Abstract
The eighteenth and nineteenth centuries marked a sweeping transition from manual to automated manufacturing on the macroscopic scale. This enabled an unmatched period of human innovation that helped drive the Industrial Revolution. The impact on society was transformative, ultimately yielding substantial improvements in living conditions and lifespan in many parts of the world. During the same time period, the first manual syntheses of organic molecules was achieved. Now, two centuries later, we are poised for an analogous transition from highly customized crafting of specific molecular targets by hand to the increasingly general and automated assembly of many different types of molecules with the push of a button. Automation of customized small molecule synthesis pathways is already enabling safer, more reproducible, and readily scalable production of specific targets, and general machines now exist for the synthesis of a wide range of different peptides, oligonucleotides, and oligosaccharides. Creating general machines that are similarly capable of making many different types of small molecules on-demand, akin to that which has been achieved on the macroscopic scale with 3D printers, has proven to be substantially more challenging. Yet important progress is being made toward this potentially transformative objective with two complementary approaches: (1) automation of customized synthesis routes to different targets via machines that enable use of many different reactions and starting materials, and (2) automation of generalized platforms that make many different targets using common coupling chemistry and building blocks. Continued progress in these exciting directions has the potential to shift the bottleneck in molecular innovation from synthesis to imagination, and thereby help drive a new industrial revolution on the molecular scale.
Introduction
We have been making macroscopic tools to enhance our lives for at least 3 million years, beginning with hammerstones and hand axes.[1] For most of this history, we made our tools by hand. Over the past two millennia there was sporadic progress in the use of machines to advance the process of toolmaking, which progressively yielded key elements of industrialization.[2] Then in the eighteenth and nineteenth centuries the power of automated manufacturing hit full stride, human innovation reached an inflection point, and the Industrial Revolution transpired. This marked a turning point in history that transformed societies and ultimately improved the quality and span of human life in many parts of the world.[3]
Around the same time, the first organic molecules were synthesized by hand, opening the possibility of rational toolmaking on the molecular scale.[4] Over the past nearly 200 years, there has been tremendous progress on this front,[5] and small molecules have made many important impacts. Such compounds serve as most of our best medicines, biological probes, crop protectants, food preservatives, as well as key components in organic materials, dyes, paints, perfumes, flavorants, and lotions. Given all this impact already, it is exciting to consider that most of the functional potential that small molecules possess likely remains untapped.[6] This is because this class of chemical matter can also perform many frontier functions that have been minimally utilized to date. These include modulating protein-protein interactions,[7] allosterically modifying protein function,[8] acting as prostheses on the molecular scale,[9] serving as next-generation biological probes,[10] enabling miniaturized diagnostics,[11] harvesting sunlight.[12] transducing energy,[13] emitting light,[14] initiating self-healing,[15] acting as molecular magnets,[16] acting as redox flow batteries,[17] enabling next generation computing,[18] and superconducting.[19] Harnessing this untapped functional potential on the molecular scale could help address some of the most important problems facing societies today. However, the hand-crafted and thus inherently slow and specialist-dependent process via which most small molecules are still synthesized represents a significant bottleneck in such efforts. The automation of small molecule synthesis has the potential to eliminate this bottleneck, and thereby enable widespread innovation on the molecular scale.
The potential benefits of automating small molecule synthesis were sought by even some of the earliest pioneers. Figure 1 shows a wonderful photograph of Albert Eschenmoser and R.B. Woodward observing what was dubbed a “B12- and B13-making machine” purportedly under development at the ETH around 1979.[20]
Figure 1.
The structure of Vitamin B12 (1) and Albert Eschenmoser and R.B. Woodward with what was dubbed a “B12- and B13-making machine”.
Substantial progress has since been made towards the automated synthesis of many small molecule targets.[21] These advances fall into two general categories. The first category includes highly optimized machines for the customized synthesis of one specific small molecule. This category is comparable to an optimized industrial process which exclusively manufactures one macroscopic product. This approach is now widely pursued in industry to maximize efficient access to specific small molecules in sufficient quantities and with reproducible quality, and involves a wide range of both batch and/or flow-based processes.
The second category alternatively aims to accelerate discovery of new small molecule function and/or make small amounts of known-to-be-useful small molecules, on-demand. This requires general machines that can synthesize many different types of small molecules. This goal is akin to achieving on the molecular scale what 3D printers have enabled on the macroscopic scale.[22] This has proven to be a much more challenging goal. However, important progress is being made with two complementary approaches. The first aims to automatically execute customized synthesis routes to each target by constructing a flexible synthesis machine capable of performing many different types of reactions and employing many different starting materials. This approach has the advantage of leveraging a large body of precedent, because it mirrors the highly customized approach that organic chemists have primarily used for the past two centuries to make small molecules by hand. The second approach alternatively aims to make most small molecules using common coupling chemistry and building blocks, similar in concept to creating many different structures from the same bucket of Lego® bricks. While this approach requires new ways of thinking about small molecule synthesis and the development of advanced strategies and methods, it has the major advantage of potentially enabling broad access to small molecule chemical space with one simple machine and one shelf of building blocks. Continued progress in all of these exciting directions will unleash the extraordinary untapped functional potential that small molecules possess, thereby driving a new industrial revolution on the molecular scale.
One machine – one small molecule
The capacity for automation to improve the scalability, safety, efficiency and reproducibility of manufacturing a specific macroscopic tool are clear and even quantifiable. The first industrial spinning machine, called the “spinning jenny”, could produce 4–8 times the amount of yarn a human spinner could produce by hand in the same timeframe.[23] The Fourdrinier Machine enabled the continuous manufacturing of paper with consistently high quality, and was also faster than the traditional manual production of mold-made paper.[24] The automation of glass bottle production made the process safer, because the hot glass is completely handled and shaped by machines.[25] Transferring the concept of automation to specific chemical processes can lead to similar improvements in scalability, safety, efficiency and reproducibility. Such automation has been pursued and successfully applied for decades, and advancing the capacity for such automation remains the focus of many current research groups.[26] Continued progress in this field will continue to have a major impact on society by improving access to specific molecules known to have a useful function.
Scalability
At least partial automation of customized syntheses of useful molecules has become common in industry, and a large driver of this is the positive impact automation can have on scalability.[27] Chemists and engineers at Eli Lilly recently demonstrated an end-to-end fully automated synthesis of 24 kg of prexasertib monolactate monohydrate (2) for use in human clinical trials (Scheme 1). This process involved combination of batch and multistep continuous flow techniques under CGMP conditions, and the entire apparatus fit inside a standard sized fume hood. This synthesis also exemplified many additional advantages of automated manufacturing: the product was produced in overall high yields (75–85 %), high purity (99.72–99.82 % HPLC-area) and greater efficiency in comparison to a manual batch process. Moreover, performing the first step, a pyrazole formation, in an automated flow-based format, keeps the overall hydrazine present at any time low, eliminating an important safety hazard. Safety was further increased by eliminating the need for human handling of the potent biologically active intermediates and final product.[28]
Scheme 1.
Eli Lilly’s process to synthesize prexasertib monolactate monohydrate (2).
Another example showing the advantages of automation for scale-up was reported by the Cork group. They were able to automatically produce on kilogram scale a key boronic acid intermediate (3) for the manufacturing of TAK-117, a selective PI3Kα inhibitor currently in Phase 1b clinical trials (Scheme 2). In the original batch process, the boronic acid moiety was introduced via an expensive Pd-catalyzed borylation.[29] Additionally, the bis-boronic acid starting material was shown to be prone to oxygen-mediated degradation and a source of potentially genotoxic impurities.[30] Thus, alternative to the Pd-catalyzed reaction, synthesizing the key boronic acid intermediate via a lithiation-borylation procedure had many potential advantages, including increased efficiency and safety, but manually performing the corresponding lithiation chemistry on scale was associated with important challenges. Automation of this process using batch conditions proved to be difficult, due to clogging issues. However, this reaction sequence was successfully automated under continuous flow conditions in conjunction with a batch workup and isolation of the desired intermediate on kilogram scale. Even though the raw material costs of the continuous flow method exceed the costs of the Pd-catalyzed batch version due to the necessary Boc-protection, the flow process has potential benefits arising from avoiding the use of Pd and potentially mutagenic boron reagents. By switching to a flow process this reaction was also made safer and more efficient due to a short residence time in the flow reactor and the use of less toxic chemicals.[31]
Scheme 2.
Flow production of the boronic acid key intermediate (3) for the manufacturing process of TAK-117.
Safety
Safety can also be a primary advantage of automating a specific chemical process. For example, safety is an important concern when working with radioactive materials, and automation of customized batch synthesizers on laboratory scale can offer a safer way to synthesize radiolabeled substances. PET imaging in particular has become an important diagnostic tool in oncology, but in addition to safety, a short 18F half-life makes the synthesis and distribution of these compounds challenging. Automation can address these problems by allowing dose-on-demand preparation of these small molecules on-site.[32] Scheme 3 shows the automated synthesis of the 18F-labeled hypoxia imaging positron emission tomography (PET) tracer [18F]FAZA (4). In the first step, the [18F]fluoride was flushed from an ion exchange cartridge into the reaction vial containing potassium carbonate Kryptofix 2.2.2. Labeling was carried out by adding the acetylated precursor under stirring. In the second step the product was hydrolyzed, and unreacted [18F]fluoride was removed by passing the reaction solution through an Al2O3-cartridge.[33] Further examples for the automated synthesis of radiolabeled compounds include Pittsburg compound-B (11C-labeled derivative),[34] Paclitaxel (18F-labaled derivative)[35] and other 18F as well as 68Ga- labeled compounds.[36]
Scheme 3.
Example for a batch processes for the synthesis of the radiolabeled drug molecule [18F]FAZA (4).
In addition to the safety hazards associated with certain chemical substances, extreme conditions and exothermic reactions can also be especially dangerous on large scale, and these risks can be mitigated by automation. For example, Jamison recently reported a three-step process for automatically manufacturing Ibuprofen (5) which includes the automation of a key step that involves an exothermic reaction and quench (Scheme 4). Specifically, the first step is a Friedel-Crafts acylation performed neat at 90 °C with AlCl3 as Lewis acid and quenched with HCl, resulting in neat product separating from an aqueous solution containing aluminum byproducts. The risks normally associated with running this type of exothermic reaction and quench in batch mode were circumvented by using automated flow chemistry, since only a small amount of material is utilized at any given time. The same advantages were observed for the second step where neat, melted ICl induced an oxidative 1,2-aryl rearrangement to form the targeted methyl ester, which was then saponified in the last chemical transformation of this sequence. Utilizing this process, up to 70 kg Ibuprofen (5) could be safely synthesized per year with equipment that fits in a standard laboratory fume hood.[37]
Scheme 4.
Flow process for the 3 step synthesis of Ibuprofen (5).
