Abstract
In light of expanding legalization of cannabis and swelling debate about the potential risks, particularly for younger users, understanding acute cannabis effects among adolescents and emerging adults is more important than ever. Contemporary models of addiction development identify subjective drug responses as central to the developmental unfolding of drug use disorders. Despite this, surprisingly little is known about cannabis’s acute subjective effects in human youth. This research utilized ecological momentary assessment (EMA) in the natural environment to identify the typical situational context of cannabis use among 85 frequent cannabis users, ages 15–24 years (M=19.8, SD=2.0; 48.2% female). Study aims were to (1) characterize momentary changes in several subjective states (i.e., stimulation, sedation, tension, craving, and ‘high’) when not using, just before cannabis use, and after use, and (2) evaluate whether cannabis responses varied with cannabis use disorder (CUD) severity or across the transition from adolescence to emerging adulthood in a correlational manner. Use of cannabis produced measurable reductions in craving and tension, as well as increases in stimulation, sedation, and ‘high.’ Participants with more CUD symptoms reported greater relief of craving and increased stimulatory response and ‘high’ following use. In contrast, emerging adults reported diminished stimulatory response and ‘high’ following use, relative to adolescents. Results highlight the utility of EMA for characterizing cannabis response as this behavior unfolds in daily life, during a key developmental timeframe in the pathogenesis of cannabis-use pathology.
Keywords: cannabis use disorder, adolescence, emerging adulthood, subjective response
Cannabis users who develop addiction typically first exhibit problems during their adolescent and emerging-adult years. In the United States, first use of cannabis emerges, on average, at age 13 years and peaks before age 20, with an estimated 3.2 million youth ages 18–25 using cannabis each day (National Survey on Drug Use and Health, 2012; Substance Abuse and Mental Health Services Administration, 2014). Epidemiologic research shows that risk of developing cannabis dependence is greatest within the first five years of use, with peak risk at age 17, and most cases of dependence are observed between the ages of 15 to 25 (Wagner & Anthony, 2002). Similar patterns are observed across the world (Coffey, Carlin, Lynskey, Li, & Patton, 2003; Swift, Coffey, Carlin, Degenhardt, & Patton, 2008; Von Sydow et al., 2001).
Conceptual models of addiction posit that abuse liability for cannabis and other substances depends, in part, on their ability to alter subjective affective states and elicit craving (Carter & Griffiths, 2009). Cannabis use produces a host of pharmacological effects that cause acute subjective changes in affect and cognition, and these effects, in turn, predict future use (de Wit & Phillips, 2012). Survey data indicate that adults commonly report using cannabis to enhance positive affect and relieve stress, and that individuals who experience more positive effects are more likely to be repeat users (de Wit & Phillips, 2012). Data from laboratory studies with adults show that cannabis administration reliably produces dose-response changes in subjective responses, such that active delta(9)-tetrahydrocannabinol (THC) produces more positive effects than placebo (Chait & Perry, 1994; Haney, 2008; Hunault et al., 2014). In particular, cannabis use in the human laboratory enhances self-reported alertness and stimulation, high, and sedation among adults in a dose-response manner, and these effects are similar for both smoked and oral administration (Chait & Zacny, 1992; Haney, 2008; Haney, Ward, Comer, Foltin, & Fischman, 1999; Hart et al., 2002; Hart, Gorp, Haney, Foltin, & Fischman, 2001).
Comparatively little research has investigated the subjective effects of cannabis in adolescents and emerging adults, and examination of how these effects relate to the severity of addiction is also limited. Neurodevelopmental changes extending from adolescence through emerging adulthood are linked to heightened substance-misuse vulnerability among youth (Lubman, Cheetham, & Yücel, 2015; Meier et al., 2012; Volkow et al., 2016), and compelling evidence from animal research suggests this liability stems, in part, from youths’ unique sensitivities to the acute effects of substances. Evidence from cannabis administration studies in animals suggests that adolescents are differently sensitive to the acute effects of cannabis (Cha, White, Kuhn, Wilson, & Swartzwelder, 2006; Quinn et al., 2008), and one recent human laboratory study also suggests cannabis-response differences in adolescent males (Mokrysz, Freeman, Korkki, Grif, & Curran, 2016). In addition, adolescence is marked by heightened sensitivity to general reward, i.e., not drug specific, and reduced sensitivity to punishment (Doremus-Fitzwater, Varlinskaya, & Spear, 2010; Spear & Varlinskaya, 2010). Taken together, there is sufficient evidence that adolescents may be differentially sensitive to the acute effects of cannabis, and, thus, advancing our understanding of the unfolding of addiction requires examining subjective effects during this developmentally important period.
Adolescent subjective response, moreover, is key to contemporary etiological models of addiction. Sensitization and allostatic models, for instance, conceptualize acute subjective effects of substances as clinically relevant endophenotypes—that is, reliably measured dynamic neurobiological processes underlying addiction liability. A common premise across these models is that repeated drug use produces neurobiological changes in the brain that heighten the individual’s sensitivity to acute substance-use rewards (Koob and LeMoal, 2001; Robinson & Berridge, 1993, 2008). This potentiation in the drug’s reinforcing effects, in turn, promotes rapid emergence and strengthening of motivation for drug rewards (i.e., craving) in non-use moments (Berridge, 2007; Robinson & Berridge, 1993, 2008). From this perspective, differences in drug response are expected as addiction develops, such that rewarding drug responses and craving in non-use moments should be heightened among youth with more severe addiction pathology. But, despite strong support from animal research, investigations of these core tenets of neuroadaptive models of addiction in humans are scant, and less than a handful of studies examine cannabis response, more generally, among adolescents or emerging adults. Advancing our understanding of the etiology of cannabis use disorder (CUD) requires leveraging newly developed technologies and ecological methods to test current theories of addiction during a pivotal period for addiction risk.
The lack of empirical testing of subjective responses to cannabis among adoelscents and emerging adults is a key research gap that stems, in part, from prohibitions on real-time examination of these effects in the human laboratory with youth in the United States. A primary focus of the current investigation was to characterize subjective responses to cannabis among adolescents and emerging adults in natural settings. This was achieved by utilizing ecological momentary assessment (EMA), a real-time in vivo approach that is well-suited to capture discrete, episodic events that are frequent and variable across situations and context (Shiffman, 2009). EMA has been applied to study subjective responses to several drugs of abuse among adults (Buckner, Zvolensky, & Ecker, 2013; Piasecki et al., 2011; Piasecki, McCarthy, Fiore, & Baker, 2008; Ray et al., 2010; Serre et al., 2012; Treloar, Piasecki, McCarthy, & Baker, 2014), and to a lesser extent among youth (Gwaltney, Bartolomei, Colby, & Kahler, 2008; Miranda et al., 2014). In adult cannabis users, Buckner and colleagues showed in vivo decreases in withdrawal, craving, and negative affect at an assessment point following cannabis use (Buckner et al., 2015). Only one other research group, to our knowledge, has studied cannabis response among younger users, ages 15–24, in vivo. Schrier and colleagues found a trend toward greater ratings of ‘highest high’ following cannabis use for younger participants (Shrier et al., 2013).
In the present research, we used EMA to capture subjective states previously shown to be affected by cannabis among adolescent animals and human adults (i.e., craving, stimulation, sedation, ‘high,’ and tension). Momentary data, including contextual information (e.g., location, presence of peers), was collected at: (a) randomly selected times within 3-hour time blocks throughout the day, (b) immediately prior to ad-lib cannabis-use episodes, and (c) just after ad-lib cannabis use episodes. Our primary objectives were twofold. First, we sought to characterize the subjective effects of ad-lib cannabis use among adolescents and emerging adults. Drawing from cannabis administration studies with adults, we anticipated that stimulation, sedation, and ‘high’ would be greater following cannabis use, relative to other times, whereas craving and tension would be reduced by cannabis use. Second, we sought to conduct the first human test of two key tenets of etiological theories of addiction, namely that adolescents and emerging adults with more severe CUD pathology will show heightened sensitivity to the rewarding subjective effects of cannabis use and higher levels of craving. Specifically, we examined whether subjective responses and craving varied across severity of CUD pathology in a correlational manner by using a symptom count of currently endorsed CUD critiera. We hypothesized that positive subjective effects of cannabis (i.e., increases in stimulation and ‘high’) would be enhanced for participants with more CUD symptoms. We also hypothesized that participants with more CUD symptoms would experience higher levels of craving during non-use moments and show greater relief (reduction) of craving following cannabis use than those with less progressed symptomatology. In addition, we anticipated a positive association between age and CUD severity, which may confound results. Thus, we explored whether our hypothesized associations were specific to CUD severity or applied, more generally, to age-related differences associated with the transition from adolescence to emerging adulthood.
