Abstract
Adolescent psychotic experiences increase risk for schizophrenia and other severe psychopathology in adulthood. Converging evidence implicates urban and adverse neighborhood conditions in the aetiology of adolescent psychotic experiences, but the role of young people’s personal perceptions of disorder (i.e., physical and social signs of threat) in their neighborhood is unknown. This was examined using data from the Environmental Risk (E-Risk) Longitudinal Twin Study, a nationally-representative birth cohort of 2,232 British twins. Participants were interviewed at age 18 about psychotic phenomena and perceptions of disorder in the neighborhood. Multilevel, longitudinal, and genetically-sensitive analyses investigated the association between perceptions of neighborhood disorder and adolescent psychotic experiences. Adolescents who perceived higher levels of neighborhood disorder were significantly more likely to have psychotic experiences, even after accounting for objectively/independently measured levels of crime and disorder, neighborhood- and family-level socioeconomic status, family psychiatric history, adolescent substance and mood problems, and childhood psychotic symptoms (OR=1.62, 95% CI=1.27–2.05, p<0.001). The phenotypic overlap between adolescent psychotic experiences and perceptions of neighborhood disorder was explained by overlapping common environmental influences (rC=0.88, CI=0.26–1.00). Findings suggest that early psychological interventions to prevent adolescent psychotic experiences should explore the role of young people’s (potentially modifiable) perceptions of threatening neighborhood conditions.
Keywords: adolescence, neighborhood disorder, perceptions, psychosis, twins
Introduction
Up to a third of youth in the general population report subclinical psychotic experiences such as hearing voices, having visions, being extremely paranoid, and other unusual thoughts and beliefs (Horwood et al., 2008; Kelleher et al., 2012a; Newbury et al., 2017; Spauwen et al., 2004; Yoshizumi et al., 2004). Though early psychotic phenomena are usually transitory (Kelleher et al., 2012a; Scott et al., 2006), adolescents who report these experiences have a significantly elevated adulthood risk for schizophrenia (Fisher et al., 2013; Poulton et al., 2000) and other serious psychiatric problems such as depression, substance dependence, and suicide attempts (Dhossche et al., 2002; Fisher et al., 2013; Kelleher et al., 2012b). Late adolescence heralds the peak age of risk for a first episode of psychosis (Häfner et al., 1994), a diagnosis which increases young people’s risk of death within a year by over 20-fold (Schoenbaum et al., 2017). Subclinical psychotic experiences during this period have also been shown to be more clinically-relevant than at earlier ages (Kelleher et al., 2012c). It is therefore crucial to improve our understanding of the mechanisms leading to psychotic experiences during adolescence – from genetic influences through to the wider built and social environment – in order to develop more targeted and effective preventative interventions (Millan et al., 2016).
Adolescent psychotic experiences share similar familial and social risk factors to adult psychosis – such as family history of mental illness, marijuana use, and low socioeconomic status (SES) (Kelleher & Cannon, 2011; Polanczyk et al., 2010). Emerging research now implicates adverse wider environmental factors in the aetiology of subclinical psychotic phenomena and clinical psychosis. Compared to youth living in rural settings, young people in cities are exposed to higher neighborhood levels of fragmentation, crime, and disorder (Goldman-Mellor et al., 2016; Newbury et al., 2017; Office for National Statistics, 2012). Neighborhood disorder is a sociological construct which refers to physical and social signs of threat and danger in the neighborhood, such as vandalism, gang activity and burglaries (Sampson & Raudenbush, 1999). Youth and young adults who live in these kinds of urban, fragmented and threatening settings are more likely to have prodromal symptoms, persistent psychotic experiences, and a first episode of psychosis (Bhavsar et al., 2014; Kirkbride et al., 2015; Spauwen et al., 2006; Wilson et al., 2016), and there is evidence that symptom severity among adults with clinical psychosis is exacerbated after brief exposure to a densely populated urban environment (Ellett, Freeman & Garety, 2008; Freeman et al., 2014). Furthermore, we recently identified higher rates of psychotic phenomena among children and adolescents living in cities in the UK (Newbury et al., 2016; Newbury et al., 2017). Our analyses showed that threatening and adverse neighborhood social conditions, as reported by mothers and residents, explained up to half of this association between urbanicity and early psychotic phenomena (Newbury et al., 2016; Newbury et al., 2017). There is now a growing consensus that urban and adverse neighborhood conditions increase risk for psychotic phenomena by elevating background and acute sources of social stress, particularly during upbringing (Heinz, Deserno & Reininghaus, 2013; Lederbogen, Haddad & Meyer-Lindenberg, 2013; Selten et al., 2013). Notably, this proposed mechanism requires that young people in cities and adverse neighborhood settings are themselves perceiving their neighborhoods as stressful and threatening.
Existing studies of neighborhood conditions and psychosis (both subclinical and clinical phenotypes) have typically derived neighborhood measures from official data assigned to broad geostatistical units. Whilst being objective, these types of measures do not establish the extent to which the neighborhood feature(s) in question was personally experienced or perceived by the individuals under study (the ecological fallacy). Individuals can and do differ in how they perceive the same environment or experience, but we currently know very little about the potential role of young people’s personal perceptions of threat in their immediate neighborhood in the aetiology of early psychotic phenomena. That is, it is unknown whether personal perceptions of neighborhood conditions are important over and above objectively measured neighborhood conditions. Considering that urban and adverse neighborhood conditions putatively increase risk for psychotic phenomena via a social stress pathway, and delusions and hallucinations involve altered perceptions of reality, we might expect personal perceptions of the neighborhood (e.g., “my neighborhood is dangerous”) to play a crucial role in the association between adverse neighborhood conditions and psychotic experiences. Recent research has shown that perceptions of neighborhood disorder are associated with common mental health problems and psychological distress among youth, above and beyond the effects of official levels of crime (Goldman-Mellor et al., 2016; Polling et al., 2014). These findings also parallel a body of research documenting stronger associations between childhood trauma and psychiatric problems when childhood trauma is retrospectively self-reported rather than obtained from objective or independent sources (Brown, Berenson & Cohen, 2005; Reuben et al., 2016; Widom & Morris, 1997; Widom, Weiler & Cottler, 1999). Examining the role of young people’s personal perceptions of threatening neighborhood conditions in early psychotic experiences could not only elucidate the mechanisms underlying previous findings on neighborhood adversity and psychotic experiences, but it might also highlight potential new avenues for interventions. For example, targeted cognitive behavioral interventions have been shown to alleviate the paranoia and distress caused by busy urban settings among patients with clinical psychosis (Freeman et al., 2015).
A number of potential methodological issues must be considered when examining the role of perceived neighborhood conditions in early psychotic phenomena. Similarly to self-report measures of childhood trauma (Hardt & Rutter, 2004), self-report measures of adverse neighborhood conditions could be subject to shared method and mood-congruent recall biases, whereby an individual’s contemporaneous mental health influences their perception and memory. It is particularly important to consider this potential confounding mechanism when investigating psychotic experiences, which involve altered perceptions of reality, such as paranoia and threat detection bias (Freeman et al., 2002; Garety et al., 2001). It is therefore useful to establish the construct validity of personal perceptions of neighborhood adversity by comparing self-reports to objective and independent measures of the neighbourhood. Moreover, given the potential bidirectional relationship between psychotic experiences and perceptions of the neighborhood, longitudinal designs are needed to examine the temporality of the association. It is also crucial to consider a range of factors which might simultaneously influence both adolescents’ perceptions of neighborhood adversity and their psychotic experiences, such as family SES, substance use, earlier psychotic symptoms in childhood – and genetic influences. Emerging behavioral genetics research suggests that overlapping genes may partly explain the correlation between psychotic phenomena and certain putatively environmental exposures, such as stressful life events (Shakoor et al., 2016) and neighborhood-level deprivation (Sariaslan et al., 2016). It is plausible that shared genetic influences might also contribute to covariance between psychotic phenomena and perceptions of neighborhood adversity. The classical twin design allows the covariance between two variables to be partitioned into genetic and environmental sources, thus providing an ideal technique for exploring this issue.
Using data from a longitudinal cohort of over 2,000 British twin children, the present study adopts a multilevel approach – spanning the wider built and social environment, family-level characteristics, and individual-level factors including genetic influences – to investigate the role of personal perceptions of threatening neighborhood conditions in the development of adolescent psychotic experiences. A comprehensive battery of data has been collected at several time-points across early development. Psychotic phenomena were measured in both childhood (age 12) and adolescence (age 18). Urbanicity, neighborhood-level SES, and neighborhood crime rates were obtained from detailed geodemographic and official data sources. Resident surveys of over 5,000 immediate neighbors of E-Risk participants provided an independent measure of neighborhood disorder. Personal perceptions of neighborhood disorder were self-reported by the participants themselves in private interviews at age 18. All neighborhood measures had high resolution (i.e., street- or postcode-level). The twin sample afforded us the opportunity to estimate the genetic versus environmental sources of covariance between perceptions of neighborhood disorder and adolescent psychotic experiences. With these measures, we firstly investigated the construct validity of adolescents’ personal perceptions of neighborhood disorder by correlating these self-reports with objective/independent measures of neighborhood adversity. We then asked: (1) Do higher perceived levels of neighborhood disorder among adolescents in urban (versus rural) settings explain the association between urbanicity and adolescent psychotic experiences? (2) (i) Is the association between perceptions of neighborhood disorder and adolescent psychotic experiences robust to neighborhood-, family-, and individual-level confounders (official neighborhood crime rates, resident-reported neighborhood disorder, neighborhood-level SES, family SES, family psychiatric history, maternal psychotic symptoms, adolescent marijuana dependence, alcohol dependence, anxiety, depression, and childhood psychotic symptoms)? (ii) Are twins who perceive higher levels of neighborhood disorder than their co-twin also more likely to have psychotic experiences (this within-family co-twin control analysis holds neighborhoods constant and accounts more robustly for unmeasured genetic and environmental factors shared between twins)? (3) Do childhood perceptions of neighborhood safety predict adolescent psychotic experiences after considering childhood psychotic symptoms; and do childhood psychotic symptoms predict adolescent perceptions of neighborhood disorder after considering childhood perceptions of neighborhood safety? (i.e., what is the temporality of the association between perceptions of neighborhood disorder and early psychotic phenomena?) And (4), [i] to what extent do genetic versus environmental factors contribute to perceptions of neighborhood disorder and adolescent psychotic experiences? [ii] To what extent do overlapping genetic versus environmental factors contribute to the covariance between perceptions of neighborhood disorder and adolescent psychotic experiences?
