Abstract
Background
Constraint-Induced Movement therapy (CIMT) is a method of physical rehabilitation that has demonstrated clinical efficacy in patients with chronic stroke, cerebral palsy, and multiple sclerosis (MS).
Objective
This pilot randomized controlled trial tested whether CIMT can also induce increases in white matter integrity in patients with MS.
Methods
Twenty adults with chronic hemiparetic MS were randomized to receive either CIMT or Complementary and Alternative Medicine (CAM) treatment (reported in first paper of this pair). Structural white matter change was assessed by Tract-Based Spatial Statistics (TBSS); measures included fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD).
Results
CIMT and CAM groups did not differ in pre-treatment disability or expectancy to benefit. As noted in the companion paper, the Motor Activity Log (MAL) improved more after CIMT than CAM (p < 0.001); the within-group effect size for CIMT was 3.7 (large d′ = 0.57), while for CAM it was just 0.7. Improvements in white matter integrity followed CIMT and were observed in the contralateral corpus callosum (FA, p < .05), ipsilateral superior occipital gyrus (AD, p < 0.05), ipsilateral superior temporal gyrus (FA, p < 0.05), and contralateral corticospinal tract (MD and RD, p < 0.05).
Conclusion
CIMT produced a very large improvement in real-world limb use and induced white matter changes in patients with hemiparetic MS when compared to CAM. The findings suggest in preliminary fashion that the adverse changes in white matter integrity induced by MS might be reversed by CIMT.
Keywords: multiple sclerosis, rehabilitation, magnetic resonance imaging, white matter, Constraint-Induced Movement therapy, Complementary and Alternative Medicine
Introduction
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system. Among many clinical consequences, MS may manifest as a disabling hemiparesis.1 White matter integrity of the central nervous system is reduced progressively in MS patients.2–5 The motor disability correlates with the white matter tract injury as indexed by markers of white matter tract integrity including fractional anisotropy.6
Multiple studies have shown that some physical rehabilitation procedures can have a positive effect on impaired motor function in patients with MS, though the treatment effects are usually not large and their retention over time and transfer to spontaneous movement in real-world situations has rarely been tested.7, 8 Several different rehabilitation procedures have also been found to preserve or increase white matter integrity. Temporarily improved white matter structure and postural control has been shown to follow balance training for persons with MS.9 Two months of limb stretching combined with resistance training in moderately impaired persons has been associated with significant corpus callosum structural improvement and nonsignificant improvement on the Expanded Disability Status Scale.10 Task-oriented training has been shown to be followed by preserved white matter structure and improved finger tapping, in comparison to passive arm mobilization, which instead was followed by deterioration of both white matter structure and finger tapping over the same interval.11
Constraint-Induced Movement therapy (CIMT) is a behavior-based neurorehabilitation derived from basic research with monkeys by Taub and colleagues12 that has been shown to produce large, clinically-significant improvements in motor function following different types of central nervous system injuries, including stroke,13–15 traumatic brain injury,16 cerebral palsy,17, 18 focal hand dystonia in musicians,19 and post-stroke nonfluent aphasia.20, 21 CIMT has been found to be particularly effective in improving the spontaneous real-world use of a more-affected extremity.22, 23 It is thought that the treatment effect of CIMT involves two primary mechanisms: (1) a behavioral mechanism that involves overcoming learned nonuse;12, 22 and (2) a neurological mechanism involving facilitating neuroplastic change24 (e.g., changes in gray matter structure in both adults with chronic stroke25 and children with cerebral palsy26). Of importance, CIMT is a promising treatment for improving arm function in individuals with hemiparesis due to MS.27 Other physical rehabilitation treatments have been employed with MS and have been reported to improve within-laboratory motor capabilities, but little attention has been paid to real-world function and community participation, which is commonly considered to be the critical objective of physical rehabilitation.
The aim of this study was to determine whether CIMT improves white matter integrity in MS patients. We compared whole-brain white matter fractional anisotropy of patients with chronic MS, along with three other indices of white matter health, in a pilot randomized controlled trial of CIMT vs. a program of Complementary and Alternative Medicine (CAM) interventions that are highly desired by patients. We hypothesized that improvements in spontaneous real-world use of the more-affected upper extremity produced by CIMT (Part I) would be paralleled by improvements in white matter integrity.
Methods
For additional details to those given below on patients, CIMT, and clinical outcome measures, refer to Part I.
Patients
Twenty adult participants with MS and mild-moderate upper extremity hemiparesis were randomized to receive either CIMT (n = 10) or a set of CAM treatments (n = 10). Inclusion/exclusion criteria are described in the companion article (Part I). Written informed consent was obtained from each participant, and the Institutional Review Board at the University of Alabama at Birmingham approved the study procedures.
