Abstract
Metal transport from the cytosol to the vacuole is thought to be an important component of ion tolerance and of a plant's potential for use in phytoremediation. The Arabidopsis antiporter CAX2 (calcium exchanger 2) may be a key mediator of this process. CAX2 expression in yeast suppressed both Ca2+ and Mn2+ growth defects. A peptide-specific antibody to the antiporter reacted with a 39-kD protein from plant vacuolar membranes. Tobacco (Nicotiana tabacum) plants expressing CAX2 accumulated more Ca2+, Cd2+, and Mn2+ and were more tolerant to elevated Mn2+ levels. Expression of CAX2 in tobacco increased Cd2+ and Mn2+ transport in isolated root tonoplast vesicles. These results suggest that CAX2 has a broad substrate range and modulation of this transporter may be an important component of future strategies to improve plant ion tolerance.
Plants are susceptible to toxicity from most essential and nonessential ions. The concentration causing toxicity varies with the ion type, ion concentration, plant type, and conditions of growth. Tolerance to metals is thought to be based on multiple mechanisms, one of which is vacuolar sequestration (Cunningham et al., 1995; Kumar et al., 1995; Salt et al., 1995, 1998; Tomsett and Thurman, 1998). Vacuolar transporters may provide an important mechanism for metal sequestration into vacuoles (Salt and Wagner, 1993; Salt and Rauser, 1995; Shaul et al., 1999). In fact, a concentration gradient of Cd2+ and Mn2+ is established across the oat root tonoplast by Cd2+/H+ and Mn2+/H+ exchange activities (Salt and Wagner, 1993; Gonzales et al., 1999); however, the genes encoding these biochemical activities have not yet been identified.
Manipulation of vacuolar exchange activity may be an important component of genetic modifications to improve plant productivity and ion tolerance. Overexpression of an Arabidopsis vacuolar Na+/H+ antiporter in plants increased salinity tolerance (Apse et al., 1999). Expression of CAX1, a putative vacuolar Ca2+/H+ antiporter from Arabidopsis, in tobacco (Nicotiana tabacum) increases Ca2+ accumulation and Ca2+-related stress sensitivities (Hirschi, 1999). Ectopic expression in tobacco of AtMHX, an Arabidopsis Mg2+ and Zn2+ vacuolar antiporter, increases sensitivity to Mg2+ and Zn2+ (Shaul et al., 1999). Thus, dysregulated expression of vacuolar antiporters can impart positive (salinity tolerance) or negative (ion sensitivity) effects on plant growth.
Previously, two Arabidopsis genes, CAX1 (for calcium exchanger 1) and CAX2 were identified by their ability to suppress mutants of yeast defective in vacuolar Ca2+ transport (Hirschi et al., 1996). CAX1 biochemical activities in yeast vacuoles correlate well with those described for plant vacuolar Ca2+/H+ antiport activities, and recent evidence suggests that CAX1 plays a role in plant Ca2+ homeostasis (Hirschi, 1999); however, the role of CAX2 in plant growth and ion homeostasis is unknown. Biochemical activities of CAX2 in yeast suggest that this gene product has a low affinity for Ca2+ (Hirschi et al., 1996).
In yeast, either CAX1 or CAX2 can compensate for the absence of the endogenous vacuolar Ca2+/H+ antiporter (Hirschi et al., 1996). The functional redundancy of CAX1 and CAX2 suggests that loss-of-function Ca2+ antiporter mutations may not reveal a perceived phenotype. Ectopic expression of CAX1 in tobacco causes Ca2+ deficiency-like symptoms (Hirschi, 1999), suggesting that heterologous CAX2 expression might provide useful insights into CAX2 function (Diener and Hirschi, 2000).
Here, we take three different approaches to further ascertain the function of CAX2 in plants. First, we describe the growth characteristics of yeast strains expressing CAX2. In the second approach we analyze the intracellular localization of CAX2 and the influence of various metal stresses on CAX2 expression in Arabidopsis. Our third approach is to create CAX2-expressing tobacco plants and analyze their biochemical properties. Together, these studies demonstrate the involvement of CAX2 in the transport of several divalent cations into the vacuole in yeast and higher plants.
RESULTS
CAX2 Expression Confers Mn2+ Resistance in Yeast
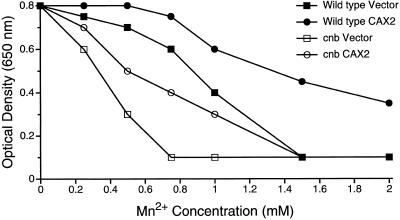
Yeast strains lacking functional calcineurin (cnb strains) display increased Mn2+ sensitivity due, in part, to decreased activity of the Golgi Ca2+-ATPase PMR1 (Farcasanu et al., 1995; Cunningham and Fink, 1996; Pozos et al., 1996; Fig. 1). Expression of the yeast vacuolar Ca2+/H+ antiporter suppresses this growth defect (Pozos et al., 1996). We therefore tested whether CAX2 expression in yeast could improve the growth of the calcineurin mutant strain on medium containing MnCl2. As shown in Figure 1, CAX2 expression increases the Mn2+ tolerance of both cnb mutant strains and isogenic wild-type parent strains.
Figure 1.
Mn2+ tolerance assay of yeast strains expressing vector or CAX2. All strains were grown to saturation in selection media at 30°C and diluted 500-fold into fresh media containing a range of MnCl2 concentrations and incubated for 1 d (wild-type strains) or 2 d (cnb strains) at 30°C in flat-bottom 96-well dishes (0.2 mL/well). Optical density at 650 nm was measured for each resuspended culture and plotted directly (Matheos et al., 1997).
