Abstract
Polymorphisms in the gene coding for the adhesion G-protein coupled receptor LPHN3 are a risk factor for attention-deficit/hyperactivity disorder (ADHD). Transient down-regulation of latrophilin3.1 (lphn3.1), the zebrafish LPHN3 homologue, causes hyperactivity. Zebrafish injected with a lphn3.1-specific morpholino are hyperactive and display an impairment in dopaminergic neuron development. In the present study we used lphn3.1 morphants to further characterize the changes to dopaminergic signaling that trigger hyperactivity. We applied dopamine agonists (Apomorphine, Quinpirole, SKF-38393) and antagonists (Haloperidol, Eticlopride, SCH-23390) to Lphn3.1 morpholino-injected or control-injected animals. The percentage of change in locomotor activity was then determined at three different time periods (10–20 min, 30–40 min and 60–70 min). Our results show that drugs targeting dopamine receptors appear to elicit similar effects on locomotion in zebrafish larvae and mammals. In addition, we observed that lphn3.1 morphants have an overall hyposensitivity to dopamine agonists and antagonists compared to control fish. These results are compatible with a model whereby dopaminergic neurotransmission is saturated in lphn3.1 morphants.
Keywords: Zebrafish, Latrophilin 3, Dopamine, Locomotion, Behavior
Highlights
-
•
Dopaminergic signaling has an overall inhibitory effect on larval zebrafish locomotion.
-
•
Lphn3 morphants are hyposensitive to modulators of DA signaling.
1. Introduction
ADHD (ADHD; MIM#143465) is a common, early onset and enduring neuropsychiatric disorder characterized by developmentally inappropriate inattention, hyperactivity and impulsivity as well as motivational dysregulation. ADHD has a complex etiology caused by a combination of environmental and genetic factors (Biederman, 2005). Genetic, imaging and pharmacological data suggest an impairment of dopaminergic (DA) neurotransmission in ADHD patients (Del Campo et al., 2011). However, the neurobiological basis of the disease is not fully understood and the precise role of DA in ADHD remains unclear.
The ADHD-risk gene Latrophilin 3 (LPHN3; recently renamed Adhesion G protein-coupled receptor L3 (ADGRL3)) was identified by linkage analysis followed by fine mapping of human patients (Arcos-Burgos et al., 2010; Domené et al., 2011; Martinez et al., 2016). A polymorphism in LPHN3 has been found to predict the efficacy of ADHD treatment drugs (Arcos-Burgos et al., 2010; Labbe et al., 2012) and the LPHN3 risk haplotype alters cognitive control in human patients (Fallgatter et al., 2013). LPHN3 is an adhesion G protein-coupled receptor that mediates α-latrotoxin-stimulated neurotransmitter release from presynaptic vesicles (Silva et al., 2011). The endogenous function of LPHN3 and its related family members LPHN1 and LPHN2 are not well understood. Recent studies have identified families of ligands for LPHN3: the Teneurin-2 splice variant Lasso, the Fibronectin leucine-rich repeat transmembrane proteins (FLRTs) and the Neurexins (NRXN) (Lu et al., 2015; O'Sullivan et al., 2012; O'Sullivan, Martini, Daake, Comoletti, & Ghosh, 2014). For example, trans-synaptic complexes formed between Lasso and LPHN1 are capable of increasing presynaptic Ca2+ and modulating neurotransmitter release (Langenhan et al., 2009). FLRT3 and LPHN3 act as a ligand-receptor pair to regulate the development and function of glutamatergic synapses (O'Sullivan et al., 2012, O'Sullivan et al., 2014; Ranaivoson et al., 2015). In addition to the crucial role of Latrophilins at the synapse, the Caenorhabditis elegans LPHN1 ortholog LAT-1 is involved in embryonic morphogenesis (Langenhan et al., 2009). The structural conservation of Latrophilins across species makes it likely that these proteins have a conserved function.
In a previous study we reported that transient down-regulation of lphn3.1, one of two zebrafish LPHN3 orthologs, induces ADHD-like locomotor endophenotypes including hyperactivity and motor impulsivity. The hyperactivity of lphn3.1 morphants (Lphn3-MO, compared to controls, Lphn3-CO) was rescued by applying the ADHD treatment drugs methylphenidate and atomoxetine. We also demonstrated a role for lphn3.1 in the development of DA neurons. lphn3.1 morphants displayed a reduction and displacement of DA neurons in the ventral diencephalon (Lange et al., 2012). The hyperactivity of lphn3.1 morphants was recently confirmed in an independent study (Reuter et al., 2016). Furthermore, knockout of Lphn3 in mouse induced hyperactivity, increased DA and 5-HT in the striatum, changed DA and 5-HT receptor expression and increased locomotor sensitivity to cocaine (Wallis et al., 2012). Transcriptomic analysis of Lphn3 null mice identified differential expression of genes coding for calcium signaling proteins and cell adhesion molecules consistent with the hyperactivity (Orsini et al., 2016). There was also an overexpression of Slc6a3 (Dat, coding for the DA transporter) in the striatum. This area of the brain is critical for motor control and reward, two DA-dependent behaviors which are perturbed in ADHD patients (Bush, 2010). Whilst these studies highlight links between Lphn3 and DA neuron development, alterations to DA neurotransmission and its consequences on behavior have not been studied in detail.
DA receptors belong to a large family of postsynaptic G protein-coupled receptors. Stimulation of D1-like receptors (D1 and D5 in humans) activates adenylyl cyclase (AC) and leads to cyclic adenosine monophosphate (cAMP) production and activation of protein kinase A (PKA) (Enjalbert and Bockaert, 1983; Kebabian and Calne, 1979; Kebabian and Greengard, 1971). In contrast, activation of D2-like receptors (D2, D3 and D4 in humans) either modulates calcium or potassium channels or inhibits PKA and Akt signaling (Beaulieu and Gainetdinov, 2011; Missale et al., 1998). In mammals, DA is the main regulator of locomotor activity via both D1 and D2 receptors. Activation of D1 receptors has a stimulatory effect on locomotion. Conversely, activation of D2 receptors has a more complex effect due to their widespread expression in several brain regions including both pre- and postsynaptic areas (De Mei et al., 2009). In rodents, low doses of D2 agonists primarily activate presynaptic receptors, reducing DA release and decreasing locomotion. At high concentrations D2 agonists activate postsynaptic neurons and induce hyperactivity (Beaulieu and Gainetdinov, 2011; Missale et al., 1998).
DA can also regulate locomotor behavior in zebrafish larvae (Ek et al., 2016; Irons et al., 2013; Lambert et al., 2012; Souza et al., 2011; Thirumalai and Cline, 2008) and D1- and D2-like receptors are expressed in the brain at larval stages (Boehmler et al., 2007, Boehmler et al., 2004; Yamamoto et al., 2013). Endogenous DA is required for the initiation of movement (Thirumalai and Cline, 2008) and, as in mammals, D1-like activation generally increases motor behavior. D2-like activation has the opposite effect and decreases locomotion (Irons et al., 2013; Souza et al., 2011).
In this study we investigated the effects of several DA receptor agonists and antagonists on locomotion in Lphn3-MO and Lphn3-CO larvae. We tested drugs at several concentrations to reveal dose-dependent changes in control and morphant behavior. To demonstrate the effect of immediate and long-term drug application we also conducted our analysis at three time-windows following drug treatment.
2. Materials and methods
2.1. Animal strains and maintenance
Zebrafish were maintained using standard fish-keeping protocols and in accordance with institutional and national guidelines for animal welfare. Tanks were connected to a continuous water system that exchanged about 10% of the total water each day. The facility was maintained at 28 °C with a 14-h day/10 h night light cycle. All experiments were performed on embryos of the AB zebrafish wildtype strain. After collection and injection (see below) eggs were kept in embryo medium in groups of 40 embryos per Petri dish. Fish were grown in a 28.5 °C incubator with a 14-h day/10 h night light cycle (lights on at 8 am and off at 10 pm). On day 6, the locomotor behavior of injected larvae was tested between 1 pm and 5 pm, in a room with an ambient temperature of approximately 27 °C.
