Abstract
Objective: The differentiation program for human thyroid follicular cells (TFCs) relies on the interplay between sequence-specific transcription factors and transcriptional co-regulators. Transcriptional co-activator with PDZ-binding motif (TAZ) is a co-activator that regulates several transcription factors, including PAX8 and NKX2-1, which play a central role in thyroid-specific gene transcription. TAZ and PAX8/NKX2-1 are co-expressed in the nuclei of thyroid cells, and TAZ interacts directly with both PAX8 and NKX2-1, leading to their enhanced transcriptional activity on the thyroglobulin (TG) promoter and additional genes.
Methods: The use of a small molecule, ethacridine, recently identified as a TAZ activator, in the differentiation of thyroid cells from human embryonic stem (hES) cells was studied. First, endodermal cells were derived from hES cells using Activin A, followed by induction of differentiation into thyroid cells directed by ethacridine and thyrotropin (TSH).
Results: The expression of TAZ was increased in the Activin A–derived endodermal cells by ethacridine in a dose-dependent manner and followed by increases in PAX8 and NKX2-1 when assessed by both quantitative polymerase chain reaction and immunostaining. Following further differentiation with the combination of ethacridine and TSH, the thyroid-specific genes TG, TPO, TSHR, and NIS were all induced in the differentiated hES cells. When these cells were cultured with extracellular matrix–coated dishes, thyroid follicle formation and abundant TG protein expression were observed. Furthermore, such hES cell–derived thyroid follicles showed a marked TSH-induced and dose-dependent increase in radioiodine uptake and protein-bound iodine accumulation.
Conclusion: These data show that fully functional human thyroid cells can be derived from hES cells using ethacridine, a TAZ activator, which induces thyroid-specific gene expression and promotes thyroid cell differentiation from the hES cells. These studies again demonstrate the importance of transcriptional regulation in thyroid cell development. This approach also yields functional human thyrocytes, without any gene transfection or complex culture conditions, by directly manipulating the transcriptional machinery without interfering with intermediate signaling events.
Keywords: : thyroid follicular cells, human embryonic stem cells, NK2 homeobox 1 (NKX2-1), Paired box gene 8 (PAX8), transcriptional co-activator with PDZ-binding motif (TAZ), ethacridine
Introduction
The differentiation program for human thyroid follicular cells (TFCs) relies on the interplay between sequence-specific transcription factors and transcriptional co-regulators. The well-characterized thyroid transcription factors PAX8, NKX2-1 (TTF1), FOXE1 (TTF2), and HEX are only expressed simultaneously in thyroid cell precursors, and they appear to drive commitment toward a differentiated thyroid cell fate. However, PAX8 and NKX2-1 are reported to play a predominant role in thyroid cell differentiation and thyroid gene transcription (1–3). Since embryonic stem (ES) cells have the capacity to differentiate into any tissue, it has demonstrated that mouse and human ES (hES) cells can be differentiated effectively into functional thyrocytes by activating the key transcription factors PAX8 and NKX2-1 (4–6). These ES cell–derived thyrocytes expressing specific transcription factors are capable of forming three-dimensional, functional thyroid follicles (4–6). However, the initial approach of transfecting PAX8 and NKX2-1 into ES cells to initiate the differentiation process may also induce uncharacterized epigenetic changes, and tumor formation may be seen after transplantation in vivo. However, the identification of a variety of regulatory growth factors inherent in the thyroid differentiation process has allowed the development of a non-transfection approach to thyroid cell differentiation (7). A different approach to inducing PAX8 and NKX2-1 has been explored by using a key transcriptional regulator of these genes.
Transcriptional co-activator with PDZ-binding motif (TAZ) is a co-activator for PAX8 and NKX2-1. These three proteins are co-expressed in the nucleus of differentiated thyroid cells. TAZ can interact with both PAX8 and NKX2-1, and this interaction can lead to the enhancement of their transcriptional activity inducing thyroid gene expression and implicating TAZ as a major factor in thyroid differentiation (8). Indeed, co-localization of TAZ, PAX8, and NKX2-1 is observed during thyroid development and in adult thyroid tissue (8).
TAZ is known to be important for the development of a variety of mammalian tissues. Structurally, the TAZ protein has an N-terminal TEAD-binding domain, one or two WW domains, and a transcriptional activation domain (9,10). The WW domain within TAZ facilitates interaction with several L/PPXY motif–containing transcription factors, such as runt-related transcription factor 2 (RUNX2) (11,12), polyomavirus T antigens (13), the TEF1 gene family (14), TBX5 (15), and PAX3 (16), which in turn modulates their transcriptional activities. TAZ is phosphorylated at residue S89 and is sequestered by the 14-3-3 protein in the cytosol. Conversely, de-phosphorylation of TAZ by protein phosphatase 1 leads to its nuclear localization and modulation of its activity (9).
