, or decrease, and how hyperglycemia exposure, severe hypoglycemic events, and age of onset influence the developmental trajectories of cognitive processes. Some of the few existing pediatric longitudinal studies (see 20,21 for investigations of longitudinal changes in cognition in T1DM between adolescence and adulthood) suggest that greater hyperglycemia exposure is associated with worse verbal and visual-spatial memory over time (22,23), more hypoglycemic events are associated with lower intelligence, verbal memory performance, and visual-spatial learning over time (18,22), and that earlier age of onset predicts less positive change in intelligence and visual-spatial ability than later age of onset (16,18,22). However, a recent study did not find any significant relationships between these clinical characteristics and longitudinal changes in cognition (19). In addition, prior pediatric longitudinal studies have not directly investigated whether within-person improvements in glycemic control can lead to within-person improvements in cognitive function.
The current study explored longitudinal change in cognitive function in youth with T1DM as compared to non-diabetic sibling controls, and how glycemic control and age of onset influence cognitive performance over time. Groups were assessed on the same tests of crystallized intelligence, visual-spatial ability, delayed memory, and processing speed at three time points, and exposure to hyperglycemia and severe hypoglycemia were ascertained prospectively. Hierarchical linear modeling (HLM) allowed us to examine effects of diabetes status, mean hyperglycemia exposure, and age of diabetes onset on mean cognitive performance over the course of 5.5 years as well as the degree of linear increase or decrease in cognitive performance trends. We also examined whether individual fluctuation in HbA1c from one occasion to the next resulted in improvements (or decrements) in cognitive function.
Methods
Study Design
Participants were tested three times with measures designed to address the cognitive domains most consistently shown to be affected by pediatric T1DM: crystallized intelligence, visual-spatial ability, memory, and processing speed. There were 2 years between time points 1 and 2 and 2-3 years between time points 2 and 3. Diabetes variables were acquired retrospectively before time point 1 and prospectively between time point 1 and the end of the study. Cross-sectional analyses of time point 1 cognitive data have been previously published (10,11).
Participants
Children and adolescents diagnosed with T1DM and their non-diabetic siblings aged 4 – 16 were recruited from the Pediatric Diabetes Clinic at Washington University in St. Louis and St. Louis Children’s Hospital. Participants were screened for significant neurological history unrelated to diabetes complications, psychiatric disorders, psychoactive medications, mental retardation, premature birth (<36 weeks gestation) with complications, and chronic diseases other than T1DM (e.g., hypothyroidism). All individuals with T1DM had received insulin therapy for at least two years before time point 1 and had not been diagnosed with retinopathy, nephropathy, or neuropathy. Research procedures were approved by the Washington University School of Medicine’s Human Studies Committee and were conducted in accordance with the Declaration of Helsinki.
Ascertainment of glycemic history
Glycemic control for individuals with T1DM was estimated from HbA1c test results available in their medical records. The percentage of time covered by HbA1c tests between diagnosis and time point 3 was calculated for each participant by multiplying the total number of HbA1c tests available during this time period by three (the number of months roughly reflected by each test) and then dividing by the duration of this time period in months. Less than 100% coverage of the time between diagnosis and time point 3 by HbA1c tests resulted from having clinical appointments more than three months apart, transferring from another diabetes clinic before time point 1, and/or use of total glycated hemoglobin tests instead of HbA1c tests. Mean HbA1c values were calculated for three time periods: diabetes diagnosis to time point 1, time points 1 to 2, and time points 2 to 3.
History of severe hypoglycemic events was ascertained by detailed parent and child interviews. Severe hypoglycemic events were defined as events with neurological dysfunction (seizure, loss of consciousness, inability to arouse from sleep) and/or that required assistance from someone else to treat (24).
Blood glucose was measured using glucose meters immediately before and after each cognitive testing session. Data from participants with meter readings below 60 or above 375 mg/dL were excluded from analyses. Pre- and post-testing meter readings were averaged to create mean blood glucose values for each testing session.
Cognitive Testing
Detailed descriptions of the cognitive tasks used in this study can be found in a prior report of cross-sectional analyses of time point 1 data (11).
Crystallized Intelligence
Raw scores from the General Information subtest of the Woodcock-Johnson III (25) were used to estimate crystallized intelligence.
Visual-Spatial Ability
Raw scores from the Spatial Relations subtest of the Woodcock-Johnson III (25) were used to estimate visual-spatial ability.
Memory
Delayed verbal memory was measured using the number of words correctly recalled on the Delayed Recall Trial of the Word Lists subtest from the Children’s Memory Scale (26). Initial learning was controlled for by including the number of words correctly recalled on the last learning trial (Trial 4) as an independent control variable in HLM analyses.