2.2. Morpholino injection
We used an lphn3.1 splice morpholino oligonucleotide (MO) to block splicing at the exon2/intron2 boundary of lphn3.1 (sequence ATGTGAGTGTATCTCTGTACCTGAA). A control MO (Lphn3-CO) with 5 bp mismatches was also used (ATcTGAcTGTATgTCTcTACCTcAA; mismatches in lower case) (Lange et al., 2012). Morpholino oligonucleotides were purchased from GeneTools LLC. AB embryos were injected with either 500 μM Lphn3-MO or Lphn3-CO. RNA was extracted from 20 two day-old embryos using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). To confirm splicing defects, we performed nested RT-PCR using the Superscriptase II kit (Invitrogen). Aberrant splicing was confirmed using the following primers MO1Forward: GTCGAGAGCTGTCGTGTGAG, MO1Reverse1 ATTCGGCCATTGTTCTGTTC. See Lange et al. (2012) for more information about the injection of Lphn3-MO.
2.3. Drug treatment and analysis of locomotion
Drugs were freshly prepared on the day of the experiment by dissolving them in embryo medium. All drugs were purchased from Sigma-Aldrich (St. Louis, MO, USA) and included a non-specific dopamine receptor agonist, apomorphine hydrochloride hemi-hydrate (Apo, A493); a D1-like receptor agonist, SKF-38393 (SKF, S101); a D2-like receptor agonist, quinpirole (Qui, Q111); a non-specific dopamine receptor antagonist, haloperidol (Halo, H1512); a D1-like receptor antagonist, eticlopride (Etic, E101); and a D2-like receptor antagonist, SCH-23390 (SCH; D054). Haloperidol was dissolved in 0.4% dimethyl sulfoxide (DMSO) and further diluted in embryo medium. There is almost no information available regarding effective doses for these drugs in larval zebrafish (Ek et al., 2016). We therefore conducted preliminary experiments to determine the lowest concentration of drug that affected locomotion and the highest concentration that we could use before we observed toxicity after 70 min of exposure (data not shown). We then chose several concentrations to use in our experiments.
Locomotor activity was recorded using ZebraLab software (Videotrack; ViewPoint Life Sciences, France). We first tested the locomotion of 6 days post fertilization (dpf) larvae for 10 min without drug treatment. Larvae were placed into separate wells of a 24-well plate inside a ZebraBox (ViewPoint Life Sciences, France). Fry were allowed to habituate to the plate without drug for one hour before the first 10-min recording started. Larvae were then transferred into the plate containing drugs and locomotion was recorded between 10–20 min, 30–40 min and 60–70 min. Each time point and concentration was repeated in three independent experiments.
2.4. Data analysis
Untreated larvae showed a spontaneous decrease in locomotion by 30–40 min (mean Lphn3-MO1 = −14.5% and mean Lphn3-CO = −4.5%) and 60–70 min (mean Lphn3-MO = −24.5% and mean Lphn3-CO = −17.6%) in the ZebraBox (Fig. 1). To account for the habituation observed without drug treatment we applied a correction to the distance swum after treatment. We calculated a habituation coefficient, defined as the absolute value of the mean percentage of change in locomotion during the recording period in the absence of any treatment (see Results paragraph 1 and Supp. Table1 for examples of calculations in Lphn3-CO larvae). Next, for each drug concentration, the mean distance swum recorded for an individual fry was adjusted using the habituation coefficient at each necessary time point using the following formula:
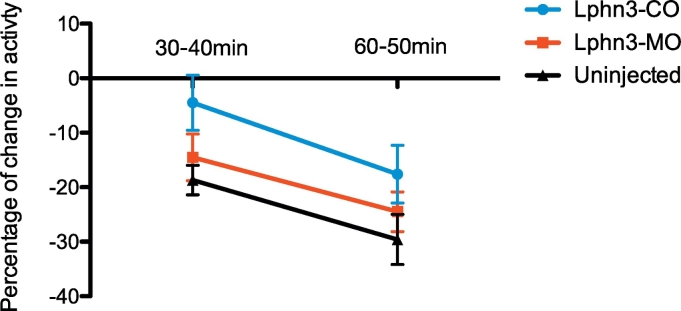
Fig. 1.
Zebrafish larval locomotion habituates to the recording apparatus. Habituation of Lphn3-MO, Lphn3-CO and uninjected larvae to the ZebraBox after a 30–40 min period and a 60–70 min period. The three populations displayed habituation that increased with time. Lphn3-MO n = 30, Lphn3-CO n = 39, Uninjected n = 42.
After calculation of the adjusted distance swum after treatment, the percentage of change in activity was determined for individual fish as follows:
The distance swum before treatment was recorded for 10 min before drugs were applied to the fry. An example calculation (adjustment of the distance swum after treatment, followed by determination of the percentage of change in activity), is provided in Sup. Table 2. As a further example, Sup. Table 3 provides all values for all control animals treated with 10 μM apomorphine. The significance of all comparisons was established using a two-way repeated measures ANOVA with the percentage of change in activity after treatment as the dependent variable and with drug treatment and time point as the independent variables. The Bonferroni post-hoc test was then applied to compare the percentage of change in activity after treatment between Lphn3-MO and Lphn3-CO. Significance was set to p ≤ 0.05 after Bonferroni correction. Two-way ANOVAs were performed on the raw data (i.e on the distance travelled). All ANOVAs and supplementary graphs were performed using Prism5 (Graphpad Software, Inc.). For figures to compare the habituation coefficient between Lphn3-MO and Lphn3-CO we used a t-test. Statistical significance was depicted as follows: *p < 0.05, **p < 0.01, ***p < 0.001. In all cases the number of animals tested is denoted by n.
3. Results
3.1. Determination of the habituation coefficient
Zebrafish larvae have already been observed to habituate to environmental stimuli (Burgess and Granato, 2007). We first tested whether the locomotion of each group of larvae was stable in our setup for the entire 70 min duration of the experiment. After an initial measurement of locomotion, 6 dpf larvae were gently transferred into a 24-well plate without addition of drug. The plates were then placed into the ZebraBox and the mean distance swum recorded at 10–20 min, 30–40 min and 60–70 min. The percentage of change in activity was calculated at each time point compared to the initial measurement. No change in locomotion was observed at 10–20 min for either genotype (not shown). However, we observed that 6 dpf Lphn3-CO and Lphn3-MO larvae spontaneously decreased their locomotion by 30–40 min (mean Lphn3-MO1 = −14.5% and mean Lphn3-CO = −4.5%) (Fig. 1). This habituation phenomenon increased over time (at 60–70 min, mean Lphn3-MO = −24.5% and mean Lphn3-CO = −17.6%). At both time points the morphants showed a greater level of habituation than Lphn3-CO controls (30–40 min: Lphn3-MO vs Lphn3-CO p = 0.02; 60–70 min Lphn3-MO vs Lphn3-CO p = 0.1). Likewise, non-injected fry exhibited habituation at 30–40 min and 60–70 min (Fig. 1); accordingly, the habituation was a general characteristic of locomotion not induced by the injection. We established a habituation coefficient for each time point and each treatment group (see Supp. Table 1 for examples on Lphn3-CO larvae). This was defined as the absolute value of the mean percentage of habituation for the recording period (30–40 min: Lphn3-MO = 0.145 and Lphn3-CO = 0.045; 60–70 min Lphn3-MO = 0.245 and Lphn3-CO = 0.176). In all subsequent experiments, we adjusted the distance swum after drug treatment, at 30–40 mn and 60–70 mn, using the habituation coefficient. The adjusted value was then used to determine the percentage of change in activity for individual zebrafish, and finally the mean change in activity of the different treated population was plotted (Sup. Table 2 and Sup. table 3).