Methods
Study Cohort
Participants were members of the Environmental Risk (E-Risk) Longitudinal Twin Study, which tracks the development of a nationally-representative birth cohort of 2,232 British twin children. The sample was drawn from a larger cohort of twins born in England and Wales in 1994-1995 (Trouton, Spinath & Plomin, 2002). Full details about the sample are reported elsewhere (Moffitt & The E-Risk Study Team, 2002). Briefly, the E-Risk sample was constructed in 1999-2000, when 1,116 families with same-sex 5-year-old twins (93% of those eligible) participated in home-visit assessments. This sample comprised 56% monozygotic (MZ) and 44% dizygotic (DZ) twin pairs; sex was evenly distributed within zygosity (49% male). Families were recruited to represent the UK population of families with newborns in the 1990s, based on residential location throughout England and Wales and mothers’ age (teenaged mothers with twins were over-selected to replace high-risk families who were selectively lost to the register through non-response. Older mothers having twins via assisted reproduction were under-selected to avoid an excess of well-educated older mothers). All families were English speaking, and the majority (93.7%) were White. Follow-up home-visits were conducted when children were aged 7, 10, 12 and 18 (participation rates were 98%, 96%, 96% and 93%, respectively). Home visits at ages 5, 7, 10, and 12 years included assessments with participants as well as their mother (or primary caretaker); the home visit at age 18 included interviews only with the participants. Each twin participant was assessed by a different interviewer. The average age of the twins at the time of the age 18 assessment was 18.4 years (SD=0.36); all interviews were conducted after the 18th birthday. At age 18, the E-Risk sample comprised 2,066 participants. There were no differences between those who did and did not take part at age 18 in terms of age–5 socioeconomic status (SES) (χ2=0.86, p=0.65), age–5 IQ scores (t=0.98, p=0.33), or age–5 internalizing or externalizing behavior problems (t=0.40, p=0.69 and t=0.41, p=0.68, respectively). The Joint South London and Maudsley and the Institute of Psychiatry Research Ethics Committee approved each phase of the study. Parents gave informed consent, and participants gave assent at ages 5–12 and informed consent at age 18.
Measures
Adolescent psychotic experiences
At age 18, E-Risk participants were privately interviewed by a research worker about 13 psychotic experiences occurring since age 12. Seven items pertained to delusions and hallucinations, with items including “have other people ever read your thoughts?”, “have you ever thought you were being followed or spied on?”, and “have you ever heard voices that other people cannot hear?”. Six items pertained to unusual experiences which drew on item pools since formalized in prodromal psychosis instruments including the PRIME-screen and SIPS (Loewy et al., 2011). These included “I worry that my food may be poisoned” and “My thinking is unusual or frightening”. Interviewers coded each item 0, 1, 2 indicating respectively “not present”, “probably present” and “definitely present”. All 13 items were summed to create a psychotic experiences scale (range=0–18, M=1.19, SD=2.58). Scores were placed into an ordinal scale. All but three participants completed the psychotic experiences interview at age 18 (N=2,063). Just over 30% of participants had at least one psychotic experience between ages 12 and 18: 69.8% reported no psychotic experiences (coded 0; N=1,440), 15.5% reported 1 or 2 psychotic experiences (coded 1; n=319), and 14.7% reported 3 or more psychotic experiences (coded 2; n=304). This 30% prevalence is similar to the prevalence of self-reported psychotic experiences in other community samples of teenagers and young adults (Horwood et al., 2008; Kelleher et al., 2012a; Spauwen et al., 2004; Yoshizumi et al., 2004).
Childhood psychotic symptoms
Childhood psychotic symptoms were used as a control and to investigate the temporality of the association between psychotic phenomena and perceptions of neighborhood conditions. This interview has been described in detail previously (Polanczyk et al., 2010). Briefly, E-Risk families were visited by mental health trainees or professionals when children were aged 12. Each child was privately interviewed about seven psychotic symptoms pertaining to delusions and hallucinations (these same delusion/hallucination items were used at age 18 as described above). The item choice was guided by the Dunedin Study’s age-11 interview protocol (Poulton et al., 2000) and an instrument prepared for the Avon Longitudinal Study of Parents and Children (Schreier et al., 2009). Interviewers coded each experience 0, 1, 2, indicating respectively “not a symptom”, “probable symptom” and “definite symptom”. A conservative approach was taken in designating a child’s report as a symptom. First, the interviewer probed using standard prompts designed to discriminate between experiences that were plausible (e.g., “I was followed by a man after school”) and potential symptoms (e.g., “I was followed by an angel who guards my spirit”), and wrote down the child’s narrative description of the experience. Second, items and interviewer notes were assessed by a psychiatrist expert in schizophrenia, a psychologist expert in interviewing children, and a child and adolescent psychiatrist to verify the validity of the symptoms. Third, because children were twins, experiences limited to the twin relationship (e.g., “My twin and I often know what each other are thinking”) were coded as “not a symptom”. Children were only designated as experiencing psychotic symptoms if they reported at least one definite symptom. At age 12, 5.9% (N=125) of children reported at least one clinically-verified psychotic symptom.
Personal perceptions of neighborhood disorder
During the age 18 interviews, participants reported on social characteristics of their immediate neighborhoods, including neighborhood disorder (Sampson & Raudenbush, 1999). We were interested in perceptions of neighborhood disorder based on previous research linking residents’ independent assessments of neighborhood disorder with psychotic phenomena in both childhood and adolescence (Newbury et al., 2016; Newbury et al., 2017), and because adolescents’ personal perceptions of threat and danger could plausibly influence (or be influenced by) psychotic phenomena. Neighborhood disorder was assessed by asking participants about whether six problems affected their neighborhood, including: litter, broken glass, and rubbish in public places; run-down buildings, abandoned cars, wasteland or vacant shop fronts; people being drunk or unruly in public; people selling or using drugs; groups of young people hanging out and causing trouble; and homes getting broken into or burgled (each coded 0, 1, 2, indicating respectively “not true”, “sometimes true”, and “ often true”). Item responses were averaged for each participant (M=0.52, SD=0.49, range=0–2).
At age 12, participants also reported on neighborhood safety as part of a computer-based self-report stress questionnaire. Children indicated whether the statement “You feel unsafe in your neighborhood” was true or false. At age 12, 12.3% (N=260) of children reported that they felt their neighborhood was unsafe.
Urbanicity
Our measure of urbanicity was derived from the Office of National Statistics’ (ONS) Rural-Urban Definition for Small Area Geographies (RUC2011) classifications. The ONS RUC2011 rural-urban classification utilized 2011 census data, and was designed for application to small statistical units (e.g. Output Areas, Super-Output Areas, Wards). Detailed information on the creation of RUC2011 is available on the ONS webpages (https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/239477/RUC11methodologypaperaug_28_Aug.pdf.). Briefly, RUC2011 was created by laying a grid of hectare cells (100m2) over England and Wales. Postcode addresses were assigned to cells, providing an indication of residential density surrounding every individual residential property. Residential densities were then calculated for increasing radii around each cell, providing each residential property with a “density profile”. This measure was combined with Output Area and contextual data, allowing each settlement to be assigned to one of ten categories of increasing urbanicity (rural categories: sparse/non-sparse hamlets and isolated dwellings, sparse/non-sparse villages, sparse/non-sparse rural towns and fringes; urban categories: sparse/non-sparse cities and towns, and minor/major conurbations). Urbanicity scores for the E-Risk participants were then created by identifying the ONS RUC2011 classification for each participant’s postcode at age 18. Given the low numbers within some rural categories, urbanicity was collapsed into three levels (1=rural: all rural settings; 2=intermediate: urban cities and towns; and 3=urban: major and minor conurbations [conurbations are densely populated, large urban regions resulting from the expansion and coalescence of adjacent cities and towns]). E-Risk families are nationally-representative in terms of level of urbanicity. For example, 31.9% of E-Risk participants lived in the most highly urbanized settings at age 18 compared to 36.1% nationwide; 48.4% vs 45.0% lived in intermediate settings; and 19.7% vs 18.9% lived in rural settings (Office for National Statistics, 2013).
Official neighborhood crime rates
Associations between perceptions of neighborhood disorder and adolescent psychotic experiences were adjusted for official rates of crime in the neighborhood to isolate the associations arising from perceived versus objectively measured threat in the neighborhood. Street-level crime data, including information on the type of crime, date of occurrence, and approximate location, were accessed online as part of an open data sharing effort about crime and policing in England and Wales. An Application Program Interface (API) was used to extract street-level crime data for each of the geospatial coordinates marking the family’s home (For a full description see: https://data.police.uk/about/#location-anonymisation). Neighborhood crime rates were calculated by mapping a one mile radius around each E-Risk Study participant’s home and tallying the total number of crimes that occurred in the area each month (M=247, SD=274, range=1–1868). Scores were computed for 2011 (the year prior to age 18 assessments), the first year for which full street-level data was available. These scores were then collapsed into quartiles. This measure covers various forms of crime, including violent offenses (e.g., assaults), sexual offenses (e.g., rape), robberies, burglaries, theft, arson, and vandalism.
Resident-reported neighborhood disorder
Associations between participants’ perceptions of neighborhood disorder and adolescent psychotic experiences were also adjusted for independently-rated neighborhood conditions as reported by immediate neighbors of the E-Risk participants, to further isolate the effects of adolescent’s personal perceptions of neighborhood disorder. Neighborhood conditions were estimated via a postal survey sent to residents living alongside E-Risk families in 2008 (Odgers et al., 2012a; Odgers et al., 2009). Survey respondents, who were typically living on the same street or within the same apartment block as the participants in our study, reported on various characteristics of their immediate neighborhood, including levels of neighborhood disorder. Surveys were returned by an average of 5.18 (SD=2.73) respondents per neighborhood, and there were at least two responses for 95% of neighborhoods (N=5,601 respondents). For neighborhood disorder, residents were asked whether fourteen problems affected their neighborhood, including muggings, assaults, vandalism, graffiti and deliberate damage to property, etc., which were each coded 0–2 (the same or very similar items were included in the 6 items used at age 18 to measure E-Risk participants’ perceptions of neighborhood disorder). Items were averaged to create summary scores for each of the 5,601 resident respondents. Neighborhood disorder scores for each E-Risk family were then created by averaging the summary scores of respondents within that family’s neighborhood. The resulting variable approached normal distribution across the full potential range (M=0.49, SD=0.34, range=0–1.93).