Method
Participant Selection
Participants (n=85) were recruited from the community to participate in a study of whether a medication affects cannabis use. Recruitment efforts included posting advertisements in settings frequented by youth, such as recreational settings, public buses, and high schools, as well as advertising on social media. Study staff also set up informational tables at schools, sporting events, festivals, and public beaches. Eligible participants were 15–24 years of age who used cannabis at least twice weekly in the past 30 days. This criterion was used to increase the likelihood of capturing marijuana use in the natural environment during the study. Those seeking formal cannabis treatment in the past 30 days were excluded. Additional exclusion criteria included current Axis I psychopathology (other than cannabis, alcohol, nicotine, or disruptive behavior disorders), active suicidality or psychotic symptoms, and medical conditions or medications that contraindicated taking study medication. Females were ineligible if they were pregnant, nursing, or unwilling to use birth control.
Procedure
This study was part of a larger clinical trial registered at http://clinicaltrials.gov (NCT01110434) and described in Miranda and colleagues (2016). Participants who met provisional eligibility criteria based on a brief telephone screening completed a comprehensive, in-person interview to confirm eligibility. Written informed consent was obtained from 18–24-year-old participants; assent was obtained from minors, and their parents provided written informed consent. The Brown University Institutional Review Board approved the study protocol (#0903992676).
Data for the present study were from a pre-randomization, pre-medication EMA period of approximately one week. Participants were not instructed to reduce or otherwise alter substance use patterns. Participants completed assessments in their usual settings via handheld wireless devices (Omnia; Samsung Electronics, Ridgefield Park, NJ) with software developed for this study. Instructions were in simple English and participants recorded data by tapping directly on the screen. Participants recorded responses at several times each day, and a combination of randomly prompted and user-initiated reports ensured adequate coverage of focal variables. Participants were compensated $10 per day for complying with the EMA protocol.
Baseline Assessments
Demographic information, including participant sex (0=male; 1=female), age, and racial/ethnic background was collected at a baseline session.
Cannabis Use Disorder
A symptom count based on participant responses to the Kiddie Schedule for Affective Disorders for School-Age Children, a clinician-administered interview based on Diagnostic and Statistical Manual (DSM-IV-TR) criteria (Kaufman et al., 1997), quantified the severity of CUD. Although abuse and dependence diagnoses were determined through case consensus, a symptom count was used to more closely match the DSM-5 diagnostic system, which excludes legal problems and assesses CUD on a continuum with mild (2–3 symptoms) moderate (4–5 symptoms), and severe (6+ symptoms) specifiers. Participants could meet up to 10 DSM-IV-TR criteria, and all criteria were represented in this sample.
Cannabis Use
A 90-day timeline follow-back interview (TLFB; Sobell & Sobell, 1992) assessed cannabis use prior to the EMA period. To facilitate accurate reporting of the quantity of cannabis use on a specific day, participants estimated how much cannabis they used by weighing a surrogate substance (i.e., oregano). When participants shared cannabis with others the total weight was divided by the number of users. This method of estimating daily quantities of cannabis use has shown evidence of reliability and validity (Mariani, Brooks, Haney, & Levin, 2011; Norberg, Mackenzie, & Copeland, 2012), and the TLFB is shown to correlate strongly with plasma THC levels (Hjorthøj, Fohlmann, Larsen, Arendt, & Nordentoft, 2012).
Momentary Assessments
Participants received random prompts each day, delivered by the device within 3-hour time blocks. Device-delivered random prompts “timed out” after two minutes; however, participants had the option to the delay the completion of random prompt assessments for up to 20 minutes. Prompts that were not completed in that timeframe were marked as missed. Other features made it ‘user-friendly,’ such as an alarm set by participants to avoid assessments while sleeping.
Cannabis Use
Participants were instructed to initiate a “begin-pot report” just before starting to use cannabis and an “end-pot report” as soon as they finished smoking. Participants were trained to complete cannabis reports for every joint, blunt, bong, bowl, or any other way cannabis was used during the study. Participants estimated how many grams of cannabis they used and reported how many people they shared cannabis with. When participants shared cannabis with others the total weight was divided by the number of users.
Other Substance Use
Recent nicotine use was assessed for all record types with the single question, “When did you last smoke a cigarette?” with response options reflecting current smoking, last 15 minutes, last hour, last two hours, more than two hours ago, or no cigarette smoking yet that day. Participants self-initiated alcohol reports with a parallel procedure as for cannabis use reports, before and after each alcoholic beverage consumed.
Subjective States
Visual analog bars (converted to discrete, 11-point scales) assessed a number of acute cannabis effects and mood states. Prompts stated, “How __________ do you feel right now?” with end-point anchors “not at all” and “extremely.” Subjective responses included energized, excited, sedated, sluggish, and high. Energized and excited were assessed for all record types, whereas acute effects of cannabis (i.e., sedated, sluggish, and ‘high’) were assessed only for cannabis reports. Items were averaged to form stimulation (energized, excited: α=.81 non-use, α=.73 begin-cannabis, α=.74 end-cannabis), tension (tense, stressed: α=.82 non-use, α=.54 begin-cannabis, α=.59 end-cannabis), and sedation (sedated, sluggish: α=.53 begin-cannabis, α=.66 end-cannabis) composites. Strong correlations between these items (rs=.66, .58, and .48, for stimulation, tension, and sedation, respectively, ps<.001) further supported their combination.
Craving/Urge
Visual analog bars (converted to discrete, 11-point scales) assessed urge to use cannabis. Prompts stated “How strong is your urge to use pot right now?” with end-point anchors “no urge” and “strongest ever.”
Contextual Variables
Participants indicated their present company using multiple checkboxes. Categories of friends and boy/girlfriend were combined to form a “presence of peers” variable (0=peers not present; 1=peer present). Current location was assessed with forced choices (choose only one); see Table 2 for a list of descriptors.
Table 2.