Delayed visual-spatial memory was assessed using the Spatial Delayed Response (SDR) task (9–11,15,27,28). After a 60 second distractor-task filled delay, participants pointed to the location on a computer screen where a dot had appeared. The mean error in millimeters between the original and remembered dot location was analyzed.
Processing Speed
Basic processing speed was measured by a Speed task that required participants to press a button as quickly as possible whenever a plus sign appeared on a computer screen (29). Processing speed in situations that require inhibitory control was assessed by a Go-No-Go task (29). Median reaction time for correct trials was analyzed for each task.
Analyses
Analyses were conducted using HLM 6.08 (significance threshold p < .05) (30). Briefly, the HLM approach decomposes variability into two ‘levels’, in this case between- and within-person. Between-person independent variables have a single value for each individual and represent a stable attribute (e.g., diabetes versus control group membership) or overall standing on a given variable over time (e.g., mean glycemic control over a given time period). Within-person independent variables (e.g., mean glycemic control between two time points, age since enrollment) are measured at multiple occasions and can vary from time-to-time for a given person. In the current study, between-person main effects can be interpreted as the mean effect of an independent variable on a given dependent variable across all time points, while within-person main effects can be interpreted as a measure of the relationship between within-person variation in an independent variable and within-person changes in a dependent variable from one occasion to the next. We also examined whether the degree of linear increase or decrease in cognitive performance trends (i.e., slopes) varied as a function of between-person variables, an effect often referred to as a cross-level interaction.
Using this approach we examined the effects of group membership, and within the T1DM group the effects of mean glycemic control, intraindividual variation in glycemic control, and age of onset on cognitive performance as follows:
Effects of Group
HLMs with group as an independent variable and task performance as the dependent variable were created for each cognitive task to test for group differences in cognitive performance across all three time points (i.e., between-person group main effects). Age at time point 1 was also included as an independent variable in these models to control for its effects on mean cognitive performance. In addition, years since enrollment was entered as a within-person predictor in each model to estimate the rate of linear change in cognitive performance. Therefore, we were able to estimate whether group had a significant influence on within-person rates of change in cognitive performance across time (i.e., cross-level interactions).
Effects of Glycemic Control and Age of Diabetes Onset
Global mean HbA1c was calculated for each participant by averaging their mean HbA1c from three time periods: diabetes diagnosis to time point 1, time points 1 to 2, and time points 2 to 3. HLMs were created for each cognitive task with age of diabetes onset (between-person), global mean HbA1c (between-person), mean HbA1c from diabetes diagnosis to time point 1, time points 1 to 2, and time points 2 to 3 (within-person), and years since enrollment (within-person) as independent variables and task performance as the dependent variable. Age at time point 1 and mean blood glucose meter readings from the cognitive testing sessions were also included as independent control variables. These models were used to test whether global mean HbA1c and age of diabetes onset made significant contributions to between-person differences in cognitive performance across all three time points (i.e., HbA1c and age of diabetes onset between-person main effects). They were also used to examine whether individual variation in HbA1c from one measurement occasion to the next influenced cognitive performance (within-person main effects). In addition, they were used to examine whether global mean HbA1c and age of diabetes onset influenced within-person changes (i.e., slopes) in cognitive performance across time (i.e., cross-level interactions). Finally, age at diabetes onset × global mean HbA1c interaction terms were subsequently added to these models to examine whether age at diabetes onset affects the strength of the relationship between mean glycemic control and mean cognitive performance across all three time points (i.e., between-person interaction effects).