Inclusion criteria were: 1) mild/moderate upper extremity hemiparesis, defined as the ability to lift the more-affected arm from a table to at least shoulder height, extend the digits and wrist at least 10 degrees, and grasp and release a small object (e.g., a hand towel); 2) upper extremity deficit resulting entirely from MS as diagnosed by the primary physician and confirmed by the project neurologist (V.M.); 3) no relapses for at least the past 3 months; 4) Motor Activity Log (see below) score less than 3/5, demonstrating significantly reduced use of the more-affected arm in the real-life situation; 5) MiniMental State Exam score ≥ 23/30; and 6) ability to tolerate 3.5 hours/day of intensive physical therapy for 10 consecutive weekdays. Patients were excluded if they: 1) had ferromagnetic metal in their body or any other factor that precluded MRI; 2) were participants in concurrent physical therapy/movement research; or 3) had been previously exposed to CIMT.
Interventions
Constraint-Induced Movement Therapy (CIMT)
Patients randomized to CIMT received its four major components of treatment23: 1) intensive training of the more-impaired arm administered in the laboratory for 3 hours/day for 10 consecutive weekdays; 2) training by the behavioral technique termed shaping; 3) a “transfer package” of behavioral procedures to facilitate transfer of the motor improvement to everyday life situations (an additional 0.5 hr/d) and 4) a heavily padded restraining mitt worn for a target of 90% of waking hours to reduce use of the less affected arm. (See 23 for further details)
Complementary and Alternative Medicine (CAM)
The participants in the comparison group received a set of CAM treatments administered by a recreational therapist for the same duration that participants in the CIMT group received therapy (i.e., 3.5 hours/day for 10 consecutive weekdays). Patients were given relaxation exercises, aquatic therapy, massage, music therapy, and yoga. These specific techniques were chosen because they are commonly sought by adults with MS,28 thus permitting control for expectancy to benefit.
Clinical Outcome Measures
Patients were administered the Motor Activity Log and the Wolf Motor Function Test before and after the therapy course to assess treatment efficacy. For participants who were assigned to CIMT, the Motor Activity Log was also administered daily to aid therapists in tracking progress during treatment. The Motor Activity Log administered daily is a reliable and valid scripted, structured interview that measures spontaneous functional use of the more-affected arm outside the treatment setting.13, 29 The Wolf Motor Function Test is a reliable and valid laboratory motor function test.30–32 It measures the speed of movement by the more-impaired arm on 15 standard, simple movements or tasks made on request.
Magnetic Resonance Imaging (MRI)
Anatomical Images
T1-weighted 3-dimensional whole brain anatomical images were acquired on a 3 Tesla Siemens Magnetom MRI system using the Magnetization Prepared Rapid Acquisition Gradient-Echo (MPRAGE) sequence in the week prior to the start of treatment and again in the week after the end of treatment. Imaging parameters were: field of view FOV (FOV) = 240 × 240 mm, 176 sagittal partitions, slice thickness = 1 mm gapless, voxel size = 1.3 × 1.0 × 1.0 mm3, flip angle = 8°, repetition time (TR) = 2080 ms, echo time (TE) = 3.93 ms. The T1 scan was performed both at the start and at the end of each scan session and the best-quality image was used.
Diffusion Tensor Imaging
During the same pre- and post-treatment sessions in which the T1 scan was performed, whole-brain diffusion-weighted images were also acquired using single shot echo planar imaging with the following parameters: 38 slices 3 mm thick with a 1 mm gap between slices, TR = 5600 ms, TE = 96 ms, voxel size = 1.6 × 1.6 × 3.0 mm3, FOV = 210 × 210 mm, diffusion-weighted images in 32 distinct directions with b-value = 1250 s/mm2 and one image with no diffusion weighting and b-value = 0 s/mm2.
MRI Data Pre-processing
Investigators were blinded to group membership and pre- or post-treatment status. Images were first converted from Digital Imaging and Communications in Medicine (DICOM; http://medical.nema.org/) to the Neuroimaging Informatics Technology Initiative (NifTI-1; http://nifti.nimh.nih.gov/) data format which required using the dcm2niigui program in MRIcron.33 Since the diffusion weighted images were acquired one gradient direction at a time, the resulting data were separated into thirty-three separate images (32 images with diffusion weighting and one image with no diffusion weighting). The 3-dimensional images were converted to a four-dimensional image with the gradient direction as the fourth dimension. Next, skull tissue from the four-dimensional image was removed using the 3DSkullStrip tool in the Analysis of Functional NeuroImages (AFNI) software package.34 The anatomical images were also skull-stripped using this method. Data alignment of the T1 and diffusion-tensor imaging scans was performed for each subject also using AFNI. Diffusion weighted images for both pre- and post-therapy sessions were motion-corrected and averaged for alignment with the T1 using the local Pearson correlation method.35 Eddy current distortions were corrected via 3-dimensional affine registration to the first-acquired diffusion weighted image with no diffusion weighting; further polar decomposition was performed using Octave (www.octave.org) and gradient corrections along with head motion were corrected as previously described.36 While fractional anisotropy measures the directionality of water movement, mean diffusivity, axial diffusivity, and radial diffusivity represent the average amount of water diffusion, parallel diffusivity, and perpendicular diffusivity, respectively. Myelin gain decreases mean diffusivity and radial diffusivity values, while compensatory mechanisms after white matter damage increase axial diffusivity values.37 Fractional anisotropy and the diffusion magnitudes (eigenvalues λ1, λ2, and λ3) were derived from pre-processed diffusion tensor imaging data using AFNI’s 3dDWItoDT program and used to determine mean diffusivity ([λ1+ λ2+ λ3]/3), axial diffusivity (A1), and radial diffusivity ([λ2+ λ3]/2).