Logistic Multilevel Models Evaluating Momentary, Contextual Correlates of Engagement in Cannabis Use, Accounting for Aggregate Contextual Variables and Other Person-level Influences
| Variable | β | SE | 95% Confidence Limits OR | UCL | p | |
|---|---|---|---|---|---|---|
|
| ||||||
| OR | LCL | |||||
| Time-varying Contextual Variables | ||||||
|
|
||||||
| Time of day | ||||||
| 6a.m.–Noon | −39.32 | 5.24 | 0.10 | 0.06 | 0.18 | <.001 |
| Noon–6p.m. | −25.46 | 5.22 | 0.29 | 0.17 | 0.48 | <.001 |
| 6p.m.–Midnight | −1.91 | 4.84 | 0.91 | 0.56 | 1.47 | .694 |
| Midnight–6a.m. | ||||||
| Location | ||||||
| Other | 2.27 | 2.84 | 1.30 | 0.68 | 2.46 | .425 |
| Vehicle | 2.97 | 3.07 | 1.27 | 0.78 | 2.06 | .334 |
| Public place | −14.08 | 3.84 | 0.31 | 0.17 | 0.58 | <.001 |
| Work | −13.94 | 5.64 | 0.13 | 0.03 | 0.66 | .014 |
| School | −12.96 | 4.67 | 0.27 | 0.11 | 0.68 | .006 |
| Other’s house | 1.36 | 3.30 | 1.14 | 0.61 | 2.11 | .680 |
| Friend’s house | 3.83 | 2.98 | 1.32 | 0.86 | 2.03 | .200 |
| Home | ||||||
| Weekend | 1.64 | 2.81 | 1.09 | 0.82 | 1.45 | .560 |
| Peers present | 17.26 | 3.53 | 2.28 | 1.64 | 3.17 | <.001 |
| Person-level Aggregate Contextual Variables1 | ||||||
|
|
||||||
| Reports completed for each time of day relative to Midnight–6 a.m. | ||||||
| % 6a.m.–Noon | 9.72 | 7.22 | 1.01 | 1.00 | 1.02 | .183 |
| % Noon–6p.m. | 11.29 | 9.88 | 1.02 | 0.99 | 1.05 | .257 |
| % 6p.m.–Midnight | −10.69 | 8.92 | 0.98 | 0.96 | 1.01 | .235 |
| Reports completed in each location relative to Home | ||||||
| % Other | −6.84 | 7.10 | 0.99 | 0.98 | 1.01 | .339 |
| % Vehicle | 10.61 | 6.60 | 1.01 | 1.00 | 1.03 | .113 |
| % Public place | −8.36 | 6.34 | 0.99 | 0.97 | 1.01 | .192 |
| % Work | −2.58 | 6.22 | 0.99 | 0.97 | 1.02 | .680 |
| % School | 17.11 | 7.37 | 1.02 | 1.00 | 1.03 | .023 |
| % Other’s house | −7.32 | 6.31 | 0.99 | 0.98 | 1.01 | .250 |
| % Friend’s house | −2.08 | 6.43 | 1.00 | 0.98 | 1.01 | .747 |
| % reports completed on Weekends | −12.95 | 5.40 | 0.97 | 0.95 | 1.00 | .019 |
| % reports in presence of Peers | 5.47 | 6.30 | 1.01 | 0.99 | 1.02 | .388 |
| Person-level Individual Difference Variables1 | ||||||
|
|
||||||
| Age | − 3.70 | 5.80 | 0.96 | 0.83 | 1.10 | .525 |
| Female relative to Male | 9.81 | 5.77 | 1.60 | 0.92 | 2.77 | .094 |
| Percent use days2 | 27.93 | 6.51 | 1.02 | 1.01 | 1.04 | <.001 |
| Grams per use day2 | 11.84 | 5.77 | 1.72 | 1.01 | 2.92 | .044 |
| Cannabis Use Disorder Symptoms2 | −1.01 | 6.36 | 0.99 | 0.85 | 1.14 | .875 |
Note. β=standardized estimate; SE=standard error; OR=odds ratio; LCL=lower confidence limit; UCL=upper confidence limit. Twenty-one location reports were missing (1.2%). Thus, 491 end-cannabis reports were compared to 1264 reports from non-use times. Missing data, albeit very minimal, occurred when the device malfunctioned and prematurely exited a report.
Time-invariant, person-level continuous variables were centered at the grand mean.
These variables were based on reports from the baseline 90-day timeline follow-back.
Time-of-Day and Social Day
All reports were date-time stamped and coded into four 6-hour blocks (e.g., 6am to noon) as a time-of-day covariate. Nesting of reports within days was identified according to participants’ individual social schedules (e.g., 8am to 4am) rather than calendar days.
Analytic Approach
Two-level, random-intercept models with momentary reports (level 1) nested within participants (level 2) were estimated in SAS 9.3 (SAS Institute Inc. 2012) PROC GLIMMIX (categorical outcomes) and PROC MIXED (continuous outcomes), with restricted maximum likelihood estimation. This approach is robust to unique timing of reports and variable numbers of reports for each participant in the study (Gibbons, Hedeker, & DuToit, 2010; Raudenbush & Bryk, 2002; Singer & Willett, 2003). The inclusion of a level indicating social day in additional model tests did not alter the pattern of fixed effects, and, thus, this level was not included.
An initial model predicted the log odds of engagement in cannabis use from several time-varying contextual variables, person-level aggregates of contextual variables, and time-invariant, person-level covariates (see Table 2 for descriptors). Next, a series of models addressed the first study aim: characterizing the effects of cannabis on subjective responses (i.e., stimulation, tension, sedation, ‘high,’ and craving) in the natural environment. A categorical variable indicated report type to examine the differences in subjective states for non-use random prompts, begin-cannabis reports, and end-cannabis reports. The reference contrasts compared begin- and end-cannabis reports to non-use reports, as well as end-cannabis reports to begin-cannabis reports.
Next, main and interactive effects of the CUD-symptom-count variable with report type were added to test whether differences in subjective effects varied as a function of CUD severity. Main effects represented the effect of disorder severity on subjective responses, in general, for the reference category. Interactive effects with report type evaluated the influence of disorder severity on differences in subjective responses across report types. All contextual and person-level variables from initial analyses were included in final models to evaluate whether these covariates accounted for the pattern of results, with continuous covariates person- and grand-mean centered, respectively. Last, main and interactive effects of age with report type were added to test whether the same pattern of differences in subjective effects would be found for CUD and age.
Results
Participant Characteristics
Eighty-five participants (48.2% female; Mage = 19.75 years, SD=2.0, range = 15–24) provided EMA data. The majority self-identified as White or Caucasian (48.2%) or Black or African American (29.4%); 17.6% indicated Hispanic or Latino ethnicity. At baseline, participants reported using marijuana on 69.91% of the past 90 days (SD=27.04) and used an average of 0.65 grams of marijuana (SD=0.53) per use day. See Table 1 for a summary of participant characteristics across varying severity of CUD symptom counts. CUD groups were created for descriptive purposes, but all other analyses used the original CUD symptom count, ranging from 0 to 9 in this sample, with the average participate reporting moderate severity CUD (M=4.4; SD=2.1).
Table 1.
Summary of Baseline Characteristics by Cannabis Use Disorder Severity
| Variable | Cannabis Use Disorder (CUD) Symptoms | Overall (N=85) | F or χ2 | p | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| None (0–1 symptom) (n=5) | Mild (2–3 symptoms) (n=25) | Moderate (4–5 symptoms) (n=28) | Severe (6+ symptoms) (n=27) | ||||
| Age | |||||||
| Observed Range | 18–24 | 18–24 | 15–24 | 15–24 | 15–24 | ||
| M (SD) | 20.0 (2.3) | 20.3 (1.7) | 20.1 (1.9) | 18.9 (2.3) | 19.8 (2.1) | 2.67 | .053 |
| Sex, # (%) female | 3 (60.0) | 15 (60.0) | 10 (35.7) | 13 (48.1) | 41 (48.2) | 3.42 | .331 |
| Race, # (%)a | |||||||
| White or Caucasian | 3 (60.0) | 13 (52.0) | 20 (71.4) | 11 (40.7) | 47 (55.3) | 17.02 | .318 |
| Black or African-American | 1 (20.0) | 7 (28.0) | 6 (21.4) | 11 (40.7) | 25 (29.4) | ||
| Other | 1 (20.0) | 4 (16.0) | 1 (3.6) | 1 (3.7) | 7 (8.2) | ||
| Hispanic, # (%) | 1 (20.0) | 5 (20.0) | 1 (3.6) | 8 (29.6) | 15 (17.6) | 6.60 | .086 |
| Baseline, 90-day cannabis-use levelsb | |||||||
| % use days, M (SD) | 36.7 (27.12) | 58.6 (29.5) | 76.8 (19.8) | 79.4 (21.5) | 69.9 (27.0) | 6.87 | <.001 |
| Grams per use day, M (SD) | 0.25 (0.17) | 0.64 (0.73) | 0.68 (0.43) | 0.70 (0.43) | 0.65 (0.53) | 1.05 | .374 |
Note.
Six participants did not identify with a race option, including “other.” Of these, 5 indicated Hispanic or Latino ethnicity.
Baseline cannabis use derived from the 90-day Timeline Follow-Back interview administered prior to in vivo assessments.