Calcineurin mutants display growth defects under a variety of conditions: for example, they have increased salt sensitivity (Pozos et al., 1996). In contrast to the Mn2+ sensitivity of cnb strains, these growth defects were unchanged by CAX2 expression (data not shown). Thus, CAX2 specifically increased tolerance to Mn2+ but could not substitute generally for a lack of calcineurin in vivo.
CAX2 expression did not alter the tolerance of wild-type yeast strains to any additional ions that were tested (Cd2+, Cu2+, Na+, Mg2+, and Zn2+; data not shown). Furthermore, CAX2 expression also did not suppress the Cd2+ sensitivity of a yeast strain defective in vacuolar Cd2+ sequestration (data not shown; Li et al., 1996).
CAX2 Is Localized in the Plant Vacuolar Membrane
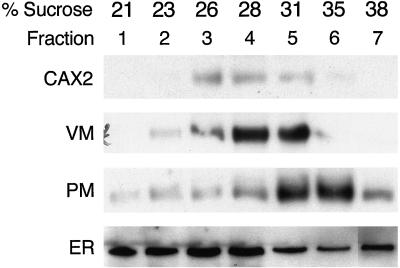
CAX2 contains 11 putative transmembrane domains and has a predicted molecular mass of 39 kD (Hirschi et al., 1996). The amino acid sequence of CAX2 lacks any special sequences that could suggest the cellular membrane to which it is targeted. However, in yeast this protein appears to function at the tonoplast membrane (Hirschi et al., 1996). To identify the cellular localization of CAX2 in plants, we produced polyclonal antibodies against a peptide from the deduced amino acid sequence of the central non-membranal loop. The antibody did not cross-react with yeast proteins; however, it did react with a 39-kD protein in yeast strains expressing CAX2 (data not shown). As shown in Figure 2, western-blot analysis of Arabidopsis membranes fractionated on Suc gradients show that CAX2 cofractionates with the vacuolar membrane marker tonoplast intrinsic protein, and not with plasma membrane or endoplasmic reticulum markers. Differential centrifugation similarly indicated that CAX2 did not cofractionate with mitochondria, plastids, or nuclei (data not shown). Thus, CAX2 is predominately localized in the vacuolar membrane. This localization is supported by ion-transport studies of tonoplast vesicles isolated from tobacco plants transformed with CAX2 (see below).
Figure 2.
Intracellular localization of CAX2 in wild-type Arabidopsis plants. Arabidopsis membranes were extracted and fractionated in a Suc gradient as previously described (Schaller and DeWitt, 1995). The fractions (fraction 1 = 21%; fraction 7 = 38% [v/v] Suc) were subjected to western-blot analyses using the following antibodies: CAX2, affinity-purified antibodies against a peptide from CAX2 deduced amino acid sequence; VM, antibodies against a vacuolar membrane marker VM23, a homolog of tonoplast intrinsic protein from radish (Raphanus sativus), which is a species closely related to Arabidopsis (Maeshima, 1992); PM, antibodies against the Arabidopsis plasma membrane marker protein RD-28 (Yamaguchi-Shinozaki et al., 1992); ER, antibodies against the endoplasmic reticulum yeast BiP protein that specifically recognize plant endoplasmic reticulum BiP (Shimoni et al., 1995).
CAX2 Expression in Arabidopsis
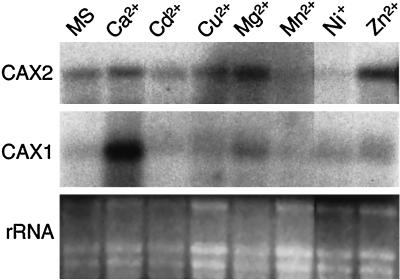
CAX2 RNA and CAX2 protein could be detected at low levels in all Arabidopsis tissues (data not shown). Northern analyses were performed to determine how ion imbalances and a variety of other stresses induced CAX2 RNA accumulation. As shown in Figure 3, CAX2 RNA was not greatly induced by any of the tested treatments; however, there may be a slight induction by Zn2+ treatment. For purposes of comparison, we also probed this blot with CAX1. The levels of CAX2 protein also did not appear to significantly increase after these treatments (data not shown). The plant hormones, abscisic acid, auxin, and gibberellin, at concentrations of 0.1 μm, also did not induce CAX2 RNA or protein expression after a 16-h incubation (data not shown).
Figure 3.
Expression of CAX2 and CAX1 in Arabidopsis. CAX RNA expression in response to ion imbalances. RNA is from whole Arabidopsis plants 16 h after treatment with various ions (Murashige and Skoog-nutrient media; Ca2+, 80 mm CaCl2; Cd2+, 0.01 mm CdCl2; Cu2+, 0.1 mm CuCl2; Mg2+, 50 mm MgCl2; Mn2+, 0.5 mm MnCl2; Ni+, 0.1 mm NiCl; Zn2+, 1 mm ZnCl2). The blot was hybridized with either the CAX2 or CAX1 cDNA. Ethidium bromide-stained rRNA before transfer is shown in the bottom panel.
