Abstract
Introduction/Background
Studies have shown that young patients with early-stage breast cancer (BC) are increasingly getting mastectomy instead of breast conserving therapy (BCT) consisting of lumpectomy and radiation. We examined the difference between outcomes in young women (age<40) treated with BCT versus mastectomy.
Materials and Methods
The Surveillance, Epidemiology, and End Results database was queried for women <40 years of age with stage I–II invasive BC treated with surgery from 1998–2003. Breast cancer specific survival (BCSS) and overall survival (OS) were evaluated by Kaplan-Meier survival analysis and the log–rank test between treatment types.
Results
Of the 7665 women, 3249 patients received BCT, while 2627 patients had mastectomy and no radiation. When separated by stage (I, IIA, and IIB), with median follow-up of 111 months, the BCT and mastectomy only groups showed no statistically significant differences in the BCSS and OS. Overall, age group 35–39 (66% of total) was associated with better 10-year BCSS (88%) and OS (86.1%) compared to younger patients aged 20–34 (34% of total), who had 10-year BCSS and OS of 84.1% and 82.3%, respectively (P<0.001 for both BCSS and OS). However, when patients of each age group were further subdivided by stages, the BCT group continued to show non-inferior BCSS and OS compared to the mastectomy group in all of the subgroups.
Conclusion
Our study suggests that while young age may be a poor prognostic factor for BC, there is no evidence that these patients have better outcome with mastectomy over BCT.
Keywords: Young women, mastectomy, breast conserving therapy, radiation, SEER, breast cancer
Introduction
In women with early stage breast cancer, breast conserving therapy (BCT) consisting of lumpectomy and radiation therapy has been shown to be equivalent to mastectomy in multiple randomized controlled trials.1, 2, 3 BCT allows for sparing of the normal breast tissue and results in better cosmesis and fewer side effects.4, 5, 6 Consequently, BCT results in better physical and psychological well-being and has been shown to improve patients’ overall quality of life and has become a standard treatment for breast cancer. 7
However, the use of BCT in young women under 40 with early stage breast cancer has been questioned due to studies showing more aggressive disease with more likelihood of local recurrence in these patients. 8 Because of the rarity of breast cancer in young patients, only a small percentage of patients included in major randomized trials have been younger than 40. 9, 10
This study examined the outcome of young women with early stage breast cancer treated with either mastectomy or BCT in the United States by using the Surveillance, Epidemiology, and End Results (SEER) database.
Materials and Methods
Patient information in the SEER database was extracted using the SEERStat software. The study included all women with malignant breast cancer as their first primary treated with surgery between 1998 and 2003. Only those with recorded American Joint Committee on Cancer stages I and II (T1-2N0-1 or T3N0 and M0) diseases were included to limit the sample to early stage breast cancer.
The type of surgery (lumpectomy vs. mastectomy) and vital status of the patients were gathered, along with length of follow up. Cause of death codes were used to identify breast cancer mortality as well as deaths resulting from other causes. Patients were further separated by whether they received “Beam radiation” or no radiation; those who received brachytherapy were not included in this study. To better compare the standard BCT and mastectomy approaches, analysis focused on patients who had lumpectomy plus radiation and those who had mastectomy and no radiation.
Statistical Analysis
Descriptive statistics were calculated to characterize the study cohort in relation to demographic, prognostic, and treatment factors of interest. Breast cancer specific survival (BCSS) was ascertained by selecting BC as the cause of death and recording the length of follow-up, censoring deaths due to other causes. Kaplan-Meier survival analysis was performed to evaluate BCSS and the log-rank test was employed to compare BCSS between demographic, prognostic, and treatment characteristics of interest. Similar analyses were performed for overall survival (OS), including all causes of deaths, and Non-BCSS, which included all causes of death other than BC, which was censored. All P-values are two-sided with statistical significance evaluated at the 0.05 alpha level. Chi-square test, where appropriate, was used to assess mortality rates and distribution of patients with various factors between different treatment groups. All data organization and statistical analyses were done using SPSS Version21.0 (SPSS Inc., Chicago, IL), and Microsoft ® Office Excel ® 2010 (Microsoft Corporation; Redmond, WA).
Results
Patient Characteristics
Of the 7665 women identified, 3249 (42%) patients received BCT, while 2627 (34%) patients had mastectomy and no radiation. There were also 994 (14%) patients who had lumpectomy but no radiation, and 795 (10%) treated with mastectomy plus radiation. The various patient and disease characteristics are detailed in Table 1.
Table 1.