EMA Compliance
Participants completed from 11–73 combined random prompt, begin-cannabis, and end-cannabis reports during the study (M=33.9, SD=14.1) and from 1–16 of these reports per day (M=6.0, SD=2.6). Devices delivered 2,065 random prompts, of which 1,730 were completed (83.8%). The average number of device-delivered prompts per day was 3.7 (SD=1.6). The majority of participants (89.3%) missed 10 or less random prompts. The average number of missed random prompts across all study days, per participant, was 5.2 (SD=3.6). The number of missed random prompts was correlated with the baseline percent cannabis-use days, r=.22, p<.001, but was not correlated with CUD symptom count, age, or grams of cannabis smoked per use day, ps=.802, .872, and .119, respectively. Males also had more missed random prompts than females, Mdifference=2.11, t(333)=6.26, p<.001. Length of participation was targeted for 1 week; however, due to scheduling of appointments, up to 14 days of data were collected per participant (M=7.4, SD=1.6). In this timeframe, participants reported, on average, 6.9 cannabis-use episodes (SD=5.3). The person-average number of grams reported per use event in the natural environment was correlated with baseline reports of grams per use day, r= .43, p<.001, as were percent use days, r=.39, p<.001.
Descriptive Information for Reports in the Natural Environment
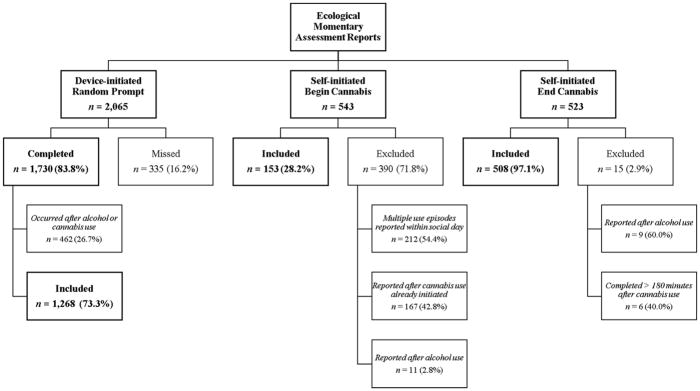
Figure 1 illustrates the flow of EMA data management. Random prompts delivered after substance use were removed to avoid confounding with acute responses (n=462). Cannabis reports occurring after alcohol use, which occurred infrequently (n=20), were also removed to disentangle cannabis and alcohol effects; reports involving concurrent cannabis and alcohol use were retained (see below). Although multiple end-cannabis reports were permissible, only the first begin-cannabis report within the participant’s social day was included to exclude carryover effects from previous use (n=212 excluded second begin-cannabis reports). A substantial proportion of begin-cannabis reports were completed after smoking had already been initiated (n=167). Over half of these were within 5 minutes of initiating smoking (n=99; range=1–122 minutes). THC is detectable in plasma seconds after first inhalation “puff” of a cannabis cigarette, however, and peak levels are observed between 3–10 minutes (Grotenhermen, 2003); therefore, these analyses followed the most stringent approach of removing any begin-cannabis reports recorded after initiation of use. End-cannabis reports occurring >3 hours (180 minutes) after finishing smoking were also excluded (n=6); this cutoff was based on literature showing declining THC concentrations between 2–3 hours after inhalation of cannabis, with the THC concentration diminished by 3 hours (Grotenhermen, 2003). This resulted in 1,268 non-use, random-prompt reports, 153 begin-cannabis reports, and 508 end-cannabis reports (see Figure 1).
Figure 1.
Ecological momentary assessment report flow.
Characterizing Cannabis Use
Participants used, on average, 0.73 grams of cannabis on days they smoked in the natural environment, (SD=0.63; Median=0.56). The average time of initiating cannabis use was 4:18pm. The primary methods of administration was via a blunt (52.6%) or bowl (28.1%), with less common use via bong (8.9%), joint (7.3%), or other (3.2%). Although uncommon, participants indicated concurrent alcohol use for 17 end-cannabis reports (3.4%) that had been retained in analyses because no alcohol use was reported earlier that day or at a begin-cannabis report. Concurrent use of nicotine cigarettes was more common (n=73; 14.5% of end-cannabis reports). Exclusion of alcohol and nicotine co-use reports did not alter the pattern of results that follows.
Table 2 presents odds ratios from mixed-effects models evaluating the situational context of cannabis use (i.e., comparing non-use and begin-cannabis reports, n=1,264, to end-cannabis reports, n=491). Predictors were contextual variables (e.g., present company, location), person effects (e.g., age, sex), and person-level variables that account for percent of time each participant was in a particular context (e.g., percentage of reports completed in the presence of peers). Being in the presence of peers when a report was completed was associated with a two-fold increase in the odds of reporting concurrent cannabis use, OR=2.28, p<.001, whereas simply completing more reports in the presence of peers was not associated with use, p=.388. Cannabis use was less likely in the morning and afternoon, relative to midnight to 6a.m, ORs=0.10 and 0.29, respectively, ps<.001. Cannabis use was also less likely in public places, including work or school, relative to being at home, ps<.014. Although participants who made more reports on weekends were less likely to report cannabis use, OR=0.97, p=.019, the likelihood of a particular report indicating cannabis use was not related to whether that report was made on a weekend or weekday, p=.560. Greater baseline reports of cannabis use were related to increased engagement in cannabis use during the EMA period (percent use days: OR=1.02, p<.001; grams per use day: OR=1.72, p=.044).
Craving and Subjective Responses
Within- and between-person variability
A primary aim of this work was to capture the subjective effects of cannabis use in the usual settings of adolescents and emerging adults. An important step toward that goal was to evaluate whether there was variability in subjective states across EMA moments and persons. Table 3 shows random person effects (intercept variances) and random momentary effects (error variances) from unconditional means models (i.e., models prior to entry of any predictor variables). An intraclass correlation coefficient (ICC) can be calculated as the ratio of these partitioned variances to reflect the relative variance between and within persons (i.e., σ/σ+ε). The ICCs were as follows: craving ICC=.32; stimulation ICC=.34; tension ICC=.38; sedation ICC=.49; high ICC=.17. ICCs can be multiplied by 100 to identify the percent variance due to between-person factors, relative to all variance. In the case of subjective ‘high,’ 17% of the variance in ‘high’ was due to person-level factors, leaving 83% of the variance in ‘high’ due to moment-to-moment factors. For sedation, about half of the variance (49%) was due to person-level factors. For the remaining subjective states, about one-third of the variability in craving, stimulation, and tension was attributable to person-level influences.
Table 3.