Ethacridine, a widely used small molecule with antiseptic ability, has also been shown to enhance the expression of unphosphorylated and nuclear TAZ (17). How ethacridine achieves this influence remains uncertain. Here, it is confirmed that ethacridine increases nuclear TAZ in Activin A–derived human endoderm cells, and that it induces PAX8 and NKX2-1. It is shown that this kick starts the thyroid differentiation program. Further differentiation of progenitor thyrocytes with thyrotropin (TSH) allows the induction of thyroid-specific genes (TG, TPO, TSHR, and NIS) in the hES cells. Furthermore, when these cells are cultured with extracellular matrix, there is thyroid follicle formation and abundant TG protein expression within the lumen of these follicles. Such hES cell–derived thyroid follicles showed TSH-enhanced 125I uptake and protein-bound 125I (PB125I). These data show that activation of TAZ subsequently induces thyroid-specific gene expression and promotes thyroid cell differentiation from hES cells.
Materials and Methods
Cell culture and differentiation
The hES cells (H9) were maintained in feeder-free culture conditions with mTeSR medium (Stemcell Technologies) on six-well plates coated with Matrigel (BD Biosciences). The culture medium was changed daily, and the cells were passaged every four to five days at ratios of 1:3 to 1:6. Cells were cultured in a humidified chamber in a 5% CO2-air mixture at 37°C.
For thyroid cell differentiation, hES cells were passaged into culture dishes for two days. To initiate endoderm differentiation, hES cells were washed once with PBS and cultured in RPMI 1640 medium (Life Technologies) containing 1% B27 (Life Technologies) supplemented with 100 ng/mL of Activin A (R&D Systems) for four days. Subsequently, the supplements were changed to ethacridine (Sigma; concentration as indicated in each experiment) to enhance the transcriptional activity of PAX8 and NKX2-1. After validating the enhancement of gene expression by real-time polymerase chain reaction (PCR), the cells were further exposed to ethacridine (5 μM) and TSH (1 mIU/mL) for the induction of differentiation up to 21 days. Then, the cells were embedded in growth factor–restricted Matrigel (BD Biosciences) and placed into six-well plates in RPMI 1640 containing 1% B27 (Life Technologies) supplemented with 1 mIU/mL TSH to form thyroid follicle. Cells were harvested for analysis at different time points.
RNA isolation and reverse transcription PCR
Total RNA was extracted from cultured cells using the RNeasy Mini Kit Isolation System (Qiagen Ltd.), which includes a digestion step with DNase I. RNA quantity and quality were assessed by UV spectrophotometry. cDNA synthesis was performed using the SuperScript® III First-Strand Synthesis System (Invitrogen Corp.). The real-time quantitative reverse transcription PCR (qRT-PCR) was carried out using predesigned qPCR Assays (intercalating dye-based assays; Integrated DNA Technologies) and employing the StepOnePlus RT-PCR system (Applied Biosystems). Relative expression levels of each gene in real time were analyzed using the 2−ΔΔCt method and normalized to the expression of the housekeeping gene GAPDH. Data presented (M ± standard error of the mean [SEM]) are from three independent experiments in which all sample sets were analyzed in triplicate.
Immunodetection
Cells were grown in Delta T culture dishes, fixed for 15 min with 4% paraformaldehyde (PFA) and rinsed three times (5 min each) with phosphate-buffered saline (PBS), and blocked for 60 min at room temperature in 5% bovine serum albumin (BSA) and 5% horse serum in PBS. Depending on the analyzed marker, 0.3% Triton X-100 was added into the blocking buffer for cell permeability in order to detect intracellular antigens. Cells were incubated with appropriate primary antibodies diluted in a solution of PBS containing 1% BSA, 1% horse serum, and 0.1% Triton X-100 overnight at 4°C. Cells were rinsed three times (5 min each) with PBS and incubated with the appropriate secondary antibody for 1 h at room temperature. After that, cells were rinsed three times with PBS and mounted using hard-set mounting media containing DAPI (Vector Laboratories).
Radioactive iodine uptake and PB125I
The differentiated hES cells were seeded in a 24-well plate at a density of approximately 0.5 × 106 cells/well in 1 mL of culture medium (RPMI 1640 with B27 and 6H) and allowed to become 70–80% confluent. Undifferentiated hES cells were used as control. After washing the cells twice with pure medium, the cells were treated with 1 mL of medium containing TSH (0.01, 0.1, 1.0 mIU/mL) + 20 μM of non-radioactive NaI +0.1 μCi carrier-free Na125I with and without 100 μM of NaClO4 (sodium perchlorate) for 24 h at 37°C in a humidified atmosphere. The radioactive solution was aspirated and washed twice with ice-cold Hanks' balanced salt solution to terminate uptake reactions. To determine the amount of 125I uptake by the cells, 500 μL of 95% ethanol was added to each well for 20 min and incubated at 4°C. The supernatants were aspirated and quantitated for radioactivity in a γ-counter (Iso-Data distributed by Polymedco, Inc.). The DNA content was measured on the material not extracted by ethanol after trichloroacetic (TCA) acid precipitation. The 125I uptake was expressed as pmol/μg of DNA.