Patient Characteristics
| Overall (n=7655) | BCT (n=3249) | Mastectomy (n=2627) | |
|---|---|---|---|
| Median Age Group | 35–39 | 35–39 | 35–39 |
| Median Follow up | 111 months | 119 months | 108 months |
| Ages 20–24 | 68 (1%) | 27 (1%) | 21 (1%) |
| 25–29 | 530 (7%) | 215 (7%) | 173 (7%) |
| 30–34 | 1991 (26%) | 828 (25%) | 688 (26%) |
| 35–39 | 5076 (66%) | 2179 (67%) | 1745 (66%) |
| White | 5806 (76%) | 2491 (77%) | 1993 (76%) |
| Black | 971 (13%) | 395 (12%) | 322 (13%) |
| Other ethnicity | 848 (11%) | 349 (11%) | 299 (11%) |
| Stage I* | 3054 (40%) | 1579(49%) | 1000 (38%) |
| Stage IIA* | 3009 (39%) | 1171 (36%) | 1086 (41%) |
| Stage IIB* | 1602 (21%) | 499 (15%) | 541 (21%) |
| T1* | 4391 (57%) | 2070 (64%) | 1442 (55%) |
| T2* | 3112 (41% | 1143 (35%) | 1139 (43%) |
| T3 | 162 (2%) | 36 (1%) | 46 (2%) |
| N0* | 4888 (64%) | 2295 (71%) | 1690 (64%) |
| N1* | 2777 (36%) | 954 (30% | 937 (36%) |
| ER+† | 4103 (54%) | 1791 (55%) | 1419 (54%) |
| ER− | 2636 (34%) | 1168 (36%) | 805(31%) |
Abbreviations: ER = Estrogen Receptor, BCT = Breast conserving therapy (lumpectomy+radiation)
P<0.05 by Chi-square test
12% of the patients’ ER statuses were unknown
The BCT and mastectomy only groups showed no significant differences in terms of age, follow up time, and race. However, compared to patients who underwent mastectomy alone, those treated with BCT were more likely to have smaller tumor (T1), negative lymph nodes (N0), and thus more stage I disease (P<0.0001 in all three factors). There was no statistically significant difference in the number of estrogen receptor (ER) positive and negative disease between these two groups.
Patient outcome
Overall, with median follow-up of 111 months (based on living patients), 1013 (13.2%) BC deaths had occurred. On multivariate analysis, higher stage, ER negative status, and mastectomy plus radiation were found to have higher risk of BC specific mortality (Table 2).
Table 2.
Multivariate Analysis of Breast Cancer Specific Mortality Risk Factors*
| Variable | P-Value | Adjusted Hazard Ratio | 95.0% CI for HR | |
|---|---|---|---|---|
| Lower | Upper | |||
| Stage=2 † | <0.0001 | 2.081 | 1.812 | 2.389 |
| ER=Negative ‡ | <0.0001 | 1.385 | 1.23 | 1.56 |
| BCT § | 0.025 | 0.81 | 0.673 | 0.974 |
| Mast. + No RT§ | 0.935 | 1.008 | 0.84 | 1.209 |
| Mast.+ RT§ | 0.004 | 1.373 | 1.107 | 1.704 |
Abbreviations: ER = Estrogen Receptor, BCT = Breast conserving therapy (lumpectomy+radiation), Mast. = Mastectomy, RT = radiation therapy
Adjusted for age group, stage, ER status, and treatment type
Referent: Stage I
Referent: ER positive
Referent: Lumpectomy only no RT
The BCT group had 10-year BCSS of 87.7% (95% CI=86.5–88.9%) compared to 85.4% (95% CI=83.8–87.0%) in mastectomy only group (Log-rank P=0.009). The 10-year OS between BCT and mastectomy groups were 85.9% (95% CI=84.5–87.3%) and 83.5% (95% CI=81.9–85.1%), respectively (Log-rank P=0.01). The 10-year BCSS in BCT and mastectomy patients with ER+ disease were 89.2% (95% CI=87.5–90.7%) vs 87.2% (95% CI=85.2–89.0%) (P=0.08) and for ER− disease were 85.7% (95% CI=83.7–87.6%) vs 82.1% (95% CI=79.5–83.5%) (P=0.02). There was no statistically significant difference in the non-BCSS between the two groups.
