Abstract
NGF is a trophic and survival factor for cholinergic neurons, and it induces the expression of several genes that are essential for synthesis and storage of acetylcholine, specifically choline acetyltransferase (ChAT), vesicular acetylcholine transporter (VAChT) and CHT (choline transporter). We have found previously that the PI3K pathway, but not the MEK/MAPK pathway, is the mediator of NGF-induced cholinergic differentiation. Here we demonstrate, in the rat pheochromocytoma cell line PC12 and in primary mouse neuronal cultures, that NGF-evoked upregulation of these three cholinergic-specific genes is mediated by the anti-apoptotic signaling molecule Akt/protein kinase B (PKB). Inhibition of Akt activation by the pharmacological inhibitor HIMO, or by a peptide fragment derived from the proto-oncogene TCL1, eliminated NGF-stimulated increases in cholinergic gene expression, as demonstrated by RT-PCR and reporter gene assays. Moreover, treatment with HIMO reversed NGF-evoked increases in ChAT activity and ACh production. In co-transfection assays with the reporter construct, a dominant-negative Akt plasmid and Akt1-specific siRNA also attenuated NGF-induced cholinergic promoter activity. Our data indicate that, in addition to its well-described role in promoting neuronal survival, Akt can also mediate signals necessary for neurochemical differentiation.
Keywords: acetylcholine, choline acetyltransferase, choline transporter, protein kinase B, PC12 cells, septum
INTRODUCTION
NGF-TrkA signaling is important for the proper development and functioning of the cholinergic system (Yuen et al. 1996). In addition to its role as a trophic and survival factor for cholinergic neurons, NGF regulates the expression of several genes that are essential for synthesis and storage of acetylcholine (ACh), specifically choline transporter (CHT), choline acetyltransferase (ChAT), and the vesicular acetylcholine transporter (VAChT) (Li et al. 1995; Tian et al. 1996; Pongrac and Rylett 1998; Berse et al. 1999; Oosawa et al. 1999; Szutowicz et al. 2004; Berse et al. 2005). We have been studying the intracellular signaling pathways that mediate these biological actions of NGF. NGF stimulates the MEK/MAPK pathway and this signaling is important for cholinergic function in vivo, as the suppression of ERK-mediated NGF signaling correlates with cholinergic deficits in aging (Williams et al. 2006; Williams et al. 2007). However, we demonstrated that induction of cholinergic gene expression occurs when the MEK/MAPK pathway is blocked (Madziar et al. 2005). In contrast, pharmacological inhibition of phosphatidylinositol 3’-kinase (PI3K) prevents NGF-induced cholinergic promoter activity, ChAT, VAChT and CHT mRNA accumulation, and ACh production (Berse et al. 2005; Madziar et al. 2005). Thus, the PI3K pathway is required for the expression of essential cholinergic markers. However, the downstream effectors of PI3K involved in this process remain undetermined.
PI3K can phosphorylate both lipids and proteins, but the majority of research focuses on its biological activity as a lipid kinase (Cantley 2002). The phosphorylated lipid products of PI3K function as docking sites to recruit to the membrane many proteins containing pleckstrin homology (PH) domains, including Akt/protein kinase B (Akt/PKB) and phosphoinositide-dependent kinase 1 (PDK1) (reviewed in Woodgett 2005; Franke 2008). Following binding via its PH domain to membrane PIP3, Akt becomes phosphorylated on Thr308 by the membrane-bound PDK1 and on Ser473 by the SIN1-rictor-mTOR complex (Hresko and Mueckler 2005; Sarbassov et al. 2005; Jacinto et al. 2006). Upon activation, Akt phosphorylates numerous downstream targets, both in the cytoplasm and in the nucleus. Through these targets, Akt participates in the regulation of cell survival/apoptosis, cell cycle progression and glucose metabolism (reviewed in Brazil et al. 2004; Manning and Cantley 2007). In neurons, the PI3K/Akt pathway is the major signaling route mediating neuronal survival either by inducing the transcription of survival genes or by inhibiting members of the apoptotic machinery (Kaplan and Miller 2000; Brunet et al. 2001; Fukunaga et al. 2005). Phosphorylation of various neuron-specific targets allows Akt to control different aspects of neuronal function, e.g. GABA receptor stabilization and memory consolidation, dopamine receptor-mediated responses, neuroprotection and neurodegeneration (Brazil et al. 2004; Beaulieu et al. 2007).