For radioactive PBI, the cells were lysed by adding 400 μL of 1 × RIPA buffer and incubated on ice for 10 min, and the cell content scraped off and treated with DNase for 1 h. The cell lysate was centrifuged at 0.8 g for 10 min at 4°C, and the supernatant was collected. Further, one volume of neat TCA stock (100% (w/v)) was added to four volumes, the cell lysates were incubated for 10 min at 4°C. After spinning again at 16.1 g for 5 min, the supernatants were removed, leaving the protein pellet intact. The pellets were washed with 200 μL of cold acetone, and the tubes microfuged again at 16.1 g for 5 min. The pellet was placed in a 95°C heat block to remove the acetone, the protein content was measured in the pellet after adding 2 × sample buffer (2 × Laemmli Sample Buffer from Bio-Rad) to dissolve the pellet, and the 125I incorporation in the precipitated protein counted and expressed as pmol/μg of protein.
Data analysis
All values are expressed as mean ± SEM. All samples were analyzed by Student's t-test or analysis of variance as described. A p-value of <0.05 was considered significant.
Results
Upregulating nuclear TAZ using ethacridine
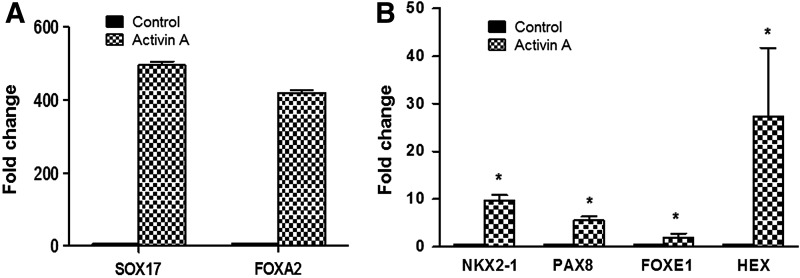
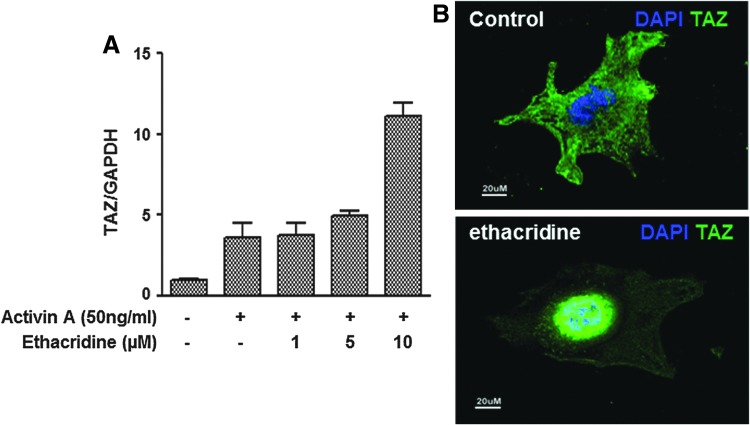
Although TAZ itself has no regular DNA binding domain, TAZ can bind to DNA binding transcription factors such as PAX8 and NKX2-1 to stimulate their transcriptional activity, including their influence on thyroid gene expression (8). While ES cells serve as an excellent in vitro model system for thyroid cell differentiation (4–6), activin/nodal signaling is required first to derive definitive endoderm, the precursor cell type that gives rise to all endoderm-derived cell lineages, including those of the thyroid (18), and much progress has been made in efficiently generating definitive endoderm from hES cells using Activin A (19,20). As previously described (4), first hES cells were exposed to Activin A to induce such endoderm formation, which was confirmed by induction of the endoderm markers SOX17 and FOXA2, which showed 400- to 500-fold changes in comparison with the untreated cells (Fig. 1A). At the same time, the basal levels of thyroid transcription factors PAX8, NKX2-1, FOXE1, and HEX were also induced. The fold changes were 5.52, 9.64, 1.8, and 27.4 for PAX8, NKX2-1, FOXE1, and HEX, respectively, in comparison with the untreated cells (Fig. 1B). In addition, Activin A induced TAZ expression, and this was markedly enhanced with ethacridine in a dose-dependent manner when assessed by qRT-PCR analysis and Western blot (Fig. 2A). There was also a marked translocation of TAZ into its nuclear location (Fig. 2B).
FIG. 1.
