Abstract
Background: Pregnancy may be associated with an increased risk of recurrence/progression of differentiated thyroid cancer (DTC). However, it is unclear if the impact of pregnancy would differ based on pre-pregnancy response to therapy status. The objective of this study was to investigate the risk of recurrence/progression of DTC, applying the response to therapy assessments to pre-pregnancy status as recommended by the 2015 American Thyroid Association thyroid cancer guidelines.
Methods: This was a retrospective review of 235 women followed at Memorial Sloan Kettering Cancer Center for DTC who had a term pregnancy after initial treatment for DTC between 1997 and 2015.
Results: Structural disease recurrence/progression after pregnancy was documented in 5% (11/235) of the patients. When evaluated 3–12 months after delivery, patients who had an excellent, indeterminate, or biochemical incomplete response before pregnancy continued to show no evidence of structurally identifiable disease. Conversely, in women with a structural incomplete response to therapy prior to pregnancy, structural progression (defined as ≥3 mm increase in the size of known disease or identification of new metastatic foci) was identified after delivery in 29% (11/38). However, additional therapy was recommended during the first postpartum years in only 8% (3/38) of those patients who had a structural incomplete response to therapy prior to pregnancy, while the remainder (92%) continued to be followed with observation.
Conclusion: None of the patients with an excellent, indeterminate, or biochemical incomplete response to therapy prior to pregnancy developed structurally identifiable disease after a full-term delivery. Even though structural disease progression was seen in almost a third of the patients with known structural disease prior to pregnancy, only a minority of these patients had changes sufficient to warrant additional therapy. These data confirm that pre-pregnancy response to therapy status is an excellent predictor of pregnancy-associated disease progression in women previously treated for DTC.
Keywords: : pregnancy, response to therapy, differentiated thyroid cancer
Introduction
Surveillance recommendations in the 2015 American Thyroid Association (ATA) thyroid cancer guidelines rely on response to therapy re-staging assessments, which modify the initial risk of recurrence estimates based on data that are accumulated during follow-up (1,2). While this dynamic risk-stratification system has been validated in multiple studies (3–7), it has not been evaluated in the context of a pregnancy after initial thyroid cancer therapy is complete.
Since differentiated thyroid cancer (DTC) is very common in women of childbearing age, the potential adverse impact of pregnancy on the risk of thyroid cancer recurrence or disease progression is an important survivorship issue (8,9). Previous studies have shown that pregnancy has little impact on the risk of recurrence in women with no biochemical or structural evidence of disease prior to pregnancy. However, minor progression of disease has been noted with pregnancy in women with evidence of a biochemical or structural incomplete response (10–12). While not formally evaluated previously, these findings do suggest that the response to therapy status prior to pregnancy may be an important predictor of the risk of pregnancy-associated disease progression.
Therefore, the aim of this study was to evaluate the risk of structural disease recurrence and/or progression within each of the response-to-therapy categories (excellent, biochemical incomplete, structural incomplete, and indeterminate) in women who had a full-term pregnancy after initial treatment of DTC.
Materials and Methods
Study population
This was a retrospective evaluation of all women who were followed at Memorial Sloan Kettering Cancer Center for DTC and who had a full-term pregnancy after initial treatment for DTC between January 1997 and September 2015. To be included in the study, subjects were required to have structural imaging (at least cervical ultrasound or computed tomography [CT] scan of the neck or radioactive iodine scans; if available, body imaging was also included for full assessment), clinical evaluation, and serum thyroglobulin (Tg) or Tg antibody measurements within 12 months before and after pregnancy. Only one pregnancy was included for each patient (most commonly, the first pregnancy after diagnosis).
Data collection
The medical records were reviewed, and the following data were collected: age at diagnosis of DTC, duration to pregnancy of interest after diagnosis of DTC, maternal age at delivery, tumor-node-metastasis staging, 2009 ATA initial risk stratification, subtype of DTC and treatment, thyrotropin (TSH), Tg and Tg antibody levels before and after pregnancy, and disease-related imaging before and after pregnancy.