Neighborhood-level SES
Associations between perceptions of neighborhood disorder and adolescent psychotic experiences were also adjusted for neighborhood-level SES to check that associations were not explained simply by poverty. Neighborhood-level SES was constructed using A Classification of Residential Neighborhoods (ACORN), a geodemographic discriminator developed by CACI Information Services (http://www.caci.co.uk/). Detailed information about ACORN’s classification of neighborhood-level SES has been provided previously (Caspi et al., 2000; Odgers et al., 2012b; Odgers et al., 2009). Briefly, CACI utilized over 400 variables from 2001 census data for Great Britain (e.g., educational qualifications, unemployment, housing tenure) and CACI’s consumer lifestyle database. Following hierarchical-cluster-analysis, CACI created five distinct and homogeneous ordinal groups ranging from “Wealthy Achiever” (coded 1) to “Hard Pressed” (coded 5) neighborhoods. Neighborhood-level SES scores for the E-Risk families were then created by identifying the ACORN classifications for the E-Risk families’ postcodes when children were aged 12. E-Risk families are representative of UK households across the spectrum of neighborhood-level SES: 25.6% of E-Risk families live in “wealthy achiever” neighborhoods compared to 25.3% of households nation-wide; 5.3% vs. 11.6% live in “urban prosperity” neighborhoods; 29.6% vs. 26.9% live in “comfortably off” neighborhoods; 13.4% vs. 13.9% live in “moderate means” neighborhoods; and 26.1% vs. 20.7% live in “hard-pressed” neighborhoods (CACI Information Services, 2006; Caspi et al., 2000). E-Risk underrepresents “urban prosperity” neighborhoods because such households are likely to be childless.
Family- and individual-level covariates
Analyses were also adjusted for a range of family- and individual-level characteristics to account for potential compositional effects and biases due to co-occurring substance and mood problems. Family SES was measured via a composite of parental income, education, and occupation when participants were aged 5. The latent variable was categorized into tertiles (i.e., low–, medium–, and high–SES) (Trzesniewski et al., 2006). Family psychiatric history and maternal psychotic symptoms were both assessed when participants were aged 12. In private interviews, the mother reported on her own mental health history and the mental health history of her biological mother, father, sisters, brothers, as well as the twins’ biological father (Milne et al., 2008; Weissman et al., 2000). This was converted to the proportion of family members with a history of any psychiatric disorder (coded 0–1.0; M=0.37, SD=0.27). For maternal psychotic symptoms, mothers were interviewed using the Diagnostic Interview Schedule (DIS) (Robins et al., 1995) for DSM-IV (American Psychiatric Association, 1994) which provides a symptom count for characteristic symptoms of schizophrenia (e.g. hallucinations, delusions, anhedonia): 16.6% of mothers had at least one symptom of schizophrenia. We interviewed participants when they were aged 18 for the presence of marijuana dependence, alcohol dependence, generalized anxiety disorder, and major depressive episode, according to DSM-IV criteria. Assessments were conducted in face-to-face interviews using the DIS (Robins et al., 1995). At age 18, 4.3% (N=89) of participants met criteria for marijuana dependence, 12.8% (N=263) met criteria for alcohol dependence, 7.4% (N=153) met criteria for anxiety, and 20.1% (N=414) met criteria for depression. Longitudinal analyses were adjusted for potential confounders measured at age 12 or earlier including resident-reports of neighborhood disorder, neighborhood-level SES, family-level confounders (SES, psychiatric history, maternal psychotic symptoms), and also for childhood anxiety and depression at age 12. Childhood anxiety was assessed via private interviews using the 10–item version of the Multidimensional Anxiety Scale for Children (MASC) (March et al., 1997). An extreme anxiety group was formed with children who scored at or above the 95th percentile (N=129, 6.1%). Childhood depression was also assessed at age 12 using the Children’s Depression Inventory (CDI) (Kovacs, 1992). Children who scored 20 or more (Rivera, Bernal & Rosello, 2005) were deemed to have clinically significant depressive symptoms (N=74, 3.5%).
The twin design
The classical twin design compares the phenotypic correlation between MZ twin pairs to that between DZ twin pairs, and allows the variation/covariation in observed traits to be partitioned into additive genetic (A), common environmental (C), and unique environmental (E) components. This is because MZ twins share ~100% of their segregating DNA, whereas DZ twins share on average 50% of their segregating DNA. In contrast, MZ and DZ reared-together twin pairs both share 100% of their common environmental influences. The twin design methodology depends on the equal environment assumption, which assumes that MZ twin pairs and DZ twin pairs do not differ in the extent that they share environmental factors (Plomin et al., 2013). In univariate analyses (variance in one trait), genetic influences on a trait are inferred if MZ correlations are greater than DZ correlations as this increased similarity between MZ twin pairs can only be accounted for by their increased genetic resemblance. Within-pair similarity that is not due to genetic factors is attributed to common environmental influences and would be implicated if the DZ correlation is greater than half that of the MZ correlation for a given trait. Unique environment accounts for individual-specific environmental factors that create differences among siblings from the same family. These are estimated from within-pair differences between MZ twins as E is the only influence that makes MZ twins different from one another. Measurement error is also included in E. Similarly, in bivariate analyses (covariance between two traits), higher cross-twin cross-trait correlations between MZ twin pairs versus DZ twin pairs suggests genetic sources of correlation between two traits (i.e. overlapping genetic influences on two traits). Maximum-likelihood estimation in OpenMx handles missing data and provides confidence intervals in addition to parameter estimates. Structural equation model fitting is used to estimate A, C, and E sources of phenotypic correlation and select the most parsimonious model (ACE, AE, CE, or E compared to the saturated model which describes the data perfectly) according to fit statistics, including minus two log likelihood and Akaike’s Information Criterion.
Statistical analysis
Analyses were conducted using STATA 14.2 and OpenMx. First, we investigated the construct validity of participants’ perceptions of neighborhood disorder by calculating the correlations of their personal perceptions with objectively/independently measured neighborhood conditions including official neighborhood crime rates, resident-reports of neighborhood disorder, and neighborhood-level SES. Second, we calculated the mean levels of perceived neighborhood disorder among adolescents in urban, intermediate, and rural settings, and used KHB pathway decomposition (Breen, Karlson & Holm, 2013) to test whether perceptions of neighborhood disorder mediated the effect of urbanicity on adolescent psychotic experiences. Third, we used ordinal logistic regression to test whether participants’ perceptions of neighborhood disorder were associated with adolescent psychotic experiences. Regression models were adjusted for official crime rates, resident-reports of neighborhood disorder, neighborhood-level SES, family-level factors (family SES, family psychiatric history, maternal psychotic symptoms), adolescent substance and mood problems (marijuana dependence, alcohol dependence, anxiety, depression), childhood psychotic symptoms, and for all potential confounders simultaneously. As an additional control step, we conducted co-twin control analyses to compare twin pairs in the same family and neighborhood who differed in their perceptions of neighborhood disorder. For this analysis we used all complete twin pairs and calculated the differences between twins (i.e., twin 1 perceived neighborhood disorder – twin 2 perceived neighborhood disorder; twin 1 psychotic experiences – twin 2 psychotic experiences). Using ordinal logistic regression we then regressed twin differences in perceptions of neighborhood disorder onto twin differences in adolescent psychotic experiences. Fourth, we used ordinal logistic regression to test whether participants who perceived their neighborhoods as unsafe at age 12 were more likely to subsequently report psychotic experiences at age 18 – after considering childhood psychotic symptoms at age 12 and perceptions of neighborhood disorder at age 18; and whether participants who reported psychotic symptoms at age 12 were subsequently more likely to perceive their neighborhoods as disordered at age 18 – after considering perceptions of neighborhood unsafety at age 12 and adolescent psychotic experiences at age 18. This step was conducted to investigate the temporality of the association between early psychotic phenomena and perceptions of neighborhood conditions. Steps 2 to 4 accounted for the non-independence of twin observations using the “CLUSTER” command in STATA. Fifth, cross-trait (the within-individual correlations between trait 1 and trait 2), cross-twin (the within-trait correlations between twin 1 and twin 2), and cross-twin cross-trait (the correlations between trait 1 in twin 1 and trait 2 in twin 2) phenotypic correlations for and between adolescent psychotic experiences and perceptions of neighborhood disorder were calculated in OpenMx (note: analyses were restricted to the 80.3% of participants who lived with their co-twin at age 18 to ensure that twin pairs were reporting on the same neighborhood). Univariate (cross-twin) and bivariate (cross-twin cross-trait) ACE models were fitted and compared to the saturated model to estimate the extent that variation/covariation in adolescent psychotic experiences and perceptions of neighborhood disorder was attributable to A, C, and E influences. For adolescent psychotic experiences, a liability-threshold ACE model was fitted because this variable was on an ordinal scale. Because adolescent psychotic experiences were on an ordinal scale whereas perceptions of neighborhood disorder were on a quasi-continuous scale, bivariate ACE models were conducted using a combined continuous-ordinal approach. As is common practice in behavioural genetics analysis, sex was regressed out of variables and model fitting was conducted using the standardized residuals.
Results
Are participants’ personal perceptions of neighborhood disorder consistent with objective/independent measures of neighborhood adversity?
Correlations between participants’ personal perceptions of neighborhood disorder and objectively/independently measured neighborhood conditions were computed to investigate the construct validity of self-reports of neighborhood disorder. Personal perceptions of neighborhood disorder were significantly positively correlated (all p’s<0.001) with official neighborhood crime rates (r=0.18), resident-reported neighborhood disorder (r=0.33), and neighborhood-level SES (r=0.35). Thus, participants’ perceptions of neighborhood disorder were consistent with more objective measures of neighborhood disorder and crime.
Do higher perceived levels of neighborhood disorder among adolescents in urban (versus rural) settings explain the association between urbanicity and adolescent psychotic experiences?
Table 1 shows the mean levels of perceived neighborhood disorder in urban, intermediate and rural settings. Consistent with previous research, participants living in urban and intermediate (versus rural) settings perceived significantly higher levels of neighborhood disorder (B=0.13, 95% CI=0.10–0.17, p<0.001). Also in keeping with previous analyses in this cohort using independent reports of neighborhood disorder (Newbury et al., 2016; Newbury et al., 2017), mediation analysis showed that participants’ personal perceptions of neighborhood disorder explained 42% of the effect of the most urban residency at age 18 on adolescent psychotic experiences (total effect of urbanicity on adolescent psychotic experiences: OR=1.81, 95% CI=1.29–2.53, p=0.001; direct effect of urbanicity: OR=1.41, 95% CI=1.00–1.98, p=0.049; indirect effect of urbanicity mediated via perceptions of neighborhood disorder: OR=1.28, 95%=1.16–1.42, p<0.001).
Table 1.