Whole brain diffusion tensor imaging data were further analyzed by employing a voxelwise approach using Tract-Based Spatial Statistics (TBSS, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS)38 within the FMRIB software library (FSL)39. Before loading patient scans into the TBSS pipeline, hemispheres were flipped in MATLAB (http://www.mathworks.com/products/matlab/) so that the hemisphere ipsilateral to the trained arm was always on the same side of the brain. To carry out within-participant comparisons from pre-treatment to post-treatment and between-group comparisons at pre-treatment, a mean fractional anisotropy image from the sample of participants in the current study was created and thinned to make a mean fractional anisotropy skeleton. This skeletonized fractional anisotropy image represents the centers of all white matter tracts common to the group. However, before a skeletonized image could be represented, each fractional anisotropy image from each subject and each session (pre- and post-treatment) was aligned to every other fractional anisotropy image to identify a within-groups specific target image, which then underwent affine transformation into 1 mm3 MNI152 standard space. Each participant’s aligned fractional anisotropy data were projected onto the fractional anisotropy skeleton and the resulting data were analyzed using voxelwise statistics. Fractional anisotropy values at each voxel could then be calculated for each participant’s pre-treatment and post-treatment data. The fractional anisotropy transformations described above were applied to mean diffusivity, axial diffusivity, and radial diffusivity and projected onto the fractional anisotropy skeleton; paired t-tests were then performed using the skeletonized medial diffusivity, axial diffusivity, and radial diffusivity maps.
Statistical Analyses
To compare voxelwise white matter changes from pre- to post-treatment across treatment conditions, we tested the within-group simple main effect of time-point using paired t-tests within the full model (i.e., the values for each index from the full sample). Because we hypothesized a positive treatment change based on prior data, all TBSS analyses were one-tailed, conducted at α = 0.05, and fully corrected for multiple comparisons across space. Structures were identified using the MNI atlas of human white matter.40
Pearson correlations were used to quantify the correspondence between (a) changes in FA in regions in which significant pre- to post-treatment white matter changes were observed and (b) changes in the clinical measures, i.e., the Motor Activity Log and Wolf Motor Function Test. Fractional anisotropy values for the corticospinal tract and superior temporal gyrus were transformed because their distributions violated normality [corticospinal tract fractional anisotropy values, W(20) = 0.58, p < 0.001; superior temporal gyrus white matter fractional anisotropy values, W(20) = 0.85, p < 0.01]. Since there was a single outlying value from different participants for each variable (corticospinal tract outlier: z = 3.87; superior temporal gyrus outlier: z = 3.21), these outlying values were truncated to 3 SD. A square root transformation was applied to the superior temporal gyrus values in addition to render their distribution normal.
Results
Clinical
As noted in Paper I, treatment groups were similar in age, MS chronicity, and pre-treatment motor ability (all t(18) < 1.66 and p > 0.1). There was a very large treatment effect in favor of CIMT on the Motor Activity Log [F(1,17) = 31.3, p < 0.001, covariate-adjusted between-group effect size = 1.6]. The mean improvement on the Motor Activity Log after CIMT was 2.7±0.7 points (within-group effect size = 3.7), whereas after CAM it was only 0.5±0.8 points (within-group effect size = 0.7). Mean improvement on the Wolf Motor Function Test was similar for participants in both groups [F(1, 17) = 0.25,n.s.]: after CIMT it was 6.4±5.4 repetitions per minute; after CAM it was 6.3±7.5.
Imaging
We observed significant pre- to post-treatment white matter improvements for the CIMT group but not the CAM group. In the CIMT group, clusters of fractional anisotropy increases were observed in the posterior corpus callosum near the callosal-septal interface contralateral to the trained upper extremity (corrected p < 0.05), as well as the white matter of the superior occipital gyrus (p < 0.05) ipsilateral to the trained upper extremity (Figure 1). For the other diffusion indices (Figure 2), axial diffusivity increases were seen in the ipsilateral superior temporal gyrus (corrected p < 0.05), and mean diffusivity and radial diffusivity decreases were seen in the contralateral corticospinal tract (corrected p < 0.05). Due to the relatively small treatment groups, and the likelihood that there may be more subtle changes in neuronal plasticity, we lowered the family-wise threshold for a Type I statistical error, i.e., α, to 0.1 to examine additional regions showing group differences in white matter integrity. Additional clusters of fractional anisotropy increases were observed in the corticospinal tract contralateral to the trained upper extremity (corrected p < 0.1) and the superior temporal gyrus white matter ipsilateral to the trained upper extremity (corrected p < 0.1). There were also axial diffusivity increases in the corpus callosum (p < 0.1). No significant pre- to posttreatment changes were observed in the CAM group in any of the indices (data not shown).