Speed
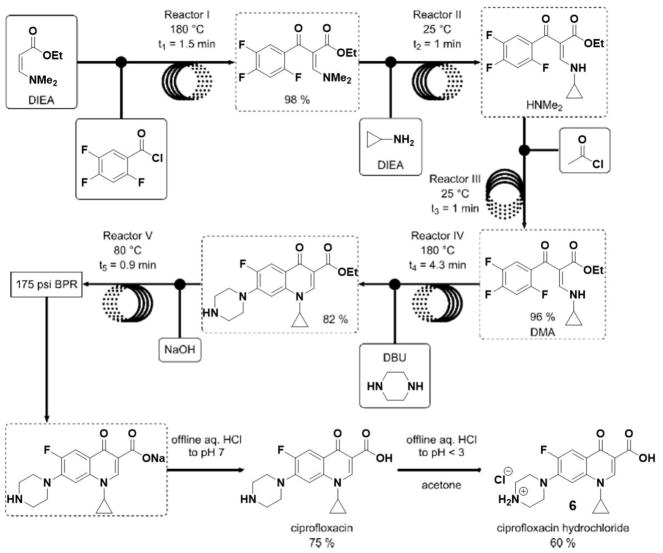
Automated synthesis platforms can also increase the speed of a chemical process, as was recently demonstrated by Jamison and coworkers with an automated flow-based platform. Specifically, the total synthesis of ciprofloxacin hydrochloride (6) was achieved in 9 minutes under continuous flow conditions via a linear reaction sequence of six chemical reactions in five flow reactors, followed by offline acidification and filtration (Scheme 5). The final product was isolated in 60% yield after the two additional offline steps, making this an overall very time-efficient process.[38] As described above, flow reactors can also enable safer handling of sensitive chemicals and dangerous reactions as well as fast and accurate temperature control, often leading to cleaner formation of even highly complex products.[39] Flow synthesizers can also have limitations, such as clogging or insufficient mixing of suspensions.[40–43]
Scheme 5.
Flow processes for the synthesis of ciprofloxacin hydrochloride (6).
Reproducibility
Another important advantage of automated synthesis can be improved reproducibility, because automation can eliminate sources of human error and enable strict process control end-to-end. In 2013 the Trout group presented an example of an end-to-end, integrated continuous manufacturing plant for the pharmaceutical aliskiren hemifumarate (7).[44] The process includes all reactions, separations, crystallizations, drying, and formulation procedures, and results in aliskiren hemifumarate (7) formulated as a tablet (Scheme 6). The entire sequence runs continuously and can produce 2.7 million tablets per year with each tablet containing precisely 112 mg of the free base form of aliskiren.[45]
Scheme 6.
Schematic process for the automated synthesis and formulation of aliskiren hemifumarate (7); R reactor, S separation, Cr crystallization, W filter/wash, D dilution tank, E extruder, MD mold.
In another example, the Ley group reported a highly reproducible and robust route for the synthesis of 5-methyl-4-propylthiophene-2-carboxylic acid (8), an important precursor for the anticancer drug candidate AZ82 (Scheme 7). This work demonstrated that a combination of flow and batch conditions can be exceptionally effective to optimize time and yield.[40] Other groups have also recently emphasized the advantages of combining batch and continuous flow synthesizers.[41–43]
Scheme 7.
An examples for integrated batch and flow systems for the Synthesis of 5-methyl-4-propylthiophene-2-carboxylic acid (8) [React. Chem. Eng. 2016, 1, 629–635] - Published by The Royal Society of Chemistry.
Leveraging all of these advantages, it is now increasingly commonplace for pharmaceuticals and other specific small molecules known to have useful functions to be manufactured using at least partially automated customized synthesis routes. In contrast, the use of automated synthesis in the process of discovering new small molecule function is much less common. Harnessing the power of automation in this context will require more general synthesizers that have the capacity to automatically prepare many different types of small molecule targets. This is an exceptionally challenging, but also potentially transformative objective.
One machine – many small molecules
One of the most important benefits of automated manufacturing is the power to enable innovation by accelerating and expanding access to the process of prototype creation. On the macroscopic scale, generalized machines that can make many different types of tools have been a major driver of such innovation. The ultimate example is the 3D printer, which enables production of an extraordinary breadth of different macroscopic objects, ranging from prosthetic limbs, to replacement parts for specialized instrumentation, to fully-functioning cameras, all by non-specialists in fabrication, and all at the push of a button.[46]
General synthesizers for similarly enabling on-demand access to certain classes of organic molecules, namely peptides,[47,48] oligonucleotides,[49] and increasingly oligosaccharides[50] have been successfully developed, and the impact on science, medicine, and technology has been transformative. To highlight just a few examples, automatically synthesized proteins,[51] genes,[52] and even complete genomes[53] pushed the development of the field of synthetic biology. New classes of therapeutics emerged due to easier access and optimization of peptide- and oligonucleotide-based drug candidates,[54] and substantial recent progress in automated oligosaccharide synthesis has led to important impacts in vaccine development.[55]
The achievement of generalized synthesis platforms for making many different types of small molecules has proven to be substantially more challenging. However, exciting progress is being made via two complementary approaches: (1) automation of customized synthesis routes to each target via flexible machines that enable the use of many different reactions and starting materials, and (2) automation of generalized synthesis platforms that make many different small molecule targets using common coupling chemistry and building blocks.
Automation of customized synthesis routes
Organic chemists typically design and develop customized routes to synthesize each small molecule target through a systematic planning algorithm known as retrosynthetic analysis.[56–59] In this inherently customized approach, one theoretically considers use of all chemical reactions in the reverse synthetic direction to systematically deconstruct a specific target into a set of starting materials. Once a best customized path for deconstructing that specific target has been identified, forward execution of the corresponding customized synthesis route is manually attempted in the lab. If the goal is to make as efficiently as possible one specific molecule, or a collection of structurally very similar molecules known to have an important function, this customized approach has substantial advantages, because it allows each synthetic solution to be highly optimized for each specific problem.
There are also, however, important disadvantages of this customized approach, especially when it comes to the goal of generalized automation. Specifically, it usually requires the de novo design a new synthetic route for each target, the development and/or optimization of many different types of reactions, access to a unique set of starting materials, a range of different equipment, and distinct safety concerns. Collectively, these features tend to make the design and execution of customized synthetic routes slow and specialist-dependent. Synthesis thus remains an important bottleneck when the goal is to discover, understand, and optimize new molecular function.
One approach for generally automating small molecule synthesis aims to build highly versatile machines that are capable of automatically replicating this classic customized approach for designing and executing synthesis pathways.
In theory, the design process could be automated via computerized retrosynthetic analysis. This is a challenging problem, but important progress has recently been made in this direction.[56–58,60–62] As such algorithms and broad access to the required computing power continue to advance, and new opportunities opened up by recent advances in artificial intelligence/machine-learning are leveraged, it is likely that a substantial portion of such customized synthetic planning can be achieved automatically.[60,62,63]
A potentially even more challenging problem associated with this approach is that it requires creating a machine that can perform as many different types of reactions as possible, is compatible with many different types of starting materials and reagents, and can execute many different types of purifications. Each of these goals alone is challenging, and in combination they represent a formidable task.
Nonetheless, important progress has been made in the development of automated platforms that aim to replicate the customized approach for small molecule synthesis.[64] As described below, handling air sensitive reactions, on-demand synthesis of multiple different pharmaceuticals, and high throughput synthesis of various targets have all been achieved with this approach.
In an early example, Bolla introduced an automatic apparatus capable of performing a number of different chemical reactions on the laboratory scale in 1985 (Figure 2A). The principle application of this machine was the optimization of reaction parameters when transferring from laboratory scale to pilot plant. A computer interface and customized programming language was developed that enabled chemists to readily utilize the system.[65]
Figure 2.
Examples of early automated synthesis systems.
Additional early prototypes were developed by Okamota and Deuchi (Figure 2B and Figure 2C). These flexible robotic workstations were similarly designed and utilized to enable the transition from laboratory scale to pilot plant and even full-scale manufacturing. It was also demonstrated that these automated synthesis systems can perform a variety of common organic reactions on gram-scale, and enable material throughput by running 24 h a day.[66]
In 2000, the Otera group developed MEDLEY, an automated one-pot synthesizer capable of conducting a variety of air sensitive chemical reactions (Scheme 8). The completely sealed apparatus can perform reactions under inert atmosphere from −78 °C to room temperature. MEDLEY can also perform a sequence of multiple chemical transformations within one reaction vessel.[67] The reservoirs of the synthesizer can be charged with different reagents, and addition, reaction time, and temperature can be controlled by the automated reaction system. This automated reaction system also initiates the quenching process after the reaction sequence is finished. Work-up, purification, and analysis of the product must still be done by hand.[67] The Otera group utilized MEDLEY to carry out a variety of chemical transformations including air-sensitive organolithium and Grignard chemistry as well as transition metal catalyzed cross-coupling reactions.[68] They also synthesized 3-substituted 5-(beta-sulfonyl vinyl)indoles by in situ generation of toxic vinyl sulfone.[69]
Scheme 8.
Syntheses performed with MEDLEY and a schematic diagram of MEDLEY (Reprinted (adapted) with permission from Org. Proc. Res. Dev. 2000, 4, 333–336. Copyright 2000 American Chemical Society).
Another batch-based automated synthesizer for air sensitive reactions, called ChemKonzert (Scheme 9), was introduced by the Takahashi group in 2010. In addition to having similar features as MEDLEY, ChemKonzert contains an additional centrifugal separator to efficiently achieve liquid-liquid extraction where the phases are separated by using the different electric conductivity of the two layers. It has also been demonstrated that ChemKonzert can perform two-step reactions between 0 °C and reflux temperature, and is able to handle air and moisture sensitive reagents. The instrument can perform many of the typical solution-phase reactions used in the pharmaceutical and chemical industries that do not require the use of high pressure or extensive cooling. In contrast to MEDLEY, the workup is also included in the automated process. A variety of different reactions were performed on ChemKonzert including introduction of protecting groups to alcohols as well as amines, a Masamune reaction to get access to β-keto esters, an O-alkylation reaction, Weinreb amidation followed by Grignard addition to synthesize ketones,[70] a formal total synthesis of Taxol (9)[71] and a total synthesis of Spiruchostatin B (10).[72] Recently, the Takahashi group also demonstrated the automated synthesis of α-amino aldehydes in a three step process exclusively performed by ChemKonzert.[73]
Scheme 9.
Synthesis performed with ChemKonzert and a schematic diagram of ChemKonzert including two reaction vessels (RF1 and RF2), a centrifugal separator (SF, 700 mL), two receivers (SF1 and SF2, 500 mL), two glass filters (FF1 and FF2, 500 and 100 mL), 12 substrate and reagent reservoirs (RR1–RR12) (Reprinted (adapted) with permission from Org. Process Res. Dev. 2009, 13, 1111–1121. Copyright 2009 American Chemical Society).