In the present study, we explore the possible role of Akt/PKB in regulating cholinergic gene expression in the pheochromocytoma cell line PC12 and in primary septal neurons. By applying several methods of inhibiting Akt activity (a pharmacological inhibitor, an engineered derivative of a regulatory peptide, a dominant-negative construct and siRNA), we demonstrate that blocking Akt eliminates NGF-evoked cholinergic promoter activity, cholinergic-specific mRNA accumulation, and ACh production. We conclude that Akt plays an essential role in the stimulation of cholinergic gene expression by NGF.
EXPERIMENTAL PROCEDURES
Materials
All reagents were purchased from Sigma-Aldrich, unless otherwise specified.
Cell culture and treatments
Pheochromocytoma (PC12) cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco-Invitrogen) containing 10% horse serum (HS) and 5% fetal bovine serum (FBS), at 37 °C in 5% CO2. One day prior to treatment, the cells were trypsinized and an appropriate number of cells was plated on 35 mm tissue culture dishes so that 20 – 30% confluence was reached on the next day. The treatments with 100 ng/ml murine NGF 2.5S (Alomone Labs) were conducted in the presence of serum. For inhibition of Akt/PKB activity, cultures were pre-treated with 10 µM HIMO or 50 µM TAT-Akt-in (both from Calbiochem) for 12 h, before addition of NGF. For prolonged treatments, a fresh aliquot of the appropriate inhibitor was added to the culture after 36 h, followed by addition of NGF, without changing the medium. After 72 h of treatment, the cells were photographed and/or collected for RNA preparation. Cell morphology images were taken using a Nikon phase contrast microscope and a SPOT RTKE camera (Diagnostic Instruments, Inc).
Primary septal cultures were prepared from embryonic day 18 (E18) CD-1 mice (Charles River Laboratories) as described previously (Madziar et al. 2005). Animal experimental protocols were approved by the Boston University School of Medicine Institutional Animal Care and Use Committee and met NIH and USDA guidelines. The cells were plated on poly-L-lysine/laminin-coated dishes in DMEM containing 10% heat-inactivated FBS. After 24 h in culture, the medium was changed to Neurobasal Medium containing B27 supplement (Gibco-Invitrogen). Approximately 12 hours later, 5 µM cytosine arabinoside (AraC) was added to the dishes in order to prevent the growth of non-neuronal cells. Cell treatments with the PI3K inhibitor LY294002 or the Akt inhibitor HIMO and/or NGF were started 12–24 h later (i.e. 48–60 h after plating). The cells were pre-treated with 5 µM LY294002 or 10 µM HIMO for 1 h or 12 h, respectively, before addition of 100 ng/ml NGF. The inhibitors and NGF were supplemented at 24 h intervals without medium change. The cells were collected after 72 hours of treatment.
Biochemical measurements
ChAT activity and intracellular ACh measurements were conducted as described previously (Madziar et al. 2005). Protein content was determined using the bicinchoninic acid assay (Smith et al. 1985) with bovine serum albumin as the standard.
Western blotting
Whole-cell extracts were prepared and Western blotting was conducted as described previously (Madziar et al. 2005). All primary antibodies were from Cell Signaling Technology and were used at a 1:2000 to 1:5000 dilution. Reactive bands were visualized by chemiluminescence using SuperSignal West Femto substrate (Pierce) and Kodak Image Station 440 with Kodak 1D Image Analysis Software.