Figure 1. Changes in Fractional Anisotropy (FA) following CI Movement therapy (CIMT).
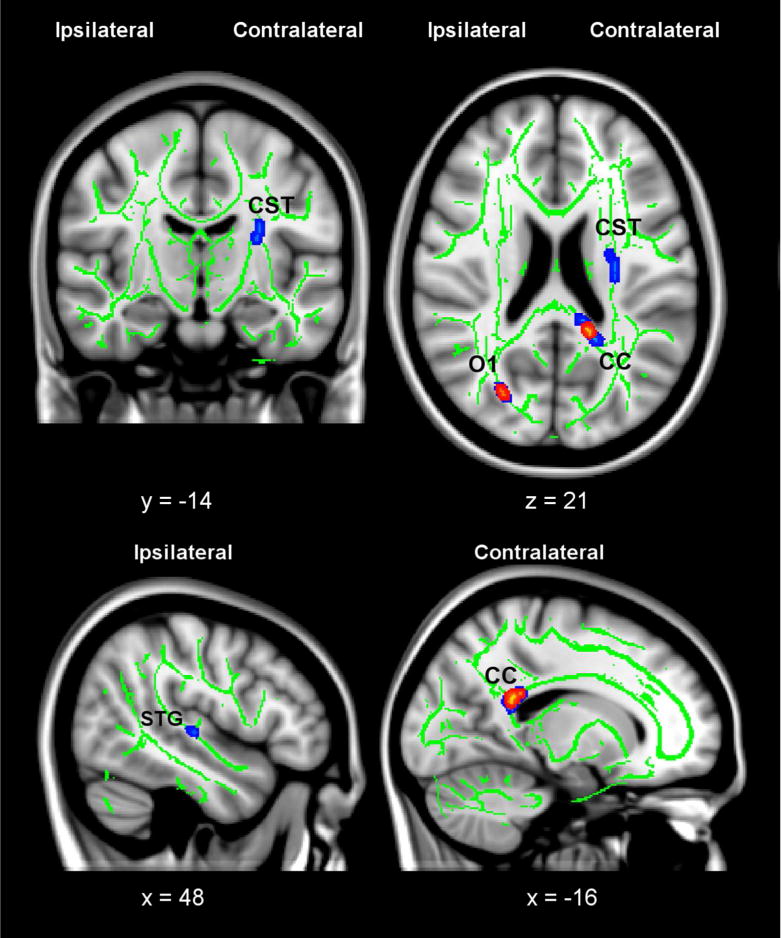
Clusters with significant pre- to post-treatment increases in FA are thickened and shown in red/yellow (corrected p < 0.05) and blue (corrected p < 0.1). In the coronal and axial slices, right on the images corresponds to the side of the brain contralateral to the trained upper extremity. Significant clusters of FA increases were observed in the ipsilateral posterior corpus callosum (CC) and contralateral superior occipital gyrus white matter (O1). After lowering the statistical threshold after correction to p = 0.1, additional clusters of FA increases were observed in the ipsilateral corticospinal tract (CST) and contralateral superior temporal gyrus white matter (STG). The green skeleton represents the center of white matter tracts common to all participants (i.e., both CIMT and CAM groups) with FA ≥ 0.2; the skeleton is superimposed on the standard MNI152 T1-weighted anatomical image. Inlaid white text indicates MNI coordinates of the slice.
Figure 2. Changes in diffusivity indices (AD, MD, RD) following CI Movement therapy (CIMT).