Patient Outcome by Stage
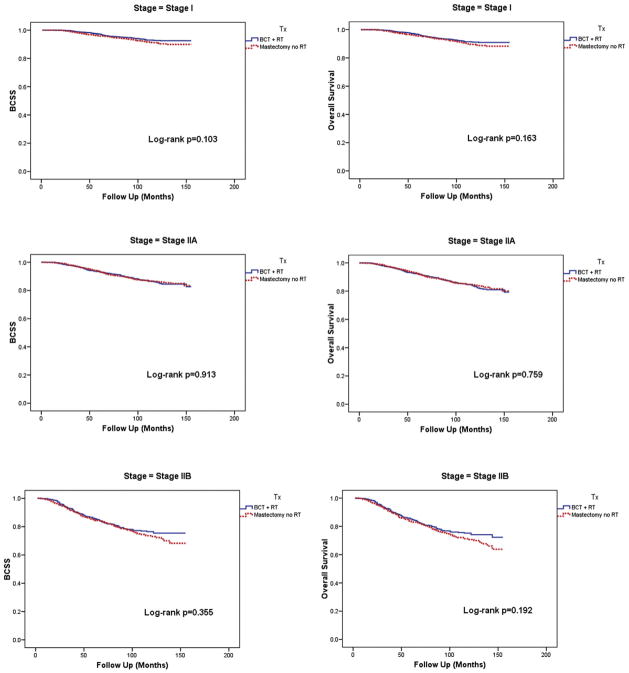
Since the BCT group had more T1 and N0 disease compared to the mastectomy group, the BCSS and OS were also compared separating the patients into Stages I, IIA, and IIB. When separated by stage, the two treatment groups showed no statistically significant difference in the BCSS and OS (Figure 1). There was also no difference in the non-BCSS in any stage disease between the two treatment groups.
Figure 1.
Patient outcome by treatment type, stratified by stage
Abbreviations: BCSS = Breast Cancer Specific Survival, BCT = Breast conserving therapy, RT = Radiation therapy
Patient Outcome and Age
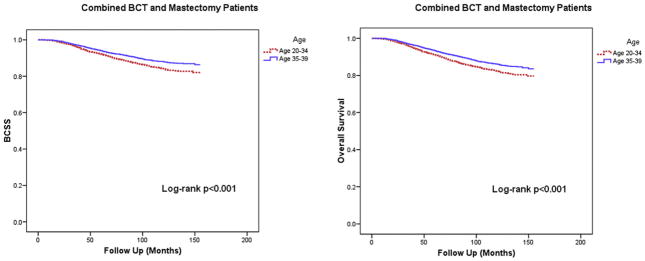
The BCSS and OS of the patients in BCT and mastectomy groups were also analyzed by separating those who were 20–34 years old at time of diagnosis with those who were 35–39. As shown Figure 2, age group 35–39 (pooled sample of BCT and mastectomy groups) was associated with better 10-year BCSS (88% (95% CI=86.8–89.2%)) and OS (86.1% (95% CI=84.9–87.3%)) compared to younger patients aged 20–34, who had 10-year BCSS and OS of 84.1% (95% CI=82.3–85.9%) and 82.3% (95% CI=80.5–84.1%), respectively (P<0.001 for both).
Figure 2.
Patient outcome by age group and treatment type
Abbreviations: BCSS = Breast Cancer Specific Survival, BCT = Breast conserving therapy, RT = Radiation therapy
When patients of each age group were further subdivided into stages I, IIA, and IIB, there was no statistically significant difference in BCSS or OS between the BCT and mastectomy groups for all stages in age 35–39 as well as stages I and IIA in the age 20–34 group. However, as shown in Figure 3, in patients between ages 20–34 with stage IIB disease only, the mastectomy only group (n=183) had significantly inferior 10-year BCSS (64% (95% CI=55.8–72.2%) vs. 79% (95% CI=72.5–85.5%), P=0.004) and OS (61% (95% CI=52.8–69.2%) vs. 77% (95% CI=70.5–83.5%), P=0.002) compared to the BCT group (n=183). The proportion of T3N0 and T2N1 diseases was similar between the mastectomy and BCT groups and was also similar to the distribution observed in stage IIB patients aged 35–39. There was also no difference in the non-BCSS in any of the subgroups.
Figure 3.