Perceptions of neighborhood disorder according to level of urbanicity.
| Level of urbanicity | Levels of perceived neighborhood disorder according to level of urbanicity
|
|
|---|---|---|
| Perceptions of neighborhood disorder
|
||
| M | SD | |
| Rural | 0.35 | 0.41 |
| Intermediate | 0.52 | 0.49 |
| Urban | 0.63 | 0.51 |
| Association between urbanicity and perceptions of neighborhood disorder | B = 0.13 (95% CI = 0.10 – 0.17, p < 0.001), B = 0.19 | |
Note: B = unstandardized beta coefficient; B = standardized beta coefficient; CI = confidence interval. The standardized (B) beta coefficient indicates the unit standard deviation change in perceptions of neighborhood disorder given one unit standard deviation change in urbanicity. Standardized betas provide exactly the same point estimates as correlation coefficients and may be interpreted as correlations, with a score of +1.0 indicating a 100% positive correlation. Beta (B) regression coefficients account for the non-independence of twin observations.
Is the association between perceptions of neighborhood disorder and adolescent psychotic experiences robust to neighborhood-, family-, and individual-level confounders?
Model 1 in Table 2 shows that psychotic experiences were significantly more common among adolescents who perceived higher levels of neighborhood disorder (i.e., physical and social signs of threat, such as vandalism, gang activity and burglaries) in their immediate neighborhood (OR=2.52, 95% CI=2.07–3.06, p<0.001). This association was slightly attenuated but remained highly significant (all p’s<0.001) after considering official neighborhood crime rates (Model 2), resident-reported neighborhood disorder (Model 3), neighborhood-level SES (Model 4), family-level characteristics including SES, psychiatric history, and maternal psychotic symptoms (Model 5), adolescent substance and mood problems including marijuana dependence, alcohol dependence, anxiety and depression (Model 6), childhood psychotic symptoms at age 12 (Model 7); as well as after considering all potential confounders simultaneously (Model 8: OR=1.62, 95% CI=1.27–2.05, p<0.001).
Table 2.
The unadjusted and adjusted association of perceptions of neighborhood disorder with adolescent psychotic experiences
| Model specification | Association between perceptions of neighborhood disorder and adolescent psychotic experiences | ||
|---|---|---|---|
|
| |||
| OR | 95% CI | P value | |
|
|
|||
| Model 1 – Unadjusted | 2.52 | 2.07 – 3.06 | <0.001 |
| Model 2 – Adjusted for official neighborhood crime rates | 2.39 | 1.96 – 2.91 | <0.001 |
| Model 3 – Adjusted for resident-reported neighborhood disorder | 2.43 | 1.98 – 2.98 | <0.001 |
| Model 4 – Adjusted for neighborhood-level SES | 2.31 | 1.87 – 2.86 | <0.001 |
| Model 5 – Adjusted for family-level characteristics | 2.20 | 1.79 – 2.70 | <0.001 |
| Model 6 – Adjusted for adolescent substance and mood problems | 1.94 | 1.57 – 2.39 | <0.001 |
| Model 7 – Adjusted for childhood psychotic symptoms | 2.43 | 2.00 – 2.96 | <0.001 |
| Model 8 – Adjusted for all covariates simultaneously | 1.62 | 1.27 – 2.05 | <0.001 |
| Official neighborhood crime rates | 1.13 | 1.01 – 1.26 | 0.035 |
| Resident-reported neighborhood disorder | 1.08 | 0.73 – 1.61 | 0.700 |
| Neighborhood-level SES | 1.02 | 0.92 – 1.12 | 0.715 |
| Family socioeconomic status | 1.17 | 0.99 – 1.39 | 0.072 |
| Family psychiatric history | 1.27 | 0.81 – 1.99 | 0.299 |
| Maternal psychotic symptoms | 1.06 | 0.92 – 1.21 | 0.448 |
| Adolescent marijuana dependence | 3.29 | 2.01 – 5.36 | <0.001 |
| Adolescent alcohol dependence | 1.58 | 1.16 – 2.15 | 0.004 |
| Adolescent anxiety | 2.56 | 1.74 – 3.76 | <0.001 |
| Adolescent depression | 3.05 | 2.33 – 3.99 | <0.001 |
| Childhood psychotic symptoms | 2.20 | 1.38 – 3.49 | 0.001 |
Note: CI = confidence interval; OR = odds ratio from ordinal logistic regression; SES, socioeconomic status. Model 1 – the unadjusted association between adolescents’ perceptions of neighborhood disorder and adolescent psychotic experiences. Model 2 – adjusted for official neighborhood crime rates. Model 3 – adjusted for resident-reported neighborhood disorder. Model 4 – adjusted for neighborhood-level SES. Model 5 – adjusted for family-level characteristics (family SES, family psychiatric history, and maternal psychotic symptoms). Model 6 – adjusted for adolescent substance and mood problems (marijuana dependence, alcohol dependence, anxiety, and depression). Model 7 – adjusted for childhood psychotic symptoms at age 12. Model 8 – adjusted simultaneously for all covariates. All analyses account for the non-independence of twin observations.
As an additional control step, we investigated whether participants who perceived higher levels of neighborhood disorder than their co-twin were also more likely to score higher for adolescent psychotic experiences. The co-twin control design controls both the predictor and outcome for within-family environmental influences and partially for genetic influences. By restricting analyses to the 80.3% of twin pairs who lived together at age 18, this analysis also holds the actual neighborhood conditions constant by design, thus providing a more stringent test of whether perceived levels of neighborhood disorder are independently associated with adolescent psychotic experiences. Among twin pairs living together, twins who perceived a higher level of neighborhood disorder than their co-twin were also significantly more likely to report more psychotic experiences than their co-twin (OR=1.34, 95% CI=1.05–1.82, p=0.036). This effect is smaller than that yielded for the entire sample from regression models of the association between perceived neighborhood disorder and adolescent psychotic experiences (adjusted OR=1.62, 95% CI=1.27–2.05, p<0.001). Nevertheless, the statistically significant associations in both the regression and co-twin control models demonstrates that perceptions of neighborhood disorder were independently associated with adolescent psychotic experiences, net of a range of measured and unmeasured genetic, individual-level and family-level potential confounders.
What is the temporality of the association between early psychotic phenomena and perceptions of neighborhood disorder?
Consistent with the association between perceptions of neighborhood disorder and adolescent psychotic experiences at age 18, children’s own perceptions that their neighborhoods were unsafe were significantly associated with childhood psychotic symptoms at age 12 (unadjusted OR=2.88, 95% CI=1.88–4.44, p<0.001). These earlier age-12 measures of psychotic symptoms and perceived neighborhood conditions were used to investigate the temporality of the association between early psychotic phenomena and perceptions of neighborhood disorder.
Model 1 in Table 3 shows that participants who had perceived their neighborhoods as unsafe at age 12 were significantly more likely to report adolescent psychotic experiences at age 18, even after taking into account earlier childhood psychotic symptoms at age 12 (OR=2.02, 95% CI=1.51–2.71, p<0.001). The association between children’s perceptions of neighborhood unsafety and adolescent psychotic experiences remained significant after additionally considering perceptions of neighborhood disorder at age 18 (Model 2), as well as after additionally considering other potential confounders listed under Table 3 (Model 3). Model 1 in Table 3 also shows that participants who reported childhood psychotic symptoms at age 12 were significantly more likely to perceive their neighborhood as disordered at age 18, even after considering earlier perceptions of neighborhood unsafety at age 12 (OR=1.59, 95% CI=1.16–2.18, p=0.004). However, the association between childhood psychotic symptoms at age 12 and perceptions of neighborhood disorder at age 18 was attenuated to below conventional levels of significance after additionally considering adolescent psychotic experiences at age 18 (Model 2) and other potential confounders (Model 3).
Table 3.
The longitudinal associations of perceptions of neighborhood safety and psychotic symptoms at age 12 with subsequent psychotic experiences and perceptions of neighborhood disorder at age 18.
| Age 12 measures | Longitudinal associations of childhood perceptions of neighborhood safety/psychotic symptoms at age 12 with adolescent psychotic experiences/perceptions of neighborhood disorder at age 18 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Model 1 | Model 2 | Model 3 | |||||||
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
|
|
|||||||||
| Adolescent psychotic experiences at age 18a | |||||||||
| Perceptions of neighborhood as unsafe at age 12 | 2.02 | 1.51 – 2.71 | <0.001 | 1.72 | 1.27 – 2.32 | <0.001 | 1.45 | 1.06 – 1.99 | 0.021 |
| Perceptions of neighborhood disorder at age 18b | |||||||||
| Childhood psychotic symptoms at age 12 | 1.59 | 1.16 – 2.18 | 0.004 | 1.31 | 0.93 – 1.84 | 0.125 | 1.19 | 0.83 – 1.70 | 0.338 |
Note: CI = confidence interval; OR = odds ratio from ordinal logistic regression.
The association of childhood perceptions of neighborhood unsafety at age 12 with adolescent psychotic experiences at age 18.
The association of childhood psychotic symptoms at age 12 with perceptions of neighborhood disorder at age 18. Model 1 = the association of childhood perceptions of neighborhood unsafety with adolescent psychotic experiences was adjusted for childhood psychotic symptoms. The association of childhood psychotic symptoms with perceptions of neighborhood disorder was adjusted for childhood perceptions of neighborhood unsafety. Model 2 = the association between perceptions of neighborhood unsafety and adolescent psychotic experiences was additionally adjusted for perceptions of neighborhood disorder at age 18. The association between childhood psychotic symptoms and perceptions of neighborhood disorder was additionally adjusted for adolescent psychotic experiences. Model 3 = both regression models were adjusted additionally for resident-reports of neighborhood disorder, neighborhood-level socioeconomic status (SES), family SES, family psychiatric history, maternal psychotic symptoms, and childhood anxiety and depression. All analyses account for the non-independence of twin observations.
To what extent do genetic versus environmental factors contribute to perceptions of neighborhood disorder and adolescent psychotic experiences?
Using the classical twin design and maximum-likelihood estimation in OpenMx, we further examined the genetic and environmental contributions to adolescent psychotic experiences and participants’ perceptions of neighborhood disorder at age 18 (note: analyses were again restricted to the 80.3% of participants who lived with their co-twin at age 18, to ensure that twins were reporting on the same neighborhoods and therefore only perceptions of neighborhoods varied between twin pairs). Table 4 shows the cross-trait, cross-twin, and cross-twin cross-trait phenotypic correlations of adolescent psychotic experiences and perceptions of neighborhood disorder, stratified by zygosity. Consistent with the logistic regression results for the entire sample in Table 2, Table 4 shows that there was a significant cross-trait correlation between adolescent psychotic experiences and perceptions of neighborhood disorder for the 80.3% of participants who lived with their co-twin (r=0.27, CI=0.21–0.33).