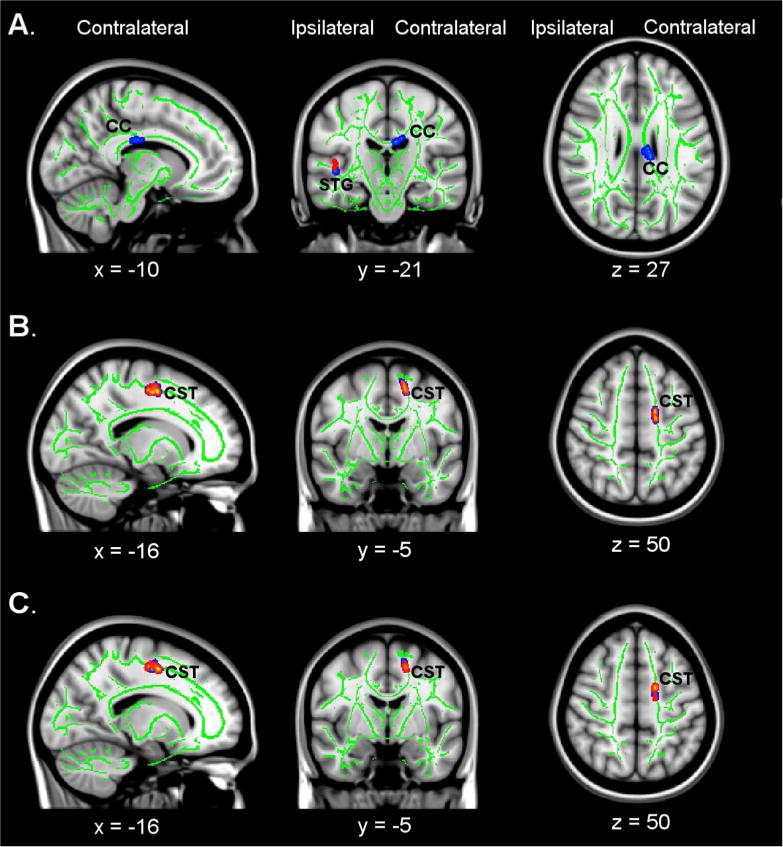
Clusters with significant pre- to post-treatment changes are thickened and shown in red/yellow (corrected p < 0.05) and blue (corrected p < 0.1). A) Increases in axial diffusivity (AD) were seen in the ipsilateral STG (p < 0.05) and the contralateral CC (p < 0.1). B) Decreases in mean diffusivity (MD) were seen in the contralateral corticospinal tract (CST) (p < 0.05). C) A decrease in radial diffusivity (RD) was seen in the contralateral CST (p < 0.05). Coordinates are given for the standard MNI152 T1-weighted anatomical image.
Improvements in real-world arm use, as measured by the Motor Activity Log, were strongly correlated with increased white matter integrity in the corpus callosum (r = 0.61, p < 0.01) and occipital gyrus (r = 0.57, p < 0.01). For the corticospinal tract, the correlation was moderate but fell below the threshold for statistical significance by a small margin (r = 0.42, p = 0.07). For the superior temporal gyrus, the correlation was small and was not significant (r = 0.27, p = 0.24). A multivariate approach to the analysis yielded a parallel result. Changes in motor capacity of the more-affected arm, as measured by the Wolf Motor Function Test, were not correlated with changes in white matter integrity.
Discussion
In this study we sought to determine whether CIMT improves white matter integrity in MS patients. The present results indicate that CIMT produced changes in white matter characteristics in patients with MS in the corticospinal tract and in temporal, callosal, and visual areas. The CIMT group also showed very large improvement in real- world arm use of the more-affected extremity (Motor Activity Log scores) relative to the CAM group (described in the companion study, Part I). This is consistent with previous demonstrations of the efficacy of CIMT in improving motor function after other types of central nervous system damage such as stroke,13–15 traumatic brain injury,16 and cerebral palsy17, 18. Similar white matter integrity changes, i.e., increases in fractional anisotropy of the contralateral corticospinal tract, were observed after CIMT in six of eight children with cerebral palsy.41 Though this change was not statistically significant, power to detect changes was limited in that study by the small sample size, relatively weak magnet (i.e., 1.5 Tesla), and large lesion size; this was also the case for a small sample of stroke patients in the same paper.41
Diffusion tensor analyses have previously detected whole brain and tract-specific changes in the white matter integrity of individuals with MS due to disease progression that are not present in healthy controls.5,42,43 Diffusion tensor imaging has also shown that lesion overlap with the corticospinal tract is related to poorer motor function in individuals with MS44 and that the diffusion integrity of the corticospinal tract was compromised in patients with MS relative to controls.45–47 The present study extends this line of investigation by suggesting that these adverse structural white matter changes can be reversed by CIMT.
The white matter changes observed here occurred within the two weeks that CI therapy was administered. While this is a relatively brief period, it is consistent with the time frame over which large neuroplastic changes were produced by somatosensory training in healthy new world monkeys by Merzenich and co-workers,48 and by motor training by Nudo and co-workers49 in new world monkeys given partial motor cortex ablations. The speed with which the neuroplastic change took place in the latter study49 was probably facilitated by the fact that increased use of the more-affected upper extremity was not confined to the 10 three-hour training sessions in the laboratory, but also included the increased use of that limb during waking hours in the real-world life situation produced by the consistent use of a restraining device on the less-affected arm.
The factors that governed the location and laterality of the white matter changes in our CIMT patients can only be speculated on at this time. The structural increase in the corticospinal tract contralateral to the trained arm is plausible in view of the concentrated practice that was required of that extremity.