RT/PCR
Total RNA was isolated from PC12 cells and primary septal cultures by guanidium isothiocyanate lysis followed by binding to silica-gel-based membrane using the RNeasy Mini Kit (Qiagen). To exclude DNA contamination, an optional on-column DNase digest was performed for most of the samples using the RNase-free DNase Set (Qiagen). Alternatively, a DNase digest was done on already purified RNA preparations using the TURBO DNA-free™ kit (Ambion, Austin, TX, USA) according to the manufacturer’s instructions. Reverse transcription/polymerase chain reactions (RT/PCR) were conducted with the SuperScript One-Step RT-PCR kit with Platinum Taq (Invitrogen), according to the manufacturer’s instructions. Briefly, reaction mixtures, containing 10–100 ng of total RNA and both reverse transcriptase and Platinum Taq Polymerase, were incubated at 48 °C for 45 min for first-strand DNA synthesis and then subjected to between 25 to 40 cycles of PCR. The annealing temperature was 57 °C for ChAT and 58 °C for VAChT and CHT. At the end of the last cycle, the samples were incubated for an additional 7 min. at 72 °C (the optimal elongation temperature) to ensure the completion of synthesis. The reaction products were separated on 1.5% agarose or 10% acrylamide gels, stained with ethidium bromide, and visualized using Kodak Image Station 440 with Kodak 1D Image Analysis software.
The following primers were used:
| for VAChT: | forward: 5'-AGC GGG CCT TTC ATT GAT CG-3' |
| reverse: 5'-GGC GCA CGT CCA CCA GAA AGG-3' | |
| for ChAT: | forward: 5’-CGG GAT CCT GCC TCA TCT CTG GTG T-3’ |
| reverse: 5’-GGC GGA ATT CAA TCA CAA CAT C-3’ | |
| for CHT: | forward: 5'-CGG GGA ACC ATT GAA TTC GTT GAA GTC TAC 3' |
| reverse: 5'-GGG GCA AGC TTC CAC TTT CAA ATA GAT ACT 3' |
Mouse β-actin control primers were obtained from Clontech.
Forward and reverse primers for ChAT and CHT were derived from different exons in order to avoid amplification of genomic DNA. Since the VAChT gene is devoid of introns, this strategy could not be employed, and therefore control amplification reactions were conducted with VAChT primers, containing Taq polymerase, but no reverse transcriptase. If DNA amplification was detected, the samples were purified by RNase-free DNase digestion using the TURBO DNA-free kit (Ambion) followed by purification on a Qiagen column in order to remove DNase from the samples. Alternatively, RNA preparations were digested during purification on silica-gel membrane with the RNase-free DNase Set (Qiagen). RT/PCR with β-actin primers was performed after DNase digestion to ensure equal loading and RNA integrity.
Real-time PCR was performed with mouse ChAT primers designed by Applied Biosystems. The primers are located in exons 15–16 of the mouse ChAT gene and amplify a fragment 70-bp in length. Total RNA was converted into cDNA with the use of the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Aliquots of the cDNA were used for PCR reactions with mouse ChAT primers and FAM-labeled TaqMan probes in the 7500 Real-Time PCR System and analyzed with SDS software (both from Applied Biosystems). The results were normalized to β-actin amplification.
Transfection and reporter gene analysis
PC12 cells were transfected with the cholinergic promoter-luciferase reporter construct pGL3Hind4.2 (Madziar et al. 2005) using LipofectAMINE 2000 (Invitrogen) in serum-free DMEM. After 4 h incubation with LipofectAMINE-DNA complexes, the medium was changed to DMEM with 10% HS, and 5% FBS with or without Akt inhibitors (10 µM HIMO or 50 µM TAT-Akt-in) followed by 100 ng/ml NGF. Sixty to 72 hours post-transfection, the cells were lysed, and luciferase activity was measured with the Luciferase Assay System (Promega), according to the manufacturer's protocol, adapted for 96-well plates as described previously (Madziar et al. 2005). In co-transfection experiments, pGL3Hind4.2 was mixed with variable amounts of a dominant-negative (DN) Akt construct or an empty expression vector. For siRNA treatments, the reporter plasmid was co-transfected with annealed Akt1 or control siRNA duplexes (Dharmacon) using the general DNA transfection protocol.