Results
Subjects
One hundred and nineteen youth with T1DM and 59 non-diabetic siblings were enrolled in this study at time point 1. Data from the 61 youth with T1DM and 28 siblings from the initial cohort who completed cognitive testing at all three study time points, had mean blood glucose meter readings between 60 and 375 mg/dL during all cognitive testing sessions, and did not develop new diagnoses that met exclusion criteria during follow-up were included in the present analyses. There were no significant differences in sex, parental education, age at study enrollment, age at diabetes diagnosis (T1DM group only), or mean HbA1c between diagnosis and time point 1 (T1DM group only) for T1DM or control group youth who completed all three study time points versus those who did not (all Χ2 and t-test Ps > 0.05). The demographic and clinical characteristics of the T1DM and control participant groups in this study are presented in Supplementary Table 1. There were no significant T1DM/non-T1DM group differences in sex (Χ2 = 1.23, P = 0.268), parental education (t = −0.55, P = 0.584), or age at study enrollment (time point 1; t = 0.91, P = 0.366). There were also no significant T1DM/non-T1DM group differences in the number of months between time points 1 and 2 (Sibling: Mean = 24.1, SD = 0.3, Range 23.5 – 24.8; T1DM: Mean = 24.1, SD = 0.3, Range 23.5 – 25.3; t = 0.35, P = 0.728) or between time points 2 and 3 (Sibling: Mean = 33.7, SD = 4.5, Range 23.9 – 41.7; T1DM: Mean = 32.4, SD = 6.3, Range 21.1 – 42.3; t = −1.10, P = 0.273). Even though the number of months between time point 1 and time point 3 varied substantially across participants, the HLM data analysis technique that we used allowed us to model cognitive performance over the course of five and a half years (the maximum time between time points 1 and 3 for a participant this study) using data from all study participants. There were no ceiling or floor performance effects on any cognitive test, indicating that the tests were valid assessments of cognitive function. Four percent of diabetes group and three percent of control group cognitive testing data was missing due to participant fatigue, time constraints, or experimenter error. For analytic purposes, missing data was treated as missing at random (MAR). HLM and other random-coefficient regression modeling techniques allow for unbiased parameter estimates using the available data under conditions of ignorable missingness, with MAR having the least restrictive assumptions (31–33).
Mean testing session blood glucose meter readings for time points 1-3 were 184 (SD = 54, range 83 – 324), 182 (SD = 59, range 73 – 312), and 182 (SD = 53, range 78 – 296) mg/dL, respectively. Mean HbA1c test coverage for the diabetes group from diagnosis to time point 3 for participants included in data analyses was 64% (SD = 12%; range 33 – 93%). Mean HbA1c values from diagnosis to time point 1, between time points 1 and 2, and between time points 2 and 3 were 8.2% (SD = 1.0%, range 6.6 – 12.7%) (66 mmol/mol, SD = 11 mmol/mol, range 49 – 115 mmol/mol), 8.3% (SD = 1.3%, range 6.4 – 12.6%) (67 mmol/mol, SD = 14 mmol/mol, range 46 – 114 mmol/mol), and 8.8% (SD = 1.3%, range 6.6 – 13.3%) (73 mmol/mol, SD = 14 mmol/mol, range 49 – 122 mmol/mol), respectively. Fifty-one percent (n = 31) of participants in the diabetes group experienced 1 or more severe hypoglycemic events between diagnosis and time point 1. However, only 13 percent (n = 8) and 15 percent (n = 9) of participants in the diabetes group experienced 1 or more severe hypoglycemic events between time points 1 and 2 and time points 2 and 3, respectively. Due to these low numbers, we were not able to reliably analyze the longitudinal effects of severe hypoglycemia exposure on cognitive function.
Cognitive performance for youth at the mean age of study enrollment is modeled in Figures 1 and 2 to illustrate developmental trends in the diabetes and control groups. All HLM analyses controlled for age at study enrollment. Therefore, all significant results indicate patterns of cognitive function that were present regardless of the enrollment age of study participants.
Figure 1.
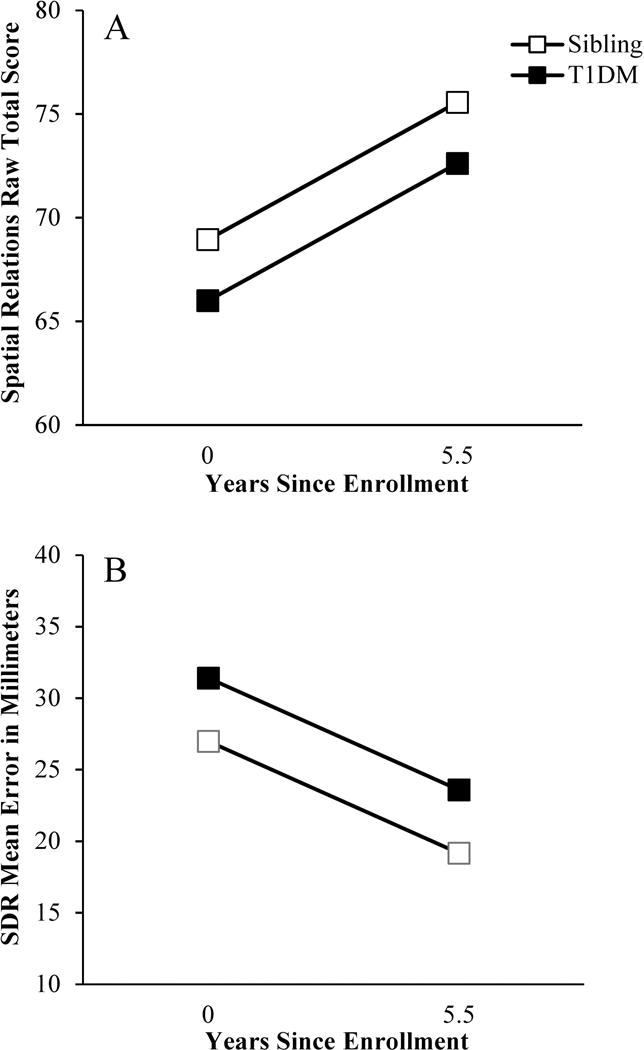
Group differences in visual-spatial cognitive performance. A: Individuals with T1DM scored lower on the Spatial Relations task than sibling controls throughout the duration of this study. B: Individuals with T1DM were also less accurate in their ability to remember dot locations on the Spatial Delayed Response (SDR) task than sibling controls across all three study time points.