Stratification based on response to therapy assessments
After initial treatment, thyroid cancer patients were stratified into the following response-to-therapy assessments established within one year prior to pregnancy and up to one year postpartum: excellent (no structural or biochemical evidence of disease with undetectable Tg antibodies and Tg levels <0.6 ng/mL); biochemical incomplete (no structural evidence of disease and Tg level >1 ng/mL or increasing Tg antibody levels); structural incomplete (persistent locoregional or distant metastases with or without detectable Tg levels or Tg antibodies); or indeterminate (Tg level >0.6 ng/mL and <1 ng/mL, Tg antibodies that are stable or declining, or non-specific structural findings on imaging). Response to therapy classification for patients treated with less than total thyroidectomy was done using definitions previously published (13).
Definitions of structural disease progression and increasing Tg values
Structural progression of DTC was defined as ≥3 mm increase in the size of known disease or identification of new metastatic foci on imaging. The abnormal cervical lymph nodes were identified with the use of ultrasound or CT, and some were confirmed by cytology. Structural disease progression that was either symptomatic or that led to additional treatment in the first 18 months after delivery was classified as clinically significant. Documented asymptomatic structural disease progression that did not prompt additional therapy was classified as minor disease progression.
Biochemical disease progression was defined as an increase in Tg levels or Tg antibodies if detected in the absence of structural disease progression. The postpartum non-stimulated Tg level was considered to be elevated above the baseline pre-pregnancy value if Tg increased by >0.2 ng/mL above a baseline Tg of <1 ng/mL, by >1 ng/mL above a baseline Tg of 1.1–10 ng/mL, or by >20% above a baseline Tg of >10 ng/mL. These definitions are used in the authors' practice and were applied in this study after careful consideration of the available Tg assays and the studied cohort.
Laboratory measurements of Tg and TSH
Serum Tg levels collected before March 2015 were measured with an immunoradiometric assay (Dynotest Tg-S; Brahms, Inc., Berlin, Germany), which in the 1990s had a functional sensitivity cutoff of 0.6 ng/mL. Serum Tg levels collected after March 2015 were measured with a solid-phase, chemiluminescent immunometric assay (Beckman Coulter, Brea, CA). All Tg values were normalized to the CRM 457 reference standard. TSH was determined by a heterogeneous sandwich immunoassay on an Immuno 1 System (Bayer Corp., Tarrytown, NY).
Statistical analysis
Continuous data are presented as means and standard deviations (SD) or median and ranges, as appropriate for each variable. Categorical comparisons were performed with the chi-square test, and continuous variables were compared using Student's t-test or one way analysis of variance, as appropriate.
Results
Clinical characteristics
A total of 235 women with a history of DTC who became pregnant and had a full-term delivery were included in this study (Table 1). At the time of delivery, the median age of the cohort was 34 years (M ± SD = 34 ± 0.4 years; range 20–45 years). The delivery occurred a median of 3 years (M ± SD = 4.9 ± 0.3 years; range 0.1–23 years) after initial treatment for DTC. The treatment for DTC included total thyroidectomy in 210 (89%) women, with 144 (61%) receiving radioactive iodine ablation (RAI). In accordance with the 2009 ATA Guidelines, 184/235 (78%) women were classified as having an intermediate risk of recurrence.
Table 1.