Statistical Analysis
One-way analysis of variance (ANOVA) and Dunnett’s post hoc comparison test (Dunnett 1955) were performed using the data analysis function of KaleidaGraph v. 3.6 (Synergy Software, Reading, PA).
RESULTS
1. Akt inhibitor HIMO eliminates the NGF effect on ChAT activity and ACh production
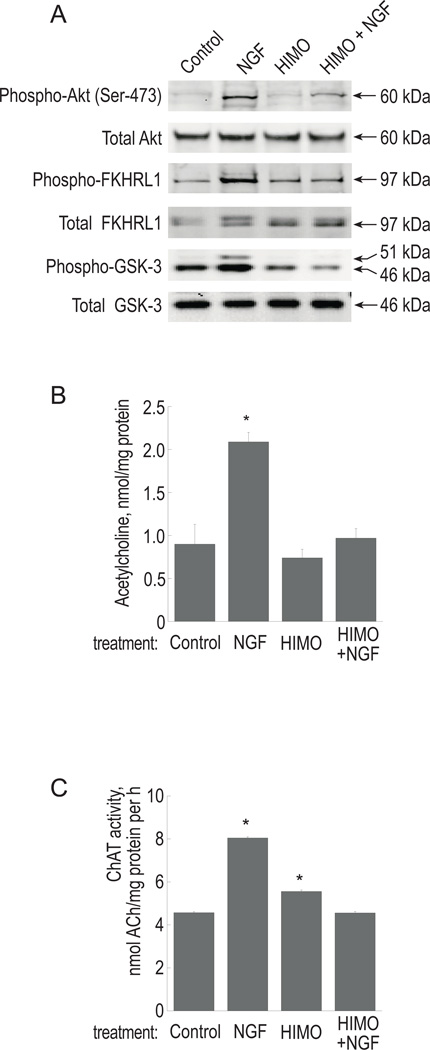
To evaluate the involvement of Akt in cholinergic function, we used 1L-6-hydroxymethyl-chiro-inositol 2(R)-2-O-methyl-3-O-octadecylcarbonate (HIMO), a 3’-modified phosphatidylinositol analogue shown to be an effective Akt inhibitor (Hu et al. 2000; Kozikowski et al. 2003). HIMO has a marked preference for Akt (IC50 of 5.0 µM) over PI3K (IC50 of 83.0 µM). PC12 cells were pre-treated with 10 µM HIMO for 12 hours, and then stimulated with 100 ng/ml NGF. The inhibition of Akt activity was demonstrated by Western blotting with phospho-specific antibodies against Akt and its downstream effectors GSK-3 and FKHRL1. HIMO markedly reduced phosphorylation of Akt and GSK-3 in response to NGF and completely prevented the NGF effect on FKHRL1 phosphorylation (Figure 1A). In order to establish whether NGF-stimulated ACh production was affected in the presence of Akt inhibitors, the cells were treated with HIMO and NGF as described above and ChAT activity and intracellular ACh content were measured after 3 days. NGF increased ChAT activity and the accumulation of intracellular ACh by 1.8 and 2.3 fold, respectively (Figure 1B–C). Pretreatment with HIMO completely eliminated those increases. Treatment with HIMO alone did not affect intracellular ACh, while in some experiments, the cells treated with HIMO alone showed a small, but statistically significant, increase in ChAT activity as compared to control (by approximately 15%). However, in the presence of the combination of HIMO and NGF, ChAT activity was at control levels. Thus, inhibition of Akt prevents NGF from upregulating ACh production in PC12 cells.
Figure 1. Effect of the Akt inhibitor HIMO on protein phosphorylation, ChAT activity and ACh production.
PC12 cells were treated with 10 µM HIMO for 12 h prior to stimulation with 100 ng/ml NGF as described in Experimental Procedures.