Figure 2.
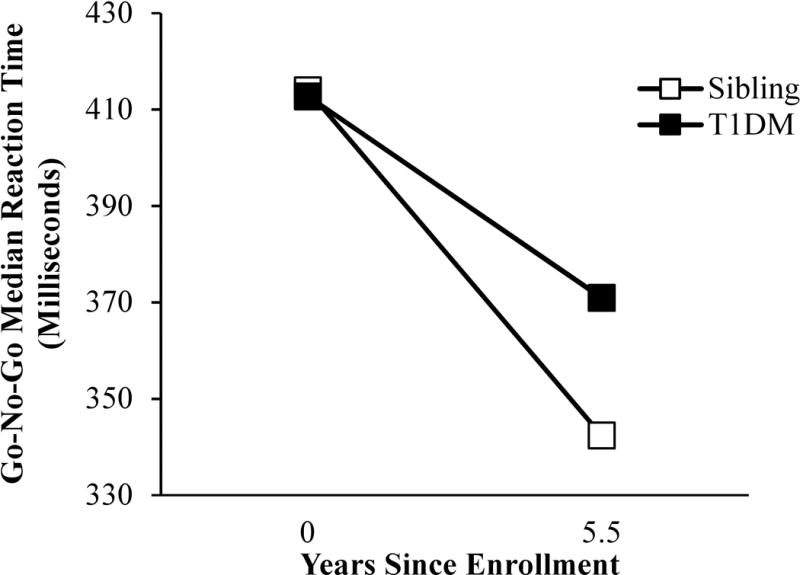
Group differences in within-person changes in Go-No-Go task median reaction times. The median reaction times of individuals with T1DM did not improve as much over time as their siblings’.
Effects of Group
Between-Person
Analyses revealed significant main effects of group on visual-spatial ability and delayed memory (Figure 1; see Table 1 for a summary of all HLM analysis results). The T1DM group scored an average of 2.95 points lower on the Spatial Relations task (B = −2.95, SE = 1.03, P = 0.006), and was an average of 4 mm less accurate in recalling dot locations on the SDR task (B = 4.42, SE = 2.19, P = 0.046), than the control group across all three time points.
Table 1.
Results (P values) of Hierarchical Linear Modeling (HLM) analyses of the effects of diabetes status, hyperglycemia, and age of diabetes onset on cognitive performance
| Group | HbA1c | Age of Onset | Age of Onset × HbA1c | ||||
|---|---|---|---|---|---|---|---|
| Between | Cross-Level | Between | Within | Between | Cross-Level | Between | |
| General Information | 0.184 | 0.638 | 0.016* | 0.659 | 0.900 | 0.647 | 0.793 |
| Spatial Relations | 0.006* | 0.918 | 0.130 | 0.004* | 0.176 | 0.130 | 0.027* |
| Word Lists Delayed Recall | 0.304 | 0.923 | 0.001* | 0.563 | 0.813 | 0.918 | 0.628 |
| SDR Delayed Recall | 0.046* | 0.427 | 0.119 | 0.085 | 0.766 | 0.936 | 0.593 |
| Speed Median RT | 0.750 | 0.369 | 0.285 | 0.437 | 0.612 | 0.476 | 0.372 |
| GNG Median RT | 0.186 | 0.037* | 0.008* | 0.026* | 0.114 | 0.188 | 0.600 |
P < 0.05.
Between, between-person main effects. Cross-Level, cross-level interaction effects. Within, within-person main effects. RT, reaction time in milliseconds. SDR, Spatial Delayed Response memory test. GNG, Go-No-Go response inhibition test.