Clinical Characteristics of 235 Women with DTC
| Clinical characteristics | ||
|---|---|---|
| Age at diagnosis (years) | Mean ± SD | 28 ± 7 |
| Median | 28 | |
| Range | 16–43 | |
| Age at delivery (years) | Mean ± SD | 34 ± 0.4 |
| Median | 34 | |
| Range | 20–45 | |
| Race/ethnicity | Caucasian | 84.3% (198) |
| Asian | 11.9% (28) | |
| Hispanic | 1.3% (3) | |
| African American | 1.3% (3) | |
| Unknown | 1.3% (3) | |
| Time between diagnosis and subsequent pregnancy (years) | Mean ± SD | 4.9 ± 0.3 |
| Median | 3 | |
| Range | 0.1–23 | |
| Initial surgery | Total thyroidectomy | 89.4% (210) |
| Thyroid lobectomy | 10.6% (25) | |
| RAI ablation | Yes | 61.3% (144) |
| No | 38.7% (91) | |
| Histology | Classic papillary | 73.6% (173) |
| Follicular variant, papillary | 19.6% (46) | |
| Poorly differentiated | 3.4% (8) | |
| Follicular | 2.6% (6) | |
| Hürthle cell | 0.9% (2) | |
| Primary tumor size (cm) | Mean ± SD | 2.1 ± 0.1 |
| Median | 1.8 | |
| Range | 0.2–8.5 | |
| Nodal status at diagnosis | N0/Nx | 48.5% (114) |
| N1 | 48.9% (115) | |
| Metastases at diagnosis | M0 | 95.7% (225) |
| M1 | 4.3%(10) | |
| 2009 ATA risk | Low | 15.7% (37) |
| Intermediate | 78.3% (184) | |
| High | 6% (14) | |
| AJCC stage | I | 95.7% (225) |
| II | 4.3% (10) | |
DTC, differentiated thyroid cancer; SD, standard deviation; RAI, radioactive iodine; ATA, American Thyroid Association; AJCC, American Joint Committee on Cancer.
The response-to-therapy assessments prior to pregnancy were as follows: 63% with an excellent response, 16% with a structural incomplete response, 12% with an indeterminate response, and 9% with a biochemical incomplete response. There were no significant differences between the percentages of patients within each of the response to therapy categories when the pre-pregnancy status was compared with the post-pregnancy status (Table 2).
Table 2.
Summary of Cohort Based on Response-to-Therapy Assessments Before and After Pregnancy (n = 235)
| Before pregnancy (n) | After pregnancy (n) | |
|---|---|---|
| Excellent | 63% (148) | 68% (159) |
| Indeterminate | 12% (29) | 11% (26) |
| Biochemical incomplete | 8.5% (20) | 6% (13) |
| Structural incomplete | 16% (38) | 16% (37) |
Pregnancy-associated structural disease recurrence/progression
Overall, structural disease recurrence/progression after pregnancy was documented in only 5% (11/235) of the cohort. Importantly, none of the women with an excellent, indeterminate, or biochemical incomplete response to therapy prior to pregnancy developed structurally identifiable disease when evaluated after delivery (Table 3). Structural disease progression was documented in 29% (11/38) of women demonstrating a structural incomplete response to therapy prior to pregnancy (five patients with a median increase in size of 4 mm in known abnormal lymph nodes, three patients with newly identified abnormal lymph nodes ranging in size from 0.5–1.8 cm, two patients with both an increase in size of known abnormal lymph nodes and newly identified abnormal lymph nodes, and one patient with progression of known distant metastases with a history of poorly differentiated thyroid cancer). One to two years prior to pregnancy, 36% (4/11) of these patients had non-progressive structural disease, and 64% (7/11) were noted to have progression of structural disease. Additionally, two out of seven patients who had progression of disease prior to pregnancy were noted to have more accelerated disease during pregnancy, as evidenced by imaging done during and within two months after delivery (Table 4).
Table 3.
Analysis of the Structural and Biochemical Recurrence/Progression Based on Pre-Pregnancy Clinical Status
| Pre-pregnancy clinical status | n | Clinically significant structural disease recurrence/progression leading to additional therapy | Minor structural disease recurrence/progression not requiring additional therapy | Postpartum Tg elevated above pre-pregnancy baseline |
|---|---|---|---|---|
| Excellent | 148 | 0% | 0% | 6.8% (10/148) |
| Indeterminate | 29 | 0% | 0% | 6.9% (2/29) |
| Biochemical incomplete | 20 | 0% | 0% | 20% (4/20) |
| Structural incomplete | 38 | 8% (3/38) | 21% (8/38) | 31.6% (12/38) |
Tg, thyroglobulin.
Table 4.