In addition to these automated batch synthesizers, a variety of flow chemistry-based generalized automated platforms have been pursued as well. Three groups from MIT developed a reconfigurable flow-reactor for on-demand synthesis of various pharmaceuticals (Scheme 10). The system is assembled by modules with special functions (reactors and separators) which can be used or skipped in the synthesis process as needed. Continuous drug-manufacturing on a reconfigurable flow-system could be particularly useful for pharmaceuticals with a short shelf-life and or to provide cheaper access to pharmaceuticals for small patient populations. To demonstrate the flexibilty of this on-demand flow-reactor, four different pharmaceuticals, diphenhydramine hydrochloride (11), lidocaine hydrochloride (12), diazepam (13), and fluoxetine hydrochloride (14), were produced using this reconfigurable system.[74] Additional efforts have been directed toward the development of high throughput micro flow-reactors, which can be used for rapid method optimization by integrating the flow-reactor into a chip, followed by automated HPLC/LC-MS analysis.[75]
Scheme 10.
Reconfigurable modules of a flow reactor and their assembly for the synthesis of different compounds.
Over the past decade, Eli Lilly has developed an automated synthesis lab designed to enable high throughput drug discovery (Scheme 11).[76] This remote-controlled automated lab can perform many different types of chemical transformations and can be accessed globally via the internet. All steps are controlled via customized software which runs continuously. The workspace is composed of a room filled with a suite of modules for different operations, such as heating, cooling, work up, sample preparation and analytics. Flow and microwave reactors further expand the range of chemical reactions possible with this set up. In 2011, 16,349 reactions on an average scale of 100 mg were performed with the automated synthesis lab. This remarkable reaction throughput highlights the potential impact of automation in discovery-based organic synthesis.
Scheme 11.
Synthesis performed with the automated synthesis lab developed by Eli Lilly and schematic diagram with (1) Input/output device (2) Bench for heated reactions (3) Bench with special functions as cooling, microwave and flow, (4) work-up bench (5) analytics.
Taking an alternative direction to creating a flexible synthesizer, the Cronin group has combined a robotic platform with a 3D printer to print optimized reaction vessels for different reactions and scales. Such a system automatically synthesized Ibuprofen (5) on three different reaction scales controlling the parameters of the synthesis with custom software. In addition, catalyst-silicone materials were incorporated into the 3D-printed device, allowing a versatile use of the reaction vessels based on the specific needs for the particular experiment.[77] Recent advances demonstrate progress toward digitization of a synthesis process with 3D printable series of vessels for reactions and purifications.[78] Targeted further developments in this field include enabling robotic platforms to handle complete synthetic routes, including work-up and purification steps.[79]
Automation of generalized synthesis processes
Given some of the inherent limitations of the customized approach for small molecule synthesis, particularly when it comes to automation, it is exciting to consider whether alternative, and more generalized strategies can be developed. Inspiration for such an approach can be found in the history of building block-based construction on the macroscopic scale. In the mid-eighteenth century Samuel Bentham pioneered the concept of interchangeable parts in the form of pulley blocks for sailboats. Automated manufacturing was recognized as critical for the success of this approach, as it maximized standardization of size and quality of these parts as well as scalability and capacity for on-demand production.[80] Half a century later, Honore LeBlanc and Eli Whitney pioneered the idea of interchangeable parts in the manufacturing of muskets.[81],[82] Soon thereafter, Henry Ford added the concept of the assembly line to similarly accelerate iterative modular manufacturing of automobiles.[81]
This type of building block-based construction not only enables efficient, reproducible, and automated assembly of a specific object, but such platforms are also inherently flexible. That is, they can make an extraordinary range of different targets via common assembly processes and the simple mixing and matching of common building blocks. This enables on-demand production of new prototypes, even by non-specialists, which powerfully and broadly enables innovation. A simple example of this can be seen in the large number of different structures a child can build with a bucket of Lego® bricks. Building block-based approaches have similarly enabled consumer-driven personalized design and construction of new homes, desktop computers, cars, etc., and new manufacturing execution systems have been developed to facilitate such on-demand customized production.[83] The ability to produce many different products with the same manufacturing process set the stage for rapid discovery of new tools and machines, which was a hallmark of the Industrial Revolution.
Encouragingly, a similar type of building block-based assembly has been realized on the molecular scale for certain subsets of targets, as best demonstrated with automated synthesis of peptides,[47] oligonucleotides,[49] and, increasingly, oligosaccharides.[50] Given all the major advantages and remarkable impact that these platforms have made already, it is exciting to ask whether many, or even most, small molecules might be accessed via an analogous building block-based approach.
Increasing evidence suggests that this goal is achievable. First, despite their substantial diversity, many types of small molecules are inherently modular. This includes natural products, most of which are biosynthesized via 5 major pathways that each employ a small number of common reactions and only a handful of building blocks (Figure 3). Consistent with this inherent modularity, a recent study revealed that more than 75 % of all polyene natural product motifs can be prepared via iterative assembly of just 12 building blocks.[84] An ongoing similar analysis of most natural products suggests that such modularity may be more pervasive.[85] Moreover, a recent analysis of all 1086 approved small molecule drugs also found a high degree of redundancy at the fragment level. Most common were heterocyclic motifs including piperidine (72 drugs), pyridine (62 drugs), piperazine (59 drugs), cephem (41 drugs), pyrrolidine (37 drugs), thiazole (30 drugs), and 16 additional heterocycles were found in over 10 drugs.[86] Many different materials are highly modular as well, often being constructed from iterative units of heterocycles, aryl rings, and/or double bonds. This collective inherent modularity suggests that a wide range of different functions can be achieved from common subunits, and that many different types of small molecules and corresponding functions should be accessible from common sets of building blocks.
Figure 3.
Small molecules are inherently modular.
It is also encouraging that many different types of iterative synthesis methods have been developed that enable the modular construction of certain types of small molecules.[87] These include early pioneering examples such as Yoshida and Isoe’s iterative route to optically active polyols via iterative addition of alpha-alkoxysilane building blocks,[88] Evans’ iterative application of diastereoselective aldol reactions to generate polyketide motifs,[89] and Moore’s development of iterative Sonagashira couplings to generative poly-arylalkynes.[90] Most of these platforms target the synthesis of specific classes of small molecule structures, but increasing evidence suggests that iterative platforms can also be developed that are both general and automated.
For example, an iterative synthesis platforms based on the assembly of MIDA boronate building blocks has been used to prepare a rapidly growing list of structurally distinct small molecules by many different groups worldwide (Figure 4).[84,91,92,93,94] This platform leverages the capacity of the trivalent MIDA-ligand to reversibly block the reactivity of a boronic acid via rehybridization of the boron center from sp2 to sp3. The MIDA ligand can also be removed under aqueous or mild aqueous basic conditions which reveals the free boronic acid.[95] This permits iterative assembly of bifunctional halo MIDA boronate building blocks via recursive cycles of boronic acid-selective coupling followed by boron deprotection.[94,96,97] This methodology is also suitable to couple highly unstable boronic acids, since reactive boronic acid species can be slowly released during the reaction from the typically stable, crystalline MIDA boronate form.[98–101]
Figure 4.
Small molecules made via iterative cross coupling from MIDA-boronate building blocks.
To implement this modular approach, practical access to the corresponding MIDA-boronate building blocks is required. Encouragingly, hundreds of MIDA-boronates are already commercially available and widely utilized.[102–106] Moreover, recent reports from many different research groups demonstrate that a wide range of otherwise challenging-to-access MIDA boronate building blocks can be prepared from MIDA boronate starting materials.[91,93,96,99–106,107] Leveraging the advances described below, there is also ample opportunity to automate the process of building block synthesis, which has the potential to substantially increase the efficiency with which new building blocks can be sustainably accessed on-demand.
It was also recently reported that this increasingly general platform for building block-based small molecule synthesis can be automated (Scheme 12).[108] Specifically, a small molecule synthesizer that assembles bifunctional MIDA-boronate building blocks via an automated deprotection, cross-coupling, and purification sequence was achieved. Most small molecule targets lack a common handle for covalent attachment to a solid support, precluding the use of solid-phase synthesis as a generalized purification technique. Alternatively, it was discovered that MIDA boronate-containing intermediates can be generally purified by taking advantage of a unique catch-and-release phenomenon on silica gel. Specifically, a wide range of different MIDA boronates show no movement on silica gel with certain eluents (Et2O/MeOH), but are rapidly eluted with others (THF). This enabled the development of a novel type of generalized and automated purification process that was coupled to automated iterative MIDA boronate assembly.
Scheme 12.
Automated Synthesis Machine (A) linear structures prepared by the automated synthesizer. (B) Cyclic scaffolds prepared with the automated synthesizer as linear precursors and cyclized by hand.
A broad range of small molecules from various classes have been assembled with this small molecule synthesizer (Scheme 12). Examples range from pharmaceuticals to materials to natural products, with the latter class including some highly complex macrocyclic or polycyclic natural products or natural product motifs. In all of these cases, all of the required functional groups, oxidation states, and stereochemistry were pre-installed into the corresponding building blocks, most of which were commercially available.[108] Hinting at the potential of such technology to bring the power of synthesis to non-specialists, a news reporter recently used this prototypical synthesizer to prepare a stereochemically complex natural product,[109] and a group of high school students recently used it to make organic light emitting diodes.[110]
There are many challenges that need to be overcome in order to maximize the generality of this platform and thereby gain on-demand automated access to large portions of targeted small molecule chemical space. These include expanding the scope of cross-coupling to include a wide range of Csp3 and Xsp3 coupling partners while maintaining excellent control of stereochemistry. As mentioned above, increased off-the-shelf access to building blocks that collectively cover large portions of small molecule chemical space is also required. There is substantial reason for optimism that such challenges can be solved, however, and the impact of achieving such generalized automated small molecule synthesis platform would be substantial.
Automating reaction optimization, molecular design, and functional discovery
A powerful series of advances in online reaction monitoring, machine self-optimization, and machine learning/artificial intelligence algorithms, are rapidly emerging in parallel and have the potential to substantially enable the development of synthesizers that automate both customized and generalized synthesis routes.[111]
End-to-end robot-mediated discovery has already been achieved for biological systems (Figure 5). Robot-scientist “Adam” can autonomously generate hypotheses about gene-function relationships in the yeast Saccharomyces cerevisiae and experimentally test these hypotheses by using laboratory automation.[112] “Eve”, an automated early-stage drug testing device, tests subsets of compounds and identifies positive results using statistics and machine learning, and autonomously predicts new small molecule structures that are likely to return better results.[113]
Figure 5.
Robot-scientists (A) Adam and (B) Eve [J. R. Soc. Interface, 2015, 12, 20141289] - Published by The Royal Society of Chemistry.