Induction of endoderm and thyroid transcriptional factors by Activin A. Induction of endodermal cells by Activin A leads to the upregulation of endodermal markers and also a basal increase in thyroid-specific transcription factors. Representative quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis for (A) endoderm markers SOX17 and FOXA2 and (B) thyroid transcriptional factors PAX8, NKX2-1, FOXE1, and HEX on Activin A–treated and control (untreated) cells. Data are expressed as mean ± standard error of the mean (SEM), and represent one of three separate experiments. Statistical analysis by unpaired two-tailed Student's t-test gave *p < 0.0001 for each gene in the Activin A–treated cells compared with the untreated controls.
FIG. 2.
Enhancement of transcriptional co-activator with PDZ-binding motif (TAZ) in Activin A–treated human embryonic stem (hES) cells by ethacridine. Dose-dependent increase in TAZ and its translocation into the nucleus after treatment is shown here. (A) Representative qRT-PCR analysis for TAZ of ethacridine-treated endoderm cells derived from hES cells by Activin A after two days at 37°C. All data are expressed as mean ± SEM and represent one of three separate experiments. (B) The nuclear translocation of TAZ detected by immunostaining. Nuclear localization of TAZ after 10 μM of ethacridine treatment is indicated by TAZ staining as the green color located in the nucleus in the lower panel in comparison to the control (untreated) cells where TAZ is located in the cytoplasm as shown in the upper panel. Scale = 20 μm.
Enhancing the expression of PAX8 and NKX2-1
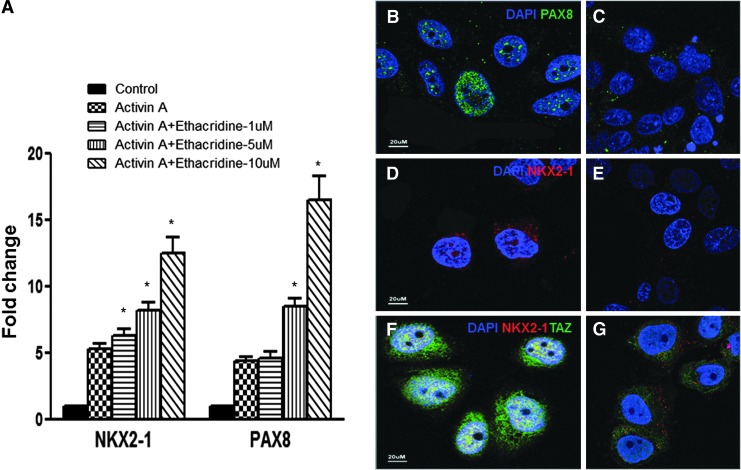
Studies in mouse ES and hES cells showed that expression of only PAX8 and NKX2-1 is sufficient to commit undifferentiated cells to thyroid cells (4–6). Furthermore, expression of PAX8 and NKX2-1 induced expression of HEX and FOXE1 in pluripotent cells, which implies thyroid differentiation (4–6). All four of these thyroid transcription factors regulate thyroid genes including TG, SLC5A5 (NIS), and TPO gene expression (21). Induction of TAZ, a co-activator of PAX8 and NKX2-1, should promote/contribute to thyroid specification in thyroid progenitor cells, and thus, next, the effects of ethacridine on PAX8 and NKX2-1 in the Activin A–derived endoderm cells were examined. The expression of both factors was further increased in a dose-dependent manner after treatment with ethacridine, as analyzed by qRT-PCR. Compared with only Activin A–treated cells, NKX2-1 was increased 20.5%, 56.45%, and 138.33% in response to 1, 5, and 10 μM ethacridine treatment, respectively, while PAX8 increased 4.48%, 90%, and 269.95% in response to the same doses of ethacridine treatment (Fig. 3A). The expression of PAX8 and NKX2-1, as well as TAZ, was also easily detected by immunostaining (Fig. 3B, D, and F), which showed that all three factors are expressed within the nuclei of these cells. The control samples not exposed to ethacridine are shown in the right panels (Fig. 3C, E, and G). These results indicate that ethacridine had the potential to enhance thyroid cell differentiation.
FIG. 3.
Enhancement of PAX8 and NKX2-1 in Activin A–treated hES cells by ethacridine. (A) Representative qRT-PCR analysis for thyroid transcription factors PAX8 and NKX2-1 on ethacridine-treated endoderm cells derived from hES cells by Activin A. Data are expressed as mean ± SEM and represent one of three separate experiments. Statistical analysis by analysis of variance (ANOVA) gave a p-value of *p < 0.0001 for NKX2-1 and PAX8 in each of the Activin A + ethacridine-treated cells compared with only Activin A–treated cells. (B–G) Immunostaining of PAX8 (B, staining as green), NKX2-1 (D, staining as red), NKX2-1 (red), and TAZ (green) (F) after treating with 10 μM of ethacridine. Cells without ethacridine treatment served as control and are shown in the right panels (C, E, and G). Scale = 20 μm.