Expression of CAX2 in Transgenic Tobacco
In previous work, CAX2 was partially characterized as its ability to suppress defects in vacuolar Ca2+ transport in yeast. However, CAX2 appears to have biochemical properties in yeast that are inconsistent with its involvement in transport of Ca2+ into the vacuole (Hirschi et al., 1996). To examine the role of CAX2 in ion homeostasis, we expressed CAX2 driven by the cauliflower mosaic virus 35S promoter (35S) in Arabidopsis and tobacco plants.
Transgenic expression of CAX2 in Arabidopsis plants was expected to either attenuate endogenous transcript levels of CAX2 by a gene-silencing phenomenon or exaggerate CAX2 expression. By northern analysis, we found that CAX2 overexpression in Arabidopsis augmented normal CAX2 expression. However, this overexpression did not result in measurable changes in CAX2 protein levels (data not shown).
As an alternative approach, we took advantage of heterologous expression and expressed the Arabidopsis CAX2 gene in tobacco (cv KY160). We generated transgenic lines of tobacco with a CAX2 open reading frame (ORF) expressed in either the sense or antisense orientation, driven by the 35S promoter. As controls, transgenic lines were prepared that harbored only the expression vector.
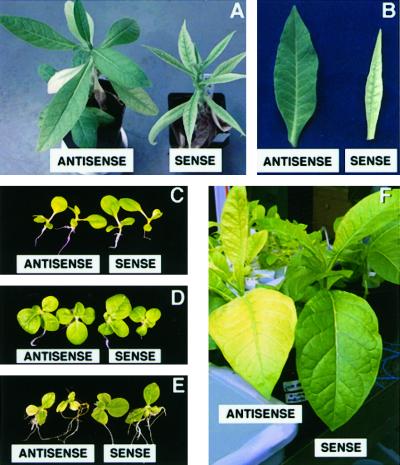
Preliminary examination of CAX2 expression in tobacco suggested that CAX2 was affecting plant growth. Figure 4A demonstrates that after several weeks, some of the primary transformants expressing the sense-oriented CAX2 displayed altered leaf morphology. This was observed in 10 of the 70 primary transformants. After several weeks, the leaves were spindle-shaped and chlorotic (Fig. 4B). In these 10 plants and an additional 10 plants, there appeared to be a reduction in root mass (data not shown). The remaining sense lines and the 50 transgenic plants expressing antisense-oriented CAX2 displayed growth phenotypes indistinguishable from the 10 vector control transgenic plants.
Figure 4.
Phenotypes of tobacco plants expressing CAX2 genes. Sense lines denote expression of the CAX2 ORF. Antisense lines contain the ORF in the opposite orientation. A, Phenotype of primary CAX2-expressing transformants lines using a 35S promoter. B, Leaf phenotype of primary CAX2-expressing transformants lines using a 35S promoter. C, CAX2-expressing seedlings grown in standard media immediately after transfer to various media (pretreatment). D, CAX2-expressing seedlings transferred to standard media and grown for 10 d. E, CAX2-expressing seedlings transferred to standard media supplemented with 0.5 mm MnCl2 and grown for 10 d. F, Phenotype of transgenic plants grown for 1 week in a hydroponic solution containing 0.5 mm MnCl2.
All 10 of the chlorotic lines failed to produce seeds. The other 60 35S::CAX2-expressing lines, the antisense-oriented CAX2 lines, and vector-containing transgenic lines all possessed about 90% fertility. The reduction in root mass revisited the majority of T2 plants from the 10 original transformants, which displayed this phenotype. Approximately 10% of the T2 plants from the remaining CAX2-expressing lines had a slight reduction in root mass; however, the majority of the plants appeared normal.
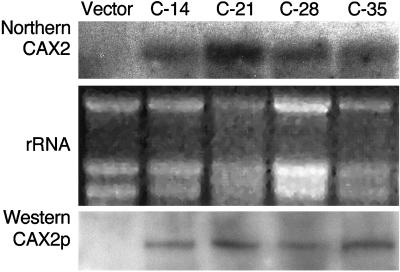
We selected four independent transgenic lines (C-14, C-21, C-28, and C-35) that displayed normal growth (no reduction in root mass) for further study. The expression of CAX2 RNA was measured in these lines by northern analysis. As shown in Figure 5, CAX2 RNA accumulates in all 35S::CAX2 transgenic lines. CAX2-specific RNA could also be detected in all antisense lines tested (data not shown). The inability to detect an endogenous transcript of the tobacco CAX2 homolog in the vector transgenic lines attests to the high stringency of our hybridization.
Figure 5.
Expression of CAX2 in transgenic tobacco plants. Ten micrograms of total RNA extracted from fully expanded leaves of 6-week-old T2 plants was analyzed by RNA gel blotting. The blot was hybridized with the CAX2 cDNA probe. Transgenic lines expressing the vector alone do not express CAX2 RNA. Sense lines (C-14, C-21, C-28, and C-35) denote 5′-3′ expression of the CAX2 ORF using the 35S promoter (35S::CAX2). Ethidium bromide-stained rRNA before transfer is shown. A protein gel blot of fractionated transgenic tobacco plants probed with Arabidopsis anti-CAX2 antiserum. Protein was extracted from 6-week-old T2 plants and 10 μg of protein was transferred to each lane of a SDS-polyacrylamide gel, blotted to nitrocellulose, and probed with the anti-CAX2 antibody.
The expression of CAX2 protein could also be verified in the transgenic plants. The antibody reacted with a protein with the expected molecular mass of 39 kD, which did not appear in vector only plants (Fig. 5B).