3.2. The effects of DA receptor agonists on locomotion is more moderate in Lphn3.1 morphants than in control larvae
3.2.1. Non-selective DA receptor agonists
To quantify the effect of different drugs on locomotion we recorded the change of activity after treatment. For each drug, time and genotype we plotted the percentage of change in activity after treatment (Fig. 2A,C,E). We also generated behavioral barcodes for each concentration of drug tested in each recording window to more visually represent the effect of a drug on both control and morphant larvae (Fig. 2B,D,F). All the mean distances travelled by the different treated groups (following adjustment for habituation for the time windows 30-40mn and 60-70mn) are indicated in Sup table 4.
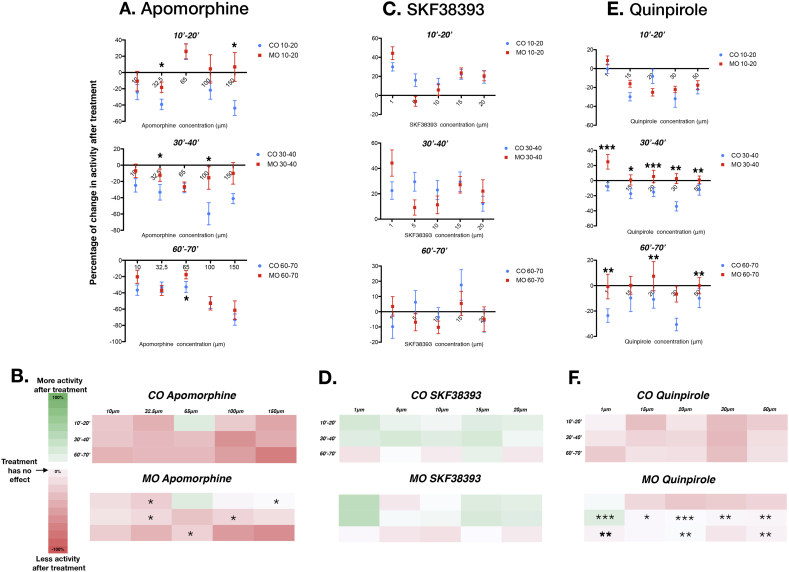
Fig. 2.
Non-specific and specific activation of DA receptors elicits a different response in Lphn3-MO and Lphn3-CO animals. A. The effects of Apomorphine, a non-selective DA agonist, in the percentage of activity after treatments, on Lphn3-CO (blue circle) and Lphn3-MO (red square) at three time periods after drug application (10–20 min top panel, 30–40 min middle panel, 60–70 min bottom panel). Lphn3-CO 10 μM n = 24, 32.5 μM n = 28, 65 μM n = 36, 100 μM n = 23, 150 μM n = 15; Lphn3-MO 10 μM n = 29, 32.5 μM n = 57, 65 μM n = 65, 100 μM n = 26, 150 μM n = 8. B. Heat map color key. Colors represent the percentage of change in locomotion after treatment normalized with the habituation coefficient. Heat map representation of the Apomorphine effects on Lphn3-CO (top) and Lphn3-MO (bottom) at three time periods after drug application (10–20, 30–40 and 60–70 min) and percentage of activity after treatments. C. Control and morphants larvae were incubated in different concentrations of the D1-like agonist SKF38393. Until at least 40 min, control larvae display moderately increased locomotion, whereas after 60 min there is a small non-dose-dependent decrease in locomotion in the two populations. Lphn3-CO 1 μM n = 36, 5 μM n = 40, 10 μM n = 44, 15 μM n = 36, 20 μM n = 43; Lphn3-MO 1 μM n = 30, 5 μM n = 33, 10 μM n = 36, 15 μM n = 34, 20 μM n = 38. D. Heat map of the D1-like agonist SKF-38393 on locomotion for Lphn3-MO and Lphn3-CO. E. Locomotion is moderately reduced in Lphn3-CO larvae in response to Qui (a D2-like agonist), while the locomotion of Lphn3-MO is not affected except at an early time-period (10–20 min). Lphn3-CO 1 μM n = 31; 15 μM n = 32; 20 μM n = 36; 30 μM n = 22; 50 μM n = 30; Lphn3-MO 1 μM n = 27, 15 μM n = 50, 20 μM n = 42; 30 μM n = 47; 50 μM n = 26. F. Heat map of the effect of the D2-like agonist Qui on locomotion for Lphn3-MO and Lphn3-CO.
All error bars are ± SEM. The statistics are the results of the two-way ANOVA followed by Bonferroni post-hoc test and represent the change in activity between Lphn3-MO and Lphn3-CO after drug application. *p < 0.05, ** p < 0.01 ***p < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We found that Apomorphine (Apo), a non-selective DA agonist, induced an inverted U shape dose-dependent early effect (10–20 min-period) in controls, with a decrease in the percentage of activity at low (10 μM and 32.5 μM) and high (100 μM and 150 μM) doses. Intermediate doses (65 μM) increased activity by 25.49 ± 15.8% (SEM) (Fig. 2A,B). After longer application times, Apo had a tendency to decrease the percentage of activity in a moderate, dose-dependent manner (60–70 min period: 10 μM, Lphn3-CO activity = −36.6% SEM ± 6.3; 150 μM, Lphn3-CO activity = −72.8% SEM ± 6.69).
Overall, Lphn3-MO larvae responded to Apo with similar trends as Lphn3-CO animals, although the effect on morphant locomotion was weaker. In the 10–20 min-period, 32.5 μM Apo decreased Lphn3-MO larval locomotion to a lesser extent than in Lphn3-CO animals (ANOVA 32.5 μM Lphn3-CO vs Lphn3-MO *p < 0.05) suggesting that Lphn3-MO are less sensitive to Apo than Lphn3-CO larvae (Fig. 2A,B). These observations can be extended to the 30–40 min period, where Apo also had a much smaller effect on Lphn3-MO locomotion, inducing only a slight decrease of locomotion compared to controls at 32.5 μM (ANOVA 32.5 μM Lphn3-CO vs Lphn3-MO *p < 0.05) and 100 μM (ANOVA 100 μM Lphn3-CO vs Lphn3-MO *p < 0.05). Finally, during the 60–70 min period Apo displayed a similar effect on both MO and Lphn3-CO with a significant difference observed at 65 μM (ANOVA 65 μM Lphn3-CO vs Lphn3-MO *p < 0.05). Overall, the non-specific activation of DA receptors by Apo tended to inhibit locomotion. This effect initially appeared weaker in Lphn3-MO compared to control animals suggesting hyposensitivity to the effects of DA.
3.2.2. D1 receptor agonists positively modulate locomotion in Lphn3.1 morphants and control larvae
Application of the D1-like agonist SKF-38393 (SKF) to Lphn3-CO larvae increased activity by 15–30% after a 10–20 min treatment at the five concentrations tested (Fig. 2C,D and Sup table 5 for raw data). This increase was not dose-dependent. After 30–40 min of treatment, the effect of D1 activation was similar with a positive effect on locomotion. Here again, no dose-dependent effects were observed. At 60 min post-incubation, there was a decrease of up to −9.8 ± 7.6% at 1 μM, whereas at 15 μM SKF had a positive effect on locomotion (17.5 ± 10%). Thus, in control animals, stimulation of D1 receptors only increased activity in a non-dose dependent manner during the first 40 min of treatment. Application of intermediate concentrations of SKF affected lphn3.1 morphant locomotion less than control animals (Fig. 2C,D). Long treatment, during the 60–70 min period, had a small negative effect on morphant animals. However, the morphant and control data were quite similar with no significant differences in behaviour.