Parameter Estimates (and Standard Errors) From Multilevel Models Predicting Subjective Responses From Cannabis Use, Cannabis Use Disorder (CUD) Symptom Count, and Age
| Craving | Stimulation | Tension | Sedation | High | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Est. | (SE) | Est. | (SE) | Est. | (SE) | Est. | (SE) | Est. | (SE) | |
| Random person variance (σ) | 4.26*** | (0.72) | 2.38*** | (0.40) | 2.08*** | (0.35) | 3.24*** | (0.61) | 2.20*** | (0.58) |
| Random momentary variance (ε) | 8.88*** | (0.29) | 4.68*** | (0.15) | 3.37*** | (0.11) | 3.43*** | (0.20) | 10.42*** | (0.61) |
| Change in Subjective States | ||||||||||
|
|
||||||||||
| Non-use average (intercept) | 3.39*** | (0.24) | 4.49*** | (0.18) | 2.25*** | (0.17) | ||||
| Begin-cannabis average (intercept) | 6.51*** | (0.32) | 5.52*** | (0.25) | 1.87*** | (0.22) | 2.40*** | (0.27) | 1.26*** | (0.23) |
| Report-type contrast | ||||||||||
| Begin-cannabis vs. non-use | 2.58*** | (0.25) | 1.04*** | (0.19) | −0.38* | (0.16) | ||||
| End-cannabis vs. non-use | −1.69*** | (0.23) | 0.68*** | (0.18) | −0.73*** | (0.15) | ||||
| End-cannabis vs. begin-cannabis | −4.27*** | (0.30) | −0.36 | (0.23) | −0.35 | (0.20) | 1.28*** | (0.21) | 6.10*** | (0.22) |
| Grams | −0.76† | (0.44) | 0.41 | (0.34) | 2.05 | (2.00) | −0.04 | (0.33) | 0.55 | (0.35) |
| Person-average grams | 6.44* | (2.75) | 1.05 | (2.03) | 0.01 | (1.93) | 0.59 | (2.53) | 2.05 | (2.00) |
| Time spent smokinga | 0.70 | (0.46) | 0.05 | (0.36) | −0.15 | (0.30) | 0.00 | (0.32) | −1.17*** | (0.34) |
| Effect of CUD on Change in Subjective States | ||||||||||
|
|
||||||||||
| Non-use average (intercept) | 3.94*** | (0.24) | 4.49*** | (0.18) | 2.25*** | (0.17) | ||||
| Begin-cannabis average (intercept) | 6.50*** | (0.32) | 5.54*** | (0.24) | 1.87*** | (0.22) | 2.41*** | (0.26) | 1.25*** | (0.23) |
| Report-type contrast | ||||||||||
| Begin-cannabis vs. non-use | 2.57*** | (0.25) | 1.05*** | (0.19) | −0.38* | (0.16) | ||||
| End-cannabis vs. non-use | −1.67*** | (0.23) | 0.66*** | (0.18) | −0.73*** | (0.15) | ||||
| End- vs. begin-cannabis | −4.23*** | (0.30) | −0.39 | (0.24) | −0.35† | (0.20) | 1.28*** | (0.21) | 6.11*** | (0.22) |
| Grams | −0.65 | (0.44) | 0.35 | (0.34) | 0.14 | (0.29) | 0.00 | (0.33) | 0.49 | (0.35) |
| Person-average grams | 5.78* | (2.77) | 1.57 | (2.05) | 0.27 | (1.97) | 1.36 | (2.55) | 1.69 | (2.04) |
| Time spent smokinga | 0.60 | (0.46) | 0.10 | (0.36) | −0.14 | (0.30) | −0.04 | (0.32) | −1.12** | (0.34) |
| CUD effect on non-use | 0.32** | (0.12) | −0.16† | (0.09) | −0.07 | (0.08) | ||||
| CUD effect on begin-cannabis | 0.15 | (0.16) | −0.17 | (0.12) | −0.05 | (0.11) | −0.09 | (0.13) | −0.03 | (0.11) |
| CUD × Report-type contrast | ||||||||||
| CUD begin-cannabis vs. non-use | −0.11 | (0.12) | −0.01 | (0.09) | 0.01 | (0.08) | ||||
| CUD end-cannabis vs. non-use | −0.28*** | (0.08) | 0.13* | (0.06) | 0.03 | (0.05) | ||||
| CUD end- vs. begin-cannabis | −0.17 | (0.13) | 0.14 | (0.10) | 0.02 | (0.08) | −0.11 | (0.08) | 0.16† | (0.09) |
| Effect of Age on Change in Subjective States | ||||||||||
|
|
||||||||||
| Non-use average (intercept) | 3.94*** | (0.24) | 4.48*** | (0.18) | 2.25*** | (0.17) | ||||
| Begin-cannabis average (intercept) | 6.52*** | (0.33) | 5.52*** | (0.24) | 1.87*** | (0.22) | 2.40*** | (0.27) | 1.24*** | (0.23) |
| Report-type contrast | ||||||||||
| Begin-cannabis vs. non-use | 2.59*** | (0.25) | 1.04*** | (0.19) | − 0.38* | (0.16) | ||||
| End-cannabis vs. non-use | −1.69*** | (0.23) | 0.69*** | (0.18) | − 0.73*** | (0.15) | ||||
| End- vs. begin-cannabis | −4.28*** | (0.30) | −0.36 | (0.23) | −0.36† | (0.20) | 1.28*** | (0.21) | 6.12*** | (0.22) |
| Grams | −0.74† | (0.44) | 0.36 | (0.34) | 0.18 | (0.29) | −0.04 | (0.33) | 0.50 | (0.35) |
| Person-average grams | 6.22* | (2.77) | 1.29 | (2.02) | 0.20 | (1.95) | 0.80 | (2.56) | 1.49 | (1.96) |
| Time spent smokinga | 0.69 | (0.46) | 0.04 | (0.35) | −0.15 | (0.30) | −0.00 | (0.32) | −1.17*** | (0.34) |
| Age effect on non-use | −0.10 | (0.12) | 0.19* | (0.09) | 0.03 | (0.08) | ||||
| Age effect on begin-cannabis | −0.17 | (0.16) | 0.08 | (0.12) | −0.01 | (0.11) | 0.07 | (0.13) | −0.01 | (0.11) |
| Age × Report-type contrast | ||||||||||
| Age begin-cannabis vs. non-use | −0.06 | (0.13) | −0.11 | (0.10) | −0.04 | (0.09) | ||||
| Age end-cannabis vs. non-use | 0.05 | (0.08) | −0.18** | (0.06) | 0.09 | (0.05) | ||||
| Age end- vs. begin-cannabis | 0.11 | (0.13) | −0.08 | (0.10) | 0.12 | (0.09) | 0.00 | (0.09) | −0.22* | (0.10) |
Note. Est. = unstandardized parameter estimate; SE = standard error; CUD = Cannabis use disorder symptom count. The same pattern of results was observed when all covariates listed in Table 2 were included (see Supplemental Table 3). Italicized text reflects results when the reference coding of report type is changed to compare end-cannabis reports to begin-cannabis reports for craving, stimulation, and tension. The reference category for sedation and high is always begin-cannabis reports because these outcomes were not measured (i.e., missing by design) at other non-use times.
Elapsed time since smoking recorded as minutes and modeled as hours to adjust the scale of this variable.
p < .10;
p < .05;
p < .01;
p < .001
Table 3 presents unstandardized parameter estimates from mixed-effects models, and standardized effects are reported in text. The middle panel of Table 3 presents comparisons of subjective effects across non-use random prompts, begin-cannabis reports completed just before use, and end-cannabis reports completed after participants finished using cannabis. Tabled results accounted for the influence of momentary total grams of cannabis smoked (i.e., the momentary report of grams smoked, centered at each participant’s own average), person-average grams (i.e., the participant’s average grams per smoking event across the study, centered at the overall average for all participants), and time spent smoking (i.e., the participant-reported time since smoking, with non-use and begin-cannabis reports coded as 0). The same pattern of results was found when other covariates from Table 2 were included (see Supplemental Table 3).
Craving
Craving was elevated just before cannabis use, β=0.71, 95%CI[0.57,0.84], p<.001, and was reduced by use, relative to non-use times, β=−0.46, 95%CI[−0.59,−0.34], p<.001, and relative to begin-cannabis reports, β=−1.17, 95%CI[−1.34,−1.01], p<.001.
Subjective affective responses
Stimulation was also elevated just before use, relative to non-use times, b=1.04, β=0.38 95%CI[0.24,0.52], p<.001. Comparison of end-cannabis and non-use reports suggested increased stimulation following use, b=0.68, β=0.25, 95%CI[0.12,0.38], p<.001, although end-cannabis and begin-cannabis reports were not different in terms of stimulatory response, p=.127. Tension was lower just before use, b=−0.38, β=– 0.16, 95%CI[−0.30,−0.02], p=.022, and following use, b=−0.73, β=−0.31, 95%CI[−0.44,−0.18], p<.001, relative to non-use times. Tension was only marginally reduced just after use, relative to just before, however, b=−0.35, β=−0.14, 95%CI[−0.32,−0.02], p=.082. Sedation was increased following use, b=1.28, β=0.50, 95%CI [0.34,0.65], p<.001, and subjective ‘high’ was also increased after use, b=6.10, β=1.71, 95%CI[1.58,1.83], p<.001, both relative to just before use.