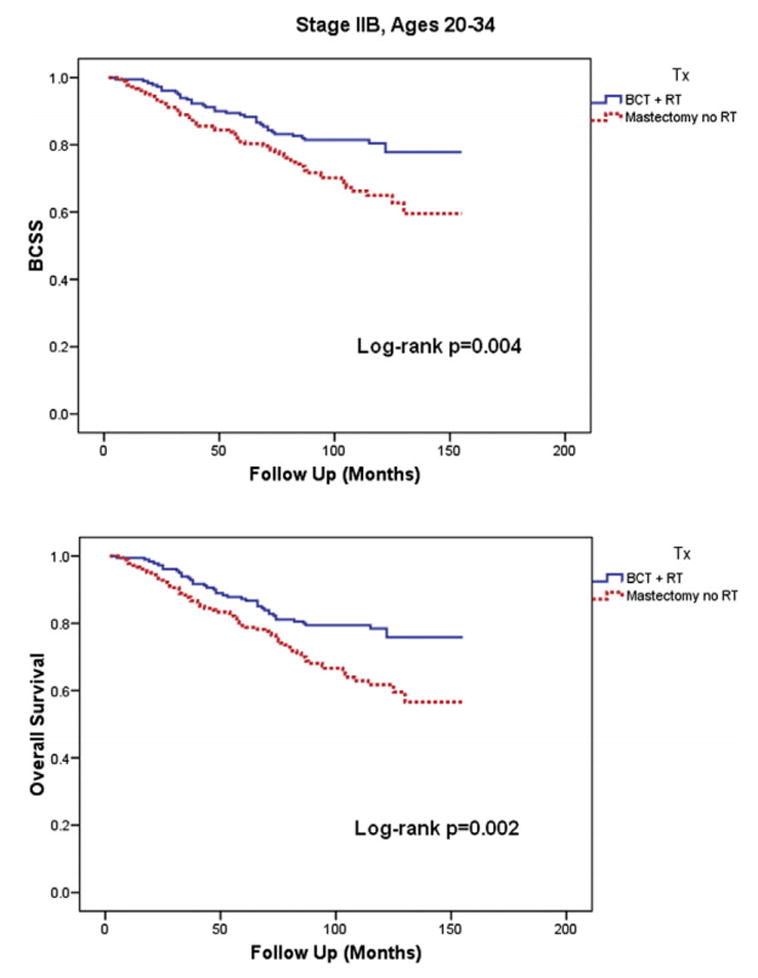
Patient outcome by treatment type in Stage IIB patients ages 20–34
Abbreviations: BCSS = Breast Cancer Specific Survival, BCT = Breast conserving therapy, RT = Radiation therapy
Note: No difference in BCSS or Overall Survival by treatment type was observed in Stages I and IIA for the same age group. There was also no difference in BCSS or Overall Survival by treatment type observed in Stages I, IIA, and IIB for patients in age group 35–39.
Summary
In women with Stage I and II breast cancer treated between 1998 and 2003 recorded in the SEER database, younger age (20–34), higher stage, and ER− disease were associated with poorer outcomes regardless of treatment received. With median follow up of nearly 10 years, patients who received BCT had similar or better BCSS and OS both for the overall sample studied, as well as in subgroups divided by age, stage, and ER status.
Discussion
The appropriate management of breast cancer in young patients is still controversial because of the notion that breast cancer in young patients tends to be more aggressive and present at more advanced stages. 11 One possible explanation for this is the lack of effective screening process due to rarity of events, resulting in higher stage at presentation similar to older patients who do not undergo screening. 12 Subset analyses of small number of patients present in available randomized data showed likely increase in local failure without a difference in survival. 8
Using the data from European Organization for Research and Treatment of Cancer early-stage breast cancer randomized controlled trials (trials 10801, 10854, 10902), a multivariable analysis showed that younger age (age <35 vs >50: HR 2.80; age 35–50 vs >50: HR 1.72) and breast conservation (HR: 1.82) were risk factors for isolated locoregional recurrence. 9 However, the recurrence risk decreased with appropriate use of perioperative chemotherapy and also did not result in difference in OS.
The Danish Breast Cancer Cooperative Group also conducted a combined analysis of their prospective randomized trials (82 TM and 89 TM). 10 In this study, younger patients were more likely to receive BCT (P<.001). Multivariable analysis revealed that those who received BCT had a 5.2-fold greater incidence of recurrence (15.4% vs 3%) in the breast within 5 years among women age <35 years compared with women aged 45–49 years. However, there was no increased risk of death observed between the treatments regardless of age.
Many retrospective studies have also failed to show a clear survival benefit to the mastectomy approach over BCT. A population based University of British Columbia study including 965 breast cancer patients aged 20–39 years treated between 1989 and 2003 showed no difference in 15-year rates of BCSS (76.0% vs 74.1%, P=.62), OS (74.2% vs 73.0%, P=0.75), local relapse free survival (85.4% vs 86.5% P=0.95), loco-regional relapse free survival (82.2% vs 81.6%, P=0.61), and distant relapse free survival (74.4% vs 71.6%, P=0.40). 13 Similarly, a population based study of 1451 women in the Netherlands showed no statistically significant different in 10-year OS between BCT and mastectomy (74.9% vs 71.2%, P=.215). 14 In a study evaluating clinico-pathological characteristics of breast cancer in women younger than 40, the authors found that while these patients present with larger tumors, more positive lymph nodes, and more stage III (vs. Stage II) diseases than the older group, the outcome in terms of death and locoregional recurrence rates was similar. 11
Despite lack of strong evidence suggesting superiority of mastectomy in this patient group, there is an increased trend in the United States for young patients to get mastectomy (unilateral or bilateral) over BCT. One National Cancer Data Base study showed that, between 2003–2010, lumpectomy in patients ≤45 years old decreased from 61.4% to 49.4%, and bilateral mastectomies increased from 9.3% to 24.1%, while unilateral mastectomies remained relatively stable from 29.3% to 26.4%. 15 This trend is in contrast to treatment trend in older patients found in the same study, along with other studies, where BCT rate is relatively stable or increased over time. 16
Barrier to receiving radiation may be a factor why young women are choosing to undergo mastectomy instead of BCT. The current commonly used conventionally fractionated breast radiation after breast conserving surgery in young patients is given daily, 5 days a week, for over 6–7 weeks, which can cause significant disruptions in daily activities. A study of women aged 20–64 years who had breast conserving surgery showed that 14% did not receive adjuvant radiation. 17 An important factor attributed to this non-compliance rate was having at least one child younger than 7, and this association was driven primarily by young women aged 20–50.