3.2.3. Lphn3.1 morphant locomotion is less sensitive to activation of D2-like signaling
Quinpirole (Qui) selectively activates D2-like receptors and displayed a moderately negative effect on locomotion in control animals (Fig. 2E/F and Sup table 6 for raw data). This negative effect was maintained at all time points analyzed with a stable peak of effect at 30 μM (10–20 min period 30 μM Lphn3-CO activity = −31.8 ± 8.8%; 30–40 min period 30 μM Lphn3-CO activity = −34 ± 5.9%; 60–70 min period 30 μM Lphn3-CO activity = −30.6 ± 4.84%). Qui application had a similar effect on Lphn3-MO larvae and on Lphn3-CO during the 10–20 min period (Fig. 2E). However, during the 30–40 min period, Qui had almost no effect on morphants, with a mild positive effect at 1 μM (24.8 ± 9.4%). The differences were significant compared to controls at all concentrations tested (ANOVA 1 μM /20 μM Lphn3-CO vs Lphn3-MO ***p < 0.001; ANOVA 5 μM Lphn3-CO vs Lphn3-MO *p < 0.05; ANOVA 30 μM/50 μM Lphn3-CO vs Lphn3-MO **p < 0.01). After a 60–70 min treatment, Qui effects on Lphn3-MO were moderate compared to Lphn3-CO, showing a small decrease in activity for the first concentration at 1 μM (−0.7 ± 3.8%) and then a small increase at 15 μM(+0.27 ± 7.04%) and 20 μM (+7.7 ± 11.5%). Compared to controls at 60–70 min there was a significant difference for 1 μM, 20 μM and 50 μM Qui (ANOVA 1 μM/20 μM/50 μM Lphn3-CO vs Lphn3-MO **p < 0.01). Overall, D2-like activation by Qui decreased locomotion in controls in a manner that was rapid and persistent over time, while it was virtually without effect on lphn3.1 morphants suggesting a saturating activation of D2-dependent signaling in these animals. The responses of morphant and control larvae were statistically different for the 30–40 min and 60–70 min time points.
3.3. DA receptor antagonists also differentially affect Lphn3.1 morphant and control larvae, with a generally smaller effect in morphants
3.3.1. Non-selective DA receptor antagonists
Haloperidol (Halo) is a non-specific broad-spectrum DA receptor antagonist which may behave as a D2-like reverse agonist in mammals. It displayed a moderate stimulatory effect on activity in control larvae at high concentrations (10 μM and 20 μM) of drug for 10–20 min (Fig. 3 A/B and Sup table 7 for raw data). Similarly, during the 30–40 min period, a moderate stimulatory effect on locomotion was observed, although low doses (1 μM) triggered a − 14.2 ± 8.2% decrease of activity. Surprisingly, after longer application times (60–70 min period), Halo displayed a non-dose-dependent reduction of activity which was strongest at 1 μM.
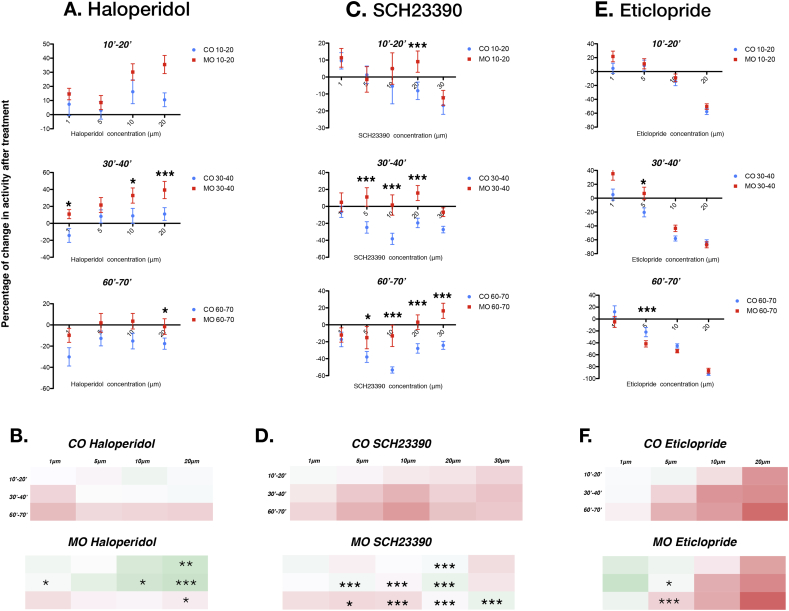
Fig. 3.
Blockade of DA receptor pathways elicits an atypical locomotion response in lphn3.1-MO.
A. Effect of the D1-like antagonist and D2-like reverse agonist Haloperidol on the percentage of activity. At early periods (10–20 min and 30–40 min) Haloperidol has almost no effect on control larvae, whereas at high doses it induces an increase in morphant locomotion. Lphn3-CO 1 μM n = 28, 5 μM n = 33, 10 μM n = 24, 20 μM n = 26; Lphn3-MO 1 μM n = 33, 5 μM n = 33, 10 μM n = 31, 20 μM n = 3 2. B. Heat map showing percentage activity following application of the D1-like antagonist and D2-like reverse agonist Haloperidol. C. The D1-like antagonist SCH23390 has a moderate effect on lphn3.1 morphants, whereas it has a negative effect on control animals after 30 min of recording. Lphn3-CO 1 μM n = 32, 5 μM n = 31, 10 μM n = 33, 20 μM n = 31, 30 μM n = 44; Lphn3-MO 1 μM n = 27, 5 μM n = 27, 10 μM n = 31, 20 μM n = 44, 30 μM n = 38. D. Heat map showing showing the effect of the D1-like antagonist SCH23390 on lphn3.1 morphants and control animals locomotion. E. The D2-like antagonist Eticlopride causes a dose-dependent decrease in locomotion at all the time-points measured for lphn3.1 morphants and controls. Lphn3-CO 1 μM n = 28, 5 μM n = 34, 10 μM n = 25, 20 μM n = 30; Lphn3-CO 1 μM n = 26, 5 μM n = 33, 10 μM n = 25, 20 μM n = 30. F. Heat map showing the effect of the D2-like antagonist Eticlopride on locomotion for Lphn3-MO and Lphn3-CO.
All error bars are ± SEM. The statistics are the results of the two-way ANOVA followed by Bonferonni post-hoc test and represent the change in activity between Lphn3-MO and Lphn3-CO after drug application. *p < 0.05, **p < 0.01 ***p < 0.001.
Application of Halo to Lphn3-MO morphants showed a dose-dependent stimulatory effect during both the 10–20 and 30–40 min period, which was clearly stronger than in controls (Fig. 3A,B). This observation is significant at the highest dose applied (20 μM) causing a 35.4 ± 6.3% increase during the 10–20 min period (ANOVA 20 μM Lphn3-CO vs Lphn3-MO **p < 0.01). A similar dose-dependent increase in the percentage of activity was seen 30–40 min after Halo treatment in a comparable range as observed at the early time point. There was a strong trend for this increase to be higher in morphants than in controls as the concentration of Halo increased. Finally, we observed a moderate negative effect of Halo on the activity of morphants after 60–70 min treatment. This effect was non-dose dependent and tended to be lower in morphants than in control larvae (ANOVA 20 μM Lphn3-CO vs Lphn3-MO *p < 0.05). Overall, Halo first exhibits a positive dose-dependent effect on locomotion which progressively decreases over time to reach a moderate inhibition of locomotion. This tendency is observed in both morphant and control larvae. However, the stimulatory effect of Halo is proportionally stronger in morphants and its later inhibitory effect much decreased. These data fit with the decreased sensitivity to DA observed with the DA receptor agonist Apo.
3.3.2. Attenuation of the effects of blocking D1-like receptors in lphn3.1 morphants
The D1-like specific antagonist SCH-23390 (SCH) exhibited a dose-dependent tendency to decrease control larvae activity during the 10–20 min period (Fig. 3C,D and Sup table 8 for raw data). During the 30–40 min period, SCH decreased activity in a U-shaped dose-dependent manner with a trend for the strongest effect at 10 μM (−38.2 ± 6.5%). A similar result was obtained at 60–70 min with the strongest effect at 10 μM (−53.2 ± 3.6%). Thus, blocking D1-like receptors decreases locomotor activity in Lphn3-CO larvae, which agrees with the stimulatory effect of the D1 receptor agonist.