Table 4.
Cross-trait, cross-twin, and cross-twin cross-trait phenotypic correlations of and between adolescent psychotic experiences and perceptions neighborhood disorder
| Type of phenotypic correlation | Phenotypic correlations of and between adolescent psychotic experiences and neighborhood disorder | |||
|---|---|---|---|---|
| MZ and DZ twins togethera | ||||
| Cross-trait phenotypic correlationsb | Correlation | CI | ||
| Adolescent psychotic experiences - Perceptions of neighborhood disorder | 0.27 | 0.21 - 0.33 | ||
| MZ | DZ | |||
| Cross-twin phenotypic correlationsc | Correlation | CI | Correlation | CI |
| Adolescent psychotic experiences | 0.46 | 0.33 - 0.58 | 0.36 | 0.21 - 0.50 |
| Perceptions of neighborhood disorder | 0.48 | 0.41 - 0.55 | 0.39 | 0.30 - 0.48 |
| MZ | DZ | |||
| Cross-twin cross-trait phenotypic correlationsd | Correlation | CI | Correlation | CI |
| Adolescent psychotic experiences - Perceptions of neighborhood disorder | 0.22 | 0.14 - 0.29 | 0.22 | 0.14 - 0.30 |
Note: CI = confidence interval; DZ = dizygotic (fraternal) twins; MZ = monozygotic (identical) twins.
All phenotypic correlation analyses in Table 4 were conducted on the subsample of twins who lived together with their co-twin at age 18 (N=1755; 85%)
The phenotypic correlation in the entire analysis sample between adolescent psychotic experiences and adolescents’ perceptions of neighborhood disorder in the immediate neighborhood.
The phenotypic correlation between twins for adolescent psychotic experiences and perceptions of neighborhood disorder, among MZ versus DZ twins. Cross-twin phenotypic correlations were also calculated for MZ and DZ males (MZm; DZm, respectively) and females (MZf; DZf, respectively) separately to check for potential sex differences (these cross-twin phenotypic correlations were calculated in STATA 14.2 without confidence intervals because of low numbers of female twin pairs concordant for 3 or more psychotic experiences when stratified by sex). Phenotypic correlations (all p’s<0.05) did not differ substantially by sex. For neighborhood disorder: MZm=0.47; DZm=0.43; MZf=0.48; DZf=0.35, for adolescent psychotic experiences: MZm=0.41; DZm=0.27; MZf=0.52; DZf=0.46. d The correlation of trait 1 in twin 1 with trait 2 in twin 2, among MZ versus DZ twins.
Cross-twin phenotypic correlations for adolescent psychotic experiences suggested some genetic contributions because MZ twin correlations (r=0.46) were slightly larger than DZ twin correlations (r=0.36); common environmental contributions (C) were also indicated because DZ correlations were greater than half that of MZ correlations; and unique environmental contributions were also indicated because MZ correlations were less than unity (Table 4). For perceptions of neighborhood disorder, cross-twin phenotypic correlations again suggested genetic contributions because MZ correlations (r=0.48) were slightly greater than DZ correlations (r=0.39); common environmental contributions (C) were indicated because DZ correlations were greater than half that of MZ correlations; and unique environmental contributions were indicated because MZ correlations were less than unity (note: cross-twin phenotypic correlations did not vary substantially between males and females [see Table 4 notes]), therefore subsequent analyses were conducted on both sexes together).
ACE estimates from univariate model fitting were consistent with the cross-twin correlations. For adolescent psychotic experiences, observed variance was mostly explained by unique environmental (55%) and common environmental (28%) factors, with genetic factors explaining a small proportion of the observed variance (17%). For perceptions of neighborhood disorder, observed variance was explained by unique environmental (50%), common environmental (24%), as well as genetic (26%) factors. Table 5 displays the fit statistics for the ACE model and nested models (AE, CE, and E). Given that the full ACE model was the best fitting model for perceptions of neighborhood disorder, we present the results from the full ACE bivariate model.
Table 5.
Fit statistics of sub-models (ACE, AE, CE, E) compared to the saturated univariate model for adolescent psychotic experiences and perceptions of neighborhood disorder
| Trait | Model | ep | minus2LL | df | AIC | diffLL | diffdf | P |
|---|---|---|---|---|---|---|---|---|
| Adolescent psychotic experiences | Sat | 10 | 2514.245 | 1630 | −745.756 | NA | NA | NA |
| ACE | 5 | 2520.850 | 1636 | −751.150 | 6.610 | 6 | 0.359 | |
| AE | 4 | 2523.643 | 1637 | −750.357 | 2.793 | 1 | 0.095 | |
| CE* | 4 | 2521.600 | 1637 | −752.400 | 0.750 | 1 | 0.386 | |
| E | 3 | 2583.039 | 1638 | −692.961 | 62.189 | 2 | 3.133-14 | |
| Perceptions of neighborhood disorder | Sat | 10 | 2048.567 | 1616 | −1183.433 | NA | NA | NA |
| ACE* | 4 | 2058.314 | 1622 | −1185.686 | 9.747 | 6 | 0.135 | |
| AE | 3 | 2064.804 | 1623 | −1181.196 | 6.490 | 1 | 0.011 | |
| CE | 3 | 2064.418 | 1623 | −1181.582 | 6.104 | 1 | 0.013 | |
| E | 2 | 2236.698 | 1624 | −1011.302 | 178.384 | 2 | 1.848 e-39 |
Note: Models include Sat = saturated model; ACE = full model testing genetic, common, and unique environmental influences compared to the saturated model; AE = model testing genetic and unique environmental influences compared to the ACE model; CE = model testing common and unique environmental influences compared to the ACE model; E = model testing unique environmental influences compared to the ACE model. ep = estimated parameters; minus2LL = minus two log likelihood; df = degrees of freedom; diff = difference; AIC = Akaike’s Information Criterion (lower values indicate a better fitting model); NA = not applicable;
Best fitting model.
To what extent do overlapping genetic versus environmental factors contribute to the covariance between adolescent psychotic experiences and perceptions of neighborhood disorder?
The cross-twin cross-trait correlations in Table 4 give an indication of the genetic, common environmental, and unique environmental sources of phenotypic correlation between adolescent psychotic experiences and perceptions of neighborhood disorder. Modest positive cross-twin cross-trait correlations between adolescent psychotic experiences and perceptions of neighborhood disorder were apparent. Correlations did not differ by zygosity, giving an initial indication that overlapping genes did not account for the phenotypic correlations.
This was supported by results from the cross-twin cross-trait bivariate model, which is presented in a pathway diagram in Figure 1 (note: ACE estimates for perceptions of neighborhood disorder from the bivariate model [i.e., A = .25; C = .25] differ slightly from those described above from the univariate model [i.e., A = .26; C = .24] because the bivariate model contains more information. However, confidence intervals for these estimates overlap). The phenotypic correlation between adolescent psychotic experiences and perceptions of neighborhood disorder was mostly explained by a large significant correlation between common environmental influences (rC=0.88), whereas A and E influences were not significantly correlated between traits. That is, a large proportion of the environmental influences that made twin siblings more similar in terms of their perceptions of neighborhood disorder also made twin siblings more similar in terms of their psychotic experiences.
Figure 1. ACE estimates and ACE correlations from cross-twin cross-trait (bivariate) model.
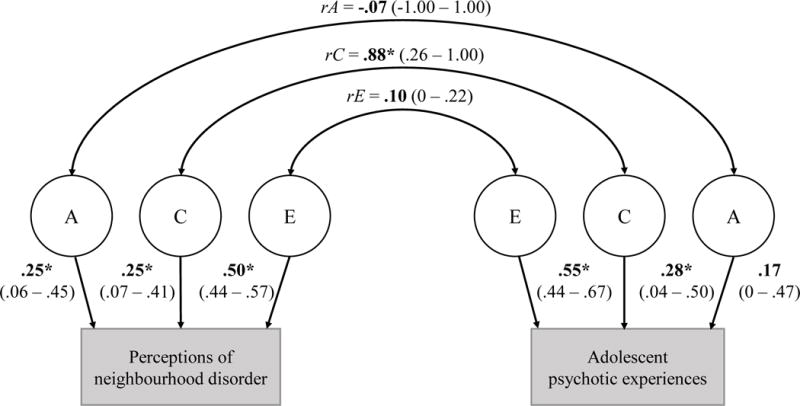
Note: A = additive genetic influences; C = common environmental influences; E = unique environmental influences; rA rC rE = genetic, common environmental, and unique environmental sources of correlation between phenotypes. The common (C) environmental contributions to variance in perceptions of neighbourhood disorder (C = .25, CI = .07 – .41) were significantly correlated with the common environmental contributions to variance in adolescent psychotic experiences (C = .28, CI = .04 – .50), yielding a large significant common environmental correlation between perceptions of neighbourhood disorder and adolescent psychotic experiences of .88 (CI = .26 – 1.00).
Discussion
This study used a multilevel, longitudinal, and genetically-sensitive design to investigate the association between individuals’ own perceptions of threatening neighborhood conditions and psychotic experiences during adolescence. Analyses revealed three main findings. First, adolescents’ personal perceptions of neighborhood disorder statistically explained 42% of the effect of the most urban residency on adolescent psychotic experiences. Second, adolescents who perceived higher levels of disorder in their immediate neighborhoods at age 18 – such as vandalism, gang activity, and burglaries – were over 60% more likely to report psychotic experiences compared to individuals who perceived their neighborhoods to be safer and less threatening, even after considering a wide range of potential neighborhood-, family-, and individual-level confounders. Third, the phenotypic correlation between adolescent psychotic experiences and perceptions of neighborhood disorder at age 18 was mostly explained by overlapping common environmental factors.
The present study’s mediation findings are consistent with previous analyses in this cohort showing that threatening and adverse neighborhood conditions (as independently-rated by mothers and residents) statistically explain up to half of the effect of urbanicity during upbringing on psychotic phenomena in childhood and adolescence (Newbury et al., 2016; Newbury et al., 2017). Our findings are also in keeping with those from recent studies documenting higher rates of psychotic phenomena, psychosis-proneness, and psychotic disorder among children, adolescents and young adults living in regions with higher fragmentation, disorder and crime as rated by independent or objective sources (Bhavsar et al., 2014; Kirkbride et al., 2015; Newbury et al., 2016; Newbury et al., 2017; Wilson et al., 2016). Here we identify a potential role for personal perceptions of threatening neighborhood conditions in early psychotic phenomena. That is, the association between adverse neighborhood conditions and early expressions of psychosis is detectable at the level of the eye of the beholder. This is consistent with psychological theories and empirical studies of psychosis aetiology which emphasize the key role played by negative beliefs about the world and other people, hostile attributions of the intentions of others, and threat anticipation (An et al., 2010; Appiah-Kusi et al., 2017; Fowler et al., 2006; Freeman, 2016; Garety et al., 2007; Noone et al., 2015) in the development of psychotic experiences, such as paranoia; together with a broader literature suggesting that subjective perceptions of early-life adversity are associated with mental health problems over and above more objective reports of adversity exposure (Brown et al., 2005; Reuben et al., 2016; Widom & Morris, 1997; Widom et al., 1999).