TAZ induction promotes thyroid gene expression and differentiation
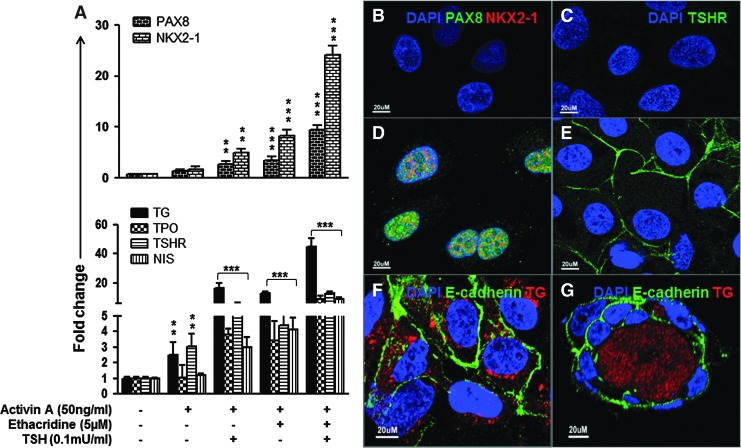
After four days of treatment with Activin A, the resulting endoderm cells were treated with ethacridine with and without TSH in order to assess whether further enhancement of the transcription activity of PAX8 and NKX2-1 occurs. Using qPCR analysis, it was found that expression of both of these thyroid transcription factors was enhanced (Fig. 4A upper), and thyroid-specific gene expression of TG, TPO, TSHR, and NIS (Fig. 4A lower) was also highly induced compared with control and single treated cells. In comparison to undifferentiated hES cells, PAX8 showed 2.65-, 3.48-, and 9.47-fold increases, while NKX2-1 showed 4.99-, 8.29-, and 24.21-fold increases in response to either TSH or ethacridine alone, or both TSH and ethacridine after Activin A treatment (Fig. 4A upper). The expression of thyroid-specific genes changed as follows in response to the same treatment: TG increased 16.56-, 12.81-, and 45.09-fold; TPO increased 3.80-, 3.41-, and 8.62-fold; TSHR increased 5.80-, 4.41-, and 12.62-fold; and NIS increased 2.99-, 4.12-, and 8.91-fold (Fig. 4A lower). Both ethacridine and TSH stimulate TG expression, and these effects seem to be amplified in the presence of both stimuli. This may be because ethacridine and TSH stimulate TG expression through different pathways. First, ethacridine enhances the unphosphorylated and nuclear TAZ, which interacts with both PAX8 and NKX2-1 to lead to a significant enhancement of the transcriptional activity of PAX8 and NKX2-1 on the thyroglobulin promoter (8). Second, the effects of TSH on TG expression are largely mediated through the cAMP-dependent protein kinase A pathway, enhancing the classical cAMP-responsive element in the upstream enhancer element of the TG promoter (22). Immunostaining of PAX8 and NKX2-1 (Fig. 4D) and TSHR (Fig. 4E) demonstrated this high expression compared with control cells (Fig. 4B and C) by immunostaining. These differentiated hES cells then formed three-dimensional follicles when exposed to extracellular matrix (Matrigel), and immunofluorescent analysis confirmed abundant E-cadherin expression in the follicular cell membranes and Tg expression in the follicular lumen and cytoplasm (Fig. 4F and G).
FIG. 4.
Analysis of differentiated hES cells. (A) qRT-PCR analysis for thyroid transcription factors (PAX8 and NKX2-1) and thyroid functional genes (TG, TPO, TSHR, and NIS) in differentiated cells. The relative expression of each transcript is presented as the fold change compared to undifferentiated ES cells (mean ± SEM) and represents one out of three separate experiments. Statistical analysis was performed by ANOVA, and the p-values were **p < 0.01 and ***p < 0.0001 for each gene when compared to untreated controls. (B–E) The immunostaining for (D) PAX8 (green) and NKX2-1(red) and (E) TSHR (green) in the differentiated hES cells and control cells (B and C). Note that the PAX8 and NKX2-1 expression is seen in the cell nuclei, and the TSHR expression is seen on the cell surface. Scale bar = 20 μm. (F) Immunodetection of TG (red) and E-cadherin (green) expression in differentiated hES cells. Note that the TG is seen in the cytoplasm, and E-cadherin is seen on the cell surface. Scale bar = 20 μm. (G) Immunodetection of TG (red) expression and E-cadherin (green) in a human thyroid neofollicle derived from differentiated hES cells. Note that the TG expression is seen in the intra-follicluar lumen, and E-cadherin expression is seen on the cell surface. Scale bar = 20 μm.