Closing the loop between automation and self-optimization in chemical small molecule synthesis is also gaining momentum. In 2008, the Engineering and Physical Sciences Research Council (EPSRC) started funding networks to provide solutions for grand challenges in chemistry. Among the funded networks is Dial-a-molecule, which targets a continuous-flow platform for synthetic chemistry with integrated analytics and automated feedback optimization to influence the reaction progress.[114]
Buchwald and Jensen recently demonstrated self-optimization and automated feedback in combination with a continuous flow reactor to find optimal conditions for Suzuki–Miyaura cross-coupling reaction improvement. They used a smart optimal design of experiments (DoE)-based algorithm to increase the turnover number and yield of the catalytic system considering both discrete variables (palladacycle and ligand) and continuous variables (temperature, time, and loading) simultaneously. All reaction data was examined via online HPLC-analysis and 96 reactions were in general necessary to find optimal conditions. With this study they gained key mechanistic insights into the coupling reactions as well as optimal conditions for each reaction tested (Scheme 13).[115]
Scheme 13.
Concept with flow diagram for automated Suzuki–Miyaura and optimized yield and TON found in some examples [React. Chem. Eng. 2016, 1, 658–666] - Published by The Royal Society of Chemistry.
Cronin also created a self-optimizing reactor system using real-time in-line NMR spectroscopy for data analysis. Utilizing 13C, 19F and 2D NMR spectra of reaction mixtures under flow conditions, this technique allows a variety of kinetic and mechanistic studies. Self-optimization in flow reactors was achieved by employing in-line 1H NMR. As model reactions an imine-formation and electrophilic fluorination were chosen (Scheme 14). Current efforts include applying a similar system for the discovery of new compounds.[116]
Scheme 14.
Scheme of the flow-NMR platform and studied model reactions [Chem. Sci. 2015, 6, 1258–1264] - Published by The Royal Society of Chemistry.
Bourne and Muller alternatively employed a combination of HPLC as well as MS in positive atmospheric pressure chemical ionization mode for online data analysis. They were able to gather multistep kinetic information about a nucleophilic aromatic substitution (Scheme 15A)[117] and showed self-optimization of different reactions with their systems (Scheme 15B and C).[118]
Scheme 15.
Three examples for self-optimization and online data analysis in flow reactors [React. Chem. Eng. 2017, 2, 103–108] and [React. Chem. Eng. 2016, 1, 366–371] - Published by The Royal Society of Chemistry.
There is even emerging support for the notion of “artificial imagination” (Figure 6) which has the theoretical potential to enable the automated design of new molecular functions. For example, last year, the Google AI AlphaGo won a game of Go against a professional human player by utilizing human-like imagination. The team used general machine learning techniques to create a system that learned on its own. It is proposed, that with enough training, processing, and search power such algorithms could outperform humans in many problem solving tasks.[119] Hinting at such potential, Aspuru-Guzik recently demonstrated the potential of machine learning to promote the discovery of next generation organic materials,[120] and there are exciting signs that such techniques can powerfully enable drug discovery.[121] It is exciting to consider the tremendous potential of creating a closed loop that interfaces such technology with automated small molecule synthesis and functional characterization.
Figure 6.

“Artificial Imagination” of AlphaGo, Published by DeepMind.
Conclusion and future outlook
Relative to the long history of toolmaking on the macroscopic scale, the history of rational small molecule synthesis is extremely brief. Viewed from this lens, the macroscopic Industrial Revolution and its transformative impact on our current world is inspiring. Major advances in the automated synthesis of certain types of biomolecules have already had a major impact, suggesting that perhaps the molecular industrial revolution has already been active for decades in certain biological contexts. Given the vast, and in many instances unique, untapped functional potential that small molecules possess, it is exciting to consider the tremendous societal impact that could achieved by likewise broadly enabling innovation in this chemical space.
Substantial progress has recently been achieved in automating customized synthesis routes to specific small molecule targets, and such technologies are already making a substantial industrial impact. Albeit more challenging, there is also encouraging progress toward generalized small molecule synthesizers. This has been achieved using two different approaches: (1) automating the classical customized approach to each target by creating highly versatile synthesis machines that can perform many different reactions and employ many different starting materials, and (2) developing more general building block-based approaches for small molecule synthesis that leverage inherent modularity and only require automating a few chemical reactions.
Parallel advances in online reaction monitoring, self-optimization, and machine learning/artificial intelligence stand to powerfully synergize with these evolving automated synthesis platforms. It is exciting to consider the prospect of generally closing the loop for design, synthesis, and testing at the molecular scale. Achieving this goal has the potential to transform automated synthesis into automated functional discovery.
Continued progress in all of these exciting directions has the potential to shift the bottleneck in molecular innovation from synthesis to imagination (human or artificial!), and thereby help drive the new industrial revolution on the molecular scale.
References
- 1.Harmand S, Lewis JE, Feibel CS, Lepre CJ, Prat S, Lenoble A, Boës X, Quinn RL, Brenet M, Arroyo A, et al. Nature. 2015;521:310. doi: 10.1038/nature14464. [DOI] [PubMed] [Google Scholar]
- 2.a) Bautista Paz E, Ceccarelli M, Echávarri Otero J, Muñoz Sanz JL. A Brief Illustrated History of Machines and Mechanisms. Springer Netherlands; Dordrecht: 2010. [Google Scholar]; b) Strandh S. A history of the machine. A & W Publ; New York: 1979. [Google Scholar]
- 3.a) Ashton TS, Hudson P. The industrial revolution, 1760–1830. Oxford University Press; Oxford: 1997. [Google Scholar]; b) Hudson P. The industrial revolution. Arnold; London: 2005. [Google Scholar]; c) Berg M. The age of manufactures, 1700–1820. Industry, innovation and work in Britain. Routledge; London: 1996. [Google Scholar]
- 4.a) Wöhler F. Ann Phys Chem. 1828;88:253. [Google Scholar]; b) Nicolaou KC. Proc R Soc London, Ser A. 2014;470:20130690. doi: 10.1098/rspa.2013.0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Nicolaou KC, Sorensen EJ. Classics in Total Synthesis. Targets, strategies, methods. WILEY-VCH; Weinheim: 1996. [Google Scholar]; b) Nicolaou KC, Snyder SA. Classics in total synthesis II. More targets, strategies, methods. WILEY-VCH; Weinheim: 2003. [Google Scholar]; c) Nicolaou KC, Chen JS. Classics in total synthesis III. Further Targets, Strategies, Methods. WILEY-VCH; Weinheim: 2011. [Google Scholar]
- 6.Palazzolo AME, Simons CLW, Burke MD. Proc Natl Acad Sci U S A. 2017;114:5564. doi: 10.1073/pnas.1706266114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Yin H, Hamilton AD. Angew Chem. 2005;117:4200. doi: 10.1002/anie.200461786. [DOI] [PubMed] [Google Scholar]; b) Yin H, Hamilton AD. Angew Chem Int Ed. 2005;44:4130. doi: 10.1002/anie.200461786. [DOI] [PubMed] [Google Scholar]; c) Villar EA, Beglov D, Chennamadhavuni S, Porco JA, Kozakov D, Vajda S, Whitty A. Nat Chem Biol. 2014;10:723. doi: 10.1038/nchembio.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Du J, Lü W, Wu S, Cheng Y, Gouaux E. Nature. 2015;526:224. doi: 10.1038/nature14853. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wellington S, Nag PP, Michalska K, Johnston SE, Jedrzejczak RP, Kaushik VK, Clatworthy AE, Siddiqi N, McCarren P, Bajrami B, et al. Nat Chem Biol. 2017;13:943. doi: 10.1038/nchembio.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Cioffi AG, Hou J, Grillo AS, Diaz KA, Burke MD. J Am Chem Soc. 2015;137:10096. doi: 10.1021/jacs.5b05765. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Grillo AS, SantaMaria AM, Kafina MD, Cioffi AG, Huston NC, Han M, Seo YA, Yien YY, Nardone C, Menon AV, et al. Science. 2017;356:608. doi: 10.1126/science.aah3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Chan J, Dodani SC, Chang CJ. Nat Chem. 2012;4:973. doi: 10.1038/nchem.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kocaoglu O, Carlson EE. Nat Chem Biol. 2016;12:472. doi: 10.1038/nchembio.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Weber J, Beard PC, Bohndiek SE. Nat Methods. 2016;13:639. doi: 10.1038/nmeth.3929. [DOI] [PubMed] [Google Scholar]; d) Schreiber SL, Kotz JD, Li M, Aubé J, Austin CP, Reed JC, Rosen H, White EL, Sklar LA, Lindsley CW, et al. Cell. 2015;161:1252. doi: 10.1016/j.cell.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Li H, Zhang P, Smaga LP, Hoffman RA, Chan J. J Am Chem Soc. 2015;137:15628. doi: 10.1021/jacs.5b10504. [DOI] [PubMed] [Google Scholar]; f) Kowalik L, Chen JK. Nat Chem Biol. 2017;13:587. doi: 10.1038/nchembio.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) van Esbroeck ACM, Janssen APA, Cognetta AB, Ogasawara D, Shpak G, van der Kroeg M, Kantae V, Baggelaar MP, de Vrij FMS, Deng H, et al. Science. 2017;356:1084. doi: 10.1126/science.aaf7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schumacher S, Sartorius D, Ehrentreich-Förster E, Bier FF. EJIFCC. 2012;23:70. [PMC free article] [PubMed] [Google Scholar]