Our adjustment for a range of potential confounders indicated that the association between personal perceptions of neighborhood disorder and adolescent psychotic experiences was i) above and beyond the effect of objectively/independently measured levels of threat in the neighborhood (associations were not explained by official neighborhood crime rates or resident-reports of neighborhood disorder), ii) not due to poverty (associations were not explained by neighborhood-level SES), iii) not explained by the composition of families living in disordered neighborhoods (associations were not explained by family SES or family history of psychiatric problems), iv) not attributable solely to substance intoxication or mood-congruent recall bias (associations were not explained by adolescent marijuana dependence, alcohol dependence, anxiety or depression), and v) was not explained by earlier childhood psychotic symptoms which might simultaneously influence participants’ subsequent perceptions of neighborhood disorder and their risk for adolescent psychotic experiences. Therefore, this association was impressively robust to a wide range of factors that typically confound such relationships. Co-twin control analyses demonstrated that the association between perceived neighborhood disorder and adolescent psychotic experiences was attenuated but remained significant after holding the family environment and neighborhood conditions (and partially genetic influences) constant by design. This approach provides strong evidence that personal perceptions of neighborhood disorder were associated with adolescent psychotic experiences above and beyond variation in the actual neighborhood conditions.
In addition, there was tentative evidence of a bidirectional relationship between perceptions of threatening neighborhood conditions and early psychotic phenomena. Individuals who had perceived their neighborhood as unsafe during childhood were subsequently more likely to have psychotic experiences during adolescence: this was not due to earlier psychotic symptoms in childhood, contemporaneous perceptions of neighborhood disorder at age 18, or a range of other potential neighborhood-, family-, and individual-level confounders. Individuals who reported psychotic symptoms at age 12 were also more likely to subsequently perceive their neighborhoods as more disordered at age 18, though this appeared to be explained by adolescent psychotic experiences at age 18 and other confounders. We could speculate that personal perceptions of threat in the neighborhood tend to precede the onset of early psychotic phenomena, rather than vice versa. However, given that psychotic experiences involve altered perceptions of reality such as threat detection biases and persecutory delusions (Freeman et al., 2002; Garety et al., 2001), it is likely that the true relationship between adolescent psychotic experiences and perceptions of neighborhood conditions is bidirectional. Psychotic experiences might intensify perceptions of neighborhood disorder, and perceptions of neighborhood disorder might exacerbate psychotic experiences.
We hypothesized that the overlap between adolescent psychotic experiences and perceptions of neighborhood disorder could be due to shared genetic factors. That is, some of the same genetic contributions to psychotic experiences could also contribute to perceptions of threatening neighborhood conditions. This hypothesis was not supported. Genetic contributions to adolescent psychotic experiences did not appear to contribute to perceptions of neighborhood disorder in this sample. Instead, common environmental factors were implicated. These environmental factors contributed to increased similarity between twin siblings both in terms of their perceptions of neighborhood disorder and their psychotic experiences. This contrasts with emerging research showing that putatively environmental risk factors for psychotic experiences, such as stressful life events (Shakoor et al., 2016) and neighborhood-level deprivation (Sariaslan et al., 2016) are associated with psychotic experiences due partly to overlapping genetic influences. One obvious environmental exposure shared between twin pairs - which could influence both adolescent psychotic experiences and perceptions of neighborhood disorder – is actual levels of neighborhood disorder. That is, threatening conditions such as vandalism, gang activity, and burglaries in the neighborhood could simultaneously influence adolescents’ perceptions of neighborhood disorder and their experience of psychotic phenomena. However, a number of alternative candidates for the overlapping common environmental influences are possible. For example, parental attitudes or family environments characterized by suspicion and fearfulness could simultaneously promote psychotic experiences and perceptions of high neighborhood disorder among offspring – though in this sample the phenotypic and longitudinal associations were not explained by family psychiatric history or maternal psychotic symptoms. Additionally, findings from the co-twin control analysis (which yielded a smaller though significant association compared to the full sample) highlight that family-wide and neighborhood-level influences did not completely explain the effect of perceived neighborhood disorder on adolescent psychotic experiences. Taken together, these findings suggests that both actual (i.e., family-level) and perceived (i.e., individual-level) neighborhood conditions contributed to risk for adolescent psychotic experiences.
Considering all the findings together – that perceptions of threatening neighborhood conditions explained part of the effect of urbanicity on adolescent psychotic experiences; were not confounded by numerous potential neighborhood-, family-, and individual-level factors; and overlapped with psychotic experiences due to environmental (rather than genetic) influences – the present study provides initial evidence implicating perceptions of disordered neighborhood conditions in the aetiology of adolescent psychotic experiences. These findings are consistent with leading aetiological models of psychosis. Growing evidence implicates psychosocial stress in the emergence of psychotic phenomena, whereby chronic, acute, and daily-life stressors (e.g., urban living, crime victimization, noisy neighbors) might promote and exacerbate psychotic phenomena. Biological and psychological mechanisms have been suggested. Chronic and acute stressors during upbringing are thought to disrupt the biological stress response (Tarullo & Gunnar, 2006; Walker, Mittal & Tessner, 2008), and in turn disrupt dopaminergic activity (van Winkel, Stefanis & Myin-Germeys, 2008). The dopaminergic system plays a key role in the brain’s attribution of salience to stimuli, and excess dopamine activity is currently the strongest biological explanation for the positive symptoms of psychosis (Howes et al., 2017; Kapur, 2003; van Winkel et al., 2008). From an adolescent’s perspective, residing in and navigating a threatening neighborhood environment could also promote or reinforce maladaptive cognitive styles such as paranoia and threat detection biases. This proposed mechanism is consistent with studies showing that the severity of persecutory delusions, anxiety, paranoia, and hallucinations among adults with schizophrenia is immediately exacerbated after brief exposure to crowded urban environments (Ellett et al., 2008; Freeman et al., 2014). The potential bidirectional relationship between perceptions of adverse neighborhood conditions and adolescent psychotic experiences is also consistent with the phenotypic overlap documented between psychosis and stress-sensitivity and -reactivity (Collip et al., 2011; Myin-Germeys, Delespaul & Van Os, 2005; Myin-Germeys et al., 2001). It is reasonable to assume that adolescents who are experiencing psychotic phenomena might be more sensitive to stressful or threatening exposures in the neighborhood.
Strengths and limitations
Combining multilevel, longitudinal and genetically-sensitive methods, this study was able to examine the association between perceptions of neighborhood adversity and adolescent psychotic experiences whilst considering a range of potential confounders including genetic influences. Nonetheless, we acknowledge several limitations. First, our self-report measure of adolescent psychotic experiences reflected the methodology widely used in the psychosis-prodrome research field. It is possible, however, that this self-report measure captured genuine experiences (e.g., being followed by a stranger) as well as psychotic phenomena (e.g., being followed by a spy). This may have led to the fairly low additive genetic estimate for adolescent psychotic experiences in this sample (17%), which is lower than that typically reported from twin analyses of more strictly defined early psychotic phenomena (Polanczyk et al., 2010; Ronald, 2015; Zavos et al., 2014). Second, the absence of overlapping genetic influences between psychotic experiences and perceptions of neighborhood disorder could also be due to the young age of the E-Risk participants. At age 18, the study individuals would have had minimal choice in the type of neighborhood they lived in compared to later in adulthood. It will be important to investigate the genetic and environmental contributions to the association between perceived neighborhood conditions and psychotic experiences later in adulthood, when individuals become more active in choosing their neighborhood environments. Furthermore, studies of adult twins living apart could investigate the genetic and environmental contributions to actual (i.e., objectively measured) neighborhood conditions as well. Third, we must interpret the longitudinal associations between perceptions of neighborhood conditions and psychotic phenomena with caution, because the age–12 measures were on binary scales measuring only neighborhood safety and the presence of at least one psychotic symptom so did not capture as much variance as the age–18 measures. Thus, we tentatively suggest that the association between perceived neighborhood adversity and psychotic phenomena is likely to be bidirectional.
Looking forward, multidisciplinary research examining the interplay between neighborhood conditions, genetic and environmental risk, and neurological and cognitive biomarkers during development is needed to establish the nature of the association between perceived neighborhood conditions and adolescent psychotic experiences. There is evidence, for example, that adults with urban versus rural upbringing differ in their neurocognitive reactivity to social stress (Haddad et al., 2015; Lederbogen et al., 2011), though little is known about the potential effects of adverse neighborhood conditions on the adolescent brain. Furthermore, future research is needed to establish whether the association between perceptions of threat and psychotic experiences is specific to neighborhood conditions, or whether this association extends to other domains such as school and work environments and social interactions.
Conclusions
Notwithstanding its limitations, the present study has clinical and public health implications. Our findings add to growing evidence that threatening and adverse neighborhood conditions during upbringing increase risk for early psychotic phenomena. This highlights potential opportunities for preventative interventions. On the one hand, our findings suggest that early interventions for psychosis (and mental health problems more generally) could reach particularly high risk groups if targeted towards adolescents living in threatening and adverse neighborhood conditions. Given the potential bidirectional relationship between psychotic experiences and perceptions of threatening neighborhood conditions, psychological therapies could incorporate strategies to help young people understand whether their perceptions of threat in the neighborhood are rational, or whether these perceptions are contributing unnecessarily to a cycle of stress, fear and psychotic experiences. On the other hand, recent findings from this team (Newbury et al., 2016; Newbury et al., 2017; Odgers et al., 2015) and others (Bhavsar et al., 2014; Goldman-Mellor et al., 2016; Kirkbride et al., 2015; Polling et al., 2014; Wilson et al., 2016) suggest a need to address whether wider physical and social environmental conditions can be improved for the benefit of young people’s mental health. Within two or three decades, 70% of the world’s population will live in cities (Dye, 2008). This figure already exceeds 80% in many developed nations including Great Britain. It is therefore likely that, as communities become more crowded and societies become more unequal (UNICEF, 2012), the neighborhoods in which young people are born and raised will become more adverse and more fragmented. We suggest that public health and urban planning initiatives aimed at increasing the safety and supportiveness (both actual and perceived) of urban communities could benefit the mental health of young people and improve mental health trajectories for a large section of society over the life course.