Clinical Characteristics of 11 Patientsa with a Structural Incomplete Response Prior to Therapy and Who Had Structural Disease Progression During Pregnancy
| Patient | Race/ethnicity | Age at diagnosis (years) | Age at delivery (years) | Histology | Primary tumor size (cm) | Known nodal involvement at diagnosis | Distant metastasis at diagnosis | ATA risk 2009 | TNM/AJCC | Structural progression prior to pregnancy | Structural progression during pregnancy | Follow-up after delivery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Caucasian | 25 | 40 | Follicular variant, papillary | 1.9 | No | No | Intermediate | 1 | No | Yes | Observation |
| 2 | Caucasian | 29 | 33 | Classic papillary | 1.9 | Yes | No | High | 1 | No | Yes | Observation |
| 3 | Caucasian | 32 | 35 | Classic papillary | 1.5 | Yes | No | Intermediate | 1 | Yes | Yes | Observation |
| 4 | Caucasian | 23 | 28 | Classic papillary | 2.5 | No | No | Intermediate | 1 | Yes | Yes | Observation |
| 5 | Caucasian | 22 | 23 | Poorly differentiated | 5.5 | Yes | Yes-lung | High | 2 | Yes | Accelerated | Chemotherapy |
| 6 | Asian | 23 | 44 | Classic papillary | 3.6 | Yes | Yes-lung | High | 2 | Yes | Yes | Observation |
| 7 | Unknown | 31 | 34 | Classic papillary | 1.6 | No | Yes-lung | High | 2 | No | Yes | Observation |
| 8 | Caucasian | 35 | 38 | Classic papillary | 5.5 | Yes | No | Intermediate | 1 | No | Yes | Observation |
| 9 | Caucasian | 41 | 44 | Classic papillary | 1.1 | Yes | No | Intermediate | 1 | Yes | Yes | Observation |
| 10 | Caucasian | 25 | 32 | Classic papillary | 2.4 | No | No | High | 1 | Yes | Yes | Neck dissection |
| 11 | Caucasian | 19 | 20 | Classic papillary | Unknown | Yes | No | Intermediate | 1 | Yes | Accelerated | Neck dissection |
All had undergone total thyroidectomy and RAI ablation at diagnosis.
TNM, tumor-node-metastasis.
Only 8% (3/38) of the women with a structural incomplete response to therapy prior to pregnancy demonstrated clinically significant structural disease progression, which led to additional treatment within the first postpartum years (two patients underwent neck dissections, one at two months and another at 15 months postpartum, and a third patient with poorly differentiated thyroid cancer received systemic therapy starting at two months postpartum). As a result, only 1.3% (3/235) of the entire cohort required additional therapy within the first postpartum years because of structural disease progression during pregnancy.
When comparing those patients who demonstrated structural disease progression (n = 11) with either the remainder of the cohort who did not demonstrate structural disease progression (n = 224) or with patients with structural incomplete response prior to pregnancy without structural disease progression (n = 27), no statistically significant differences were found with respect to race/ethnicity, histology, extent of initial surgery, RAI remnant ablation, size of primary tumor, vascular invasion, nodal status at initial therapy, distant metastases, age at surgery, age at delivery, years from initial therapy to pregnancy, pre-pregnancy non-stimulated Tg value, or postpartum non-stimulated Tg value. At least one TSH value was available during pregnancy for 10/11 women demonstrating structural disease progression. There was no indication that structural disease progression was related to TSH levels during pregnancy (all 10 had suppressed or normal TSH values when measured during pregnancy).
Impact of pregnancy on patients with distant metastases
Pulmonary metastases were present at diagnosis of DTC in 10 (4%) patients. Prior to pregnancy, a median of six years after diagnosis (range 1–21 years), seven of these patients had a structural incomplete response (all with persistent pulmonary nodules on cross-sectional imaging), one patient had a biochemical incomplete response, and two patients had an excellent response to therapy. Pregnancy was associated with structural progression of the pulmonary metastases in only 1/10 patients (an unusual young patient with a poorly differentiated thyroid cancer and known lung metastases). No patients with M1 disease arising from papillary thyroid cancer (n = 7) or follicular variant of papillary thyroid cancer (n = 2) demonstrated pregnancy-associated progressive lung metastases. Serum Tg increased in 1/10 patients with pulmonary metastases (from 39 to 60 ng/mL comparing non-stimulated Tg values three months before and three months after pregnancy) without accompanying structural disease progression.