- 12.Sokolov AN, Atahan-Evrenk S, Mondal R, Akkerman HB, Sánchez-Carrera RS, Granados-Focil S, Schrier J, Mannsfeld SCB, Zoombelt AP, Bao Z, et al. Nat Commun. 2011;2:437. doi: 10.1038/ncomms1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Hickenboth CR, Moore JS, White SR, Sottos NR, Baudry J, Wilson SR. Nature. 2007;446:423. doi: 10.1038/nature05681. [DOI] [PubMed] [Google Scholar]; b) Davis DA, Hamilton A, Yang J, Cremar LD, van Gough D, Potisek SL, Ong MT, Braun PV, Martínez TJ, White SR, et al. Nature. 2009;459:68. doi: 10.1038/nature07970. [DOI] [PubMed] [Google Scholar]; c) Mart RJ, Allemann RK. Chem Commun (Cambridge, U K) 2016;52:12262. doi: 10.1039/c6cc04004g. [DOI] [PubMed] [Google Scholar]; d) Romero NA, Nicewicz DA. Chem Rev. 2016;116:10075. doi: 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- 14.Aizawa N, Pu Y-J, Watanabe M, Chiba T, Ideta K, Toyota N, Igarashi M, Suzuri Y, Sasabe H, Kido J. Nat Commun. 2014;5:5756. doi: 10.1038/ncomms6756. [DOI] [PubMed] [Google Scholar]
- 15.Wilson GO, Caruso MM, Reimer NT, White SR, Sottos NR, Moore JS. Chem Mater. 2008;20:3288. [Google Scholar]
- 16.Thiele S, Balestro F, Ballou R, Klyatskaya S, Ruben M, Wernsdorfer W. Science. 2014;344:1135. doi: 10.1126/science.1249802. [DOI] [PubMed] [Google Scholar]
- 17.Lin K, Gómez-Bombarelli R, Beh ES, Tong L, Chen Q, Valle A, Aspuru-Guzik A, Aziz MJ, Gordon RG. Nat Energy. 2016;1:16102. [Google Scholar]
- 18.Heinrich BW, Braun L, Pascual JI, Franke KJ. Nat Phys. 2013;9:765. [Google Scholar]
- 19.a) Cui H, Kobayashi H, Ishibashi S, Sasa M, Iwase F, Kato R, Kobayashi A. J Am Chem Soc. 2014;136:7619. doi: 10.1021/ja503690m. [DOI] [PubMed] [Google Scholar]; b) Valade L, de Caro D, Faulmann C, Jacob K. Coord Chem Rev. 2016;308:433. [Google Scholar]
- 20.Benfey OT, Morris PJT, editors. History of modern chemical sciences. Chemical Heritage Foundation; Philadelphia: 2001. [Google Scholar]
- 21.a) Ley SV, Fitzpatrick DE, Ingham RJ, Myers RM. Angew Chem Int Ed. 2015;54:3449. doi: 10.1002/anie.201410744. [DOI] [PubMed] [Google Scholar]; b) Ley SV, Fitzpatrick DE, Ingham RJ, Myers RM. Angew Chem. 2015;127:3514. doi: 10.1002/anie.201410744. [DOI] [PubMed] [Google Scholar]; c) Ley SV, Fitzpatrick DE, Myers RM, Battilocchio C, Ingham RJ. Angew Chem Int Ed. 2015;54:10122. doi: 10.1002/anie.201501618. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Ley SV, Fitzpatrick DE, Myers RM, Battilocchio C, Ingham RJ. Angew Chem. 2015;127:10260. doi: 10.1002/anie.201501618. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Fitzpatrick DE, Battilocchio C, Ley SV. ACS Cent Sci. 2016;2:131. doi: 10.1021/acscentsci.6b00015. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Fitzpatrick DE, Ley SV. Tetrahedron. 2017 doi: 10.1016/j.tet.2017.08.050. [DOI] [Google Scholar]; g) Hartwig J, Kirschning A. Angew Chem. 2015;127:10554. doi: 10.1002/anie.201504615. [DOI] [PubMed] [Google Scholar]; h) Hartwig J, Kirschning A. Angew Chem Int Ed. 2015;54:10412. doi: 10.1002/anie.201504615. [DOI] [PubMed] [Google Scholar]
- 22.a) Ferdinand J-P, Petschow U, Dickel S, editors. Progress in IS. Springer International Publishing; Cham: 2016. [Google Scholar]; b) Straub J. Machines. 2015;3:55. [Google Scholar]
- 23.Guest R. A compendious history of the cotton manufacture. Cass; London: 1968. [Google Scholar]
- 24.Hills RL. Papermaking in Britain, 1488 – 1988. A short history. Athlone Press; London: 1988. [Google Scholar]
- 25.Arpe H-J, Ullmann F, editors. Ullmann’s encyclopedia of industrial chemistry. Weinheim; 1989. [Google Scholar]
- 26.a) Carneiro PF, Gutmann B, de Souza ROMA, Kappe CO. ACS Sustainable Chem Eng. 2015;3:3445. [Google Scholar]; b) Guetzoyan L, Ingham RJ, Nikbin N, Rossignol J, Wolling M, Baumert M, Burgess-Brown NA, Strain-Damerell CM, Shrestha L, Brennan PE, et al. Med Chem Commun. 2014;5:540. [Google Scholar]; c) Hayashi N, Sugawara T, Shintani M, Kato S. J Autom Chem. 1989;11:212. doi: 10.1155/S1463924689000428. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Hayashi N, Sugawara T. Tetrahedron Comput Methodol. 1988;1:237. [Google Scholar]; e) Hayashi N, Sugawara T. Chem Lett. 1988;17:1613. [Google Scholar]; f) Poh J-S, Browne DL, Ley SV. React Chem Eng. 2016;1:101. doi: 10.1039/c5re00082c. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Smith CJ, Nikbin N, Ley SV, Lange H, Baxendale IR. Org Biomol Chem. 2011;9:1938. doi: 10.1039/c0ob00815j. [DOI] [PubMed] [Google Scholar]; h) Hayashi N, Sugawara T, Kato S. J Autom Chem. 1991;13:187. doi: 10.1155/S1463924691000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.a) Bana P, Örkényi R, Lövei K, Lakó Á, Túrós GI, Éles J, Faigl F, Greiner I. Bioorg Med Chem. 2016 doi: 10.1016/j.bmc.2016.12.046. [DOI] [PubMed] [Google Scholar]; b) Battilocchio C, Bosica F, Rowe SM, Abreu BL, Godineau E, Lehmann M, Ley SV. Org Process Res Dev. 2017 doi: 10.1021/acs.oprd.7b00229. [DOI] [Google Scholar]; c) Battilocchio C, Deadman BJ, Nikbin N, Kitching MO, Baxendale IR, Ley SV. Chem - Eur J. 2013;19:7917. doi: 10.1002/chem.201300696. [DOI] [PubMed] [Google Scholar]; d) Baumann M, Baxendale IR. Beilstein J Org Chem. 2015;11:1194. doi: 10.3762/bjoc.11.134. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Baxendale IR, Deeley J, Griffiths-Jones CM, Ley SV, Saaby S, Tranmer GK. Chem Commun (Cambridge, U K) 2006:2566. doi: 10.1039/b600382f. [DOI] [PubMed] [Google Scholar]; f) Britton J, Jamison TF. Angew Chem Int Ed. 2017;56:8823. doi: 10.1002/anie.201704529. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Britton J, Jamison TF. Angew Chem. 2017;129:8949. doi: 10.1002/anie.201704529. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Ingham RJ, Battilocchio C, Fitzpatrick DE, Sliwinski E, Hawkins JM, Ley SV. Angew Chem Int Ed. 2015;54:144. doi: 10.1002/anie.201409356. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Ingham RJ, Battilocchio C, Fitzpatrick DE, Sliwinski E, Hawkins JM, Ley SV. Angew Chem. 2015;127:146. doi: 10.1002/anie.201409356. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Newton S, Carter CF, Pearson CM, de L, Alves C, Lange H, Thansandote P, Ley SV. Angew Chem Int Ed. 2014;53:4915. doi: 10.1002/anie.201402056. [DOI] [PubMed] [Google Scholar]; k) Newton S, Carter CF, Pearson CM, de L, Alves C, Lange H, Thansandote P, Ley SV. Angew Chem. 2014;126:5015. doi: 10.1002/anie.201402056. [DOI] [PubMed] [Google Scholar]; l) Peeva L, Da Silva Burgal J, Heckenast Z, Brazy F, Cazenave F, Livingston A. Angew Chem Int Ed. 2016;55:13576. doi: 10.1002/anie.201607795. [DOI] [PMC free article] [PubMed] [Google Scholar]; m) Peeva L, Da Silva Burgal J, Heckenast Z, Brazy F, Cazenave F, Livingston A. Angew Chem. 2016;128:13774. doi: 10.1002/anie.201607795. [DOI] [PMC free article] [PubMed] [Google Scholar]; n) Porta R, Benaglia M, Puglisi A. Org Process Res Dev. 2016;20:2. [Google Scholar]; o) Schuler H. at - Automatisierungstechnik. 2006;54:315. [Google Scholar]; p) Stouten SC, Noël T, Wang Q, Hessel V. Aust J Chem. 2013;66:121. [Google Scholar]; q) Webb D, Jamison TF. Chem Sci. 2010;1:675. [Google Scholar]
- 28.Cole KP, Groh JM, Johnson MD, Burcham CL, Campbell BM, Diseroad WD, Heller MR, Howell JR, Kallman NJ, Koenig TM, et al. Science. 2017;356:1144. doi: 10.1126/science.aan0745. [DOI] [PubMed] [Google Scholar]
- 29.Gurung SR, Mitchell C, Huang J, Jonas M, Strawser JD, Daia E, Hardy A, O’Brien E, Hicks F, Papageorgiou CD. Org Process Res Dev. 2017;21:65. [Google Scholar]
- 30.O’Donovan MR, Mee CD, Fenner S, Teasdale A, Phillips DH. Mutat Res. 2011;724:1. doi: 10.1016/j.mrgentox.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Usutani H, Nihei T, Papageorgiou CD, Cork DG. Org Process Res Dev. 2017;21:669. [Google Scholar]