Associations between CUD Symptoms, Craving, and Subjective Responses
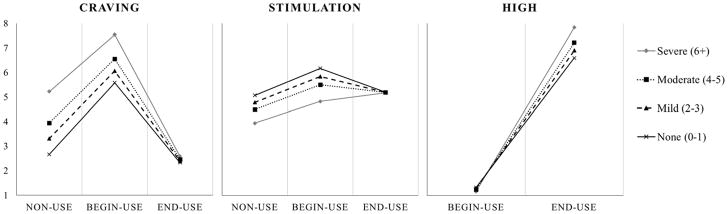
Table 3 (middle panel) presents models including main and interactive effects of CUD symptom count on subjective states. Main effects represent the effect of CUD symptoms on the intercept of subjective states (i.e., the reference category), and interactive effects represent the relation of CUD symptoms to subjective states across report types. Consistent with our hypotheses, CUD symptom count was related to greater craving at non-use times, b=0.32, β=0.15, 95%CI[0.02,0.28], p=.029, and marginally related to reduced stimulation at non-use times, b=−0.16, β=−0.12, 95%CI[−0.25,−0.01], p=.075. As predicted, CUD symptoms also affected the difference in craving and stimulation after smoking, relative to non-use times. Each additional CUD symptom was associated with an additional one-quarter to one-third of a point reduction in craving following use, b=−0.28, β=−0.16, 95%CI[−0.25,−0.07], p<.001, and CUD symptoms also enhanced differences in stimulation after use, b=0.13, β=0.10, 95%CI[0.01,0.19], p=.035. CUD symptoms did not alter the pattern of subjective tension, sedation, or ‘high.’ Figure 2 illustrates model-based (empirical Bayes) estimates of subjective craving, stimulation, and ‘high’ when not using, just before use, and after use as a function of CUD symptom count. Models were replicated including only the first, paired begin- and end-cannabis reports of each social day (see Supplemental Table 4). Additionally, a summary of differential associations of individual CUD symptoms with subjective states is provided in Supplemental Table 5.
Figure 2.
Model-based (empirical Bayes) estimates representing the conditional change in subjective states across report types as a function of Cannabis Use Disorder (CUD) symptom count. Solid black lines reflect no CUD diagnosis (0–1 symptoms), dashed lines reflect mild CUD (2–3 symptoms), dotted black lines reflect moderate CUD (4–5 symptoms), and solid gray lines indicate severe CUD (6+ symptoms).
Associations between Age and Subjective Response
The pattern of findings for age differed from that for CUD (see Table 3). Where CUD symptoms were associated with blunted stimulation when not using and enhanced stimulatory effects of use, age was associated with greater stimulation when not using, b=0.19, β=0.14, 95%CI[0.02,0.27], p=.026, and blunted stimulatory effects of use, b=−0.18, β=−0.13, 95%CI[−0.23,0.04], p=.004. Age was also associated with blunted ‘high’ following cannabis use, b=−0.22, β=−0.12, 95%CI[−0.22,−0.02], p=.021, where CUD symptoms were marginally associated with enhanced ‘high.’ Age was also not associated with craving for any report type, whereas these were some of the strongest effects of CUD symptoms.
Discussion
Findings supported our predictions regarding the effects of ad-lib cannabis use on subjective responses in adolescents and emerging adults in real-world settings. Cannabis use produced acute, measurable increases in stimulation, sedation, and ‘high’, as well as reductions in craving and tension. In addition, as predicted, participants with more CUD symptomatology reported sharper increases in stimulation and decreases in craving following use relative to those with fewer CUD symptoms. Together, these findings provide additional support for the clinical relevance of subjective responses to cannabis as factors that either underlie or coincide with the development of CUD.
Our work features EMA as a method for characterizing aspects of cannabis use among adolescents and emerging adults in real time in their natural environments during an important developmental period in the pathogenesis of CUD. That craving was heightened prior to use and reduced by use supported craving as an important proximal predictor of use. Craving ratings then converge at low levels for end-cannabis reports, regardless of CUD, suggesting that use unilaterally reduced craving for all participants. We did not anticipate that stimulation would be heightened and tension lessened prior to use. Differences emerging prior to use may indicate that some components of subjective “responses” to cannabis occur in anticipation of smoking. Pre-use changes were found regardless of whether begin-cannabis reports self-initiated after smoking had already begun were retained or excluded, and supplemental analysis of paired begin- and end-use reports replicated these findings. Nonetheless, we cannot discount that the internal monitoring of subjective states may have influenced when participants self-initiated a cannabis-use report, thus limiting the ecological validity of these assessments.
The overall finding that cannabis use paradoxically increased stimulation and sedation as well as ‘high’ is consistent with adult administration studies (Haney et al., 1999; Hart et al., 2001, 2002). But, as predicted, results also showed that participants with more severe CUD experienced greater cannabis-induced stimulation. It is noteworthy that neurocircuitry governing the reinforcing effects of cannabis and other drugs (i.e., mesocorticolimbic circuits) undergo extensive neuromaturation during adolescence and emerging adulthood. These fundamental changes heighten youths’ hedonic sensitivity and promote developmentally normative increases in impulsive and reward-seeking behavior. Taken together, the confluence of hypersensitivity to the positive reinforcing effects of cannabis and other drugs paired with dampened self-control and increased propensity for reward-seeking behavior appears to confer liability for hazardous drug use and the development of drug-related problems (Lubman, Cheetham, & Yücel, 2015; Lubman, Yücel, & Hall, 2007). This study, which found a positive association between CUD severity and subjective rewarding effects of cannabis use, but negative association between age and stimulatory response, provides cross-sectional support for these notions.
We also found that craving was elevated just prior to cannabis use and depressed following use, relative to non-use times, with effects strengthened among those with more CUD symptoms. Greater CUD pathology was also associated with higher levels of craving in non-use moments. Craving is a chief motivational determinant of drug use in most contemporary models of addiction. Laboratory studies consistently show that cannabis cues evoke craving under controlled conditions, and individuals with dependence and heavier users show stronger craving than lighter and less dependent users (Norberg, Kavanagh, Olivier, & Lyras, 2016). Findings from this study are consistent with research that shows alcohol and cannabis craving is experienced by adolescents and adults, is higher on use days than non-use days, and is reduced by use (Buckner et al., 2015; Ramirez & Miranda, 2014).
Finally, there is compelling evidence from animal models that adolescents differ from adults in how they respond the acute effects of substance use. Most of the work focused on alcohol, and findings generally show that adolescent rodents from outbred strains not only typically drink 2–3 times more alcohol than adults, but are less sensitive to the aversive, sedative, and motor impairing effects of alcohol, while showing greater sensitivity to alcohol’s stimulatory and social-facilitating effects than adults (Quoilin, Didone, Tirelli, & Quertemont, 2010; Spear, 2011). These alcohol sensitivities often persist into adulthood after chronic alcohol exposure during adolescence (see Spear & Swartzwelder, 2014, for review), perhaps contributing to the greater propensity for high levels of alcohol use in adulthood after adolescent alcohol exposure (Spear & Varlinskaya, 2010; Windle et al., 2009). Only one human laboratory study compared the acute effects of cannabis in adolescent males, ages 16–17 years, and adults, ages 24–28 (Mokrysz et al., 2016). As compared to adults, adolescents had blunted subjective ‘high’ and greater impairment of psychotomimetic and inhibitory processes, but also enhanced alertness relative to young adults. Our findings use different assessments in vivo in males and females, and thus are not directly comparable, but support the notion that adolescents have enhanced stimulatory responses to cannabis relative to emerging adults. Findings for subjective ‘high’ were contradictory to Mokrysz and colleagues (2016), with our adolescents showing greater subjective ‘high’ than their emerging-adult counterparts.