Change in serum Tg values
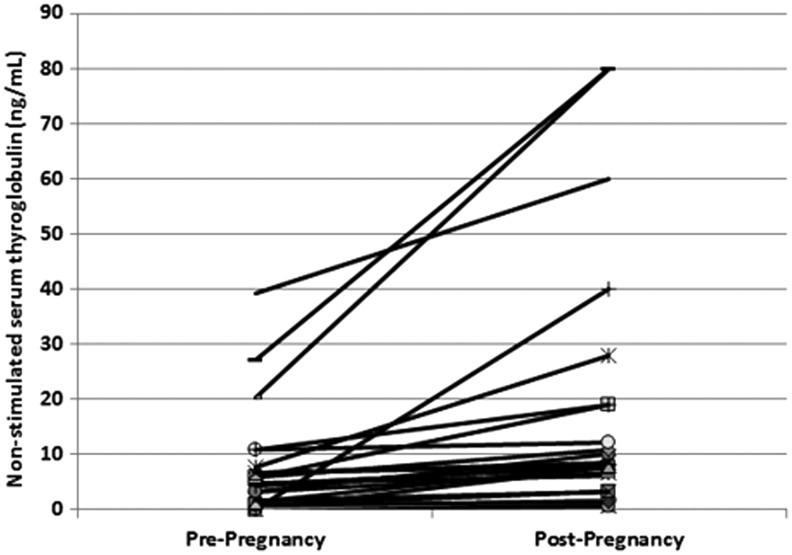
For the entire cohort, there was no significant difference in the mean serum Tg levels comparing baseline non-stimulated serum Tg levels prior to pregnancy with those obtained after pregnancy (3.8 ± 27 ng/mL before delivery vs 3.7 ± 15 ng/mL after delivery). However, 12% (28/235) of the patients demonstrated an increase in non-stimulated serum Tg levels after delivery. Sixteen (16/28) of these women had an excellent, indeterminate, or biochemical incomplete response to therapy prior to pregnancy, and none of these women had evidence of structural disease, despite an increase in Tg levels after pregnancy. On the other hand, of the 12 women who had structural evidence of disease prior to pregnancy, four women had progression of a known structural disease, and two of these women had undergone additional surgery after pregnancy (Fig. 1).
FIG. 1.
Thyroglobulin (Tg) levels of 28 women who demonstrated an increase in non-stimulated serum Tg levels after delivery. For each woman, two non-stimulated Tg levels are plotted: pre-pregnancy and post-pregnancy. All Tg levels were obtained within one year before pregnancy (pre-pregnancy) and one year after delivery (post-pregnancy).
Despite minor TSH variations throughout pregnancy, biochemical progression was seen in only 8% (16/197) of the patients in the non-structural groups. Specifically, 14/16 patients in the non-structural groups with biochemical progression had TSH levels of <0.5 mIU/L when measured after pregnancy with concomitant serum Tg levels (two patients had TSH levels of 4.0 mIU/L and 5.2 mIU/L).
Only 4/28 women with a pregnancy-associated increase in serum Tg levels demonstrated structural disease progression (all with a structural incomplete response prior to pregnancy with pre-conception non-stimulated Tg values of <0.2, <0.2, 6.6, and 27.2 ng/mL without interfering antibodies). The likelihood of structural disease progression was significantly higher in patients with biochemical disease progression (14.3%; 4/28) compared with those patients who did not show a significant change in serum Tg after pregnancy (3.4%; 7/207; p = 0.03).