- 32.a) Awasthi V, Watson J, Gali H, Matlock G, McFarland A, Bailey J, Anzellotti A. Appl Radiat Isot. 2014;89:167. doi: 10.1016/j.apradiso.2014.02.015. [DOI] [PubMed] [Google Scholar]; b) Chi YT, Chu PC, Chao HY, Shieh WC, Chen CC. BioMed Res Int. 2014;2014:680195. doi: 10.1155/2014/680195. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kuge Y, Shiga T, Tamaki N, editors. Perspectives on Nuclear Medicine for Molecular Diagnosis and Integrated Therapy. Springer; Japan, Tokyo: 2016. [Google Scholar]; d) Pascali G, Matesic L. Perspectives on Nuclear Medicine. :79–92. [Google Scholar]
- 33.Reischl G, Ehrlichmann W, Bieg C, Solbach C, Kumar P, Wiebe LI, Machulla H-J. Appl Radiat Isot. 2005;62:897. doi: 10.1016/j.apradiso.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Verdurand M, Bort G, Tadino V, Bonnefoi F, Le Bars D, Zimmer L. Nucl Med Commun. 2008;29:920. doi: 10.1097/MNM.0b013e328304e0e1. [DOI] [PubMed] [Google Scholar]
- 35.Kalen JD, Hirsch JI, Kurdziel KA, Eckelman WC, Kiesewetter DO. Appl Radiat Isot. 2007;65:696. doi: 10.1016/j.apradiso.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.a) Waldmann CM, Lebedev A, Allison N, Sadeghi S. Appl Radiat Isot. 2017;127:245. doi: 10.1016/j.apradiso.2017.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Li S, Schmitz A, Lee H, Mach RH. EJNMMI Radiopharm Chem. 2017;1:615. doi: 10.1186/s41181-016-0018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Boschi S, Lodi F, Malizia C, Cicoria G, Marengo M. Appl Radiat Isot. 2013;76:38. doi: 10.1016/j.apradiso.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Snead DR, Jamison TF. Angew Chem Int Ed. 2015;54:983. doi: 10.1002/anie.201409093. [DOI] [PubMed] [Google Scholar]
- 38.a) Lin H, Dai C, Jamison TF, Jensen KF. Angew Chem Int Ed. 2017;56:8870. doi: 10.1002/anie.201703812. [DOI] [PubMed] [Google Scholar]; b) Lin H, Dai C, Jamison TF, Jensen KF. Angew Chem. 2017;129:8996. doi: 10.1002/anie.201703812. [DOI] [PubMed] [Google Scholar]
- 39.a) Gutmann B, Cantillo D, Kappe CO. Angew Chem Int Ed. 2015;54:6688. doi: 10.1002/anie.201409318. [DOI] [PubMed] [Google Scholar]; b) Gutmann B, Cantillo D, Kappe CO. Angew Chem. 2015;127:6788. doi: 10.1002/anie.201409318. [DOI] [PubMed] [Google Scholar]; c) Malet-Sanz L, Susanne F. J Med Chem. 2012;55:4062. doi: 10.1021/jm2006029. [DOI] [PubMed] [Google Scholar]; d) McQuade DT, Seeberger PH. J Org Chem. 2013;78:6384. doi: 10.1021/jo400583m. [DOI] [PubMed] [Google Scholar]; e) Pastre JC, Browne DL, Ley SV. Chem Soc Rev. 2013;42:8849. doi: 10.1039/c3cs60246j. [DOI] [PubMed] [Google Scholar]; f) Plutschack MB, Pieber B, Gilmore K, Seeberger PH. Chem Rev. 2017;117:11796. doi: 10.1021/acs.chemrev.7b00183. [DOI] [PubMed] [Google Scholar]; g) Razzaq T, Kappe CO. Chem - Asian J. 2010;5:1274. doi: 10.1002/asia.201000010. [DOI] [PubMed] [Google Scholar]; h) Sauks JM, Mallik D, Lawryshyn Y, Bender T, Organ M. Org Process Res Dev. 2014;18:1310. [Google Scholar]; i) Shukla CA, Kulkarni AA. Beilstein J Org Chem. 2017;13:960. doi: 10.3762/bjoc.13.97. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Wegner J, Ceylan S, Kirschning A. Adv Synth Catal. 2012;354:17. [Google Scholar]
- 40.Fitzpatrick DE, Ley SV. React Chem Eng. 2016;1:629. doi: 10.1039/c5re00082c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carmona-Vargas CC, de L, Alves C, Brocksom TJ, de Oliveira KT. React Chem Eng. 2017;2:366. [Google Scholar]
- 42.Hartman RL, McMullen JP, Jensen KF. Angew Chem Int Ed. 2011;50:7502. doi: 10.1002/anie.201004637. [DOI] [PubMed] [Google Scholar]
- 43.Hartman RL, McMullen JP, Jensen KF. Angew Chem. 2011;123:7642. doi: 10.1002/anie.201004637. [DOI] [PubMed] [Google Scholar]
- 44.Heider PL, Born SC, Basak S, Benyahia B, Lakerveld R, Zhang H, Hogan R, Buchbinder L, Wolfe A, Mascia S, et al. Org Process Res Dev. 2014;18:402. [Google Scholar]
- 45.a) Mascia S, Heider PL, Zhang H, Lakerveld R, Benyahia B, Barton PI, Braatz RD, Cooney CL, Evans JMB, Jamison TF, et al. Angew Chem Int Ed. 2013;52:12359. doi: 10.1002/anie.201305429. [DOI] [PubMed] [Google Scholar]; b) Mascia S, Heider PL, Zhang H, Lakerveld R, Benyahia B, Barton PI, Braatz RD, Cooney CL, Evans JMB, Jamison TF, et al. Angew Chem. 2013;125:12585. doi: 10.1002/anie.201305429. [DOI] [PubMed] [Google Scholar]
- 46.a) Ventola CL. P & T: a peer-reviewed journal for formulary management. 2014;39:704. [PMC free article] [PubMed] [Google Scholar]; b) Dodziuk H. Polish journal of cardio-thoracic surgery. 2016;13:283. doi: 10.5114/kitp.2016.62625. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Schubert C, van Langeveld MC, Donoso LA. The British journal of ophthalmology. 2014;98:159. doi: 10.1136/bjophthalmol-2013-304446. [DOI] [PubMed] [Google Scholar]
- 47.Merrifield RB. Science. 1965;150:178. doi: 10.1126/science.150.3693.178. [DOI] [PubMed] [Google Scholar]
- 48.Mijalis AJ, Thomas DA, Simon MD, Adamo A, Beaumont R, Jensen KF, Pentelute BL. Nat Chem Biol. 2017;13:464. doi: 10.1038/nchembio.2318. [DOI] [PubMed] [Google Scholar]
- 49.Caruthers MH. Science. 1985;230:281. doi: 10.1126/science.3863253. [DOI] [PubMed] [Google Scholar]
- 50.Plante OJ, Palmacci ER, Seeberger PH. Science. 2001;291:1523. doi: 10.1126/science.1057324. [DOI] [PubMed] [Google Scholar]
- 51.Kent SBH. Chem Soc Rev. 2009;38:338. doi: 10.1039/b700141j. [DOI] [PubMed] [Google Scholar]
- 52.Khorana HG. Science. 1979;203:614. doi: 10.1126/science.366749. [DOI] [PubMed] [Google Scholar]
- 53.Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang R-Y, Algire MA, Benders GA, Montague MG, Ma L, Moodie MM, et al. Science. 2010;329:52. doi: 10.1126/science.1190719. [DOI] [PubMed] [Google Scholar]
- 54.a) Fosgerau K, Hoffmann T. Drug Discovery Today. 2015;20:122. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]; b) Khvorova A, Watts JK. Nat Biotechnol. 2017;35:238. doi: 10.1038/nbt.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seeberger PH, Werz DB. Nat Rev Drug Discovery. 2005;4:751. doi: 10.1038/nrd1823. [DOI] [PubMed] [Google Scholar]
- 56.Corey EJ. Chem Soc Rev. 1988:111. [Google Scholar]
- 57.Corey EJ. Angew Chem Int Ed. 1991;30:455. [Google Scholar]
- 58.Corey EJ, Cheng X-M. Logic of chemical synthesis. Wiley; New York: 1995. [Google Scholar]
- 59.Hendrickson JB. In: ACS Symposium Series. Lankey RL, Anastas PT, editors. American Chemical Society; Washington, DC: 2002. pp. 127–144. [Google Scholar]
- 60.Coley CW, Rogers L, Green WH, Jensen KF. ACS Cent Sci. 2017 doi: 10.1021/acscentsci.7b00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.a) Corey E, Long A, Rubenstein S. Science. 1985;228:408. doi: 10.1126/science.3838594. [DOI] [PubMed] [Google Scholar]; b) Corey EJ, Wipke WT. Science. 1969;166:178. doi: 10.1126/science.166.3902.178. [DOI] [PubMed] [Google Scholar]; c) Huang Q, Li LL, Yang SY. J Chem Inf Model. 2011;51:2768. doi: 10.1021/ci100216g. [DOI] [PubMed] [Google Scholar]; d) Law J, Zsoldos Z, Simon A, Reid D, Liu Y, Khew SY, Johnson AP, Major S, Wade RA, Ando HY. J Chem Inf Model. 2009;49:593. doi: 10.1021/ci800228y. [DOI] [PubMed] [Google Scholar]; e) Satoh K, Funatsu K. J Chem Inf Comput Sci. 1999;39:316. doi: 10.1021/ci980088o. [DOI] [PubMed] [Google Scholar]; f) Szymkuć S, Gajewska EP, Klucznik T, Molga K, Dittwald P, Startek M, Bajczyk M, Grzybowski BA. Angew Chem Int Ed. 2016;55:5904. doi: 10.1002/anie.201506101. [DOI] [PubMed] [Google Scholar]; g) Szymkuć S, Gajewska EP, Klucznik T, Molga K, Dittwald P, Startek M, Bajczyk M, Grzybowski BA. Angew Chem. 2016;128:6004. doi: 10.1002/anie.201506101. [DOI] [PubMed] [Google Scholar]; h) Gelernter H, Rose JR, Chen C. J Chem Inf Comput Sci. 1990;30:492. [Google Scholar]
- 62.Segler MHS, Waller MP. Chemistry (Weinheim an der Bergstrasse, Germany) 2017;23:5966. doi: 10.1002/chem.201605499. [DOI] [PubMed] [Google Scholar]
- 63.a) Liu B, Ramsundar B, Kawthekar P, Shi J, Gomes J, Luu Nguyen Q, Ho S, Sloane J, Wender P, Pande V. ACS Cent Sci. 2017;3:1103. doi: 10.1021/acscentsci.7b00303. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lapkin AA, Heer PK, Jacob PM, Hutchby M, Cunningham W, Bull SD, Davidson MG. Faraday discussions. 2017;202:483. doi: 10.1039/c7fd00073a. [DOI] [PubMed] [Google Scholar]
- 64.Hahm HS, Schlegel MK, Hurevich M, Eller S, Schuhmacher F, Hofmann J, Pagel K, Seeberger PH. Proc Natl Acad Sci U S A. 2017;114:E3385–E3389. doi: 10.1073/pnas.1700141114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Legrand M, Bolla P. J Autom Chem. 1985;7:31. doi: 10.1155/S1463924685000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.a) Sugawara T, Kato S, Okamoto S. J Autom Chem. 1994;16:33. doi: 10.1155/S1463924694000039. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Okamoto H, Deuchi K. Lab Robotics Autom. 2000;12:2. [Google Scholar]
- 67.Orita A, Yasui Y, Otera J. Org Process Res Dev. 2000;4:333. [Google Scholar]