Cross-Level Interaction
Analyses revealed that a diagnosis of T1DM had a significant effect on within-person changes over time in Go-No-Go task median reaction times (B = 5.38, SE = 2.54, P = 0.037). The diabetes group median reaction times did not improve as much over time as those of the control group (Figure 2).
Effects of Hyperglycemia Exposure
Between-Person
Significant main effects of HbA1c controlling for blood glucose values at time of testing were found on crystallized intelligence, delayed verbal memory, and processing speed within the diabetes group. Higher global mean HbA1c was associated with lower performance on the General Information task (B = −0.92, SE = 0.36, P = 0.016), slower median reaction times on the Go-No-Go task (B= 14.96, SE = 5.35, P = 0.008), but better delayed memory performance on the Word Lists task (B= 0.46, SE = 0.13, P = 0.001) across all three time points.
Within-Person
Within-person changes in glycemic control controlling for blood glucose values at time of testing were associated with within-person changes in visual-spatial ability and processing speed. Decreases in HbA1c from one occasion to the next were associated with performance improvements on the Spatial Relations task and faster median reaction times on the Go-No-Go task, while increases in HbA1c from one occasion to the next were associated with performance decrements on the Spatial Relations task (B = −1.46, SE = 0.49, P = 0.004) and slower median reaction times on the Go-No-Go task (B = 12.04, SE = 5.33, P = 0.026). There was also a trend for decreases in HbA1c to be associated with larger errors and increases in HbA1c to be associated with smaller errors on the delayed visual-spatial memory SDR task (B = −2.76, SE = 1.59, P = 0.085).
Effects of Age of Diabetes Onset
Between-Person
Age of diabetes onset did not have any significant between-person main effects on cognitive performance (all Ps > .05). However, there was a significant age of onset × global mean HbA1c interaction for the Spatial Relations task (B= 0.33, SE = 0.15, P = 0.027). There was a stronger negative relationship between global mean HbA1c and Spatial Relations task performance for youth with earlier compared to later onset diabetes (Figure 3).
Figure 3.
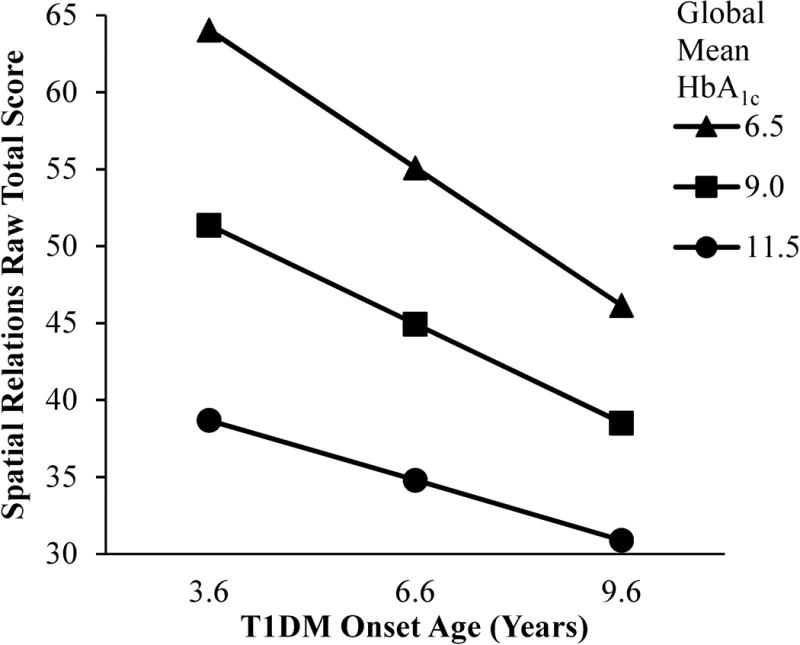
Interactive effect of age of diabetes onset and global mean HbA1c on visual-spatial ability. The relationships between Spatial Relations task performance, age of diabetes onset, and global mean HbA1c, which were all continuous variables in our HLM analyses, are modeled for individuals who were diagnosed with T1DM at an age that was one standard deviation younger than the mean age of diabetes onset, at the mean age of diabetes onset, and at an age that was one standard deviation older than the mean age of diabetes onset to illustrate the significant age of diabetes onset × global mean HbA1c interaction for the Spatial Relations task. There was a stronger negative relationship between global mean HbA1c and Spatial Relations task performance for youth with earlier compared to later onset diabetes. The between-person main effect of age of diabetes onset was not significant.
Cross-Level Interaction
Age of diabetes onset did not explain differences in within-person changes in cognitive performance over time (all Ps > .05).