(A) Following 10 min stimulation with NGF, cell extracts were analyzed by Western blotting with phospho-specific antibodies as indicated. Antibodies recognizing total proteins, regardless of phosphorylation, served as loading control.
(B) Following 72 h of NGF treatment, intracellular ACh was measured as described in Experimental Procedures. The results are presented as means ± S.E.M. of three independent cultures. (*) significantly different from control (p<0.01, ANOVA, followed by Dunnett’s test). (C) ChAT activity was measured in cell extracts as described in Experimental Procedures. ANOVA followed by Dunnett’s test determined that the NGF-treated (p<0.001) and HIMO-treated (p<0.01) groups are significantly different from controls.
2. Two distinct Akt inhibitors prevent NGF-mediated stimulation of cholinergic mRNA accumulation
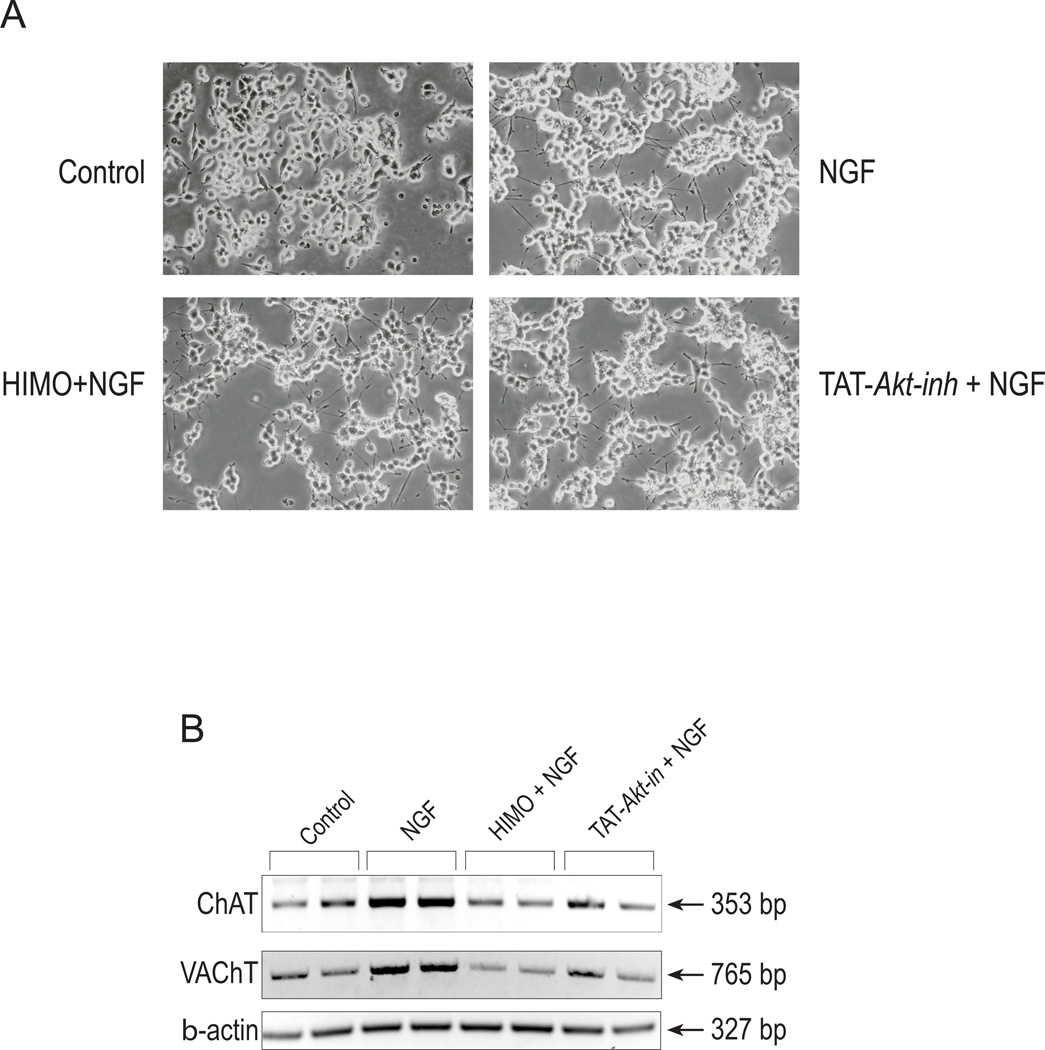
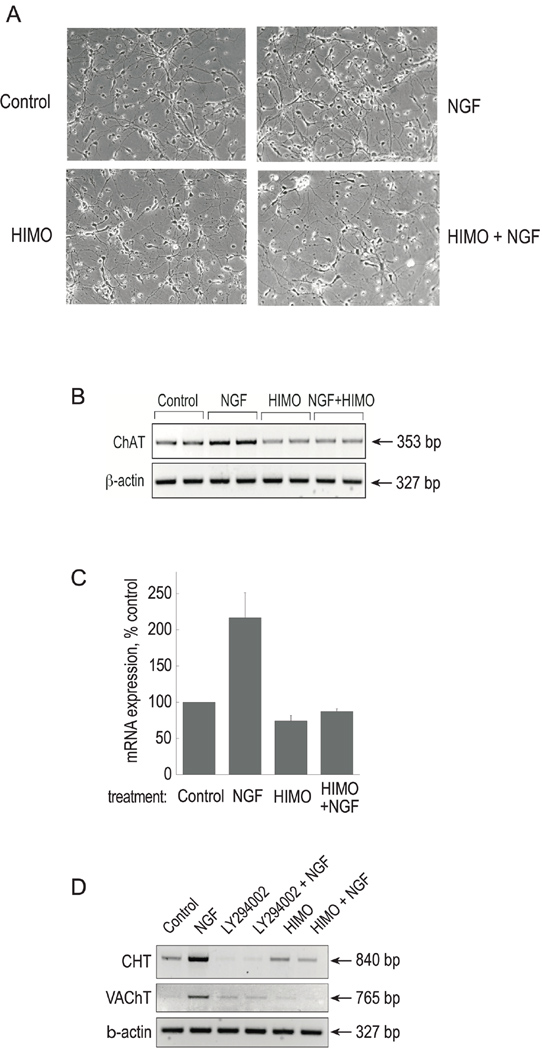
We examined the effect of Akt inhibition on the mRNA accumulation of the cholinergic-specific genes ChAT and VAChT. In this approach, in addition to HIMO, we employed the fusion peptide TAT-Akt-in, to inhibit Akt phosphorylation and activation in PC12 cells. Based on the proto-oncogene TCL1 domain that interacts with Akt (Kunstle et al. 2002), Hiromura et al (2004) identified and characterized the peptide sequence H-Ala-Val-Thr-Asp-His-Pro-Asp-Arg-Leu-Trp-Ala-Trp-Glu-Lys-Phe-OH, that