There were several limitations to this study. First and foremost, the cross-sectional nature of this work leaves unanswered the important question of directionality in the relationship between CUD symptom course and subjective responses and craving. Because we assessed cannabis problems at only one time point, our findings cannot differentiate between pre-existing vulnerability and neuroadaptations that accompany disorder progression. Additionally, feelings of sedation, sluggishness, and ‘high’ were not assessed at non-use times in this study, limiting our understanding of CUD effects on sedative and ‘high’ outcomes. Multi-wave longitudinal studies that employ intensive momentary research methods with more comprehensive assessments of subjective states at use and non-use times are necessary to track adolescents’ responses to cannabis and craving over time and prospectively evaluate the causal pathways posited to underlie addiction etiology. Short of these limitations, the present study provides a first look at subjective cannabis responses, in general, as well as how these responses may relate to the developmental unfolding of CUD.
Additional limitations include the participant selection criteria, methodological limiations, study duration, and a host of putative influences on subjective responses not directly assessed, accounted for, or tested in the present research. First, we studied adolescent and emerging-adult cannabis users who varied in terms of severity of CUD symptomatology, but may not be representative of adult users who have struggled with addiction for many years. Consequently, our findings may not generalize to older cannabis users, and this limitation may be especially salient for subjective responses to cannabis. While most addiction theories predict early years of addiction are marked by heightened sensitivity to rewarding drug effects, they differ in their predictions about how these changes evolve as individuals develop more severe addiction. Allostatic and chronic tolerance models predict that drug use produces less potent acute positive effects as people progress in the addiction pathology continuum. By contrast, sensitization theories purport that individuals experience greater stimulant effects as they develop more severe drug problems. Future research is needed to evaluate whether these patterns persist or change as individuals develop longer and more problematic cannabis-use histories.
Next, data were culled from a prerandomization, pre-medication period of a longer clinical trial evaluating a pharmacotherapeutic intervention for cannabis misuse (Miranda et al., 2016). Thus, participants were frequent cannabis users not seeking formal treatment but willing to engage in a study designed to reduce cannabis use. Inasmuch as this influenced results, findings may not generalize to other users. Additionally, participants were not drug tested for cannabis metabolites during the period comprising data for the present analyses, and cannabis potency was not assessed. Last, the 7–14 day monitoring window is short. This concern is mitigated, however, by the frequency of cannabis use in this sample. Results are from 1,929 ecological reports over 542 distinct social days and 85 cannabis-using adolescents and emerging adults who reported, on average 3.4 cannabis use days during the study period. Participant characteristics were therefore favorable, allowing for examination of several cannabis use episodes across a range of CUD symptomatology.
On the whole, research suggests that the developmental stage of adolescence is well suited to promote cannabis misuse, potentially via developmentally linked differences in subjective responses. Yet, understanding of subjective response as a phenotypic marker of risk for developing cannabis addiction in humans is lacking, particularly from adolescence through emerging adulthood—a crucial window for development of CUD. Thus, characterizing the acute effects of cannabis in youth is imperative for determining factors that confer liability to continued cannabis use and the development of CUD. Our findings provide the first human evidence, albeit correlational, that the acute subjective responses to cannabis use are associated with CUD severity.
Supplementary Material
General Scientific Summary.
Expanding legalization of cannabis and swelling debate about the potential risks highlight the importance of understanding cannabis misuse in adolescents and emerging adults. This study suggests that youth with more severe cannabis-use problems respond differently to cannabis use in daily life, supporting some models of addiction development.
Acknowledgments
The National Institute of Alcohol Abuse and Alcoholism and National Institute on Drug Abuse at the National Institutes of Health supported this research (AA007850 and DA026778, PI: Miranda; AA024808, PI: Treloar).
Footnotes
Findings reported herein were previously presented at the 39th Annual Scientific Meeting of the Research Society on Alcoholism, New Orleans, LA.
References
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. https://doi.org/10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Zvolensky MJ, Crosby RD, Wonderlich SA, Ecker AH, Richter A. Antecedents and Consequences of Cannabis Use among Racially Diverse Cannabis Users: An Analysis from Ecological Momentary Assessment. Drug and Alcohol Dependence. 2015;147:20–25. doi: 10.1016/j.drugalcdep.2014.12.022. https://doi.org/10.1016/j.drugalcdep.2014.12.022.Antecedents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Zvolensky MJ, Ecker AH. Cannabis use during a voluntary quit attempt: an analysis from ecological momentary assessment. Drug and Alcohol Dependence. 2013;132(3):610–6. doi: 10.1016/j.drugalcdep.2013.04.013. https://doi.org/10.1016/j.drugalcdep.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug and Alcohol Dependence. 2009;105(Suppl):S14–25. doi: 10.1016/j.drugalcdep.2009.04.003. https://doi.org/10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of delta 9 -THC on learning in adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2006;83:448–455. doi: 10.1016/j.pbb.2006.03.006. https://doi.org/10.1016/j.pbb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Chait LD, Perry JL. Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology. 1994;115(3):340–349. doi: 10.1007/BF02245075. https://doi.org/10.1007/BF02245075. [DOI] [PubMed] [Google Scholar]
- Chait LD, Zacny JP. Reinforcing and subjective effects of oral D–9-THC and smoked marijuana in humans. Psychopharmacology. 1992;107:255–262. doi: 10.1007/BF02245145. https://doi.org/10.1007/BF02245145. [DOI] [PubMed] [Google Scholar]
- de Wit H, Phillips TJ. Do initial responses to drugs predict future use or abuse? Neuroscience and Biobehavioral Reviews. 2012;36(6):1565–76. doi: 10.1016/j.neubiorev.2012.04.005. https://doi.org/10.1016/j.neubiorev.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: Possible implications for age differences in substance abuse and other risk-taking behaviors. Brain and Cognition. 2010;72(1):114. doi: 10.1016/j.bandc.2009.08.008. https://doi.org/10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, DuToit S. Advances in analysis of longitudinal data. Annual Review of Clinical Psychology. 2010;6:79–107. doi: 10.1146/annurev.clinpsy.032408.153550. https://doi.org/10.1146/annurev.clinpsy.032408.153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clinical Pharmacokinetics. 2003;42(4):327–60. doi: 10.2165/00003088-200342040-00003. https://doi.org/10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- Gwaltney CJ, Bartolomei R, Colby SM, Kahler CW. Ecological momentary assessment of adolescent smoking cessation: a feasibility study. Nicotine & Tobacco Research. 2008;10(7):1185–90. doi: 10.1080/14622200802163118. https://doi.org/10.1080/14622200802163118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M. Self-administration of cocaine, cannabis and heroin in the human laboratory: benefits and pitfalls. Addiction Biology. 2008;14(1):9–21. doi: 10.1111/j.1369-1600.2008.00121.x. https://doi.org/10.1111/j.1369-1600.2008.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. 1999;141(4):395–404. doi: 10.1007/s002130050849. https://doi.org/10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Hart CL, Van Gorp W, Haney M, Foltin RW, Fischman MW. Effects of Acute Smoked Marijuana on Complex Cognitive Performance. Neuropsychopharmacology. 2001;25:757–765. doi: 10.1016/S0893-133X(01)00273-1. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral D 9 -tetrahydrocannabinol in humans. Psychopharmacology. 