During the first 10 min, application of SCH on morphants had a small effect on activity (Fig. 3C,D). At 20 μM there however a significant difference compared to the control larvae (ANOVA 20 μM Lphn3-CO vs Lphn3-MO ***p < 0.001). During the 30–40 min period, the effect on locomotion was very similar with a slight positive modulation in most cases except at high concentrations where activity was decreased. In the 30–40 min period there was a significant difference for control treated fish at 5 μM, 10 μM and 20 μM (ANOVA 5 μM /10 μM/20 μM Lphn3-CO vs Lphn3-MO ***p < 0.001). During the 60–70 min periods, SCH treatment caused a smaller decrease in locomotion in Lphn3-MO. However, at higher concentrations (30 μM) SKF stimulated morphant activity to a small extent (16.4 ± 4.6%), a significant difference compared to the same SCH concentration in controls (ANOVA 30 μM Lphn3-CO vs Lphn3-MO ***p < 0.001). Overall, Lphn3-MO appeared less sensitive to the decrease in locomotion triggered by SCH than control animals, which was statistically significant for concentrations higher than 1 μM. This effect is compatible with a desensitization of D1 receptors in morphants. The results of manipulating D1-like receptors with agonists and antagonists indicate that D1-like signaling tends to increase locomotion with some desensitization occurring overtime. Furthermore, D1-like receptors have a weaker effect on n lphn3.1 morphants than control larvae.
3.3.3. Blocking D2 signaling induce a dose-dependent suppression of locomotion
We next blocked D2-like signaling with Eticlopride (Etic), which resulted in a very stable dose-dependent decrease in locomotion in Lphn3-CO larvae at the three periods recorded (10–20 min /30–40 min/60–70 min) (Fig. 3E,F and Sup table 9 for raw data). Application of 1 μM Etic had no effect on the percentage of activity over time, whereas 20 μM Etic strongly decreased locomotion. This effect is paradoxical, since D2 agonists had a similar effect at all concentrations tested. Application of Etic to lphn3.1 morphants also induced a general dose-dependent decrease in activity (Fig. 3E,F). However, at 30–40 min 5 μM Etic treatment had a stimulatory effect on Lphn3-MO locomotion which was significantly different from the response of Lphn3-CO control larvae (ANOVA 5 μM Lphn3-CO vs Lphn3-MO *p < 0.05). At 60–70 min, 5 μM Etic reduced the locomotion of morphants significantly less than in controls (ANOVA 5 μM Lphn3-CO vs Lphn3-MO ***p < 0.001). In summary, blocking D2-like signaling generally induced a robust dose-dependent decrease in activity that was comparable in control and morphant larvae. However, 5 μM Etic induced a moderate increase in locomotion in morphants during the first 40 min, a response significantly different from control fish. Overall, manipulation of D2-like receptors with both antagonists and agonists has a negative effect on locomotion which can be explained by a dual role for D2 receptors in the control of locomotion. Morphants were again less sensitive to D2-like manipulation than controls.
4. Discussion
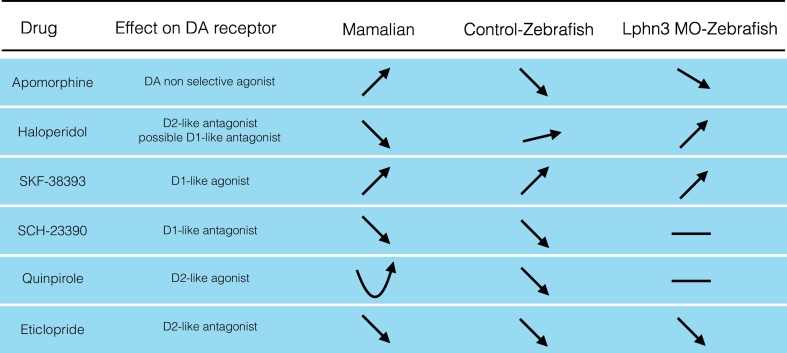
This study investigated changes to locomotion in Lphn3-MO and Lphn3-CO animals at different time points following treatment with drugs that target DA receptors. Although the drugs used in the present paper have mainly been characterized in mammals, their pharmacological activity appears to be generally similar in zebrafish (see Table 1). Our data confirm that DA receptor agonists and antagonists trigger different locomotor responses in zebrafish larvae and largely agree with previous studies (Ek et al., 2016; Irons et al., 2013; Le Crom et al., 2004; Souza et al., 2011; Tran et al., 2016; Tran et al., 2015).
Table 1.
Summary of changes in locomotion after drug treatment.
 Arrows indicate an increase in locomotion, whereas
Arrows indicate an increase in locomotion, whereas  arrows indicate decrease in locomotion after drug treatment. U-shaped
arrows indicate decrease in locomotion after drug treatment. U-shaped  arrows represent biphasic dose dependent effects, with decrease in activity at low doses and an increase in activity at high doses. Arrows with a less steep slope denote a moderate effect of the drug. Finally – indicates no effects. For references consult discussion.
arrows represent biphasic dose dependent effects, with decrease in activity at low doses and an increase in activity at high doses. Arrows with a less steep slope denote a moderate effect of the drug. Finally – indicates no effects. For references consult discussion.
4.1. Control of locomotor activity by DA signaling in the zebrafish larva
In the present report, the dominant effect of DA signaling manipulation using classical pharmacological drugs was to inhibit larval locomotion, a phenotype that primarily depends upon D2 receptors (Beaulieu and Gainetdinov, 2011). Indeed, both the non-specific DA receptor agonist Apo and the D2-specific agonist Qui had similar effects (Fig. 2A,B, Fig. 2E,F, Supp. Tables 3 and 5). In addition, blockade of the D1-like receptor with the specific antagonist SCH-23390 increased locomotion, an observation consistent with the activation of D2-like receptors by endogenous levels of dopamine (Fig. 3C,D).
For Apo, our results contrast with prior publications where Apo increases D1 and D2 receptor activity and induces a dose-dependent hyper-locomotion in rodents (Chow and Beck, 1984; Zarrindast and Eliassi, 1991). However at very low doses Apo is preferentially an agonist of D2 auto-receptors, which inhibit DA release and suppress locomotor activity (Carrera et al., 2011; Dias et al., 2006). Therefore, the dose-dependent decrease in locomotion observed in control larvae treated with Apo may be explained by the presence of both presynaptic auto-receptors and postsynaptic receptors as reported in mammals (although this has not yet been demonstrated in non-mammalian species) and by the concentration used in our study. Our data also differ from the reported increase in zebrafish locomotion after Apo treatment with a similar range of concentrations. An explanation for this difference might be that locomotion in this other study was recorded in the dark (Ek et al., 2016; Irons et al., 2013; Souza et al., 2011).
In rodents, application of a large spectrum DA receptor antagonist such as Halo decreased activity in a dose-dependent manner (Hauber, 1990; Waddington et al., 1995), whereas this compound had a more complex effect in zebrafish. Halo treatment produced a dose-dependent increase in locomotion at early time points, a moderate dose-dependent increase at intermediate time points and a small negative effect after longer exposure. A similar effect was described in adult zebrafish treated with Halo (Tran et al., 2015) and in larvae treated with either butaclamol (another non-specific DA receptor antagonist) or Halo (Irons et al., 2013). Despite the plausible presence of presynaptic autoreceptors in zebrafish, as suggested by our results, the sensitivity and expression of receptors remains unknown in fish and this could explain the contrasting zebrafish versus rodent data. This observation could also be explained by DA activating inhibitory and excitatory systems that control motor behavior with different sensitivity. The organization of the larval zebrafish motor system is likely to be significantly different from that of adult mammals and a one to one comparison between them may not be possible. In rodents, D2-like antagonists such as Etic mainly act on postsynaptic receptors to decrease locomotor activity (Schindler and Carmona, 2002). Here, we obtained similar results when we applied Etic to larval zebrafish with a strong dose-dependent decrease in locomotion following treatment. A satisfactory explanation of our results would require zebrafish D2-like receptors to be characterized in more detail (Yamamoto et al., 2015).