encompassed the A strand of TCL1, interacted with Akt, and inhibited Akt kinase activity. This peptide, referred to as Akt-in, binds to the PH domain of Akt (Kd ~18 µM) and interferes with the Akt-PtdIns interaction, hindering membrane translocation from the cytosol. It has been shown to inhibit selectively the phosphorylation of Akt in response to PDGF with no inhibition towards PKA, PKC, PDK1, p42/44 MAPK or p38 MAPK (Hiromura et al. 2004). We used TAT-Akt-in, a fusion of Akt-in with the TAT protein transduction domain, which renders it cell-permeable. PC12 cells were pre-treated with HIMO or TAT-Akt-in and then stimulated with NGF. Phase-contrast microscope pictures taken after 3 days of treatment demonstrate that, as expected, NGF caused neurite extension in PC12 cells. Neither HIMO nor TAT-Akt-in, administered alone or in combination with NGF, affected cell morphology, attachment to the substratum or neurite formation evoked by NGF (Figure 2A and data not shown). Approximately equal numbers of cells were observed in the presence and absence of the inhibitors. Also, Trypan Blue staining showed no significant difference between the groups in the number of dead cells (data not shown). Thus, treatment of PC12 cells with these Akt inhibitors does not affect cell viability, and some aspects of NGF signaling are intact in cells treated with the inhibitors.