Ethacridine differentiated thyroid cells are accumulating and organifying 125I
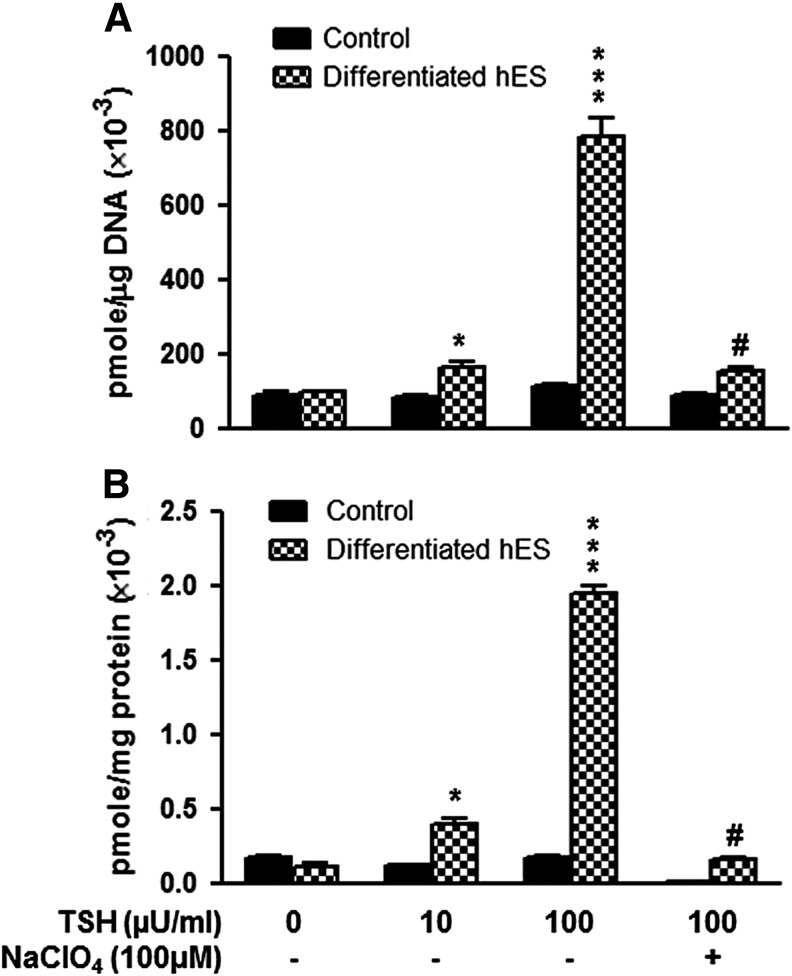
The resulting differentiated human thyroid cell follicles derived from hES cells showed a dose-related increase in radioiodine uptake and PB125I accumulation in response to TSH stimulation (Fig. 5). The radioiodine uptake in the differentiated human thyroid cells increased from 66.33% to 679.20% in response to 10–100 μIU/mL of TSH stimulation. In addition, PB125I increased 272.73% to 1672.73% in response to 10–100 μIU/mL of TSH stimulation. Evidence for the dependence of such uptake and organification on NIS function was obtained by the specific inhibition of NIS using sodium perchlorate, which almost abolished the radioiodine uptake (Fig. 5A) and protein-bound iodine formation with TSH stimulation (Fig. 5B).
FIG. 5.
Demonstration of differentiated hES cell thyroid cell function. Differentiated hES cells were exposed to Na125I as described in the Methods, and the radioactive uptake (A) and protein bound 125I (B) in the differentiated thyroid cells was measured with and without thyrotropin (TSH; 0.01 and 0.1 mIU/mL). Sodium perchlorate was used to inhibit the NIS activity. Undifferentiated hES cells were used as control. Statistical analysis was performed with ANOVA. p-Values: *p < 0.01 and ***p < 0.0001 in the TSH-treated groups compared to untreated controls, and #p < 0.0001 in the 100 μIU/mL TSH-treated cells compared to 100 μIU/mL TSH + sodium perchlorate–treated cells.
Discussion
The differentiation program of TFCs, the most abundant cell population in the thyroid gland, relies on the interplay between sequence-specific transcription factors and transcriptional co-regulators with the basal transcriptional machinery of the cell. To date, well-characterized thyroid transcription factors include PAX8, NKX2-1, FOXE1, and HEX, which are expressed simultaneously in thyroid cell precursors and drive commitment toward a differentiated thyroid fate. Persistent expression of these genes in adult thyroid cells is presumably needed to establish and maintain the differentiated thyroid phenotype (21,23,24). Among these thyroid transcription factors, it has been demonstrated that PAX8 and NKX2-1 associate biochemically and act synergistically in order to contribute to the orderly timing of the switch from progenitor cell proliferation to the initiation of terminal thyrocyte differentiation programs marked by the expression of specific genes encoding TG, TPO, TSHR, and NIS (23,25,26).
Using this information, it has been demonstrated that mouse ES cells can be differentiated effectively into functional thyrocytes by inducing two key transcription factors: PAX8 and NKX2-1 (5,6). Similarly, it has been previously shown that hES cell differentiation into thyrocytes can be achieved by manipulating their transcriptional control in the same way (4). These mouse ES and hES cell–derived thyrocytes expressing specific transcription factors are capable of forming three-dimensional, functional thyroid follicles (4–6).