A second finding from this study is the stimulatory effect of D1 receptor activation on locomotor behavior in keeping with other research in zebrafish (Irons et al., 2013). This effect is very consistent and is likely to represent an important role for dopamine in larval zebrafish motor control. Blockade of D1 receptors by SCH-23390 attenuates adult zebrafish locomotor activity (Irons et al., 2013; Tran et al., 2015). These results are similar to rodent data, in which the selective D1-like agonist SKF has been reported to increase locomotor activity, whereas antagonists of D1-like signaling decrease locomotion (Centonze et al., 2003; Mazurski and Beninger, 1991; Molloy and Waddington, 1985). In addition, SKF activation of the D1-like receptor tends to diminish at longer time periods and higher doses, an observation compatible with receptor desensitization. Desensitization is a characteristic of D1-like receptor subtypes which has been shown to occur in many vertebrate species including teleost fish (LeCrom et al., 2004).
4.2. A model to explain the impaired DA signaling resulting from the abrogation of Lphn3 function
The main aim of this study was to characterize the DA signaling deficits induced by transient down-regulation of lphn3.1. Our results reveal a general hyposensitivity of lphn3.1 morphants to modulators of DA signaling. This phenomenon can be interpreted by morphants suffering from a constitutive, saturating hyperactivation of DA signaling. Application of the D1-like agonist SKF to morphants increased their activity. However, at specific concentrations Lphn3-MO larvae were hyposensitive to SKF. This hyposensitivity was further confirmed by treatment with SCH (a D1-like antagonist). SCH had no effect in morphants whereas it decreased locomotion in control animals. Overall, the activity of D1-like drugs was decreased or abolished in morphants. Activation of D2-like receptors by Qui also had no effect in Lphn3-MO animals, whereas Qui inhibited locomotion in control larvae. Finally, blocking D2-like postsynaptic receptors with Etic generally decreased the activity of Lphn3-MO larvae, but this effect was lessened or moderately opposite at low doses compared to control larvae. Thus, lphn3.1 morphants also appear to be hyposensitive to manipulation of D2-like receptors.
A scenario that could explain the responses of Lphn3-MO larvae to our pharmacological manipulations is a global increase in DA signaling. Indeed, this would strongly activate both D1-like- and D2-like receptors and might explain the hyperactivity of morphants. Within this framework, the hyposensitivity of morphants to DA-specific drugs could result from high levels of DA at the synaptic cleft, thereby increasing DA turnover and moderating the effects of agonists and antagonists through receptor desensitization. In order to investigate this, future studies could use fast-scan cyclic voltammetry to measure the release of dopamine in the brain of morphant and control animals (Jones et al., 2015). The slight increase of activity in Lphn3-MO at low doses of Etic might be due to blockade of presynaptic D2 receptors. Therefore, high levels of DA neurotransmission might mostly act on postsynaptic receptors to induce hyperactivity. A paradox remains however, as the stronger increase of locomotion observed following Halo treatment in morphants compared to control larvae cannot be explained by our model. However, the complex effect of Halo, which antagonizes both D1- and D2-like receptors (Seeman and Ulpian, 1988) makes it difficult to interpret. Halo is generally considered to primarily inhibit presynaptic D2 receptors in keeping with its tendency to increase locomotion in control larvae. How this inhibition, which would presumably increase DA at the synapse, could result in a further increase in locomotion in morphants remains unclear.
The global increase in DA signaling suggested by our pharmacological data might be caused by several non-exclusive mechanisms in morphants. We previously demonstrated a decrease in the number of DA neurons in Lphn3-MO larvae that correlated with their hyperactivity (Lange et al., 2012); in this context, a compensatory effect by the remaining neurons is possible. In support of our hypothesis, Lphn3-null mice display significantly higher striatal DA levels than WT mice (Wallis et al., 2012). We did not find any difference in DA levels and in DA turnover (the ratio of DA/DOPAC) by HPLC measurement in Lphn3-MO larvae (Lange et al., 2012). However, HPLC was performed on the entire brain, meaning that local alterations of DA signaling in some brain areas may have been missed, and it will be important to measure DA levels with better spatial resolution. Alternatively, or in addition, downstream DA signaling components could be affected. The pathways downstream of DA receptors activation are complex and use PKA as the primary effector. The PKA substrates DARP-32, ERK or Akt regulate locomotion (Beaulieu and Gainetdinov, 2011). Furthermore, the interaction between D2 and Akt is conserved in zebrafish and plays a role in larval locomotion (Souza et al., 2011). Future studies should investigate the expression of these key intracellular signaling molecules. Finally, specific up- or down regulation of DA receptors expression may contribute to the saturating hyperactivation of DA signaling in morphants. Zebrafish possess extra copies of many DA subtypes, probably due to the teleost-specific genome duplication (Yamamoto et al., 2015), and the exact number of receptors remain unknown. The functional significance of such receptor diversity is not clear, however the duplicated paralogous genes are maintained in many species (Yamamoto et al., 2015) suggesting that their function is probably not redundant. The expression of D1- and D2- like receptor orthologues have been reported in zebrafish (Boehmler et al., 2004, Boehmler et al., 2007; Yamamoto et al., 2013), and the role of the presynaptic D2 receptors is to regulate DA release (Van der Weide et al., 1988). A possible absence of presynaptic D2-like receptors in the morphants could contribute to the increase in DA signaling. Precise studies of the localization and expression of DA receptors in zebrafish larvae are required to further understand whether such modulations could underlie the defects observed in lphn3.1 morphants.
5. Conclusions
Our data confirm that DA receptor agonists and antagonists elicit different locomotor responses in zebrafish larvae with a global inhibitory effect. This overall inhibition suggests a predominant role for D2 signaling on larval locomotion as revealed by application of the non-specific DA receptor agonist Apo and the D2-specific agonist Qui, both of which triggered a decrease in locomotion. Our results, enabled by the accessibility of zebrafish larvae to pharmacological assays, also reveal a general hyposensitivity of lphn3.1 morphants to modulators of DA signaling. This observation can be explained by a saturating hyperactivation of DA signaling in fish lacking lphn3.1 function, although the detailed mechanistic links connecting Lphn3 activity and the modulation of DA signaling remain to be established. This working model can now be further challenged by combining pharmacological studies and careful analyses of DA development and signaling in animal models lacking Latrophilin function, and should provide insights into the functional interactions between Lphn3 and DA that lead to ADHD and hyperactivity in humans.
Competing interests statement
The authors declare no competing financial interests.
Animals maintenance
Zebrafish were maintained using standard fish-keeping protocols and in accordance with institutional and national guidelines for animal welfare.
Acknowledgements
We thank members of the L. B-C lab as well as P. Vernier, K.P. Lesch and F. Reichmann for their critical input. Work in the L. B-C. lab was funded by the Agence Nationale de la Recherche (grant ANR-2012-BSV4-0004-01), the Ecole des Neurosciences de Paris (ENP), the European Research Council (AdG 322936) and the Labex Revive. Merlin Lange is supported by RIKEN's Programs for Junior Scientists. Work in the Norton lab is funded by the European Community's Seventh Framework Program (FP7/2007-2013) under grant agreement no. 602805 and the Marie Skłodowska-Curie program under grant agreement no. 643051.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pnpbp.2018.02.010.
Contributor Information
Merlin Lange, Email: merlin.lange@riken.jp.
Laure Bally-Cuif, Email: laure.bally-cuif@pasteur.fr.