Figure 2. Akt inhibitors eliminate NGF-evoked increases in cholinergic mRNA accumulation, but not neurite formation.
(A) PC12 cells were treated with 10 µM HIMO or 50 µM TAT-Akt-in for 12 h prior to stimulation with 100 ng/ml NGF as described in Experimental Procedures. Phase-contrast photographs were taken after 72 h of treatment. Objective lens 20 ×.
(B) The cells were cultured as in (A). Total RNA was prepared and RT/PCR for ChAT, VAChT and β-actin was carried out as described in Experimental Procedures. Each treatment was performed in duplicate, hence it is represented by two lanes. The results are representative of three independent experiments.
RT/PCR was used to evaluate ChAT and VAChT expression in treated cells. The two genes are contained within a single genomic locus, termed the cholinergic locus, and they share regulatory transcriptional elements. However, through differential promoter use and alternative splicing, the cholinergic locus yields discrete ChAT- and VAChT-specific mRNAs. Thus, we were able to use gene-specific PCR primer pairs to evaluate ChAT and VAChT expression separately. RT/PCR carried out for 36 cycles on total RNA preparations and subsequent analysis with the Kodak Image Station revealed that, in agreement with previously published results, NGF increased both ChAT and VAChT mRNA accumulation. In contrast, in the presence of HIMO or TAT-Akt-in, NGF-stimulated increases in ChAT and VAChT mRNA accumulation were eliminated (Figure 2B). For VAChT primers, derived from a single exon, parallel reactions with Taq polymerase in the absence of RT demonstrated no genomic DNA contamination of the RNA samples (data not shown), indicating that the PCR products are the result of RNA amplification. Expression of β-actin in the RNA prepared from each sample was used as loading control. Akt inhibition by 10 µM HIMO for three days, in the absence of NGF, did not change basal ChAT or VAChT mRNA expression (data not shown). RT/PCR experiments conducted with increasing numbers of amplification cycles (up to 40) demonstrated that the PCR reactions were not saturated after 36 cycles (data not shown). We conclude that inhibition of Akt eliminates NGF-evoked upregulation of ChAT and VAChT gene expression in PC12 cells.