Like all other transcription factors, the activity of PAX8 and NKX2-1 is regulated by various co-regulators that can function as co-activators, co-repressors, or adaptor molecules. Recently, it has been demonstrated that TAZ, together with PAX8 and NKX2-1, are co-expressed in the nucleus of differentiated thyroid cells, and TAZ interacts with both PAX8 and NKX2-1 in vitro and in vivo. Most importantly, this interaction leads to a significant enhancement of the transcriptional activity of PAX8 and NKX2-1 on the TG promoter (8). Analysis of morpholino (MO)-mediated knockdown of TAZ in zebrafish at later stages of development revealed that the number and lumen size of thyroid follicles were significantly smaller (27), further indicating that TAZ is needed for the control of genes involved in thyroid development and differentiation.
Recently, it was reported that ethacridine, a widely used antiseptic, enhances the interaction of TAZ and protein phosphatases and thus increases unphosphorylated nuclear TAZ (17). The present results also demonstrate that ethacridine strongly enhances nuclear localization of TAZ in Activin A–induced endoderm derived from hES cells, and at the same time augments the expression of the thyroid transcription factors PAX8 and NKX2-1. The fact that increases in PAX8 and NKX2-1 gene expression correlate with increases in TAZ gene expression indicates that the increase is driven by TAZ. Continued culture of these ethacridine-treated endoderm cells with ethacridine and TSH induces thyroid-specific genes, and these differentiated hES cells form three-dimensional thyroid follicles, which express high levels of TG protein, and show iodide uptake and iodine organification as PB125I accumulation in response to TSH stimulation.
The fact that it was possible to induce thyroid differentiation in thyroid progenitor cells by either forced expression of key specification transcription factors (4–6) or inducing a combination of BMP and FGF signaling, which in turn induces the transcription factors by an undefined signaling cascade (7), strongly suggests that turning on PAX8 and NKX2-1 can be achieved by multiple mechanisms. The data shown here reveal a simple way to enhance the formation of differentiated and functional thyroid follicles from hES cells by direct transcriptional stimulation of PAX8 and NKX2-1. Although the mechanism by which TAZ can achieve this effect is not fully characterized, it can be assumed that TAZ, a known coactivator with several distinct domains including WW domain, putative coiled-coiled, and PDZ-binding domains, is capable of protein–protein interaction with the PAX8 and NKX2-1 transcriptional factors to upregulate their downstream signaling activity.
In conclusion, the present data show that the TAZ activator ethacridine strongly enhances nuclear localization of TAZ in Activin A–induced endoderm cells from hES cells and augments the thyroid transcription factors PAX8 and NKX2-1 to induce thyroid gene transcription, leading to a simple and direct approach for thyroid cell differentiation. Importantly, the data strongly implicate the TAZ transcription factor as a key regulator of thyroid cell speciation in hES cells.
Acknowledgments
Supported in part by DK069713 from the National Institutes of Health, the David Owen Segal Endowment, and the VA Merit Review Program (to T.F.D.).
Author Disclosure Statement
The authors declare that no competing interests exist.
References
- 1.Di Palma T, Nitsch R, Mascia A, Nitsch L, Di Lauro R, Zannini M. 2003. The paired domain-containing factor Pax8 and the homeodomain-containing factor TTF-1 directly interact and synergistically activate transcription. J Biol Chem 278:3395–3402 [DOI] [PubMed] [Google Scholar]
- 2.Miccadei S, De Leo R, Zammarchi E, Natali PG, Civitareale D. 2002. The synergistic activity of thyroid transcription factor 1 and Pax 8 relies on the promoter/enhancer interplay. Mol Endocrinol 16:837–846 [DOI] [PubMed] [Google Scholar]
- 3.Espinoza CR, Schmitt TL, Loos U. 2001. Thyroid transcription factor 1 and Pax8 synergistically activate the promoter of the human thyroglobulin gene. J Mol Endocrinol 27:59–67 [DOI] [PubMed] [Google Scholar]