Appendix A. Supplementary data
Supplementary Table 1: Calculation of the habituation coefficient: example of Lphn3-CO larvae.
Supplementary Table 2: Calculation of the adjusted percentage of change in locomotion: example on Lphn3-CO larvae treated with Apo.
Supplementary Table 3: Examples of experimental and adjusted values for 24 control-injected animals treated with Apo and analyzed during the time window 30–40 min.
Supplementary Table 4: Mean distances swum per fish (in mm) after Apomorphine treatment.
Supplementary Table 5: Mean distances swum per fish (in mm) after SKF38393 treatment.
Supplementary Table 6: Mean distances swum per fish (in mm) after Quinpirole treatment.
Supplementary Table 7: Mean distances swum per fish (in mm) after Haloperidol treatment.
Supplementary Table 8: Mean distances swum per fish (in mm) after SCH23390 treatment.
Supplementary Table 9: Mean distances swum per fish (in mm) after Eticlopride treatment.
References
- Arcos-Burgos M., Jain M., Acosta M.T., Shively S., Stanescu H., Wallis D. A common variant of the latrophilin 3 gene, LPHN3, confers susceptibility to ADHD and predicts effectiveness of stimulant medication. Mol. Psychiatry. 2010;15(11):1053–1066. doi: 10.1038/mp.2010.6. [DOI] [PubMed] [Google Scholar]
- Beaulieu J.-M., Gainetdinov R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol. Psychiatry. 2005;57(11):1215–1220. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Boehmler W., Obrecht Pflumio S., Canfield V., Thisse C., Thisse B., Levenson R. Evolution and expression of D2 and D3 dopamine receptor genes in zebrafish. Dev. Dyn. 2004;230(3):481–493. doi: 10.1002/dvdy.20075. [DOI] [PubMed] [Google Scholar]
- Boehmler W., Carr T., Thisse C., Thisse B., Canfield V.A., Levenson R. D4 dopamine receptor genes of zebrafish and effects of the antipsychotic clozapine on larval swimming behaviour. Genes Brain Behav. 2007;6(2):155–166. doi: 10.1111/j.1601-183X.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Burgess H.A., Granato M. Sensorimotor gating in larval zebrafish. J. Neurosci. 2007;27(18):4984–4994. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. 2010;35(1):278–300. doi: 10.1038/npp.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera M.P., Carey R.J., Dias F.R.C., de Matos L.W. Reversal of apomorphine locomotor sensitization by a single post-conditioning trial treatment with a low autoreceptor dose of apomorphine: a memory re-consolidation approach. Pharmacol. Biochem. Behav. 2011;99(1):29–34. doi: 10.1016/j.pbb.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Centonze D., Grande C., Saulle E., Martín A.B., Gubellini P., Pavón N. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J. Neurosci. 2003;23(24):8506–8512. doi: 10.1523/JNEUROSCI.23-24-08506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow H.L., Beck C.H.M. The effect of apomorphine on the open-field behavior of rats: alone and in pairs. Pharmacol. Biochem. Behav. 1984;21(1):85–88. doi: 10.1016/0091-3057(84)90135-7. [DOI] [PubMed] [Google Scholar]
- De Mei C., Ramos M., Iitaka C., Borrelli E. Getting specialized: presynaptic and postsynaptic dopamine D2 receptors. Curr. Opin. Pharmacol. 2009;9(1):53–58. doi: 10.1016/j.coph.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo N., Chamberlain S.R., Sahakian B.J., Robbins T.W. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2011;69(12):e145–57. doi: 10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- Dias F.R.C., Carey R.J., Carrera M.P. Conditioned locomotion induced by unilateral intrastriatal administration of Apomorphine: D2 receptor activation is critical but not the expression of the unconditioned response. Brain Res. 2006;1083(1):85–95. doi: 10.1016/j.brainres.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Domené S., Stanescu H., Wallis D., Tinloy B., Pineda D.E., Kleta R. Screening of human LPHN3 for variants with a potential impact on ADHD susceptibility. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156B(1):11–18. doi: 10.1002/ajmg.b.31141. [DOI] [PubMed] [Google Scholar]
- Ek F., Malo M., Andersson M.Å., Wedding C., Kronborg J., Svensson P. Behavioral analysis of dopaminergic activation in zebrafish and rats reveals similar phenotypes. ACS Chem. Neurosci. 2016;7(5):633–646. doi: 10.1021/acschemneuro.6b00014. [DOI] [PubMed] [Google Scholar]
- Enjalbert A., Bockaert J. Pharmacological characterization of the D2 dopamine receptor negatively coupled with adenylate cyclase in rat anterior pituitary. Mol. Pharmacol. 1983;23(3):576–584. [PubMed] [Google Scholar]
- Fallgatter A.J., Ehlis A.-C., Dresler T., Reif A., Jacob C.P., Arcos-Burgos M., Muenke M., Lesch K.-P. Influence of a Latrophilin 3 (LPHN3) risk haplotype on event-related potential measures of cognitive response control in attention-deficit hyperactivity disorder (ADHD) Eur. Neuropsychopharmacol. 2013;23(6):458–468. doi: 10.1016/j.euroneuro.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber W. The NMDA antagonist dizocilpine (MK-801) reverses haloperidol-induced movement initiation deficits. Behav. Brain Res. 1990;41(2):161–166. doi: 10.1016/0166-4328(90)90151-4. [DOI] [PubMed] [Google Scholar]
- Irons T.D., Kelly P.E., Hunter D.L., MacPhail R.C., Padilla S. Acute administration of dopaminergic drugs has differential effects on locomotion in larval zebrafish. Pharmacol. Biochem. Behav. 2013;103(4):792–813. doi: 10.1016/j.pbb.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L., McCutcheon J., Young A., Norton W. Neurochemical measurements in the zebrafish brain. Front. Behav. Neurosci. 2015;9(74):458. doi: 10.3389/fnbeh.2015.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian J.W., Calne D.B. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. (11 January 1979) [DOI] [PubMed] [Google Scholar]
- Kebabian J.W., Greengard P. Dopamine-sensitive adenyl cyclase: possible role in synaptic transmission. Science (New York, N.Y.) 1971;174(4016):1346–1349. doi: 10.1126/science.174.4016.1346. [DOI] [PubMed] [Google Scholar]
- Labbe A., Liu A., Atherton J., Gizenko N., Fortier M.È., Sengupta S.M., Ridha J. Refining psychiatric phenotypes for response to treatment: contribution of LPHN3 in ADHD. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012;159B(7):776–785. doi: 10.1002/ajmg.b.32083. [DOI] [PubMed] [Google Scholar]
- Lambert A.M., Lambert A.M., Bonkowsky J.L., Bonkowsky J.L., Masino M.A., Masino M.A. The conserved dopaminergic diencephalospinal tract mediates vertebrate locomotor development in zebrafish larvae. J. Neurosci. 2012;32(39):13488–13500. doi: 10.1523/JNEUROSCI.1638-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M., Lange M., Norton W., Norton W., Coolen M., Coolen M. The ADHD-susceptibility gene lphn3.1 modulates dopaminergic neuron formation and locomotor activity during zebrafish development. Mol. Psychiatry. 2012;17(9):946–954. doi: 10.1038/mp.2012.29. [DOI] [PubMed] [Google Scholar]