A significant cluster of white matter integrity change was also observed in the contralateral posterior callosal-septal junction following CIMT. A previous study of 42 patients with MS found that 92% had focal lesions in the callosal-septal interface, thereby disrupting the fibers radiating to the overlying callosum.50 Another MRI study found axon damage and demyelination in the corpus callosum of MS patients.51 These findings suggest that this is an area that is susceptible to damage in MS, and hence this area might respond more vigorously to an appropriate therapeutic intervention. Consistent with this study, Ibrahim et al. found improvements in white matter integrity of the corpus callosum in eleven MS patients following facilitation physical therapy.10 Thus, it may be that the damaged brain distributes the burden of supporting the improved movement required by the therapy by recruiting the ipsilateral hemisphere, which is not typically importantly involved in producing those movements. A direct way of accomplishing this interhemispheric recruitment would be via the corpus callosum, which we observed to have white matter improvements pre- to post-CIMT (i.e., increased fractional anisotropy and axial anisotropy). The same mechanism may account for the increase in white matter integrity in ipsilateral temporal cortex.
The white matter of the superior occipital gyrus was another site of significant fractional anisotropy increases in the CIMT group in this experiment. Ungerleider and Mishkin first described the dorsal stream of visual processing connecting the V1 area to the parietal cortex.52 It has since been shown to mediate visually guided arm movements.53, 54 Because arm movements are targeted for improvement in upper extremity CIMT and were under visual guidance in our patients, the increased fractional anisotropy underlying the occipital cortex in our CIMT patients may have reflected the greater reliance on visual attention in their therapeutic activities, compared to the relative lack of specificity for hand-eye coordination in the CAM procedures that were used.
There were several limitations in the present study. The number of participants per treatment group was relatively small, and they had a variety of non-overlapping white matter lesions, making the comparisons between groups less sensitive to small changes. Thus the marginal changes in the present study (i.e., corticospinal tract and temporal lobe) should be reassessed in a follow-up study with a larger sample size. Additionally, white matter changes in the TBSS skeleton in each hemisphere might be found to be more symmetric in a larger patient sample. Moreover, the detection of changes is biased toward areas that are devoid of crossing fibers, such as the corpus callosum and the corticospinal tract,55 because diffusion tensor imaging has good resolution in such areas, while training-associated changes in other areas where diffusion tensor imaging has poor resolution may have been obscured.
Conclusion
Notwithstanding these limitations, the present experiment shows that there are positive changes in regional white matter indices and overall improvement in white matter integrity following CIMT in patients with MS that correspond to the large improvement in real-world use of a more-affected upper extremity in chronic stroke patients that has been previously observed (see Part I).
Acknowledgments
Funding. This work was supported by grant RG 4221 from the National Multiple Sclerosis Society and HD061767 from the National Institutes of Health.
Clinical trial registration number: ClinicalTrials.gov (NCT01081275)
Footnotes
Declaration of Conflicting Interests. None of the authors declare conflicting interests.
References
- 1.Bertoni R, Lamers I, Chen CC, Feys P, Cattaneo D. Unilateral and bilateral upper limb dysfunction at body functions, activity and participation levels in people with multiple sclerosis. Mult Scler. 2015;21:1566–1574. doi: 10.1177/1352458514567553. [DOI] [PubMed] [Google Scholar]
- 2.Lenzi D, Conte A, Mainero C, et al. Effect of corpus callosum damage on ipsilateral motor activation in patients with multiple sclerosis: a functional and anatomical study. Hum Brain Mapp. 2007;28:636–644. doi: 10.1002/hbm.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tallantyre EC, Bø L, Al-Rawashdeh O, et al. Clinico-pathological evidence that axonal loss underlies disability in progressive multiple sclerosis. Mult Scler. 2010;16:406–411. doi: 10.1177/1352458510364992. [DOI] [PubMed] [Google Scholar]
- 4.Ciccarelli O, Werring DJ, Barker GJ, et al. A study of the mechanisms of normalappearing white matter damage in multiple sclerosis using diffusion tensor imaging-evidence of Wallerian degeneration. J Neurol. 2003;250:287–292. doi: 10.1007/s00415-003-0992-5. [DOI] [PubMed] [Google Scholar]
- 5.Harrison DM, Caffo BS, Shiee N, et al. Longitudinal changes in diffusion tensor-based quantitative MRI in multiple sclerosis. Neurology. 2011;76:179–186. doi: 10.1212/WNL.0b013e318206ca61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filippi M, Iannucci G, Cercignani M, Assunta Rocca M, Pratesi A, Comi G. A quantitative study of water diffusion in multiple sclerosis lesions and normal-appearing white matter using echo-planar imaging. Arch Neurol. 2000;57:1017–1021. doi: 10.1001/archneur.57.7.1017. [DOI] [PubMed] [Google Scholar]