CAX2 Expression Confers Mn2+ Tolerance in Plants
Constitutive CAX2 expression might also alter the ion sensitivity of transgenic plants. As shown in Figure 4C, transgenic seeds were germinated on standard media and then transferred to various media when they were similar in size and vigor to the control plants. When the CAX2-transformed plants were allowed to grow in standard media, they were the same size as the vector controls (Fig. 4D). More than 200 T2 seeds were analyzed from 20 35S:CAX2 lines, and these plants exhibited no alterations in growth on Al3+-, Ca2+-, Cd2+-, Cu2+-, Ni2+-, Mg2+-, Na+-, or Zn2+-containing media (data not shown). CAX2-expressing plants were more tolerant to Mn2+ than the vector control (not shown) or antisense lines (Fig. 4E). This tolerance to Mn2+ could be seen in 30% of the CAX2-expressing transgenic lines. The Mn2+ tolerance could also be seen when plants were grown hydroponically in 0.5 mm MnCl2 (Fig. 4F). However, under the conditions tested, the sense CAX2-expressing plants began to exhibit similar symptoms to the vector controls after an additional 4 d (data not shown).
Metal Accumulation in CAX2-Expressing Plants
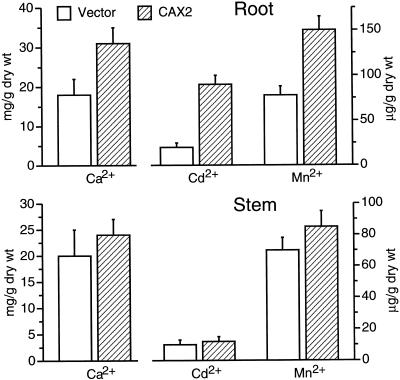
To ascertain whether CAX2 expression altered total metal accumulation, ion concentrations were measured in roots and stems of transgenic plants. As shown in Figure 6, CAX2-expressing plants contained almost three times the total Cd2+ in root tissue as the vector control plants. Stems of CAX2-expressing plants contained approximately 15% more total Cd2+ than plants expressing the vector alone. Ca2+ and Mn2+ levels were doubled in CAX2-expressing root tissues with 15% to 20% increases in the content of these ions in the stem. CAX2-expressing plants were grown in 0.1 μm AlSO4, 0.1 μm CuCl2, 10 mm MgCl2, or 0.5 mm ZnSO4; supplemented media did not show differences compared with the vector controls (data not shown).
Figure 6.
Ion concentrations in roots and stems of transgenic plants. Ion content of vector controls (V) and CAX2-expressing plants (line C-14) grown in standard media supplemented with 10 mm CaCl2, 0.1 μm CdCl2 or 0.1 mm MnCl2. Ion content was determined using atomic absorption spectrophotometry. Data represent the means (±sd) of three independent assays.
Vacuolar Transport in CAX2-Expressing Tobacco
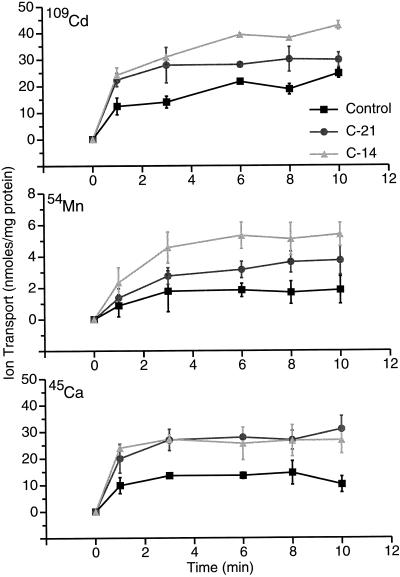
The relative 109Cd, 54Mn, and 45Ca root tonoplast transport activities of control, C-14, and C-21 lines were examined using the direct vesicle filtration assay. As shown in Figure 7, CAX2-expressing plants had higher root tonoplast transport of all three ions than the control. For Cd2+, lines C-14 and C-21 had approximately 2.1- and 1.6-fold the ion accumulation after 8 min as controls, respectively. For Mn2+, the enhancements were 3.0- and 2.2-fold, respectively. For Ca2+, C-14 and C-21 lines had similar uptake that was 1.8 times that of the control. The initial rates of uptake (0 to 1 min) of Cd2+ and Ca2+ appeared to be higher in transformed versus control plants. In the case of Mn2+, only the C-14 line suggested a clearly higher initial rate versus control. Further study is needed to substantiate and understand results regarding initial uptake rates. The real-time acridine orange fluorescence quench assay unfortunately is not useful for monitoring proton efflux in response to Mn2+ uptake (Gonzales et al., 1999). The methylamine assay for monitoring proton efflux in response to Mn2+ uptake into vesicles, like the ion transport assay used here, is not amenable to monitoring initial rates in detail. The affinity of CAX2 for Mn2+ is apparently much lower than that for Cd2+ and Ca2+. In transport assays, 10 μm Cd2+ and Ca2+ was found to be optimal, whereas for Mn2+ no activity is observed using this same concentration of Mn2+, but 100 μm Mn2+ was suitable. This observation corresponds to the fact that 100 times more Ca2+ than Mn2+ occurs in nutritionally balanced plants, and it corresponds to the earlier observation that 20-fold higher Mn2+ than Ca2+ was required to obtain the same proton efflux response (methylamine assay) in oat root tonoplast vesicles (Gonzales et al., 1999).
Figure 7.