Similarly, fear of the need for repeated surgery may also play a role in patient decision. In a survey of 1984 women aged 20–79 (mean 58.6), where 75.4% (1468) of the patients initially had lumpectomy, 37.9% (525) required additional operation, including 26% (358) re-excision and 11.9% (167) mastectomy. 18 It should be noted, however, that the survey response rate for this study was 73.1% and the actual rate of additional surgery after lumpectomy may be different due to response bias.
In addition to logistic barriers, reasons often cited for choosing mastectomy instead of BCT include patients’ desire to lower the chance of another breast cancer, “peace of mind,” and perceived chance for improved survival. 15 Younger patients are more likely to be strongly involved in their healthcare decision making, which, along with fear of disease recurrence, may cause them to choose mastectomy, which is perceived as more aggressive type of treatment. 19 In a survey of 460 patients who underwent mastectomy, 288 (62.6%) did so because of surgeon recommendation while 172 (37.4%) chose so without surgeon recommendation specifically for mastectomy, indicating that patient belief and preference are strong factors in treatment decision making in addition to physician recommendations. 18 Unfortunately, the perceived future breast cancer risks are often overestimated by the patients, who may be electing for a more radical surgery unnecessarily. 15, 20
An example of patients pursuing the most “aggressive” local treatment and their outcome is well documented by a cohort study using the California Cancer Registry. 21 Among 189,734 stage 0-III breast cancer patients, the rate of bilateral mastectomies increased over six times from 2% in 1998 to 12% in 2011. This rate of increase was the greatest in patients younger than 40 years, from 3.6% in 1998 to 33% in 2011. In the same time period, BCT in age <40 decreased from around 45% to 28% (estimated from graph). Yet, this drastic increase in more aggressive local surgery did not correlate with improved survival of the patients. On multiple regression analysis of the overall sample, compared with BCT, bilateral mastectomy had similar mortality (HR, 1.02 [95% CI, 0.94–1.11]), whereas unilateral mastectomy was associated with higher mortality (HR, 1.35 [95% CI, 1.32–1.39]). After adjusting for risk factors and treatment status in propensity weighted analysis, unilateral mastectomy was still associated with the highest 10-year mortality of 20.1% (95% CI, 19.9% – 20.4%) compared to bilateral mastectomy (18.8% (95% CI, 18.6%–19.0%)), with BCT having the most favorable outcome (16.8% (95% CI, 16.6%–17.1%)). While there was no separate analysis for the younger than 40 subset, the results do not seem to justify the trend of favoring mastectomy (unilateral or bilateral) over BCT.
This trend favoring mastectomy in younger patients is concerning because not only is there no survival benefit shown over BCT, mastectomy is associated with overall poorer quality of life. While BCT’s benefits in physical function, sexual function, and body image are immediately apparent after treatment, improved social function and global quality of life may only become apparent at follow up of 5-years or more, which is especially important in young women with early stage breast cancer, majority of whom are expected to live for a long time after diagnosis.5,7
Patients with breast reconstruction procedure after mastectomy can potentially improve some of the issues, but can still have inferior quality of life compared to BCT. Rosenberg et al. have found in a survey study for women under age of 40 undergoing contralateral prophylactic mastectomy (94% had reconstruction) that 42% of patients felt worse than expected sense of sexuality and 31% had worse than expected “self-consciousness about appearance.” 20 In reality, studies have shown that majority of mastectomy patients do not even undergo reconstruction. In the California Cancer Registry study, only 47% of the patients undergoing bilateral and 14% undergoing unilateral mastectomies had breast reconstruction, although the rate of reconstruction in the age <40 subset is unreported. 21 In another survey study of Japanese women aged 45 years and younger, breast reconstruction rate was 36.7%. 22 The most common reason for not undergoing breast reconstruction was the fear of cancer relapse. Other factors mentioned were to avoid additional distress on the body from surgery, financial reasons, and belief that breast reconstruction is unnecessary. Those who underwent breast reconstruction were more likely to rate themselves as more attractive. However, regardless of having undergone breast reconstruction or not, women who reported higher levels of self-consciousness over the treated areas showed more restrictions on activity and higher chances of a decline in psychological well-being.