- 68.Orita A, Yasui Y, Otera J. Org Process Res Dev. 2000;4:337. [Google Scholar]
- 69.Orita A, Katakami M, Yasui Y, Otera J, Kurihara A. Green Chem. 2001;3:13. [Google Scholar]
- 70.a) Machida K, Hirose Y, Fuse S, Sugawara T, Takahashi T. Chem Pharm Bull. 2010;58:87. doi: 10.1248/cpb.58.87. [DOI] [PubMed] [Google Scholar]; b) Tanaka Y, Fuse S, Tanaka H, Doi T, Takahashi T. Org Process Res Dev. 2009;13:1111. [Google Scholar]
- 71.Doi T, Fuse S, Miyamoto S, Nakai K, Sasuga D, Takahashi T. Chem - Asian J. 2006;1:370. doi: 10.1002/asia.200600156. [DOI] [PubMed] [Google Scholar]
- 72.Fuse S, Okada K, Iijima Y, Munakata A, Machida K, Takahashi T, Takagi M, Shinya K, Doi T. Org Biomol Chem. 2011;9:3825. doi: 10.1039/c0ob01169j. [DOI] [PubMed] [Google Scholar]
- 73.Masui H, Yosugi S, Fuse S, Takahashi T. Beilstein J Org Chem. 2017;13:106. doi: 10.3762/bjoc.13.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adamo A, Beingessner RL, Behnam M, Chen J, Jamison TF, Jensen KF, Monbaliu J-CM, Myerson AS, Revalor EM, Snead DR, et al. Science. 2016;352:61. doi: 10.1126/science.aaf1337. [DOI] [PubMed] [Google Scholar]
- 75.Heiland JJ, Warias R, Lotter C, Mauritz L, Fuchs PJW, Ohla S, Zeitler K, Belder D. Lab Chip. 2016;17:76. doi: 10.1039/c6lc01217e. [DOI] [PubMed] [Google Scholar]
- 76.Godfrey AG, Masquelin T, Hemmerle H. Drug Discovery Today. 2013;18:795. doi: 10.1016/j.drudis.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 77.a) Kitson PJ, Glatzel S, Chen W, Lin CG, Song YF, Cronin L. Nat Protoc. 2016;11:920. doi: 10.1038/nprot.2016.041. [DOI] [PubMed] [Google Scholar]; b) Kitson PJ, Glatzel S, Cronin L. Beilstein J Org Chem. 2016;12:2776. doi: 10.3762/bjoc.12.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kitson PJ, Marie G, Francoia J-P, Zalesskiy SS, Sigerson RC, Mathieson JS, Cronin L. Science (New York, NY) 2018;359:314. doi: 10.1126/science.aao3466. [DOI] [PubMed] [Google Scholar]
- 79.a) Monaghan T, Harding MJ, Harris RA, Friel RJ, Christie SDR. Lab Chip. 2016;16:3362. doi: 10.1039/c6lc00562d. [DOI] [PubMed] [Google Scholar]; b) Capel AJ, Wright A, Harding MJ, Weaver GW, Li Y, Harris RA, Edmondson S, Goodridge RD, Christie SDR. Beilstein J Org Chem. 2017;13:111. doi: 10.3762/bjoc.13.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coad J. The Portsmouth block mills. Benthan, Brunel and the start of the Royal Navy’s industrial revolution. English Heritage; London: 2005. [Google Scholar]
- 81.Hounshell DA. From the American system to mass production, 1800 – 1932. The development of manufacturing technology in the United States. Hopkins Univ. Press; Baltimore, ca: 1997. [Google Scholar]
- 82.Roe JW. English & American tool builders. The men who created machine tools. Lindsay; Bradley, Il: 1987. [Google Scholar]
- 83.a) Simão JM, Stadzisz PC, Morel G. J Mater Process Technol. 2006;179:268. [Google Scholar]; b) Li XL, Lu JS, Chai GZ, Tang HT. Adv Mater Res. 2010;102–104:776. [Google Scholar]; c) Filho MF, Liao Y, Loures ER, Canciglieri O. Procedia Manufacturing. 2017;11:1471. [Google Scholar]; d) Yu JG, Feng ML, Huang PP, Zhang QZ. Appl Mech Mater. 2011;121–126:4080. [Google Scholar]
- 84.Woerly EM, Roy J, Burke MD. Nat Chem. 2014;6:484. doi: 10.1038/nchem.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Service R. Science. 2017 doi: 10.1126/science.aal1073. [DOI] [Google Scholar]
- 86.Vitaku E, Smith DT, Njardarson JT. J Med Chem. 2014;57:10257. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 87.a) Balieu S, Hallett GE, Burns M, Bootwicha T, Studley J, Aggarwal VK. J Am Chem Soc. 2015;137:4398. doi: 10.1021/ja512875g. [DOI] [PubMed] [Google Scholar]; b) Brown HC, Bhat KS. J Am Chem Soc. 1986;108:5919. doi: 10.1021/ja00279a042. [DOI] [PubMed] [Google Scholar]; c) Burns M, Essafi S, Bame JR, Bull SP, Webster MP, Balieu S, Dale JW, Butts CP, Harvey JN, Aggarwal VK. Nature. 2014;513:183. doi: 10.1038/nature13711. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Crimmins MT, Chaudhary K. Org Lett. 2000;2:775. doi: 10.1021/ol9913901. [DOI] [PubMed] [Google Scholar]; e) Crimmins MT, King BW, Tabet EA, Chaudhary K. J Org Chem. 2001;66:894. doi: 10.1021/jo001387r. [DOI] [PubMed] [Google Scholar]; f) Crudden CM, Ziebenhaus C, Rygus JPG, Ghozati K, Unsworth PJ, Nambo M, Voth S, Hutchinson M, Laberge VS, Maekawa Y, et al. Nat Commun. 2016;7:11065. doi: 10.1038/ncomms11065. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Dechert-Schmitt AMR, Schmitt DC, Gao X, Itoh T, Krische MJ. Nat Prod Rep. 2014;31:504. doi: 10.1039/c3np70076c. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Leonori D, Aggarwal VK. Acc Chem Res. 2014;47:3174. doi: 10.1021/ar5002473. [DOI] [PubMed] [Google Scholar]; i) Lewis JEM, Winn J, Cera L, Goldup SM. J Am Chem Soc. 2016;138:16329. doi: 10.1021/jacs.6b08958. [DOI] [PubMed] [Google Scholar]; j) Mori Y, Nogami K, Hayashi H, Noyori R. J Org Chem. 2003;68:9050. doi: 10.1021/jo035145d. [DOI] [PubMed] [Google Scholar]; k) Myers A, Yang B, Chen H, Kopecky D. Synlett. 1997;1997:457. [Google Scholar]; l) Nicolaou KC, Hale CRH, Nilewski C, Ioannidou HA. Chem Soc Rev. 2012;41:5185. doi: 10.1039/c2cs35116a. [DOI] [PMC free article] [PubMed] [Google Scholar]; m) Nicolaou KC, Daines RA, Uenishi J, Li WS, Papahatjis DP, Chakraborty TK. J Am Chem Soc. 1988;110:4672. [Google Scholar]; n) Noble A, Roesner S, Aggarwal VK. Angew Chem Int Ed. 2016;55:15920. doi: 10.1002/anie.201609598. [DOI] [PMC free article] [PubMed] [Google Scholar]; o) Paterson I, Donghi M, Gerlach K. Angew Chem Int Ed. 2000;39:3315. doi: 10.1002/1521-3773(20000915)39:18<3315::aid-anie3315>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]; p) Paterson I, Scott JP. Tetrahedron Lett. 1997;38:7445. [Google Scholar]; q) Pulis AP, Aggarwal VK. J Am Chem Soc. 2012;134:7570. doi: 10.1021/ja303022d. [DOI] [PubMed] [Google Scholar]; r) Roesner S, Blair DJ, Aggarwal VK. Chem Sci. 2015;6:3718. doi: 10.1039/c4sc03901g. [DOI] [PMC free article] [PubMed] [Google Scholar]; s) Suzuki A, Sasaki M, Nakagishi T, Ueda T, Hoshiya N, Uenishi J-i. Org Lett. 2016;18:2248. doi: 10.1021/acs.orglett.6b00877. [DOI] [PubMed] [Google Scholar]; t) ter Horst B, Feringa BL, Minnaard AJ. Org Lett. 2007;9:3013. doi: 10.1021/ol071078o. [DOI] [PubMed] [Google Scholar]; u) Thomas SP, French RM, Jheengut V, Aggarwal VK. Chem Rec. 2009;9:24. doi: 10.1002/tcr.20168. [DOI] [PubMed] [Google Scholar]; v) Wu J, Lorenzo P, Zhong S, Ali M, Butts CP, Myers EL, Aggarwal VK. Nature. 2017;547:436. doi: 10.1038/nature23265. [DOI] [PubMed] [Google Scholar]; w) Zhang K, Cai L, Jiang X, Garcia-Garibay MA, Kwon O. J Am Chem Soc. 2015;137:11258. doi: 10.1021/jacs.5b07403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoshida J, Maekawa T, Morita Y, Isoe S. J Org Chem. 1992;57:1321. [Google Scholar]
- 89.Evans DA, Clark JS, Metternich R, Novack VJ, Sheppard GS. J Am Chem Soc. 1990;112:866. [Google Scholar]
- 90.a) Xu Z, Kahr M, Walker KL, Wilkins CL, Moore JS. J Am Chem Soc. 1994;116:4537. doi: 10.1016/1044-0305(94)80005-7. [DOI] [PubMed] [Google Scholar]; b) Zhang J, Moore JS, Xu Z, Aguirre RA. J Am Chem Soc. 1992;114:2273. [Google Scholar]; c) Zhang J, Pesak DJ, Ludwick JL, Moore JS. J Am Chem Soc. 1994;116:4227. [Google Scholar]
- 91.Gillis EP, Burke MD. J Am Chem Soc. 2008;130:14084. doi: 10.1021/ja8063759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.a) Lee SJ, Gray KC, Paek JS, Burke MD. J Am Chem Soc. 2008;130:466. doi: 10.1021/ja078129x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Woerly EM, Cherney AH, Davis EK, Burke MD. J Am Chem Soc. 2010;132:6941. doi: 10.1021/ja102721p. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Delaunay T, Es-Sayed M, Vors JP, Monteiro N, Balme G. Chem Lett. 2011;40:1434. doi: 10.1021/ol101087j. [DOI] [PubMed] [Google Scholar]; d) Fujii S, Chang SY, Burke MD. Angew Chem Int Ed. 2011;50:7862. doi: 10.1002/anie.201102688. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Fujii S, Chang SY, Burke MD. Angew Chem. 2011;123:8008. doi: 10.1002/anie.201102688. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Mohamed YMA, Hansen TV. Tetrahedron Lett. 2011;52:1057. [Google Scholar]; g) Dennis EG, Jeffery DW, Johnston MR, Perkins MV, Smith PA. Tetrahedron. 2012;68:340. [Google Scholar]; h) Fujita K, Matsui R, Suzuki T, Kobayashi S. Angew Chem Int Ed. 2012;51:7271. doi: 10.1002/anie.201203093. [DOI] [PubMed] [Google Scholar]; i) Fujita K, Matsui R, Suzuki T, Kobayashi S. Angew Chem. 2012;124:7383. doi: 10.1002/anie.201203093. [DOI] [PubMed] [Google Scholar]; j) Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, Burke MD. Proc Natl Acad Sci U S A. 2012;109:2234. doi: 10.1073/pnas.1117280109. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Grob JE, Dechantsreiter MA, Tichkule RB, Connolly MK, Honda A, Tomlinson RC, Hamann LG. Org Lett. 2012;14:5578. doi: 10.1021/ol302702q. [DOI] [PubMed] [Google Scholar]; l) Igarashi Y, Aoki K, Nishimura H, Morishita I, Usui K. Chem Pharm Bull. 2012;60:1088. doi: 10.1248/cpb.c12-00382. [DOI] [PubMed] [Google Scholar]; m) Lindsay A, Sperry J. Synlett. 2013;24:461. [Google Scholar]; n) Weber A, Dehn R, Schläger N, Dieter B, Kirschning A. Org Lett. 2014;16:568. doi: 10.1021/ol403441c. [DOI] [PubMed] [Google Scholar]; o) Li J, Grillo AS, Burke MD. Acc Chem Res. 2015;48:2297. doi: 10.1021/acs.accounts.5b00128. [DOI] [PMC free article] [PubMed] [Google Scholar]; p) Muir CW, Vantourout JC, Isidro-Llobet A, Macdonald SJF, Watson AJB. Org Lett. 2015;17:6030. doi: 10.1021/acs.orglett.5b03030. [DOI] [PubMed] [Google Scholar]; q) Seath CP, Fyfe JWB, Molloy JJ, Watson AJB. Angew Chem Int Ed. 2015;54:9976. doi: 10.1002/anie.201504297. [DOI] [PubMed] [Google Scholar]; r) Seath CP, Fyfe JWB, Molloy JJ, Watson AJB. Angew Chem. 2015;127:10114. doi: 10.1002/anie.201504297. [DOI] [PubMed] [Google Scholar]; s) Go EB, Wetzler SP, Kim LJ, Chang AY, Vosburg DA. Tetrahedron. 2016;72:3790. [Google Scholar]; t) Davoren JE, Lee CW, Garnsey M, Brodney MA, Cordes J, Dlugolenski K, Edgerton JR, Harris AR, Helal CJ, Jenkinson S, et al. J Med Chem. 2016;59:6313. doi: 10.1021/acs.jmedchem.6b00544. [DOI] [PubMed] [Google Scholar]; u) Brun E, Bellosta V, Cossy J. J Org Chem. 2016;81:8206. doi: 10.1021/acs.joc.6b01166. [DOI] [PubMed] [Google Scholar]; v) Nishioka Y, Yano Y, Kinashi N, Oku N, Toriyama Y, Katsumura S, Shinada T, Sakaguchi K. Synlett. 2017;28:327. [Google Scholar]; w) Cornil J, Echeverria PG, Phansavath P, Ratovelomanana-Vidal V, Guérinot A, Cossy J. Organic letters. 2015;17:948. doi: 10.1021/acs.orglett.5b00042. [DOI] [PubMed] [Google Scholar]
- 93.Li J, Burke MD. J Am Chem Soc. 2011;133:13774. doi: 10.1021/ja205912y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gillis EP, Burke MD. J Am Chem Soc. 2007;129:6716. doi: 10.1021/ja0716204. [DOI] [PubMed] [Google Scholar]
- 95.Gonzalez JA, Ogba OM, Morehouse GF, Rosson N, Houk KN, Leach AG, Cheong PH-Y, Burke MD, Lloyd-Jones GC. Nat Chem. 2016;8:1067. doi: 10.1038/nchem.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ballmer SG, Gillis EP, Burke MD. Org Synth. 2009;86:344. [Google Scholar]
- 97.a) Lee SJ, Anderson TM, Burke MD. Angew Chem Int Ed. 2010;49:8860. doi: 10.1002/anie.201004911. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lee SJ, Anderson TM, Burke MD. Angew Chem. 2010;122:9044. doi: 10.1002/anie.201004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Knapp DM, Gillis EP, Burke MD. J Am Chem Soc. 2009;131:6961. doi: 10.1021/ja901416p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dick GR, Woerly EM, Burke MD. Angew Chem Int Ed. 2012;51:2667. doi: 10.1002/anie.201108608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dick GR, Woerly EM, Burke MD. Angew Chem. 2012;124:2721. doi: 10.1002/anie.201108608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dick GR, Knapp DM, Gillis EP, Burke MD. Org Lett. 2010;12:2314. doi: 10.1021/ol100671v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gillis EP, Burke MD. Aldrichimica acta. 2009;42:17. [PMC free article] [PubMed] [Google Scholar]
- 103.Uno BE, Gillis EP, Burke MD. Tetrahedron. 2009;65:3130. [Google Scholar]
- 104.Struble JR, Lee SJ, Burke MD. Tetrahedron. 2010;66:4710. [Google Scholar]
- 105.Woerly EM, Struble JR, Palyam N, O’Hara SP, Burke MD. Tetrahedron. 2011;67:4333. doi: 10.1016/j.tet.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Woerly EM, Miller JE, Burke MD. Tetrahedron. 2013;69:7732. doi: 10.1016/j.tet.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.a) Brak K, Ellman JA. J Org Chem. 2010;75:3147. doi: 10.1021/jo100318s. [DOI] [PubMed] [Google Scholar]; b) Chan JMW, Amarante GW, Toste FD. Tetrahedron. 2011;67:4306. doi: 10.1016/j.tet.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Close AJ, Kemmitt P, Mark Roe S, Spencer J. Org Biomol Chem. 2016;14:6751. doi: 10.1039/c6ob01141a. [DOI] [PubMed] [Google Scholar]; d) He Z, Yudin AK. J Am Chem Soc. 2011;133:13770. doi: 10.1021/ja205910d. [DOI] [PubMed] [Google Scholar]; e) Lee CF, Holownia A, Bennett JM, Elkins JM, St Denis JD, Adachi S, Yudin AK. Angew Chem Int Ed. 2017;56:6264. doi: 10.1002/anie.201611006. [DOI] [PubMed] [Google Scholar]; f) Lee CF, Holownia A, Bennett JM, Elkins JM, St Denis JD, Adachi S, Yudin AK. Angew Chem. 2017;129:6360. doi: 10.1002/anie.201611006. [DOI] [PubMed] [Google Scholar]; g) Lv WX, Zeng YF, Li Q, Chen Y, Tan DH, Yang L, Wang H. Angew Chem Int Ed. 2016;55:10069. doi: 10.1002/anie.201604898. [DOI] [PubMed] [Google Scholar]; h) Lv WX, Zeng YF, Li Q, Chen Y, Tan DH, Yang L, Wang H. Angew Chem. 2016;128:10223. doi: 10.1002/anie.201604898. [DOI] [PubMed] [Google Scholar]; i) Quiclet-Sire B, Zard SZ. J Am Chem Soc. 2015;137:6762. doi: 10.1021/jacs.5b03893. [DOI] [PubMed] [Google Scholar]; j) Seath CP, Wilson KL, Campbell A, Mowat JM, Watson AJB. Chem Commun (Cambridge, U K) 2016;52:8703. doi: 10.1039/c6cc04554e. [DOI] [PubMed] [Google Scholar]; k) St Denis JD, He Z, Yudin AK. Org Biomol Chem. 2012;10:7900. doi: 10.1039/c2ob26503f. [DOI] [PubMed] [Google Scholar]; l) Trinchera P, Corless VB, Yudin AK. Angew Chem Int Ed. 2015;54:9038. doi: 10.1002/anie.201504271. [DOI] [PubMed] [Google Scholar]; m) Trinchera P, Corless VB, Yudin AK. Angew Chem. 2015;127:9166. doi: 10.1002/anie.201504271. [DOI] [PubMed] [Google Scholar]
- 108.Li J, Ballmer SG, Gillis EP, Fujii S, Schmidt MJ, Palazzolo AME, Lehmann JW, Morehouse GF, Burke MD. Science. 2015;347:1221. doi: 10.1126/science.aaa5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Service RF. Science. 2015;347:1190. doi: 10.1126/science.347.6227.1190. [DOI] [PubMed] [Google Scholar]
- 110.Burke Hopes to Cure Disease, “Hook” Students, Via Molecule-Making Machine. 2015 can be found under https://www.istem.illinois.edu/news/burke.html.
- 111.a) Fabry DC, Sugiono E, Rueping M. React Chem Eng. 2016;1:129. [Google Scholar]; b) Reizman BJ, Jensen KF. Acc Chem Res. 2016;49:1786. doi: 10.1021/acs.accounts.6b00261. [DOI] [PubMed] [Google Scholar]; c) Winicov H, Schainbaum J, Buckley J, Longino G, Hill J, Berkoff CE. Analytica Chimica Acta. 1978;103:469. [Google Scholar]; d) Yadav MK. New J Chem. 2017;41:1411. [Google Scholar]
- 112.a) King RD, Whelan KE, Jones FM, Reiser PGK, Bryant CH, Muggleton SH, Kell DB, Oliver SG. Nature. 2004;427:247. doi: 10.1038/nature02236. [DOI] [PubMed] [Google Scholar]; b) King RD, Rowland J, Oliver SG, Young M, Aubrey W, Byrne E, Liakata M, Markham M, Pir P, Soldatova LN, et al. Science. 2009;324:85. doi: 10.1126/science.1165620. [DOI] [PubMed] [Google Scholar]
- 113.Williams K, Bilsland E, Sparkes A, Aubrey W, Young M, Soldatova LN, de Grave K, Ramon J, de Clare M, Sirawaraporn W, et al. J R Soc, Interface. 2015;12:20141289. doi: 10.1098/rsif.2014.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.a) Kilpin KJ, Goodman JM, Johnson AP, Whitby RJ. Chem Cent J. 2015;9:49. doi: 10.1186/s13065-015-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kilpin KJ, Whitby RJ. Chem Cent J. 2015;9:43. doi: 10.1186/s13065-015-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kirkpatrick K. Commun ACM. 2015;58:13. [Google Scholar]; d) Sans V, Cronin L. Chem Soc Rev. 2016;45:2032. doi: 10.1039/c5cs00793c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reizman BJ, Wang Y-M, Buchwald SL, Jensen KF. React Chem Eng. 2016;1:658. doi: 10.1039/c6re00153j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sans V, Porwol L, Dragone V, Cronin L. Chem Sci. 2015;6:1258. doi: 10.1039/c4sc03075c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hone CA, Holmes N, Akien GR, Bourne RA, Muller FL. React Chem Eng. 2017;2:103. doi: 10.1039/c6re00109b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.a) Holmes N, Akien GR, Blacker AJ, Woodward RL, Meadows RE, Bourne RA. React Chem Eng. 2016;1:366. [Google Scholar]; b) Holmes N, Akien GR, Savage RJD, Stanetty C, Baxendale IR, Blacker AJ, Taylor BA, Woodward RL, Meadows RE, Bourne RA. React Chem Eng. 2016;1:96. [Google Scholar]
- 119.Silver D, Huang A, Maddison CJ, Guez A, Sifre L, van den Driessche G, Schrittwieser J, Antonoglou I, Panneershelvam V, Lanctot M, et al. Nature. 2016;529:484. doi: 10.1038/nature16961. [DOI] [PubMed] [Google Scholar]
- 120.a) de Luna P, Wei J, Bengio Y, Aspuru-Guzik A, Sargent E. Nature. 2017;552:23. doi: 10.1038/d41586-017-07820-6. [DOI] [PubMed] [Google Scholar]; b) Gómez-Bombarelli R, Aguilera-Iparraguirre J, Hirzel TD, Duvenaud D, Maclaurin D, Blood-Forsythe MA, Chae HS, Einzinger M, Ha DG, Wu T, et al. Nature materials. 2016;15:1120. doi: 10.1038/nmat4717. [DOI] [PubMed] [Google Scholar]
- 121.Hernandez D. Wall Street Journal. Jun 25, 2017. How AI Is Transforming Drug Creation. [Google Scholar]