Discussion
Overall, youth with T1DM had worse performance than non-diabetic sibling controls on visual-spatial ability and delayed memory tasks across all three study time points, suggesting that group differences in these tasks were present at the beginning of the study and remained stable over time. In contrast, group differences in processing speed emerged over time, with the diabetic group failing to improve as much as the control group. The fact that both patterns were found within the same study suggests that the effects of T1DM on visual-spatial processing emerge early while the effects of T1DM on processing speed emerge later in development or exposure to diabetes. Depending on the age or disease duration at which subjects are tested, cross-sectional analyses could find different or even conflicting results.
Between-person analyses of the effects of glycemic control on cognitive function revealed that greater hyperglycemia exposure was associated with learning less factual knowledge and slower processing speed across all three time points. However, within-person decreases in hyperglycemia between time points were associated with improved visual-spatial ability and faster processing speed. This pattern of results suggests that poor glycemic control can have a negative effect on cognitive function in youth with T1DM, but also suggests that treatments that lower hyperglycemia exposure may result in improvements in cognitive function. To our knowledge, this is the first study to find a significant positive relationship between within-person improvements in glycemic control and within-person improvements in cognitive function in youth with T1DM. Since there was a stronger negative relationship between global mean HbA1c and Spatial Relations task performance for youth with earlier compared to later onset diabetes in this study, it may be particularly helpful to control hyperglycemia exposure in early childhood.
Notably, greater exposure to hyperglycemia was also associated with better delayed verbal memory performance across all three study time points. Within individuals, there was also a trend for increasing hyperglycemia from one occasion to the next to be associated with better delayed visual-spatial memory. Prior studies have reported that greater hyperglycemia and severe hypoglycemia exposure are associated with worse memory performance (7,9–11,15,22,23). A possible explanation for the positive associations between hyperglycemia exposure and delayed memory seen here is that greater hyperglycemia exposure may indicate minimal exposure to severe hypoglycemia. This hypothesis could not be tested in our analyses due to the limited known exposure of our diabetic participants to severe hypoglycemia during the follow-up period. Future research is needed to directly compare the longitudinal effects of hyperglycemia and hypoglycemia exposure on delayed memory performance.
This study extends the findings of prior research that has statistically compared longitudinal change in cognitive function in youth with T1DM relative to non-diabetic peers. In a younger cohort (ages 4-9), Cato and colleagues did not find any significant group differences in change in intelligence, memory, or visuomotor processing speed over the course of 18 months (19). However, in a cohort similar to ours in age, Northam and colleagues have reported that a T1DM group had less positive change on intelligence over 2 years (16) and more decline in intelligence over the course of 12 years (18) compared to controls. However, in the Northam et al. longitudinal study, intelligence was measured using scaled scores from different tests across time points for some participants, limiting the conclusions that can be drawn. The present study analyzed raw scores from the same tests at all time points and demonstrated that visual-spatial ability and delayed memory are lower, and that group differences in visuomotor processing speed can emerge, in youth with T1DM over the course of 5.5 years.
This study is limited by the overall modest sample size, particularly for controls, due to the fact that cognitive data for all three study time points was available for only half of the participants who enrolled in the study at time point 1. In addition, few severe hypoglycemic events were observed during follow-up, limiting our ability to assess the effects of hypoglycemia exposure on cognitive trajectories and the complexity of our hyperglycemia analyses and interpretations. Given that past cross-sectional research has suggested that early age of diabetes onset has a negative effect on cognitive function in youth with T1DM (1,3,4), it was surprising that only one significant effect of age of diabetes onset on cognitive performance was found in our analyses. One possible explanation is that participants’ mean age of onset was quite young (6.4 years), which may have limited our ability to detect the effects of age of diabetes onset on longitudinal change in cognitive function. The cognitive effects observed in this study are relatively small and are unlikely to be clinically noticeable, but suggest that further exposure to hyperglycemia may lead to continued or increasing differences in cognitive function between youth with T1DM and their non-diabetic peers. Future research is needed to determine the degree to which improvements in glycemic control can enhance cognitive function in everyday life in youth with T1DM, particularly given that recent research has shown that greater hyperglycemia exposure is associated with worse academic performance in youth with T1DM (34,35).
In conclusion, the results of this study suggest that on average differences in cognitive function between youth with T1DM and non-diabetic relatives are maintained or increase during childhood and adolescence. Hyperglycemia and age of diabetes onset can have negative effects on cognitive developmental trajectories in youth with T1DM. However, at least some alterations in cognitive function may be modifiable during childhood and adolescence. Treatments that lower hyperglycemia could lead to improved function in some cognitive domains (e.g., visual-spatial ability and processing speed).