Ion uptake in root tonoplast vesicles of CAX2-expressing plants. Potassium-loaded vesicles were energized by addition of nigericin. A, 109Cd transport, 10 μm total Cd. B, 54Mn transport, 100 μm total Mn. C, 45Ca transport, 10 μm total Ca.
DISCUSSION
Properties of CAX2
In plants, the primary driving force for transport processes is the electrochemical H+ gradient, which is generated by H+-ATPases localized in both the plasma membrane and the vacuolar membrane (Ma-athuis and Sanders, 1992). The Arabidopsis CAX2 transporter appears to be localized in the vacuolar membrane (Fig. 2) and transports divalent cations into the vacuole (Fig. 7).
CAX2 was initially cloned by its ability to suppress a yeast mutant defective in vacuolar Ca2+ transport (Hirschi et al., 1996). There have been several recent reports of yeast and plant Ca2+ transporters suppressing Mn2+ growth defects (Pozos et al., 1996; Liang et al., 1997; Del Poza et al., 1999). We demonstrate here that CAX2 is also capable of suppressing Mn2+ growth defects in yeast (Fig. 1).
Various plant and yeast transporters appear to generally have a broad selectivity in ion transport (Kamizono et al., 1989). For example, the plant transporter IRT1 was initially identified as an Fe (II) transporter (Eide et al., 1996); however, this protein can also transport Mn2+ and Zn2+ (Korshunova et al., 1999). The plant transporter LCA1 mediates the uptake of Ca2+ and Cd2+ in yeast (Clemens et al., 1998). CAX2 is shown here to be able to transport Ca2+, Cd2+, and Mn2+ (Figs. 6 and 7). Future experiments will be directed at determining if CAX2 is capable of transporting other ions as well.
The relative root accumulation of Cd2+ and Mn2+ versus Ca2+ found here for CAX2 transgenic plants (Fig. 6) is similar to the relative Cd2+ and Mn2+ versus Ca2+ transport capabilities observed in root tonoplast vesicles isolated from these plants (Fig. 7). The sensitivity of our studies unfortunately did not allow us to precisely correlate the increased accumulations with increased CAX2 expression. For example, our results suggest that CAX2 is expressed at approximately equal levels in transgenic lines C-14 and C-21 (Fig. 5). However, C-14 demonstrated increased ion accumulation and Mn2+ transport compared with C-21 (Fig. 7; data not shown). Nevertheless, our findings support the conclusion that CAX2 has broad ion selectivity and that this transporter plays a role in vacuolar uptake of Cd2+ and Mn2+ in plants.
CAX2 RNA levels did not increase in response to exogenous Ca2+; however, CAX1 RNA levels increase significantly in response to Ca2+ treatment (Fig. 3; Hirschi, 1999). Plants apparently regulate these transporters through different mechanisms. Given the lack of fluctuation in CAX2 protein levels during ion imbalances, this protein may also be regulated post-translationally. In yeast, various transporters are modulated during ion imbalances. This regulation occurs through a cascade of proteins that include a transcription factor that is regulated by the phosphatase calcineurin (Matheos et al., 1997; Stathopoulos and Cyert, 1997). In plants, CAX1 and CAX2 may be part of an ensemble of transporters, which are regulated by as-yet-unidentified factors during ion imbalances.
Implications of CAX2 Expression for Enhanced Mn2+ Tolerance and Phytoremediation
At the cellular level, one component of engineering ion tolerance in plants appears to be the manipulation of plant vacuolar transporters. Increased expression of Na+/H+ antiport activity confers increased sodium accumulation in Arabidopsis and thus increased salt tolerance (Apse et al., 1999). Expression of a putative vacuolar Ca2+/H+ antiporter in tobacco increases total Ca2+ content in plants (Hirschi, 1999). Expression of a vacuolar Zn2+ and Mg2+ transporter in tobacco confers heightened sensitivity to these specific ions (Shaul et al., 1999).
We demonstrate here that expression of CAX2 in tobacco altered the Ca2+, Cd2+, and Mn2+ content of plants and made transgenic plants more tolerant to Mn2+ stress (Figs. 4 and 6). Mn2+ is a plant micronutrient that is required for many enzyme-catalyzed reactions (Marscher, 1995). Mn2+ toxicity also can be an important factor limiting plant growth, particularly in acidic, poorly drained soils (Horst, 1988). Mn2+ toxicity affects a number of agriculturally important crops; in fact, in Kentucky this problem costs growers 40 million dollars each year in yield loss (Sims et al., 1990; Marschner, 1995). Cd2+ can also be toxic to plants, but levels encountered in natural and agricultural environments are generally below toxicity levels (Wagner, 1992). Mechanisms of Cd2+ accumulation in plants have been characterized (Wagner, 1992; Rea et al., 1998). Several hypotheses concerning the physiological mechanisms of Mn2+ tolerance have also been proposed (Gonzales and Lynch, 1999). CAX2 expression in transgenic crops could potentially alleviate Mn2+ toxicity problems and aid in phytoremediation of Cd2+ through sequestration of these ions into the vacuole. However, at the stress levels tested, the Mn2+ tolerance was limited. After several days, the CAX2-expressing plants also had Mn2+ toxicity symptoms. Furthermore, the CAX2-expressing plants demonstrated only modest increases in Cd2+ and Mn2+ accumulation in the stem tissue (Fig. 6) and no enhanced Cd2+ tolerance when grown on Cd2+-containing media. This suggests that future approaches to increase Mn2+ tolerance and Cd2+ phytoremediation potential will have to also include control of root uptake, long distance metal transport, and additional tolerance factors to accommodate high concentrations of these ions (Raskin et al., 1994). Nonetheless, it will be interesting in the future to compare CAX2-like activity in naturally derived Mn2+-tolerant and sensitive plants (Burke et al., 1990).