Because younger patients are only represented in a marginal proportion of patients in the available randomized data, and a new randomized controlled trial comparing BCT and mastectomy dedicated to the younger population is unlikely to happen in the future, our current study attempted to add to the existing literature by examining the SEER national database. Some of the strengths of a SEER study include large sample size and the ability to understand the actual patient outcome in the United States outside of clinical trials, once a treatment has been widely adopted.
Our study showed that overall, young women with Stage I and II breast cancer between 1998– 2003 younger age (20–34), higher stage, and ER− disease were associated with poorer outcomes, regardless of the treatment received, consistent with risk factors reported in the literature. 9, 10, 23
The BCT group in the sample tended to have more favorable risk factors, such as more T1 disease and N0 disease, compared to the mastectomy group, which is consistent with the observation that those with poor prognostic factors are more likely to pursue unilateral or bilateral mastectomies. 15
Upon separation by stage groups, it was observed that BCT and mastectomy yielded equivalent OS and BCSS, supporting the notion that while stage is prognostic, it is not a predictive factor for mastectomy being superior to BCT in young women with early stage breast cancer. 9,10
Age, even in women younger than 40, has been known to play a role in the prognosis of breast cancer, and our study confirmed this by showing lower OS and BCSS in patients younger than 35 compared to those who were 35–39 at diagnosis. However, when controlled for stage, those who received BCT and mastectomy in either age group once again showed equivalent outcome, suggesting that age is also prognostic but not predictive for either treatment modality.
The one exception found was in stage IIB patients aged between 20–34, where those who received BCT had significantly better BCSS and OS. Subsequent analysis of this subgroup in terms of stage, tumor size, lymph node status, ER status did not show any significant difference compared to others. One possible reason for the difference in outcome is patient compliance to treatment. Perhaps many of these patients in the mastectomy only group were recommended post-mastectomy radiation but did not comply for various reasons, which is known to result in inferior outcome, but such speculations cannot be confirmed in this study. 24
Accessibility and time commitments can present as major barrier to receiving radiation. 17 Unfortunately, due to lack of sufficient number of young patients in available data, current American Society for Radiation Oncology guidelines do not recommend shorter course, lower dose radiation modalities such as hypofractionated whole breast irradiation or accelerated partial breast irradiation in these patients. 25,26 Such reasons may explain why in our study, there were 14% of the patients who received lumpectomy but did not undergo radiation.
There are several important limitations to the study that must be acknowledged in interpreting the results. Owing to the nature of SEER database, only vital status and cause of death could be gathered, and the true incidence of breast cancer recurrence is unknown in either group.
Another limitation of SEER is the lack of information regarding chemotherapy and hormonal therapies, which, if differed between the groups, may confound the study outcome. Fortunately, given the similar distribution of ER+/− patients among different treatment groups, the use of systemic therapy was likely similar after separating by stage groups with similar distribution of T and N stages. In addition, potential coding errors in the database for both disease characteristics and treatment types may also affect the findings. However, these coding errors are expected to be uncommon and distributed throughout the entire sample.
Even though our study attempted to control for difference in disease risk factors between the treatment groups by using multivariate regression analysis and sorting patients by stage, differences in the distribution of pathological details such as grade, presence of lymphovascular invasion, extracapsular extension, triple negative breast cancer, and number and size of nodes positive can still confound the results. The fact that the BCT group overall had smaller tumor with fewer nodes positive suggests that even within the stage groups there may have been differences, which could have also explained the outcome discrepancy in stage IIB age 20–34 subgroup. In addition, potential selection bias and difference in underlying medical co-morbidities can still exist that may confound the results. One such important factor not available in SEER is BRCA status. Regarding other medical comorbidities, it is encouraging that we did not find any difference in non-BCSS in any of the analyses we conducted, suggesting no baseline difference in co-morbidities among the groups studied. Owing to the potential confounding factors that cannot be completely controlled, the study results are no substitution for randomized controlled trials, and must be interpreted with caution.
In addition to survival outcomes, another important issue in addressing optimal management of breast cancer in young women involves the long term toxicity from treatment, particularly from radiation, such as secondary malignancy and cardiac risk. There is convincing evidence in younger patients (<30) who received radiation to the chest for lymphoma that there is a significant lifetime risk of developing secondary malignancies. 27 Only 8% of our study sample fell in this age group, and clinicians must appropriately counsel these patients regarding the risks of secondary cancer when recommending BCT. While there was no difference in secondary cancer (lung or other) or cardiac mortality between the BCT and mastectomy groups, the current study was not intended to study this effect because longer follow up period would be necessary.28, 29, 30, 31
Prospective randomized trials comparing BCT and mastectomy in young patients, if feasible, would be important in confirming the findings from our study, as well as in assessing potential long term toxicities associated with different treatments. Future short course radiation trials including more young patients and those with higher risk disease are also important, because if these more convenient courses of radiation are shown to be equivalent, it would eliminate some of the barriers in getting appropriate therapy.