Acknowledgments
We are grateful to the study mothers and fathers, the twins, and the twins’ teachers for their participation. Our thanks to members of the E-Risk team for their dedication, hard work and insights, and to CACI Inc. for use of their consumer lifestyle databases. We also thank Emma Hedman for geo-coding assistance. The E-Risk Study is funded by the Medical Research Council (G1002190). Additional support was provided by National Institute of Child Health and Human Development (HD077482); British Academy (SQ140024); and the Jacobs Foundation. J.B.N. and J.R.B. received Multidisciplinary Studentships from the Economic and Social Research Council (ESRC). L.A. is the Mental Health Leadership Fellow for the UK ESRC. H.L.F. is supported by an MQ Fellows Award (MQ14F40). C.L.O. is a Jacobs Foundation and Canadian Institute for Advanced Research Fellow.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- An SK, Kang JI, Park JY, Kim KR, Lee SY, Lee E. Attribution bias in ultra-high risk for psychosis and first-episode schizophrenia. Schizophrenia Research. 2010;118(1):54–61. doi: 10.1016/j.schres.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Appiah-Kusi E, Fisher HL, Petros N, Wilson R, Mondelli V, Garety P, Mcguire P, Bhattacharyya S. Do cognitive schema mediate the association between childhood trauma and being at ultra-high risk for psychosis? Journal of Psychiatric Research. 2017;88:89–96. doi: 10.1016/j.jpsychires.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Bhavsar V, Boydell J, Murray R, Power P. Identifying aspects of neighbourhood deprivation associated with increased incidence of schizophrenia. Schizophrenia Research. 2014;156(1):115–121. doi: 10.1016/j.schres.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Breen R, Karlson KB, Holm A. Total, direct, and indirect effects in logit and probit models. Sociological Methods & Research. 2013;42(2):164–191. [Google Scholar]
- Brown J, Berenson K, Cohen P. Documented and self-reported child abuse and adult pain in a community sample. Clinical Journal of Pain. 2005;21(5):374–377. doi: 10.1097/01.ajp.0000149797.16370.dc. [DOI] [PubMed] [Google Scholar]
- CACI Information Services. ACORN user guide. London: CACI; 2006. [Google Scholar]
- Caspi A, Taylor A, Moffitt TE, Plomin R. Neighborhood deprivation affects children’s mental health: Environmental risks identified in a genetic design. Psychological Science. 2000;11(4):338–342. doi: 10.1111/1467-9280.00267. [DOI] [PubMed] [Google Scholar]
- Collip D, Nicolson N, Lardinois M, Lataster T, Van Os J, Myin-Germeys I. Daily cortisol, stress reactivity and psychotic experiences in individuals at above average genetic risk for psychosis. Psychological Medicine. 2011;41(11):2305–2315. doi: 10.1017/S0033291711000602. [DOI] [PubMed] [Google Scholar]
- Dhossche D, Ferdinand R, van der Ende J, Hofstra M, Verhulst F. Diagnostic outcome of self-reported hallucinations in a community sample of adolescents. Psychological Medicine. 2002;32(04):619–627. doi: 10.1017/s003329170200555x. [DOI] [PubMed] [Google Scholar]
- Dye C. Health and urban living. Science. 2008;319(5864):766–769. doi: 10.1126/science.1150198. [DOI] [PubMed] [Google Scholar]
- Ellett L, Freeman D, Garety PA. The psychological effect of an urban environment on individuals with persecutory delusions: the Camberwell walk study. Schizophrenia Research. 2008;99(1):77–84. doi: 10.1016/j.schres.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Fisher HL, Caspi A, Poulton R, Meier MH, Houts R, Harrington H, Arseneault L, Moffitt TE. Specificity of childhood psychotic symptoms for predicting schizophrenia by 38 years of age: a birth cohort study. Psychological Medicine. 2013;43(10):2077–2086. doi: 10.1017/S0033291712003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler D, Freeman D, Smith B, Kuipers E, Bebbington P, Bashforth H, Coker S, Hodgekins J, Gracie A, Dunn G. The Brief Core Schema Scales (BCSS): psychometric properties and associations with paranoia and grandiosity in non-clinical and psychosis samples. Psychological Medicine. 2006;36(06):749–759. doi: 10.1017/S0033291706007355. [DOI] [PubMed] [Google Scholar]
- Freeman D. Persecutory delusions: a cognitive perspective on understanding and treatment. The Lancet Psychiatry. 2016;3(7):685–692. doi: 10.1016/S2215-0366(16)00066-3. [DOI] [PubMed] [Google Scholar]
- Freeman D, Emsley R, Dunn G, Fowler D, Bebbington P, Kuipers E, Jolley S, Waller H, Hardy A, Garety P. The stress of the street for patients with persecutory delusions: a test of the symptomatic and psychological effects of going outside into a busy urban area. Schizophrenia Bulletin. 2014;41(4):971–979. doi: 10.1093/schbul/sbu173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D, Garety PA, Kuipers E, Fowler D, Bebbington PE. A cognitive model of persecutory delusions. British Journal of Clinical Psychology. 2002;41(4):331–347. doi: 10.1348/014466502760387461. [DOI] [PubMed] [Google Scholar]
- Freeman D, Waller H, Harpur-Lewis RA, Moore R, Garety P, Bebbington P, Kuipers E, Emsley R, Dunn G, Fowler D. Urbanicity, persecutory delusions, and clinical intervention: the development of a brief CBT module for helping patients with persecutory delusions enter social urban environments. Behavioural and Cognitive Psychotherapy. 2015;43(01):42–51. doi: 10.1017/S1352465813000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garety P, Kuipers E, Fowler D, Freeman D, Bebbington P. A cognitive model of the positive symptoms of psychosis. Psychological Medicine. 2001;31(2):189–196. doi: 10.1017/s0033291701003312. [DOI] [PubMed] [Google Scholar]
- Garety PA, Bebbington P, Fowler D, Freeman D, Kuipers E. Implications for neurobiological research of cognitive models of psychosis: a theoretical paper. Psychological Medicine. 2007;37(10):1377. doi: 10.1017/S003329170700013X. [DOI] [PubMed] [Google Scholar]
- Goldman-Mellor S, Margerison-Zilko C, Allen K, Cerdá M. Perceived and objectively-measured neighborhood violence and adolescent psychological distress. Journal of Urban Health. 2016;93(5):758–769. doi: 10.1007/s11524-016-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad L, Schäfer A, Streit F, Lederbogen F, Grimm O, Wüst S, Deuschle M, Kirsch P, Tost H, Meyer-Lindenberg A. Brain structure correlates of urban upbringing, an environmental risk factor for schizophrenia. Schizophrenia Bulletin. 2015;41(1):115–122. doi: 10.1093/schbul/sbu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häfner H, Maurer K, Löffler W, Fätkenheuer B. The epidemiology of early schizophrenia. Influence of age and gender on onset and early course. British Journal of Psychiatry. 1994;164(23):29–38. [PubMed] [Google Scholar]
- Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. Journal of Child Psychology and Psychiatry. 2004;45(2):260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Deserno L, Reininghaus U. Urbanicity, social adversity and psychosis. World Psychiatry. 2013;12(3):187–197. doi: 10.1002/wps.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood J, Salvi G, Thomas K, Duffy L, Gunnell D, Hollis C, Lewis G, Menezes P, Thompson A, Wolke D. IQ and non-clinical psychotic symptoms in 12-year-olds: results from the ALSPAC birth cohort. British Journal of Psychiatry. 2008;193(3):185–191. doi: 10.1192/bjp.bp.108.051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, McCutcheon R, Owen MJ, Murray RM. The role of genes, stress, and dopamine in the development of schizophrenia. Biological Psychiatry. 2017;81(1):9–20. doi: 10.1016/j.biopsych.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. American Journal of Psychiatry. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Cannon M. Psychotic-like experiences in the general population: characterizing a high-risk group for psychosis. Psychological Medicine. 2011;41(1):1–6. doi: 10.1017/S0033291710001005. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Connor D, Clarke MC, Devlin N, Harley M, Cannon M. Prevalence of psychotic symptoms in childhood and adolescence: a systematic review and meta-analysis of population-based studies. Psychological Medicine. 2012a;42(9):1857–1863. doi: 10.1017/S0033291711002960. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Lynch F, Harley M, Molloy C, Roddy S, Fitzpatrick C, Cannon M. Psychotic symptoms in adolescence index risk for suicidal behavior: findings from 2 population-based case-control clinical interview studies. Archives of General Psychiatry. 2012b;69(12):1277–1283. doi: 10.1001/archgenpsychiatry.2012.164. [DOI] [PubMed] [Google Scholar]
- Kirkbride J, Stochl J, Zimbron J, Crane C, Metastasio A, Aguilar E, Webster R, Theegala S, Kabacs N, Jones P. Social and spatial heterogeneity in psychosis proneness in a multilevel case–prodrome–control study. Acta Psychiatrica Scandinavica. 2015;132(4):283–292. doi: 10.1111/acps.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Children’s Depression Inventory: Manual. Multi-Health Systems; 1992. [Google Scholar]
- Lederbogen F, Haddad L, Meyer-Lindenberg A. Urban social stress - Risk factor for mental disorders. The case of schizophrenia. Environmental Pollution. 2013;183:2–6. doi: 10.1016/j.envpol.2013.05.046. [DOI] [PubMed] [Google Scholar]
- Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, Wust S, Pruessner JC, Rietschel M, Deuschle M, Meyer-Lindenberg A. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474(7352):498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- Loewy RL, Pearson R, Vinogradov S, Bearden CE, Cannon TD. Psychosis risk screening with the Prodromal Questionnaire—brief version (PQ-B) Schizophrenia Research. 2011;129(1):42–46. doi: 10.1016/j.schres.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(4):554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Andrieux A, Bartzokis G, Cadenhead K, Dazzan P, Fusar-Poli P, Gallinat J, Giedd J, Grayson DR, Heinrichs M, Kahn R, Krebs MO, Leboyer M, Lewis D, Marin O, Marin P, Meyer-Lindenberg A, McGorry P, McGuire P, Owen MJ, Patterson P, Sawa A, Spedding M, Uhlhaas P, Vaccarino F, Wahlestedt C, Weinberger D. Altering the course of schizophrenia: progress and perspectives. Nature Reviews Drug Discovery. 2016;15(7):485–515. doi: 10.1038/nrd.2016.28. [DOI] [PubMed] [Google Scholar]