In conclusion, expression of the low-affinity Ca2+/H+ antiporter, CAX2, in transgenic plants produces phenotypes that are distinct from and less severe than those produced by expression of the high-affinity Ca2+/H+ antiporter, CAX1. CAX1-expressing plants accumulate high levels of Ca2+ but have symptoms of Ca2+ deficiency (Hirschi, 1999). In contrast, even though CAX2-expressing plants accumulated Ca2+ levels comparable with those seen with CAX1, these plants were, for the most part, as vigorous as controls. Furthermore, the broad-substrate range of the CAX2 transporter allowed plants to accumulate other metal ions and increased the tolerance of the plants to Mn2+ stress. These findings suggest that engineering the expression of vacuolar metal transporters with broad substrate ranges may have an important impact on improving plant productivity.
MATERIALS AND METHODS
Yeast Strains and Plant Materials
Yeast strains were grown in standard yeast peptone dextrose medium (2% [v/v] Difco yeast extract, 1% [v/v] bacto-peptone, and 2% [v/v] dextrose) or synthetic complete minus uracil media (Sherman et al., 1986) supplemented with the ions when indicated in the text. The wild-type yeast strain was W303-1A (Wallis et al., 1989) and the calcineurin-deficient strain was K603 (Cunningham and Fink, 1994). These strains were transformed using the lithium acetate procedure (Sherman et al., 1986) with CAX2 and vector control plasmids (Hirschi et al., 1996). Columbia was the Arabidopsis ecotype used in this study. For stress treatment, surface-sterilized seeds were grown on one-half-strength Murashige and Skoog medium (Murashige and Skoog, 1962), 2% (w/v) Suc, and 1% (w/v) agar, pH 5.7 (standard media) for 3 weeks and then transferred to a water bath containing the appropriate stress. Tobacco (Nicotiana tabacum cv KY160) was used in this study. Plants were grown in a greenhouse as previously described (Hirschi, 1999).
Surface-sterilized tobacco seeds were plated on standard media and maintained in a temperature-controlled room at 25°C with continuous cool-fluorescent illumination as previously described (Hirschi, 1999). Most experiments were carried out with the segregating T2 generations of tobacco lines C-14 and C-21. Phenotypes did not drastically differ among the 35S::CAX2-expressing plants. Antisense line D-23 was used in most experiments; however, the phenotypes displayed by antisense and vector control lines were indistinguishable in all experiments performed.
Preparation of CAX2 Antibody and Protein Gel Blots
A polyclonal antibody was raised against a synthetic peptide that was derived for the CAX2 sequence: LDEESNQNEETSAE. The peptide was linked through its N-terminal residue to the high-Mr keyhole impact hemocyanin carrier as previously described (Harlow and Lane, 1988) and injected into rabbits. The antibody was affinity purified against this peptide using the Sulfolink Coupling Gel (Pierce Chemical, Rockford, IL) according to manufacturer's instructions.
Protein gel electrophoreses and electrophoretic transfer was performed as previously described (Hirschi et al., 1998). Immunodetection was performed using a 1:1,000 dilution of CAX2 antiserum and a 1:10,000 dilution of horseradish peroxidase-coupled anti-rabbit secondary antibody (Amersham, Buckinghamshire, UK). Detection of the marker proteins was performed as previously described (Hong et al., 1999; Shaul et al., 1999). Enhanced chemiluminescence was performed, according to the instructions given by the manufacturer (Amersham). To ensure reproducibility of the results obtained from immunoblots, at least three independent experiments were performed at exposure times, which varied from 30 s to 15 min.
Membrane Fractionation
We prepared microsomal membranes according to Hong et al. (1999) and fractionated these on Suc gradients containing EDTA.
Cloning and Plant Transformations
Standard techniques of DNA cloning were performed as described by Ausubel et al. (1998). The coding region of CAX2 was cloned into pBIN19 (CLONTECH Laboratories, Palo Alto, CA), which contained the 35S fragment and nos terminator (Hull et al., 2000). The recombinant plasmids, or vector controls, were introduced in Agrobacterium tumefaciens LBA4404 (Life Technologies, Grand Island, NY). Tobacco leaf disc transformation were carried out as previously described (Hirschi, 1999). Transformants were selected on standard media containing 100 μg/mL kanamycin. Seventy primary transformants harboring the 35S::CAX2 construct were transferred to soil.
RNA Extraction and RNA Gel-Blot Analysis
RNA was isolated from Arabidopsis plants (leaves, stems, and roots) and tobacco leaves according to previously published procedures (Niyogi and Fink, 1992). After electrophoresis on a 1% (v/v) agarose gel in formaldehyde, total RNA was blotted onto nylon membranes (Hybond N+, Amersham) as recommended by the manufacturer. The full-length CAX2 cDNA was radiolabeled with [32P]dCTP by using a random primed labeling kit (Amersham). Blots were hybridized at 65°C according to the method of Church and Gilbert (1984). Blots were washed three times (15 min each) in 0.1× SSC (1× SSC is 0.15 m NaCl and 0.015 m sodium citrate) and 0.1% (v/v) SDS at 65°C, and hybridization was visualized by autoradiography.