- 7.Haselkorn JK, Hughes C, Rae-Grant A, et al. Summary of comprehensive systematic review: rehabilitation in multiple sclerosis. Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2015;85:1896–1903. doi: 10.1212/WNL.0000000000002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamers I, Maris A, Severijns D, et al. Upper limb rehabilitation in people with multiple sclerosis: a systematic review. Neurorehabil Neural Repair. 2016;30:773–793. doi: 10.1177/1545968315624785. [DOI] [PubMed] [Google Scholar]
- 9.Prosperini L, Fanelli F, Petsas N, et al. Multiple sclerosis: changes in microarchitecture of white matter tracts after training with a video game balance board. Radiology. 2014;273:529–538. doi: 10.1148/radiol.14140168. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim I, Tintera J, Skoch A, et al. Fractional anisotropy and mean diffusivity in the corpus callosum of patients with multiple sclerosis: the effect of physiotherapy. Neuroradiology. 2011;53:917–926. doi: 10.1007/s00234-011-0879-6. [DOI] [PubMed] [Google Scholar]
- 11.Bonzano L, Tacchino A, Brichetto G, et al. Upper limb motor rehabilitation impacts white matter microstructure in multiple sclerosis. Neuroimage. 2014;90:107–116. doi: 10.1016/j.neuroimage.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Taub E. Somatosensory deafferentation research with monkeys: implications for rehabilitation medicine. In: Ince LP, editor. Behavioral Psychology in Rehabilitation Medicine: Clinical Applications. Baltimore: Williams and Wilkins; 1980. pp. 371–401. [Google Scholar]
- 13.Taub E, Miller NE, Novack TA, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. [PubMed] [Google Scholar]
- 14.Taub E, Uswatte G, King DK, Morris D, Crago JE, Chatterjee A. A placebo-controlled trial of Constraint-Induced Movement therapy for upper extremity after stroke. Stroke. 2006;37:1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. [DOI] [PubMed] [Google Scholar]
- 15.Wolf SL, Winstein CJ, Miller JP, et al. Effect of Constraint-Induced Movement Therapy on upper extremity function 3 to 9 months after stroke. The EXCITE Randomized Clinical Trial. J Am Med Assoc. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 16.Shaw SE, Morris DM, Uswatte G, McKay S, Meythaler JM, Taub E. Constraint-induced movement therapy for recovery of upper-limb function following traumatic brain injury. J Rehabil Res Dev. 2005;42:769–778. doi: 10.1682/jrrd.2005.06.0094. [DOI] [PubMed] [Google Scholar]
- 17.Taub E, Ramey SL, DeLuca S, Echols K. Efficacy of Constraint-Induced Movement Therapy for children with cerebral palsy with asymmetric motor impairment. Pediatrics. 2004;113:305–312. doi: 10.1542/peds.113.2.305. [DOI] [PubMed] [Google Scholar]
- 18.Taub E, Griffin A, Uswatte G, Gammons K, Nick J, Law CR. Treatment of congenital hemiparesis with Constraint-Induced Movement therapy. J Child Neurol. 2011;26:1163–1173. doi: 10.1177/0883073811408423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Candia V, Elbert T, Altenmuller E, Rau H, Schäfer T, Taub E. Constraint-induced movement therapy for focal hand dystonia in musicians. Lancet. 1999;353:42. doi: 10.1016/S0140-6736(05)74865-0. [DOI] [PubMed] [Google Scholar]
- 20.Pulvermuller F, Neininger B, Elbert T, et al. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32:1621–1626. doi: 10.1161/01.str.32.7.1621. [DOI] [PubMed] [Google Scholar]
- 21.Johnson ML, Taub E, Harper LH, et al. An enhanced protocol for constraint-induced aphasia therapy II: a case series. Am J Speech Lang Pathol. 2014;23:60–72. doi: 10.1044/1058-0360(2013/12-0168). [DOI] [PubMed] [Google Scholar]
- 22.Taub E, Uswatte G, Mark VW, Morris DM. The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys. 2006;42:241–255. [PubMed] [Google Scholar]
- 23.Taub E, Uswatte G, Mark VW, et al. Method for enhancing real-world use of a more affected arm in chronic stroke: transfer package of Constraint-Induced Movement therapy. Stroke. 2013;44:1383–1388. doi: 10.1161/STROKEAHA.111.000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liepert J, Bauder H, Miltner WHR, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 25.Gauthier LV, Taub E, Perkins CE, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39:1520–1525. doi: 10.1161/STROKEAHA.107.502229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterling C, Taub E, Davis D, et al. Structural neuroplastic change following Constraint- Induced Movement therapy in children with cerebral palsy. Pediatrics. 2013;131:e1664– e1669. doi: 10.1542/peds.2012-2051. [DOI] [PubMed] [Google Scholar]
- 27.Mark V, Taub E, Bashir K, et al. Constraint-Induced Movement therapy can improve hemiparetic progressive multiple sclerosis. Preliminary findings. Mult Scler. 2008;14:992994. doi: 10.1177/1352458508090223. [DOI] [PubMed] [Google Scholar]