- Milne B, Moffitt T, Crump R, Poulton R, Rutter M, Sears M, Taylor A, Caspi A. How should we construct psychiatric family history scores? A comparison of alternative approaches from the Dunedin Family Health History Study. Psychological Medicine. 2008;38(12):1793–1802. doi: 10.1017/S0033291708003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, The E-Risk Study Team Teen‐aged mothers in contemporary Britain. Journal of Child Psychology and Psychiatry. 2002;43(6):727–742. doi: 10.1111/1469-7610.00082. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, Delespaul P, Van Os J. Behavioural sensitization to daily life stress in psychosis. Psychological Medicine. 2005;35(05):733–741. doi: 10.1017/s0033291704004179. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, van Os J, Schwartz JE, Stone AA, Delespaul PA. Emotional reactivity to daily life stress in psychosis. Archives of General Psychiatry. 2001;58(12):1137–1144. doi: 10.1001/archpsyc.58.12.1137. [DOI] [PubMed] [Google Scholar]
- Newbury J, Arseneault L, Caspi A, Moffitt TE, Odgers CL, Fisher HL. Why are children in urban neighborhoods at increased risk for psychotic symptoms? Findings from a UK longitudinal cohort study. Schizophrenia Bulletin. 2016;42(6):1372–1383. doi: 10.1093/schbul/sbw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury J, Arseneault L, Caspi A, Moffitt TE, Odgers CL, Fisher HL. Cumulative effects of neighborhood social adversity and personal crime victimization on adolescent psychotic experiences. Schizophrenia Bulletin. 2017 doi: 10.1093/schbul/sbx060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noone D, Ames C, Hassanali N, Browning S, Bracegirdle K, Corrigall R, Laurens K, Hirsch C, Kuipers E, Maddox L. A preliminary investigation of schematic beliefs and unusual experiences in children. European Psychiatry. 2015;30(5):569–575. doi: 10.1016/j.eurpsy.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Odgers CL, Caspi A, Bates CJ, Sampson RJ, Moffitt TE. Systematic social observation of children’s neighborhoods using Google Street View: a reliable and cost-effective method. Journal of Child Psychology and Psychiatry. 2012a;53(10):1009–1017. doi: 10.1111/j.1469-7610.2012.02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odgers CL, Caspi A, Russell MA, Sampson RJ, Arseneault L, Moffitt TE. Supportive parenting mediates neighborhood socioeconomic disparities in children’s antisocial behavior from ages 5 to 12. Development and Psychopathology. 2012b;24(03):705–721. doi: 10.1017/S0954579412000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odgers CL, Donley S, Caspi A, Bates CJ, Moffitt TE. Living alongside more affluent neighbors predicts greater involvement in antisocial behavior among low‐income boys. Journal of Child Psychology and Psychiatry. 2015;56(10):1055–1064. doi: 10.1111/jcpp.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odgers CL, Moffitt TE, Tach LM, Sampson RJ, Taylor A, Matthews CL, Caspi A. The protective effects of neighborhood collective efficacy on British children growing up in deprivation: a developmental analysis. Developmental Psychology. 2009;45(4):942. doi: 10.1037/a0016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office for National Statistics. The likelihood of becoming a victim of crime: Crime statistics, period ending March 2012. London: Office for National Statistics; 2012. [Google Scholar]
- Office for National Statistics. Urban and rural area definitions for policy purposes in England and Wales: Methodology (v1.0) London: Office of National Statistics; 2013. [Google Scholar]
- Plomin R, DeFries JC, Knopik VS, Neiderhiser JM. Behavioral Genetics. New York: Worth Publishers; 2013. [Google Scholar]
- Polanczyk G, Moffitt TE, Arseneault L, Cannon M, Ambler A, Keefe SER, Houts RM, Odgers CL, Caspi A. Etiological and clinical features of childhood psychotic symptoms: Results from a birth cohort. Archives of General Psychiatry. 2010;67(4):328–338. doi: 10.1001/archgenpsychiatry.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polling C, Khondoker M, Hatch S, Hotopf M, team, S. s. Influence of perceived and actual neighbourhood disorder on common mental illness. Social Psychiatry and Psychiatric Epidemiology. 2014;49(6):889–901. doi: 10.1007/s00127-013-0813-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton R, Caspi A, Moffitt TE, Cannon M, Murray RM, Harrington H. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Archives of General Psychiatry. 2000;57(11):1053–1058. doi: 10.1001/archpsyc.57.11.1053. [DOI] [PubMed] [Google Scholar]
- Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, Hogan S, Ramrakha S, Poulton R, Danese A. Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. Journal of Child Psychology and Psychiatry. 2016;57(10):1103–1112. doi: 10.1111/jcpp.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera CL, Bernal G, Rosello J. The Children Depression Inventory (CDI) and the Beck Depression Inventory (BDI): Their validity as screening measures for major depression in a group of Puerto Rican adolescents. International Journal of Clinical and Health Psychology. 2005;5(3):485. [Google Scholar]
- Robins L, Cottler L, Bucholz K, Compton W. Diagnostic Interview Schedule for DSM-IV (DIS-IV) St Louis, MO: Washington University School of Medicine; 1995. [Google Scholar]
- Ronald A. Recent quantitative genetic research on psychotic experiences: new approaches to old questions. Current Opinion in Behavioral Sciences. 2015;2:81–88. [Google Scholar]
- Sampson RJ, Raudenbush SW. Systematic social observation of public spaces: A new look at disorder in urban neighborhoods. American Journal of Sociology. 1999;105(3):603–651. [Google Scholar]
- Sariaslan A, Fazel S, D’Onofrio B, Långström N, Larsson H, Bergen S, Kuja-Halkola R, Lichtenstein P. Schizophrenia and subsequent neighborhood deprivation: revisiting the social drift hypothesis using population, twin and molecular genetic data. Translational Psychiatry. 2016;6(5):e796. doi: 10.1038/tp.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum M, Sutherland JM, Chappel A, Azrin S, Goldstein AB, Rupp A, Heinssen RK. Twelve-month health care use and mortality in commercially insured young people with incident psychosis in the United States. Schizophrenia Bulletin. 2017 doi: 10.1093/schbul/sbx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier A, Wolke D, Thomas K, Horwood J, Hollis C, Gunnell D, Lewis G, Thompson A, Zammit S, Duffy L. Prospective study of peer victimization in childhood and psychotic symptoms in a nonclinical population at age 12 years. Archives of General Psychiatry. 2009;66(5):527–536. doi: 10.1001/archgenpsychiatry.2009.23. [DOI] [PubMed] [Google Scholar]
- Scott J, Chant D, Andrews G, McGrath J. Psychotic-like experiences in the general community: the correlates of CIDI psychosis screen items in an Australian sample. Psychological Medicine. 2006;36(2):231–238. doi: 10.1017/S0033291705006392. [DOI] [PubMed] [Google Scholar]
- Selten JP, van der Ven E, Rutten BP, Cantor-Graae E. The social defeat hypothesis of schizophrenia: an update. Schizophrenia Bulletin. 2013;39(6):1180–1186. doi: 10.1093/schbul/sbt134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoor S, Zavos HM, Haworth CM, McGuire P, Cardno AG, Freeman D, Ronald A. Association between stressful life events and psychotic experiences in adolescence: evidence for gene–environment correlations. British Journal of Psychiatry. 2016;208(6):532–538. doi: 10.1192/bjp.bp.114.159079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spauwen J, Krabbendam L, Lieb R, Wittchen HU, van Os J. Does urbanicity shift the population expression of psychosis? Journal of Psychiatric Research. 2004;38(6):613–618. doi: 10.1016/j.jpsychires.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Spauwen J, Krabbendam L, Lieb R, Wittchen HU, van Os J. Evidence that the outcome of developmental expression of psychosis is worse for adolescents growing up in an urban environment. Psychological Medicine. 2006;36(3):407–415. doi: 10.1017/S0033291705006902. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50(4):632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Trouton A, Spinath FM, Plomin R. Twins early development study (TEDS): a multivariate, longitudinal genetic investigation of language, cognition and behavior problems in childhood. Twin Research. 2002;5(5):444–448. doi: 10.1375/136905202320906255. [DOI] [PubMed] [Google Scholar]
- Trzesniewski KH, Moffitt TE, Caspi A, Taylor A, Maughan B. Revisiting the association between reading achievement and antisocial behavior: New evidence of an environmental explanation from a twin study. Child Development. 2006;77(1):72–88. doi: 10.1111/j.1467-8624.2006.00857.x. [DOI] [PubMed] [Google Scholar]
- UNICEF. Children in an Urban World. New York: United Nations Children’s Fund; 2012. [Google Scholar]
- van Winkel R, Stefanis NC, Myin-Germeys I. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophrenia Bulletin. 2008;34(6):1095–1105. doi: 10.1093/schbul/sbn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annual Review of Clinical Psychology. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Archives of General Psychiatry. 2000;57(7):675–682. doi: 10.1001/archpsyc.57.7.675. [DOI] [PubMed] [Google Scholar]
- Widom CS, Morris S. Accuracy of adult recollections of childhood victimization, part 2: childhood sexual abuse. Psychological Assessment. 1997;9(1):34–46. [Google Scholar]
- Widom CS, Weiler BL, Cottler LB. Childhood victimization and drug abuse: a comparison of prospective and retrospective findings. Journal of Consulting and Clinical Psychology. 1999;67(6):867–880. doi: 10.1037//0022-006x.67.6.867. [DOI] [PubMed] [Google Scholar]
- Wilson C, Smith ME, Thompson E, Demro C, Kline E, Bussell K, Pitts SC, DeVylder J, Reeves G, Schiffman J. Context matters: The impact of neighborhood crime and paranoid symptoms on psychosis risk assessment. Schizophrenia Research. 2016;171(1-3):56–61. doi: 10.1016/j.schres.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Yoshizumi T, Murase S, Honjo S, Kaneko H, Murakami T. Hallucinatory experiences in a community sample of Japanese children. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(8):1030–1036. doi: 10.1097/01.chi.0000126937.44875.6b. [DOI] [PubMed] [Google Scholar]
- Zavos HM, Freeman D, Haworth CM, McGuire P, Plomin R, Cardno AG, Ronald A. Consistent etiology of severe, frequent psychotic experiences and milder, less frequent manifestations: a twin study of specific psychotic experiences in adolescence. JAMA Psychiatry. 2014;71(9):1049–1057. doi: 10.1001/jamapsychiatry.2014.994. [DOI] [PMC free article] [PubMed] [Google Scholar]