Supplementary Material
Acknowledgments
We thank members of the Hershey lab, in particular Heather Lugar, Emily Bihun, and Allison Bischoff, for assistance with data collection and management. This work was supported by the NIH (R01 DK64832, CTSA UL1 RR024992, DRTC 020579) and the Dana Foundation.
References
- 1.Gaudieri PA, Chen R, Greer TF, Holmes CS. Cognitive function in children with type 1 diabetes: a meta-analysis. Diabetes Care. 2008;31:1892–1897. doi: 10.2337/dc07-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naguib JM, Kulinskaya E, Lomax CL, Garralda ME. Neuro-cognitive performance in children with type 1 diabetes – a meta-analysis. J Pediatr Psychol. 2009;34:271–282. doi: 10.1093/jpepsy/jsn074. [DOI] [PubMed] [Google Scholar]
- 3.Tonoli C, Heyman E, Roelands B, et al. Type 1 diabetes-associated cognitive decline: a meta-analysis and update of the current literature. J Diabetes. 2014;6:499–513. doi: 10.1111/1753-0407.12193. [DOI] [PubMed] [Google Scholar]
- 4.Ryan C, Vega A, Drash A. Cognitive deficits in adolescents who developed diabetes early in life. Pediatrics. 1985;75:921–927. [PubMed] [Google Scholar]
- 5.Rovet JF, Ehrlich RM, Hoppe M. Intellectual deficits associated with early onset of insulin-dependent diabetes mellitus in children. Diabetes Care. 1987;10:510–515. doi: 10.2337/diacare.10.4.510. [DOI] [PubMed] [Google Scholar]
- 6.Rovet J, Alvarez M. Attentional functioning in children and adolescents with IDDM. Diabetes Care. 1997;20:803–810. doi: 10.2337/diacare.20.5.803. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman FR, Epport K, Engilman R, Halvorson M. Neurocognitive functioning in children diagnosed with diabetes before age 10 years. J Diabetes Complications. 1999;13:31–38. doi: 10.1016/s1056-8727(98)00029-4. [DOI] [PubMed] [Google Scholar]
- 8.Northam EA, Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA. Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care. 2001;24:1541–1546. doi: 10.2337/diacare.24.9.1541. [DOI] [PubMed] [Google Scholar]
- 9.Hershey T, Lillie R, Sadler M, White NH. Severe hypoglycemia and long-term spatial memory in children with type 1 diabetes mellitus: a retrospective study. J Int Neuropsychol Soc. 2003;9:740–750. doi: 10.1017/S1355617703950077. [DOI] [PubMed] [Google Scholar]
- 10.Hershey T, Perantie DC, Warren SL, Zimmerman EC, Sadler M, White NH. Frequency and timing of severe hypoglycemia affects spatial memory in children with type 1 diabetes. Diabetes Care. 2005;28:2372–2377. doi: 10.2337/diacare.28.10.2372. [DOI] [PubMed] [Google Scholar]
- 11.Perantie DC, Lim A, Wu J, et al. Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatr Diabetes. 2008;9:87–95. doi: 10.1111/j.1399-5448.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- 12.Patino-Fernandez AM, Delamater AM, Applegate EB, et al. Neurocognitive functioning in preschool-age children with type 1 diabetes mellitus. Pediatr Diabetes. 2010;11:424–430. doi: 10.1111/j.1399-5448.2009.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aye T, Reiss AL, Kesler S, et al. The feasibility of detecting neuropsychologic and neuroanatomic effects of type 1 diabetes in young children. Diabetes Care. 2011;34:1458–1462. doi: 10.2337/dc10-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolu-Kendir O, Kiris N, Temiz F, et al. Relationship between metabolic control and neurocognitive functions in children diagnosed with type 1 diabetes mellitus before and after 5 years of age. Turk J Pediatr. 2012;54:352–361. [PubMed] [Google Scholar]
- 15.Semenkovich K, Bischoff A, Doty T, et al. Clinical presentation and memory function in youth with type 1 diabetes. Pediatr Diabetes. 2015 doi: 10.1111/pedi.12314. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Northam EA, Anderson PJ, Werther GA, Warne GL, Adler RG, Andrewes D. Neuropsychological complications of IDDM in children 2 years after disease onset. Diabetes Care. 1998;21:379–384. doi: 10.2337/diacare.21.3.379. [DOI] [PubMed] [Google Scholar]