Conclusion
Our study, consistent with many others reported in the literature, suggests that while young age may be a poor prognostic factor for breast cancer, there is no evidence that these patients have better outcome with mastectomy over BCT, and continues to support the use of BCT. Aside from disease specific mortality, however, there are many other factors (cosmesis, quality of life, long term toxicities etc.) that must be considered in deciding between BCT and mastectomy. If there are reasons patients should not or cannot undergo radiation, patients should undergo mastectomy, as radiation has been shown to be an integral part of BCT. Clinicians must continue to educate the patients about the risks and benefits of BCT and provide evidence-based recommendations so that they can make a truly informed decision about their treatment.
Clinical Practice Points.
While younger age is a known poor prognostic factor for breast cancer (BC), available evidence shows that there is no survival benefit in mastectomies over breast conserving therapy (BCT), consisting of lumpectomy and adjuvant radiation. In addition, mastectomies have been associated with higher rate of complications, side effects, poorer cosmetic results, and overall poorer quality of life compared to BCT. Despite the lack of clear evidence to suggest its benefit, there has been a national trend in the increased use of mastectomy over BCT in young women (younger than 40) with BC. Some potential reasons for this trend include the patients’ fear of cancer recurrence, perception that mastectomy is a more aggressive local therapy and therefore more appropriate for BC in young patients, which is known to be more aggressive, and their inability to get adjuvant radiation therapy etc. The current study reviewed the national Surveillance, Epidemiology, and End Results (SEER) database and compared the overall and BC-specific survivals of women aged 20–40 with stage I–II BC treated with BCT or mastectomy alone between 1998–2003. After controlling for stage, there was no statistically significant difference in the outcome between the two treatment groups, thus further strengthening the current available evidence that mastectomy does not result in better outcome compared to BCT. Clinicians must continue to educate the patients about the risks and benefits of BCT and provide evidence-based recommendations so that they can make a truly informed decision about their treatment.
Acknowledgments
Funding Source: Dr. Paul Christos was partially supported by the following grant: Clinical Translational Science Center (UL1-TR000457-06).
References
- 1.Fisher B, Anderson S, Bryant J, et al. Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy plus Irradiation for the Treatment of Invasive Breast Cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 2.(EBCTCG) EBCTCG. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.(EBCTCG) EBCTCG. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378:1707–16. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 5.Curran D, van Dongen J, Aaronson N, et al. Quality of life of early-stage breast cancer patients treated with radical mastectomy or breast-conserving procedures: results of EORTC Trial 10801. The European Organization for Research and Treatment of Cancer (EORTC), Breast Cancer Co-operative Group. Eur J Cancer. 1998;34(3):307–14. doi: 10.1016/s0959-8049(97)00312-2. [DOI] [PubMed] [Google Scholar]
- 6.NIH Consensus Conference. Treatment of Early-Stage Breast Cancer. JAMA. 1991;265(3):391–5. [PubMed] [Google Scholar]
- 7.Arndt V, Stegmaier C, Ziegler H, Brenner H. Quality of life over 5 years in women with breast cancer after breast-conserving therapy versus astectomy: a population-based study. J Cancer Res Clin Oncol. 2008;134:1311–1318. doi: 10.1007/s00432-008-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao JQ, Olson RA, Tyldesley SK. Comparison of recurrence and survival rates after breast-conserving therapy and mastectomy in young women with breast cancer. Curr Oncol. 2013;20:e593–601. doi: 10.3747/co.20.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bock GH, van der Hage JA, Putter H, Bonnema J, Bartelink H, van de Velde CJ. Isolated loco-regional recurrence of breast cancer is more common in young patients and following breast. Eur J Ca. 2006;42:351–356. doi: 10.1016/j.ejca.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Kroman N, Holtveg H, Wohlfahrt J, et al. Effect of Breast-Conserving Therapy versus Radical Mastectomy on Prognosis for Young Women with Breast Carcinoma. Cancer. 2004 Feb;100(4):688–693. doi: 10.1002/cncr.20022. [DOI] [PubMed] [Google Scholar]