Patients with a structural incomplete response to therapy prior to pregnancy were more likely to have biochemical progression (32%; 12/38) than patients with either a biochemical incomplete response (20%; 4/20), indeterminate response (7%; 2/29), or excellent response (7%; 10/148; p = 0.001; see Table 3). No other statistically significant differences were found with respect to race/ethnicity, histology, extent of initial surgery, RAI remnant ablation, size of primary tumor, vascular invasion, nodal status at initial therapy, distant metastases, age at surgery, age at delivery, years from initial therapy to pregnancy, or pre-pregnancy non-stimulated Tg values when comparing patients with or without a pregnancy-associated increase in serum Tg.
Comparison of response to therapy status before and after pregnancy
When analyzed based on response-to-therapy status, 94% of women who had an excellent response to therapy prior to pregnancy remained in this category after pregnancy, while 5% were classified as indeterminate based on Tg values or non-specific ultrasound findings, and 1% transitioned to a biochemical incomplete response based on a postpartum Tg value of >1 ng/mL. Interestingly, 52% of the women in the indeterminate category prior to pregnancy were reclassified as having an excellent response to therapy after delivery, while 45% remained in the indeterminate group and 3% were reclassified as having a biochemical incomplete response after delivery.
Similarly, while the majority (55%) of the patients in the pre-pregnancy biochemical incomplete group remained in the same category after pregnancy, 20% were reclassified as having an excellent response to therapy, and 25% were reclassified as having an indeterminate response to therapy after delivery. As expected, 99% of the patients in the pre-pregnancy structural incomplete response group were classified as structural incomplete after delivery, with only one patient showing resolution of a small metastatic cervical lymph node over time.
Discussion
In the last decade, a few small studies have addressed the impact of pregnancy in women who were treated for DTC. This is the first retrospective study to apply response-to-therapy assessment as a predictor of postpartum clinical outcome. Consistent with these previous small studies (10–12), the present findings demonstrate that pregnancy is seldom associated with clinically significant disease progression, except in those patients with structural disease present prior to pregnancy. Even in patients with a structural incomplete response to therapy, the degree of disease progression is usually minor and generally does not require additional therapy in the first postpartum years. These data are even more reassuring considering that 78% of the patients included in this study had an ATA intermediate risk of recurrence at diagnosis, probably as a result of the referral bias at a high-volume cancer center.
As expected, the response to therapy status prior to pregnancy was an excellent indicator of structural disease progression. None of the patients with an excellent, indeterminate, or biochemical incomplete response to therapy demonstrated pregnancy-associated structural disease progression independent of the duration after initial treatment. Furthermore, only a minority of patients demonstrated a postpartum Tg that was higher than their baseline level. The evaluation of response to therapy prior to pregnancy can serve as a powerful predictor of postpartum clinical outcomes. Therefore, it is recommended that clinicians apply response-to-therapy status in their practice and reassure women with non-structural disease that pregnancy is safe and unlikely to be associated with new structural disease.
The majority (32/38) of patients with structural disease had no change in the natural course of their disease with either stable or slow progression of structural disease during pregnancy. Only a minority (6/38) of these patients had evidence of new or more aggressive structural disease progression. The noted structural disease progression in these patients could be secondary to pregnancy or may represent a natural course of the disease independent of pregnancy. Owing to the small number of patients with structural disease in the study, the understanding of the impact of pregnancy in this subgroup is limited.
Only three patients had clinically significant structural disease progression that required additional therapy such as surgery or systemic therapy within 18 months after delivery. The decision to proceed with additional therapy directly reflects the management of DTC at the authors' practice. Therefore, the percentage of patients who have clinically significant structural disease progression at other institutions may differ depending on their approach to patients with structural disease.
In patients with known pulmonary metastases, only one patient out of seven had significant progression of known distant metastases during pregnancy; of note, she was a young patient with a rare poorly differentiated thyroid cancer. No progression of known pulmonary metastases was noted in the other six patients who had papillary thyroid cancer or follicular variant of papillary thyroid cancer. These data are reassuring, and it is recommended that patients are counselled that properly treated distant metastases are not a definite contraindication to pregnancy, even though some minor biochemical or structural disease progression is possible.