Metal Analysis
Tobacco plants were grown for 50 d in the greenhouse using hydroponic conditions previously described (Hirschi, 1999). Vector control and CAX2-expressing plants of equal root mass and leaf area were grown side by side. The plants were grown in a nutrient solution containing the following macronutrients: 1.2 mm KNO3, 0.8 mm Ca(NO3)2, 0.1 mm NH4H2PO4, and 0.2 mm MgSO4. The following micronutrients were also added: 25 μm CaCl2, 2 μm MnSO4, and 2 μm ZnSO4. Nutrient solutions were changed every 15 d. Five days prior to metal analysis the nutrient solutions were supplemented with various ions. The roots and stems were harvested and treated as previously described (Hirschi, 1999). The Fruit and Vegetable Science Analytical Laboratory (Ithaca, NY) determined ion analysis.
Isolation of Sealed Tonoplast-Enriched Vesicles
Isolation of sealed tonoplast-enriched vesicles from tobacco roots was done according to previously published procedures (Hirschi, 1999), essentially as described for oat roots (Gonzales et al., 1999).
Loading of Vesicles with Potassium and Transport Assays
Vesicles were loaded with potassium and transport assays were done after establishment of a proton gradient using nigericin as previously described (Gonzales et al., 1999; Hirschi, 1999). 109Cd (1.06 × 104 MBq μg−1, NEN-DuPont, Research Products, Boston MA), 54Mn (0.52 MBq μg−1, Amersham Life Science, Arlington Heights, IL), and 45Ca (carrier free, American Radiolabeled Chemicals, St. Louis) were used in these studies.
ACKNOWLEDGMENTS
We thank Marica Miranda and Jean Sunega for technical support. We thank M.J. Chrispeels, M. Maeshima, J. Harper, and O. Shaul for antibodies. We are grateful to Bonnie Bartel and Toshiro Shigaki for critical reading of the manuscript.
Footnotes
This work was supported in part by the National Institutes of Health (grant nos. CHRC 5 P30 and 1R01 GM 57427) and by the U.S. Department of Agriculture/Agricultural Research Service under cooperative agreement (grant no. 58–6250–6001).
LITERATURE CITED
- Apse MP, Aharon GS, Snedden WA, Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science. 1999;285:1256–1258. doi: 10.1126/science.285.5431.1256. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: Greene Publishing Associates/Wiley Interscience; 1998. [Google Scholar]
- Burke D, Watkins K, Scott BJ. Manganese toxicity effects on visible symptoms, yield, manganese levels, and organic acid levels in tolerant and sensitive wheat cultivars. Crop Sci. 1990;30:275–280. [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Antosiewicz DM, Ward JM, Schachtman DP, Schroeder JI. The plant cDNA LCT1 mediates the uptake of calcium and cadmium in yeast. Proc Natl Acad Sci USA. 1998;95:12043–12048. doi: 10.1073/pnas.95.20.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KW, Fink GR. Ca2+ transport in Saccharomyces cerevisiae. J Exp Biol. 1994;196:157–166. doi: 10.1242/jeb.196.1.157. [DOI] [PubMed] [Google Scholar]
- Cunningham KW, Fink GR. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+-ATPase in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham SD, Berti WR, Huang JWW. Phytoremediation of contaminated soils. Trends Biotechnol. 1995;13:393–397. [Google Scholar]
- Del Poza L, Osaba L, Corchero J, Jimenez A. A single nucleotide change in the MNR1 VCX1/HUM1 gene determines resistance to manganese in Saccharomyces cere-visiae. Yeast. 1999;15:371–375. doi: 10.1002/(sici)1097-0061(19990330)15:5<371::aid-yea380>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- Diener A, Hirschi KD. Heterologous expression for dominant-like gene activity. Trends Plant Sci. 2000;5:10–11. doi: 10.1016/s1360-1385(99)01512-5. [DOI] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farcasanu IC, Hirata D, Tsuchiya E, Nishiyama F, Miya-kawa T. Protein phosphatase 2B of Saccharomyces cerevisiae is required for tolerance to manganese, in blocking the entry of ions into the cells. Eur J Biochem. 1995;232:712–717. [PubMed] [Google Scholar]
- Gonzales A, Koren'kov V, Wagner GJ. A comparison of Zn, Mn, Cd and Ca transport mechanisms in oat root tonoplast vesicles. Physiol Plant. 1999;106:203–209. [Google Scholar]
- Gonzales A, Lynch J. Subcellular and tissue Mn compartmentation in bean leaves under Mn toxicity stress. Aust Plant Physiol. 1999;26:811–822. [Google Scholar]
- Harlow ED, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Hirschi KD. Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell. 1999;11:2113–2122. doi: 10.1105/tpc.11.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD, Zhen R-G, Cunningham KW, Rea PA, Fink GR. CAX1 and H+/Ca2+ antiporter for Arabidopsis. Proc Natl Acad Sci USA. 1996;93:8782–8786. doi: 10.1073/pnas.93.16.8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, Rohovsky SA, D'Amore PA. PDGF, TGF-β, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong B, Ichida A, Wang Y, Gens JS, Pickard BG, Harper JF. Identification of a calmodulin-regulated Ca2+-ATPase in the endoplasmic reticulum. Plant Physiol. 1999;119:1165–1175. doi: 10.1104/pp.119.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst WJ. The physiology of manganese toxicity in soils and plants. In: Graham RD, Hannam RJ, Uven NC, editors. Manganese in Soils and Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 175–188. [Google Scholar]