- 4.Ma R, Latif R, Davies TF. 2015. Human embryonic stem cells form functional thyroid follicles. Thyroid 25:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonica F, Kasprzyk DF, Opitz R, Iacovino M, Liao XH, Dumitrescu AM, Refetoff S, Peremans K, Manto M, Kyba M, Costagliola S. 2012. Generation of functional thyroid from embryonic stem cells. Nature 491:66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma R, Latif R, Davies TF. 2013. Thyroid follicle formation and thyroglobulin expression in multipotent endodermal stem cells. Thyroid 23:385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurmann AA, Serra M, Hawkins F, Rankin SA, Mori M, Astapova I, Ullas S, Lin S, Bilodeau M, Rossant J, Jean JC, Ikonomou L, Deterding RR, Shannon JM, Zorn AM, Hollenberg AN, Kotton DN. 2015. Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells. Cell Stem Cell 17:527–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Palma T, D'Andrea B, Liguori GL, Liguoro A, de Cristofaro T, Del Prete D, Pappalardo A, Mascia A, Zannini M. 2009. TAZ is a coactivator for Pax8 and TTF-1, two transcription factors involved in thyroid differentiation. Exp Cell Res 315:162–175 [DOI] [PubMed] [Google Scholar]
- 9.Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC, Yaffe MB. 2000. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J 19:6778–6791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang K, Degerny C, Xu M, Yang XJ. 2009. YAP, TAZ, and Yorkie: a conserved family of signal-responsive transcriptional coregulators in animal development and human disease. Biochem Cell Biol 87:77–91 [DOI] [PubMed] [Google Scholar]
- 11.Cui CB, Cooper LF, Yang X, Karsenty G, Aukhil I. 2003. Transcriptional coactivation of bone-specific transcription factor Cbfa1 by TAZ. Mol Cell Biol 23:1004–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, Hopkins N, Yaffe MB. 2005. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 309:1074–1078 [DOI] [PubMed] [Google Scholar]
- 13.Tian Y, Li D, Dahl J, You J, Benjamin T. 2004. Identification of TAZ as a binding partner of the polyomavirus T antigens. J Virol 78:12657–12664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahoney WM, Jr, Hong JH, Yaffe MB, Farrance IK. 2005. The transcriptional co-activator TAZ interacts differentially with transcriptional enhancer factor-1 (TEF-1) family members. Biochem J 388:217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami M, Nakagawa M, Olson EN, Nakagawa O. 2005. A WW domain protein TAZ is a critical coactivator for TBX5, a transcription factor implicated in Holt–Oram syndrome. Proc Natl Acad Sci U S A 102:18034–18039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami M, Tominaga J, Makita R, Uchijima Y, Kurihara Y, Nakagawa O, Asano T, Kurihara H. 2006. Transcriptional activity of Pax3 is co-activated by TAZ. Biochem Biophys Res Commun 339:533–539 [DOI] [PubMed] [Google Scholar]
- 17.Kawano S, Maruyama J, Nagashima S, Inami K, Qiu W, Iwasa H, Nakagawa K, Ishigami-Yuasa M, Kagechika H, Nishina H, Hata Y. 2015. A cell-based screening for TAZ activators identifies ethacridine, a widely used antiseptic and abortifacient, as a compound that promotes dephosphorylation of TAZ and inhibits adipogenesis in C3H10T1/2 cells. J Biochem 158:413–423 [DOI] [PubMed] [Google Scholar]
- 18.Grapin-Botton A, Melton DA. 2000. Endoderm development: from patterning to organogenesis. Trends Genet 16:124–130 [DOI] [PubMed] [Google Scholar]
- 19.D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. 2005. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 23:1534–1541 [DOI] [PubMed] [Google Scholar]
- 20.Brown S, Teo A, Pauklin S, Hannan N, Cho CH, Lim B, Vardy L, Dunn NR, Trotter M, Pedersen R, Vallier L. 2011. Activin/Nodal signaling controls divergent transcriptional networks in human embryonic stem cells and in endoderm progenitors. Stem Cells 29:1176–1185 [DOI] [PubMed] [Google Scholar]
- 21.Fernandez LP, Lopez-Marquez A, Santisteban P. 2015. Thyroid transcription factors in development, differentiation and disease. Nat Rev Endocrinol 11:29–42 [DOI] [PubMed] [Google Scholar]
- 22.Berg V, Vassart G, Christophe D. 1997. A zinc-dependent DNA-binding activity co-operates with cAMP-responsive-element-binding protein to activate the human thyroglobulin enhancer. Biochem J 323:349–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Felice M, Di Lauro R. 2011. Minireview: intrinsic and extrinsic factors in thyroid gland development: an update. Endocrinology 152:2948–2956 [DOI] [PubMed] [Google Scholar]
- 24.Fagman H, Nilsson M. 2010. Morphogenesis of the thyroid gland. Mol Cell Endocrinol 323:35–54 [DOI] [PubMed] [Google Scholar]
- 25.Lazzaro D, Price M, de Felice M, Di Lauro R. 1991. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development 113:1093–1104 [DOI] [PubMed] [Google Scholar]
- 26.Pasca di Magliano M, Di Lauro R, Zannini M. 2000. Pax8 has a key role in thyroid cell differentiation. Proc Natl Acad Sci U S A 97:13144–13149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pappalardo A, Porreca I, Caputi L, De Felice E, Schulte-Merker S, Zannini M, Sordino P. 2015. Thyroid development in zebrafish lacking Taz. Mech Dev 138:268–278 [DOI] [PubMed] [Google Scholar]