The authors agree with the recommendations in the ATA guidelines that additional monitoring beyond appropriate TSH values is not required during pregnancy in patients with an excellent response to therapy prior to conception (14). Furthermore, this minimalistic approach is extended to patients with an indeterminate and biochemical incomplete response to therapy, as no evidence of structural disease recurrence was found in these patients. Since the study included a limited number of patients with structural disease, the recommendations for this diverse group remain conservative. It is recommended that neck ultrasonography is considered at the end of the first trimester and two to three months postpartum in patients with a structural incomplete response to therapy in order to assess for the presence and extent of pregnancy-associated structural disease progression.
In summary, this is the first study that formally applies response-to-therapy assessment to thyroid cancer patients who had at least one full-term pregnancy. Our analysis show that none of the patients with an excellent, indeterminate, or biochemical incomplete response to therapy prior to pregnancy developed structurally identifiable disease after a full-term delivery. Furthermore, <10% of patients with a structural incomplete response to therapy required additional treatment during the first postpartum years. These data confirm that response-to -therapy status prior to pregnancy is an excellent predictor of pregnancy-associated disease recurrence/progression in women who were previously treated for DTC.
Acknowledgment
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Author Disclosure Statement
The authors have nothing to disclose.
References
- 1.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Momesso DP, Tuttle RM. 2014. Update on differentiated thyroid cancer staging. Endocrinol Metab Clin North Am 43:401–421 [DOI] [PubMed] [Google Scholar]
- 3.Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, Brokhin M, Omry G, Fagin JA, Shaha A. 2010. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 20:1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castagna MG, Maino F, Cipri C, Belardini V, Theodoropoulou A, Cevenini G, Pacini F. 2011. Delayed risk stratification, to include the response to initial treatment (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur J Endocrinol 165:441–446 [DOI] [PubMed] [Google Scholar]
- 5.Pitoia F, Bueno F, Urciuoli C, Abelleira E, Cross G, Tuttle RM. 2013. Outcomes of patients with differentiated thyroid cancer risk-stratified according to the American Thyroid Association and Latin American Thyroid Society risk of recurrence classification systems. Thyroid 23:1401–1407 [DOI] [PubMed] [Google Scholar]
- 6.Vaisman F, Momesso D, Bulzico DA, Pessoa CHN, Dias F, Corbo R, Vaisman M, Tuttle RM. 2012. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol 77:132–138 [DOI] [PubMed] [Google Scholar]
- 7.Vaisman F, Tala H, Grewal R, Tuttle RM. 2011. In differentiated thyroid cancer, an incomplete structural response to therapy is associated with significantly worse clinical outcomes than only an incomplete thyroglobulin response. Thyroid 21:1317–1322 [DOI] [PubMed] [Google Scholar]
- 8.Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS. 2009. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev 18:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen AY, Jemal A, Ward EM. 2009. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 115:3801–3807 [DOI] [PubMed] [Google Scholar]
- 10.Leboeuf R, Emerick LE, Martorella AJ, Tuttle RM. 2007. Impact of pregnancy on serum thyroglobulin and detection of recurrent disease shortly after delivery in thyroid cancer survivors. Thyroid 17:543–547 [DOI] [PubMed] [Google Scholar]
- 11.Hirsch D, Levy S, Tsvetov G, Weinstein R, Lifshitz A, Singer J, Shraga-Slutzky I, Grozinski-Glasberg S, Shimon I, Benbassat C. 2010. Impact of pregnancy on outcome and prognosis of survivors of papillary thyroid cancer. Thyroid 20:1179–1185 [DOI] [PubMed] [Google Scholar]
- 12.Rosário PW, Barroso AL, Purisch S. 2007. The effect of subsequent pregnancy on patients with thyroid carcinoma apparently free of the disease. Thyroid 17:1175–1176 [DOI] [PubMed] [Google Scholar]
- 13.Momesso DP, Vaisman F, Yang SP, Bulzico DA, Corbo R, Vaisman M, Tuttle RM. 2016. Dynamic risk stratification in differentiated thyroid cancer patients treated with radioactive iodine. J Clin Endocrinol Metab 101:2692–26700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W. 2011. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 21:1081–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]