- Langenhan T., Prömel S., Mestek L., Esmaeili B., Waller-Evans H., Hennig C. Latrophilin signaling links anterior-posterior tissue polarity and oriented cell divisions in the C. Elegans embryo. Dev. Cell. 2009;17(4):494–504. doi: 10.1016/j.devcel.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Crom S., Sugamori K.S., Sidhu A., Niznik H.B., Vernier P. Delineation of the conserved functional properties of D1A, D1B and D1C dopamine receptor subtypes in vertebrates. Biol. Cell. 2004;96(5):383–394. doi: 10.1016/j.biolcel.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Lu Y.C., Nazarko O.V., Sando R., Salzman G.S., Südhof T.C., Araç D. Structural basis of latrophilin-FLRT-UNC5 interaction in cell adhesion. Structure. 2015;23(9):1678–1691. doi: 10.1016/j.str.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A.F., Abe Y., Hong S., Molyneux K., Yarnell D., Löhr H. An ultraconserved brain-specific enhancer within ADGRL3 (LPHN3) underpins attention-deficit/hyperactivity disorder susceptibility. Biol. Psychiatry. 2016;80(12):943–954. doi: 10.1016/j.biopsych.2016.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurski E.J., Beninger R.J. Effects of selective drugs for dopaminergic D1 and D2 receptors on conditioned locomotion in rats. Psychopharmacology. 1991;105(1):107–112. doi: 10.1007/BF02316871. (March 1991) [DOI] [PubMed] [Google Scholar]
- Missale C., Nash S.R., Robinson S.W., Jaber M., Caron M.G. Dopamine receptors: from structure to function. Physiol. Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Molloy A.G., Waddington J.L. Sniffing, rearing and locomotor responses to the D-1 dopamine agonist R-SK&F 38393 and to apomorphine: differential interactions with the selective D-1 and D-2 antagonists SCH 23390 and metoclopramide. Eur. J. Pharmacol. 1985;108(3):305–308. doi: 10.1016/0014-2999(85)90454-6. [DOI] [PubMed] [Google Scholar]
- Orsini C.A., Setlow B., DeJesus M., Galaviz S., Loesch K., Ioerger T., Wallis D. Behavioral and transcriptomic profiling of mice null for Lphn3, a gene implicated in ADHD and addiction. Mol. Genet. Genomic Med. 2016;4(3):322–343. doi: 10.1002/mgg3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M.L., de Wit J., Savas J.N., Comoletti D., Otto-Hitt S., Yates J.R., III, Ghosh A. FLRT proteins are endogenous latrophilin ligands and regulate excitatory synapse development. Neuron. 2012;73(5):903–910. doi: 10.1016/j.neuron.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M.L., Martini F., Daake Von S., Comoletti D., Ghosh A. LPHN3, a presynaptic adhesion-GPCR implicated in ADHD, regulates the strength of neocortical layer 2/3 synaptic input to layer 5. Neural Dev. 2014;9(1):7. doi: 10.1186/1749-8104-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaivoson F.M., Liu Q., Martini F., Bergami F., Daake Von S., Li S. Structural and mechanistic insights into the latrophilin3-FLRT3 complex that mediates glutamatergic synapse development. Structure. 2015;23(9):1665–1677. doi: 10.1016/j.str.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter I., Knaup S., Romanos M., Lesch K.-P., Drepper C., Lillesaar C. Developmental exposure to acetaminophen does not induce hyperactivity in zebrafish larvae. J. Nel Transmission (Vienna, Austria: 1996) 2016;123(8):841–848. doi: 10.1007/s00702-016-1556-z. [DOI] [PubMed] [Google Scholar]
- Schindler C.W., Carmona G.N. Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacol. Biochem. Behav. 2002;72(4):857–863. doi: 10.1016/s0091-3057(02)00770-0. [DOI] [PubMed] [Google Scholar]
- Seeman P., Ulpian C. Dopamine D1 and D2 receptor selectivities of agonists and antagonists. Adv. Exp. Med. Biol. 1988;235:55–63. doi: 10.1007/978-1-4899-2723-1_5. [DOI] [PubMed] [Google Scholar]
- Silva J.-P., Lelianova V.G., Ermolyuk Y.S., Vysokov N., Hitchen P.G., Berninghausen O. Latrophilin 1 and its endogenous ligand lasso/teneurin-2 form a high-affinity transsynaptic receptor pair with signaling capabilities. Proc. Natl. Acad. Sci. U. S. A. 2011;108(29):12113–12118. doi: 10.1073/pnas.1019434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza B.R., Romano-Silva M.A., Tropepe V. Dopamine D2 receptor activity modulates Akt signaling and alters GABAergic neuron development and motor behavior in zebrafish larvae. J. Neurosci. 2011;31(14):5512–5525. doi: 10.1523/JNEUROSCI.5548-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumalai V., Cline H.T. Endogenous dopamine suppresses initiation of swimming in prefeeding zebrafish larvae. J. Neurophysiol. 2008;100(3):1635–1648. doi: 10.1152/jn.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran S., Nowicki M., Muraleetharan A., Chatterjee D., Gerlai R. Differential effects of acute administration of SCH-23390, a D1 receptor antagonist, and of ethanol on swimming activity, anxiety-related responses, and neurochemistry of zebrafish. Psychopharmacology. 2015;232(20):3709–3718. doi: 10.1007/s00213-015-4030-y. [DOI] [PubMed] [Google Scholar]
- Tran S., Facciol A., Gerlai R. Alcohol-induced behavioral changes in zebrafish: the role of dopamine D2-like receptors. Psychopharmacology. 2016;233(11):2119–2128. doi: 10.1007/s00213-016-4264-3. [DOI] [PubMed] [Google Scholar]
- Van der Weide J., Tendijck M.E., Tepper P.G., De Vries J.B., Dubocovich M.L., Horn A.S. The enantiomers of the D-2 dopamine receptor agonist N-0437 discriminate between pre- and postsynaptic dopamine receptors. Eur. J. Pharmacol. 1988;146(2–3):319–326. doi: 10.1016/0014-2999(88)90309-3. [DOI] [PubMed] [Google Scholar]
- Waddington J.L., Daly S.A., Downes R.P., Deveney A.M., McCauley P.G., O'boyle K.M. Behavioural pharmacology op ‘D-1-like’ dopamine receptors: further subtyping, new pharmacological probes and interactions with ‘D-2-like’ receptors. Prog. Neuro-Psychopharmacol. Biol. Psychiatr. 1995;19(5):811–831. doi: 10.1016/0278-5846(95)00130-n. [DOI] [PubMed] [Google Scholar]
- Wallis D., Hill D.S., Mendez I.A., Abbott L.C., Finnell R.H., Wellman P.J., Setlow B. Initial characterization of mice null for Lphn3, a gene implicated in ADHD and addiction. Brain Res. 2012;1463:85–92. doi: 10.1016/j.brainres.2012.04.053. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Mirabeau O., Bureau C. Evolution of dopamine receptor genes of the D1 class in vertebrates. Mol. Biol. Evol. 2013;30(4):833–843. doi: 10.1093/molbev/mss268. (Epub 2012 Nov 28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Fontaine R., Pasqualini C., Vernier P. Classification of dopamine receptor genes in vertebrates: nine subtypes in osteichthyes. Brain Behav. Evol. 2015;86(3–4):164–175. doi: 10.1159/000441550. [DOI] [PubMed] [Google Scholar]
- Zarrindast M.R., Eliassi A. Differential effects of dopamine agonists on locomotion in intact and reserpine-treated mice. Gen. Pharmacol. 1991;22(6):1027–1031. doi: 10.1016/0306-3623(91)90573-o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Calculation of the habituation coefficient: example of Lphn3-CO larvae.
Supplementary Table 2: Calculation of the adjusted percentage of change in locomotion: example on Lphn3-CO larvae treated with Apo.
Supplementary Table 3: Examples of experimental and adjusted values for 24 control-injected animals treated with Apo and analyzed during the time window 30–40 min.
Supplementary Table 4: Mean distances swum per fish (in mm) after Apomorphine treatment.
Supplementary Table 5: Mean distances swum per fish (in mm) after SKF38393 treatment.
Supplementary Table 6: Mean distances swum per fish (in mm) after Quinpirole treatment.
Supplementary Table 7: Mean distances swum per fish (in mm) after Haloperidol treatment.
Supplementary Table 8: Mean distances swum per fish (in mm) after SCH23390 treatment.
Supplementary Table 9: Mean distances swum per fish (in mm) after Eticlopride treatment.