- Hull A, Vij R, Celenza JL. Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proc Natl Acad Sci USA. 2000;97:2379–2384. doi: 10.1073/pnas.040569997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamizono A, Nishizawa M, Teranishi T, Murata K, Kimura A. Identification of a gene conferring resistance to zinc and cadmium ions in the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1989;219:161–167. doi: 10.1007/BF00261172. [DOI] [PubMed] [Google Scholar]
- Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol Biol. 1999;40:37–44. doi: 10.1023/a:1026438615520. [DOI] [PubMed] [Google Scholar]
- Kumar PBAN, Dushenkov V, Motto H, Raskin J. Phytoextraction: the use of plants to remove heavy metals from soils. Environ Sci Technol. 1995;29:1232–1238. doi: 10.1021/es00005a014. [DOI] [PubMed] [Google Scholar]
- Li Z-S, Szczypka M, Lu Y-P, Thiele DJ, Rea PA. The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J Biol Chem. 1996;172:6509–6517. doi: 10.1074/jbc.271.11.6509. [DOI] [PubMed] [Google Scholar]
- Liang F, Cunningham KW, Harper JF, Sze H. ECA1 complements yeast mutants defective in Ca2+ pumps and encodes an endoplasmic reticulum-type Ca2+-ATPase in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1997;94:8579–8584. doi: 10.1073/pnas.94.16.8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D. Plant membrane transport. Curr Opin Cell Biol. 1992;4:661–669. doi: 10.1016/0955-0674(92)90087-s. [DOI] [PubMed] [Google Scholar]
- Maeshima M. Characterization of the major and integral protein of vacuolar membrane. Plant Physiol. 1992;98:1248–1254. doi: 10.1104/pp.98.4.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H. Mineral Nutrition of Higher Plants. San Diego: Academic Press; 1995. [Google Scholar]
- Matheos DP, Kingsbury TJ, Ahsan US, Cunningham KW. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 1997;11:3445–3458. doi: 10.1101/gad.11.24.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Niyogi KK, Fink GR. Two anthranilate synthase genes in Arabidopsis: defense-related regulation of the tryptophan pathway. Plant Cell. 1992;4:721–733. doi: 10.1105/tpc.4.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozos TC, Sekler I, Cyert MS. The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+C2+ exchangers. Mol Cell Biol. 1996;16:3730–3741. doi: 10.1128/mcb.16.7.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I, Kumar PBAN, Dushenkov S, Salt DE. Bioconcentration of heavy metals by plants. Curr Opin Biotechnol. 1994;5:285–290. [Google Scholar]
- Rea PA, Li Z, Lu Y, Drozdowicz YM. From vacuolar GS-X pumps to multispecific ABC transporters. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:727–760. doi: 10.1146/annurev.arplant.49.1.727. [DOI] [PubMed] [Google Scholar]
- Salt DE, Blaylock M, Kumar NPBA, Viatcheslav D, Ensley BD, Chet I, Raskin I. Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology. 1995;13:468–473. doi: 10.1038/nbt0595-468. [DOI] [PubMed] [Google Scholar]
- Salt DE, Rauser WE. MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiol. 1995;107:1293–1301. doi: 10.1104/pp.107.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Smith RD, Raskin I. Phytoremediation. Annu Rev Plant Physiol. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- Salt DE, Wagner GJ. Cadmium transport across tonoplast of vesicles from oat roots. J Biol Chem. 1993;268:12297–12302. [PubMed] [Google Scholar]
- Schaller GE, DeWitt ND. Analysis of the H+-ATPase and other proteins of the Arabidopsis plasma membrane. Methods Cell Biol. 1995;50:129–148. [PubMed] [Google Scholar]
- Shaul O, Hilgemann DW, de-Almeida-Engler J, Van Montagu MV, Inze D, Galili G. Cloning and characterization of a novel Mg2+/H+ exchanger. EMBO J. 1999;8:3973–3980. doi: 10.1093/emboj/18.14.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Hicks JB. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1986. [Google Scholar]
- Shimoni Y, Zhu X, Levanony H, Segal G, Galili G. Purification, characterization and intracellular localization of glycosylated protein disulfide isomerase from wheat grains. Plant Physiol. 1995;108:327–335. doi: 10.1104/pp.108.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims JL, Wells KL, Greer EC. Effect of banded fertilizer on manganese toxicity of burley tobacco. Agron Notes. 1990;23:1–4. [Google Scholar]
- Stathopoulos AM, Cyert MS. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 1997;11:3432–3444. doi: 10.1101/gad.11.24.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomsett AB, Thurman DA. Molecular biology of metal tolerances of plants. Plant Cell Environ. 1998;11:383–394. [Google Scholar]
- Wagner GJ. Accumulation of Cd in crop plants and its consequences to human health. Adv Agron. 1992;51:173–212. [Google Scholar]
- Wallis JW, Chrebet G, Brodsky G, Rolfe M, Rothstein R. A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell. 1989;58:409–419. doi: 10.1016/0092-8674(89)90855-6. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Masahiro K, Satomi U, Kazuo S. Molecular cloning and characterization of 9 cDNAs for genes that are responsive for desiccation in Arabidopsis thaliana: sequence analysis of one cDNA clone that encodes a putative transmembrane channel protein. Plant Cell Physiol. 1992;33:217–224. [Google Scholar]