2002;164:407–415. doi: 10.1007/s00213-002-1231-y. https://doi.org/10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- Hjorthøj CR, Fohlmann A, Larsen AM, Arendt M, Nordentoft M. Correlations and agreement between delta-9-tetrahydrocannabinol (THC) in blood plasma and timeline follow-back (TLFB)-assisted self-reported use of cannabis of patients with cannabis use disorder and psychotic illness attending the CapOpus randomized clin. Addiction. 2012;107(6):1123–31. doi: 10.1111/j.1360-0443.2011.03757.x. https://doi.org/10.1111/j.1360-0443.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- Hunault CC, Böcker KBE, Stellato RK, Kenemans JL, de Vries I, Meulenbelt J. Acute subjective effects after smoking joints containing up to 69 mg Δ9-tetrahydrocannabinol in recreational users: a randomized, crossover clinical trial. Psychopharmacology. 2014 doi: 10.1007/s00213-014-3630-2. https://doi.org/10.1007/s00213-014-3630-2. [DOI] [PubMed]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–8. doi: 10.1097/00004583-199707000-00021. https://doi.org/10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Cheetham A, Yücel M. Cannabis and adolescent brain development. Pharmacology and Therapeutics. 2015;148:1–16. doi: 10.1016/j.pharmthera.2014.11.009. https://doi.org/10.1016/j.pharmthera.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yücel M, Hall WD. Substance use and the adolescent brain: A toxic combination? Journal of Psychopharmacology. 2007;21(8):792–794. doi: 10.1177/0269881107078309. https://doi.org/10.1177/0269881107078309. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Brooks D, Haney M, Levin FR. Quantification and comparison of marijuana smoking practices: blunts, joints, and pipes. Drug and Alcohol Dependence. 2011;113(2–3):249–51. doi: 10.1016/j.drugalcdep.2010.08.008. https://doi.org/10.1016/j.drugalcdep.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RSE, … Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(40):E2657–64. doi: 10.1073/pnas.1206820109. https://doi.org/10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Monti PM, Ray L, Treloar HR, Reynolds EK, Ramirez J, … Magill M. Characterizing subjective responses to alcohol among adolescent problem drinkers. Journal of Abnormal Psychology. 2014;123(1):117–29. doi: 10.1037/a0035328. https://doi.org/10.1037/a0035328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Treloar H, Blanchard A, Justus A, Monti PM, Chun T, … Gwaltney CJ. Topiramate and motivational enhancement therapy for cannabis use among youth: a randomized placebo-controlled pilot study. Addiction Biology. 2016 doi: 10.1111/adb.12350. https://doi.org/10.1111/adb.12350. [DOI] [PMC free article] [PubMed]
- Miranda R, Treloar H, Blanchard A, Justus A, Monti PM, Chun T, … Gwaltney CJ. Topiramate and motivational enhancement therapy for cannabis use among youth: A randomized placebo-controlled pilot study. Addiction Biology. 2016 doi: 10.1111/adb.12350. https://doi.org/10.1111/adb.12350. [DOI] [PMC free article] [PubMed]
- Mokrysz C, Freeman TP, Korkki S, Grif K, Curran HV. Are adolescents more vulnerable to the harmful effects of cannabis than adults? A placebo-controlled study in human males. Translational Psychiatry. 2016;6:1–10. doi: 10.1038/tp.2016.225. https://doi.org/10.1038/tp.2016.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Survey on Drug Use and Health. National Survey on Drug Use and Health, 2012. 2012 https://doi.org/10.3886/ICPSR34933.v2.
- Norberg MM, Mackenzie J, Copeland J. Quantifying cannabis use with the timeline followback approach: a psychometric evaluation. Drug and Alcohol Dependence. 2012;121(3):247–52. doi: 10.1016/j.drugalcdep.2011.09.007. https://doi.org/10.1016/j.drugalcdep.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jahng S, Wood PK, Robertson BM, Epler AJ, Cronk NJ, … Sher KJ. The subjective effects of alcohol-tobacco co-use: an ecological momentary assessment investigation. Journal of Abnormal Psychology. 2011;120(3):557–71. doi: 10.1037/a0023033. https://doi.org/10.1037/a0023033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, McCarthy DE, Fiore MC, Baker TB. Alcohol consumption, smoking urge, and the reinforcing effects of cigarettes: an ecological study. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors. 2008;22(2):230–9. doi: 10.1037/0893-164X.22.2.230. https://doi.org/10.1037/0893-164X.22.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaram N, … McGregor IS. Adolescent Rats Find Repeated Δ 9-THC Less Aversive Than Adult Rats but Display Greater Residual Cognitive Deficits and Changes in Hippocampal Protein Expression Following Exposure. Neuropsychopharmacology. 2008;33:1113–1126. doi: 10.1038/sj.npp.1301475. https://doi.org/10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- Quoilin C, Didone V, Tirelli E, Quertemont E. Ontogeny of the stimulant and sedative effects of ethanol in male and female Swiss mice: gradual changes from weaning to adulthood. Psychopharmacology. 2010;212(4):501–512. doi: 10.1007/s00213-010-1971-z. https://doi.org/10.1007/s00213-010-1971-z. [DOI] [PubMed] [Google Scholar]
- Ramirez J, Miranda R. Alcohol craving in adolescents: Bridging the laboratory and natural environment. Psychopharmacology. 2014;231:1841–1851. doi: 10.1007/s00213-013-3372-6. https://doi.org/10.1007/s00213-013-3372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2. Thousand Oaks, CA: Sage Publications, Inc; 2002. [Google Scholar]
- Ray LA, Miranda R, Tidey JW, McGeary JE, MacKillop J, Gwaltney CJ, … Monti PM. Polymorphisms of the mu-opioid receptor and dopamine D4 receptor genes and subjective responses to alcohol in the natural environment. Journal of Abnormal Psychology. 2010;119(1):115–25. doi: 10.1037/a0017550. https://doi.org/10.1037/a0017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. https://doi.org/10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre F, Fatseas M, Debrabant R, Alexandre JM, Auriacombe M, Swendsen J. Ecological momentary assessment in alcohol, tobacco, cannabis and opiate dependence: a comparison of feasibility and validity. Drug and Alcohol Dependence. 2012;126(1–2):118–23. doi: 10.1016/j.drugalcdep.2012.04.025. https://doi.org/10.1016/j.drugalcdep.2012.04.025. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment. 2009;21(4):486–97. doi: 10.1037/a0017074. https://doi.org/10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrier La, Walls C, Rhoads A, Blood Ea. Individual and contextual predictors of severity of marijuana use events among young frequent users. Addictive Behaviors. 2013;38(1):1448–56. doi: 10.1016/j.addbeh.2012.05.026. https://doi.org/10.1016/j.addbeh.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis. New York, NY: Oxford University Press, Inc; 2003. [Google Scholar]
- Sobell LC, Sobell MB. In: Measuring Alcohol Consumption: Psychosocial and biochemical methods. Litten RZ, Allen JP, editors. Totowa, NJ: Humana Press; 1992. https://doi.org/10.1007/978-1-4612-0357-5. [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience. 2011;1(4):390–403. doi: 10.1016/j.dcn.2011.08.001. https://doi.org/10.1016/j.dcn.2011.08.001.Rewards. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Swartzwelder HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: A mini-review. Neuroscience and Biobehavioral Reviews. 2014;45:1–8. doi: 10.1016/j.neubiorev.2014.04.012. https://doi.org/10.1016/j.neubiorev.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: Implications for prevention science? Developmental Psychobiology. 2010;52(3):236–243. doi: 10.1002/dev.20457. https://doi.org/10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. The CBHSQ Report: A Day in the Life of Young Adults: Substance Use Facts. Rockville, MD: 2014. Retrieved April 13, 2016, from http://www.samhsa.gov/data/sites/default/files/CBHSQ-SR168-TypicalDay-2014/CBHSQ-SR168-TypicalDay-2014.htm. [PubMed] [Google Scholar]
- Treloar HR, Piasecki TM, McCarthy DE, Baker TB. Relations Among Caffeine Consumption, Smoking, Smoking Urge, and Subjective Smoking Reinforcement in Daily Life. Journal of Caffeine Research. 2014;4(3):93–99. doi: 10.1089/jcr.2014.0007. https://doi.org/10.1089/jcr.2014.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, … Baler R. Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA Psychiatry. 2016;73(3):292–297. doi: 10.1001/jamapsychiatry.2015.3278. https://doi.org/10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From First Drug Use to Drug Dependence: Developmental Periods of Risk for Dependence upon Marijuana, Cocaine, and Alcohol. Neuropsychopharmacology. 2002;26(4):479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Windle M, Spear L, Fuligni A, Angold A, Brown JD, Pine D, … Dahl RE. Transitions into underage and problem drinking: Summary of developmental processes and mechanisms: Ages 10–15. Alcohol Research & Health. 2009;32(1):30–40. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.