- 11.Thangjam S, Laishram RS, Debnath K. Breast carcinoma in young females below the age of 40 years: A histopathological perspective. South Asian Journal of Cancer. 2014 Jun;3(2):97–100. doi: 10.4103/2278-330X.130441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofvind S, Lee CL, Elmore JG. Stage-specific breast cancer incidence rates among participants and non-participants of a population-based mammographic screening program. Breast Cancer Res Treat. 2012;135:291–299. doi: 10.1007/s10549-012-2162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao JQ, Truone PT, Olivotto IA, et al. Should Women Younger Than 40 Years of Age With Invasive Breast Cancer Have a Mastectomy?: 15-Year Outcomes in a Population-Based Cohort. Int J Radiation Oncol Biol Phys. 2014;90(3):509–517. doi: 10.1016/j.ijrobp.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 14.van der Sangen MJ, van de Wiel FM, Poortmans PM, et al. Are breast conservation and mastectomy equally effective in the treatment of young women with early breast cancer? Long-term results of a population-based cohort of 1,451 patients aged. Breast Cancer Res Treat. 2011;127:207–215. doi: 10.1007/s10549-010-1110-x. [DOI] [PubMed] [Google Scholar]
- 15.Pesce CE, Liederbach E, Czechura T, Winchester DJ, Yao K. Changing Surgical Trends in Young Patients with Early Stage Breast Cancer, 2003 to 2010: A Report from the National Cancer Data Base. J Am Coll Surg. 2014 Jul;219(1):19–28. doi: 10.1016/j.jamcollsurg.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 16.Escriba JM, Pareja L, Esteban L, et al. Trends in the surgical procedures of women with incident breast cancer in Catalonia, Spain, over a 7-year period (2005–2011) BMC Research Notes. 2014;7:587. doi: 10.1186/1756-0500-7-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan IW, Smith BD, Shih YCT. Factors Contributing to Underuse of Radiation Among Younger Women With Breast Cancer. J Natl Cancer Inst. 2014;106(1):djt340. doi: 10.1093/jnci/djt340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrow M, Jagsi R, Alderman AK, et al. Surgeon Recommendations and Receipt of Mastectomy for Treatment of Breast Cancer. JAMA. 2009;302(14):1551–6. doi: 10.1001/jama.2009.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz SJ, Lantz PM, Janz NK, et al. Patient Involvement in Surgery Treatment Decisions for Breast Cancer. J Clin Onc. 2005;23(24):5526–33. doi: 10.1200/JCO.2005.06.217. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg SM, Tracy MS, Meyer ME, et al. Perceptions, Knowledge, and Satisfaction With Contralateral Prophylactic Mastectomy Among Young Women With Breast Cancer: A Cross-sectional Survey. Ann Intern Med. 2013 Sep;159(6):373–381. doi: 10.7326/0003-4819-159-6-201309170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurian AW, Lichtensztajn DY, Keegan TH, Nelson DO, Clarke CA, Gomez SL. Use of and Mortality After Bilateral Mastectomy Compared With Other Surgical Treatments for Breast Cancer in California, 1998–2011. JAMA. 2014;312(9):902–914. doi: 10.1001/jama.2014.10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nozawa K, Ichimura M, Oshima A, et al. The present state and perception of young women with breast cancer towards breast reconstructive surgery. Int J Clin Oncol. 2014 doi: 10.1007/s10147-014-0716-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Pesce C, Liederbach E, Wang C, Lapin B, Winchester DJ, Yao K. Contralateral Prophylactic Mastectomy Provides No Survival Benefit in Young Women with Estrogen Receptor-Negative Breast Cancer. Ann Surg Oncol. 2014;21:3231–3239. doi: 10.1245/s10434-014-3956-3. [DOI] [PubMed] [Google Scholar]
- 24.EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;363:2127–35. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith BD, Bentzen SM, Correa CR, Hahn CA. Fractionation for whole breast irradiation: an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiation Oncology Biol Phys. 2011;81(1):59–68. doi: 10.1016/j.ijrobp.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 26.Smith BD, Arthur DW, Buchholz TA, Haffty BG, Hahn CA. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO) Int J Radiation Oncology Biol Phys. 74(4):987–1001. doi: 10.1016/j.ijrobp.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 27.Alm El-Din MA, El-Badawy SA, Taghian AG. Breast Cancer After Treatment of Hodgkin’s Lymphoma: General Review. Int J Radiation Oncology Biol Phys. 2008;72(5):1291–7. doi: 10.1016/j.ijrobp.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 28.Ye JC, Yan W, Christos P, Nori D, Chao KC, Ravi A. Second Cancer, Breast Cancer, and Cardiac Mortality in Stage T1aN0 Breast Cancer Patients With or Without External Beam Radiation Therapy: A National Registry Study. Clin Breast Cancer. 2014 Aug; doi: 10.1016/j.clbc.2014.07.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Darby S, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300 000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–65. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 30.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 31.Henson KE, McGale P, Taylor C, Darby SC. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. British J of Cancer. 2013;108:179–82. doi: 10.1038/bjc.2012.575. [DOI] [PMC free article] [PubMed] [Google Scholar]