- 17.Fox MA, Chen RS, Holmes CS. Gender differences in memory and learning in children with insulin-dependent diabetes mellitus (IDDM) over a 4-year follow-up interval. J Pediatr Psychol. 2003;28:569–578. doi: 10.1093/jpepsy/jsg047. [DOI] [PubMed] [Google Scholar]
- 18.Lin A, Northam EA, Werther GA, Cameron FJ. Risk factors for decline in IQ in youth with type 1 diabetes over the 12 years from diagnosis/illness onset. Diabetes Care. 2015;38:236–242. doi: 10.2337/dc14-1385. [DOI] [PubMed] [Google Scholar]
- 19.Cato MA, Mauras N, Mazaika P, et al. Longitudinal evaluation of cognitive functioning in young children with type 1 diabetes over 18 months. J Int Neuropsychol Soc. 2016;21:1–10. doi: 10.1017/S1355617715001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musen G, Jacobson AM, Ryan CM, et al. Impact of diabetes and its treatment on cognitive function among adolescents who participated in the diabetes control and complications trial. Diabetes Care. 2008;31:1933–1938. doi: 10.2337/dc08-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asvold BO, Sand T, Hestad K, Bjorgaas MR. Cognitive function in type 1 diabetic adults with early exposure to severe hypoglycemia: a 16-year follow-up study. Diabetes Care. 2010;33:1945–1947. doi: 10.2337/dc10-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Northam EA, Anderson PJ, Werther GA, Warne GL, Andrewes D. Predictors of change in the neuropsychological profiles of children with type 1 diabetes 2 years after disease onset. Diabetes Care. 1999;22:1438–1444. doi: 10.2337/diacare.22.9.1438. [DOI] [PubMed] [Google Scholar]
- 23.Kent S, Chen R, Kumar A, Holmes C. Individual growth curve modeling of specific risk factors and memory in youth with type 1 diabetes: an accelerated longitudinal design. Child Neuropsychol. 2010;16:169–181. doi: 10.1080/09297040903264140. [DOI] [PubMed] [Google Scholar]
- 24.The DCCT Research Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am J Med. 1991;90:450–459. [PubMed] [Google Scholar]
- 25.McGrew KS, Woodcock RW. Technical Manual. Woodcock-Johnson III. Itasca, IL: Riverside Publishing; 2001. pp. 1–209. [Google Scholar]
- 26.Cohen MJ. Children’s Memory Scale. San Antonio, TX: Harcourt Brace and Company; 1997. pp. 1–269. [Google Scholar]
- 27.Hershey T, Bhargava N, Sadler M, White NH, Craft S. Conventional versus intensive diabetes therapy in children with type 1 diabetes: effects on memory and motor speed. Diabetes Care. 1999;22:1318–1324. doi: 10.2337/diacare.22.8.1318. [DOI] [PubMed] [Google Scholar]
- 28.Hershey T, Lillie R, Sadler M, White NH. A prospective study of severe hypoglycemia and long-term spatial memory in children with type 1 diabetes. Pediatr Diabetes. 2004;5:63–71. doi: 10.1111/j.1399-543X.2004.00045.x. [DOI] [PubMed] [Google Scholar]
- 29.Christ SE, Steiner RD, Grange DK, Abrams RA, White DA. Inhibitory control in children with phenylketonuria. Dev Neuropsychol. 2006;30:845–864. doi: 10.1207/s15326942dn3003_5. [DOI] [PubMed] [Google Scholar]
- 30.Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows. Skokie, IL: Scientific Software International Inc; 2004. [Google Scholar]
- 31.Laird NM. Missing data in longitudinal studies. Stat Med. 1988;7:305–315. doi: 10.1002/sim.4780070131. [DOI] [PubMed] [Google Scholar]
- 32.Little RJA. Modeling the dropout mechanism in repeated-measures studies. J Am Stat Assoc. 1995;90:1112–1121. [Google Scholar]
- 33.Little RJA, Schlenker N. Missing Data. In: Arminger G, Clogg CC, Sobel ME, editors. Handbook of Statistical Modeling for the Social and Behavioral Sciences. Plenum; New York: 1995. pp. 39–75. [Google Scholar]
- 34.Cooper MN, McNamara KAR, de Klerk NH, Davis EA, Jones TW. School performance in children with type 1 diabetes: a contemporary population-based study. Pediatr Diabetes. 2016;17:101–111. doi: 10.1111/pedi.12243. [DOI] [PubMed] [Google Scholar]
- 35.Semenkovich K, Patel PP, Pollock AB, et al. Academic abilities and glycaemic control in children and young people with Type 1 diabetes mellitus. Diabet Med. 2016;33:668–673. doi: 10.1111/dme.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