- 28.Bowling AC. Complementary and alternative medicine and multiple sclerosis [review] Neurol Clin. 2011;29:465–480. doi: 10.1016/j.ncl.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Uswatte G, Taub E, Morris D, Light K, Thompson PA. The Motor Activity Log-28: assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67:1189–1194. doi: 10.1212/01.wnl.0000238164.90657.c2. [DOI] [PubMed] [Google Scholar]
- 30.Morris D, Uswatte G, Crago J, Cook E, Taub E. The reliability of the Wolf Motor Function Test for assessing upper extremity function following stroke. Arch Phys Med Rehabil. 2001;82:750–755. doi: 10.1053/apmr.2001.23183. [DOI] [PubMed] [Google Scholar]
- 31.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–1639. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 32.Hodics TM, Nakatsuka K, Upreti B, Alex A, Smith PS, Pezzullo JC. Wolf Motor Function Test for characterizing moderate to severe hemiparesis in stroke patients. Arch Phys Med Rehabil. 2012;93:1963–1967. doi: 10.1016/j.apmr.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- 34.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 35.Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allendorfer JB, Storrs JM, Szaflarski JP. Changes in white matter integrity follow excitatory rTMS treatment of post-stroke aphasia. Restor Neurol Neurosci. 2012;30:103– 115. doi: 10.3233/RNN-2011-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sbardella E, Tona F, Petsas N, Pantano P. DTI measurements in multiple sclerosis: evaluation of brain damage and clinical implications [review] Mult Scler Int. 2013;2013:671730. doi: 10.1155/2013/671730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 39.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 40.Oishi K, Andreia VF, van Zijl PCM, Mori S. MRI Atlas of Human White Matter. 2. Amsterdam: Elsevier Academic Press; 2010. [Google Scholar]
- 41.Rickards T, Sterling C, Taub E, et al. a diffusion tensor imaging study of response to CI therapy of children with hemiparetic cerebral palsy and adults with chronic stroke. Arch Phys Med Rehabil. 2014;95:506–514. doi: 10.1016/j.apmr.2013.08.245. [DOI] [PubMed] [Google Scholar]
- 42.Cercignani M, Inglese M, Pagani E, Comi G, Filippi M. Mean diffusivity and fractional anisotropy histograms of patients with multiple sclerosis. AJNR Am J Neuroradiol. 2001;22:952–958. [PMC free article] [PubMed] [Google Scholar]
- 43.Bodini B, Khaleeli Z, Cercignani M, Miller DH, Thompson AJ, Ciccarelli O. Exploring the relationship between white matter and gray matter damage in early primary progressive multiple sclerosis: an in vivo study with TBSS and VBM. Hum Brain Mapp. 2009;30:2852–2861. doi: 10.1002/hbm.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pagani E, Filippi M, Rocca MA, Horsfield MA. A method for obtaining tract-specific diffusion tensor MRI measurements in the presence of disease: application to patients with clinically isolated syndromes suggestive of multiple sclerosis. Neuroimage. 2005;26:258–265. doi: 10.1016/j.neuroimage.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Ceccarelli A, Rocca MA, Valsasina P, et al. Structural and functional magnetic resonance imaging correlates of motor network dysfunction in primary progressive multiple sclerosis. Eur J Neurosci. 2010;31:1273–1280. doi: 10.1111/j.1460-9568.2010.07147.x. [DOI] [PubMed] [Google Scholar]
- 46.Reich DS, Smith SA, Zackowski KM, et al. Multiparametric magnetic resonance imaging analysis of the corticospinal tract in multiple sclerosis. Neuroimage. 2007;38:271–279. doi: 10.1016/j.neuroimage.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson M, Tench CR, Morgan PS, Blumhardt LD. Pyramidal tract mapping by diffusion tensor magnetic resonance imaging in multiple sclerosis: improving correlations with disability. J Neurol Neurosurg Psychiatry. 2003;74:203–207. doi: 10.1136/jnnp.74.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- 49.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791– 1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 50.Gean-Marton AD, Vezina LG, Marton KI, Stimac GK, Psyster RG, Taveras JM. Abnormal corpus callosum: a sensitive and specific indicator of multiple sclerosis. Radiology. 1991;180:215–221. doi: 10.1148/radiology.180.1.2052698. [DOI] [PubMed] [Google Scholar]
- 51.Ozturk A, Smith SA, Gordon-Lipkin EM, et al. MRI of the corpus callosum in multiple sclerosis: association with disability. Mult Scler. 2010;16:166–177. doi: 10.1177/1352458509353649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mishkin M, Ungerleider LG. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav Brain Res. 1982;6:57–77. doi: 10.1016/0166-4328(82)90081-x. [DOI] [PubMed] [Google Scholar]
- 53.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 54.Desmurget M, Grea H, Grethe JS, Prablanc C, Alexander GE, Grafton ST. Functional anatomy of nonvisual feedback loops during reaching: a positron emission tomography study. J Neurosci. 2001;21:2919–2928. doi: 10.1523/JNEUROSCI.21-08-02919.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taubert M, Villringer A, Ragert P. Learning-related gray and white matter changes in humans: an update. Neuroscientist. 2012;18:320–325. doi: 10.1177/1073858411419048. [DOI] [PubMed] [Google Scholar]