3. Akt inhibition interferes with cholinergic promoter activity in reporter gene assays
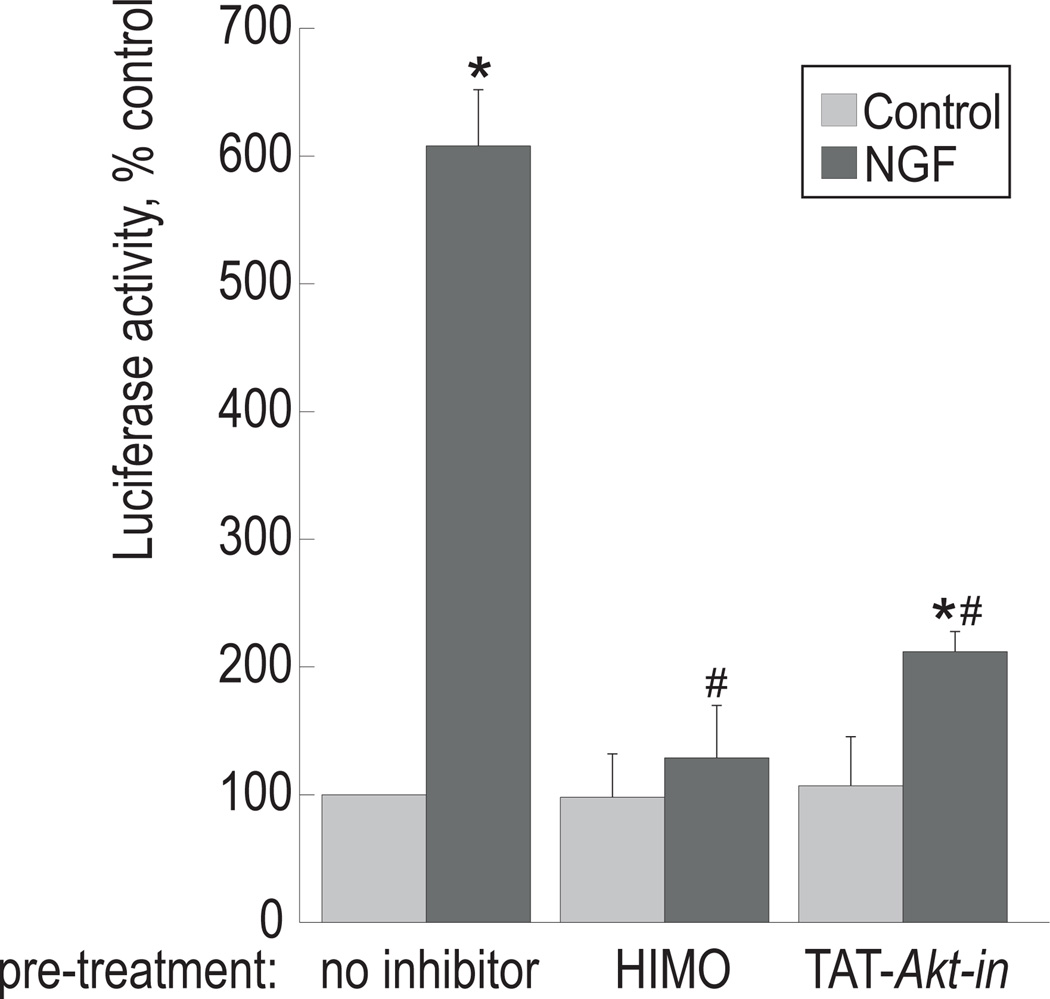
We used a reporter gene assay to evaluate the role of Akt in regulating cholinergic promoter activity. The cholinergic promoter-luciferase reporter construct pGL3Hind4.2, containing the distal promoter region of the cholinergic locus, has been described elsewhere (Madziar et al. 2005). The transcriptional activity of this reporter construct is stimulated 4- to 6-fold by NGF in PC12 cells and, to a lesser degree, in the septal cell line SN56T17 (Madziar et al. 2005 and B. Berse, unpublished observations).
In order to examine the effect of Akt inhibitors on cholinergic promoter activity, PC12 cells transfected with pGL3-Hind 4.2 were pre-treated with HIMO or TAT-Akt-in, then 100 ng/ml NGF was added and the incubation was continued for 3 days, with one more application of the inhibitors and the growth factor after 48 h. Luciferase and protein levels were assayed as described in Experimental Procedures. In agreement with our previous results (Madziar et al. 2005), NGF up-regulated ChAT promoter activity by approximately 4-fold (Figure 3). Neither HIMO or TAT-Akt-in, when administered alone, affected basal luciferase activity, but both significantly reduced luciferase expression in the presence of NGF. This suggests that Akt signaling is required for NGF-mediated stimulation of the cholinergic locus expression. The cell number and morphological appearance of the cells in all treatment groups were similar, indicating that viability was not affected by the treatments.
Figure 3. Akt inhibitors prevent induction of cholinergic promoter transcriptional activity by NGF.
PC12 cells were transfected with the reporter construct pGL3Hind4.2 and cultured with 10 µM HIMO or 50 µM TAT-Akt-in for 12 h prior to addition of 100 ng/ml NGF. Luciferase activity was measured in cell extracts 60 h post-transfection as described in Experimental Procedures. The results are presented as means ± S.E.M. of three independent cultures. (*) significantly different from untreated control, p<0.005; (#) significantly different from NGF-treated sample in the absence of the inhibitors, p<0.001 (ANOVA, followed by Dunnett’s test). The results are representative of three experiments.
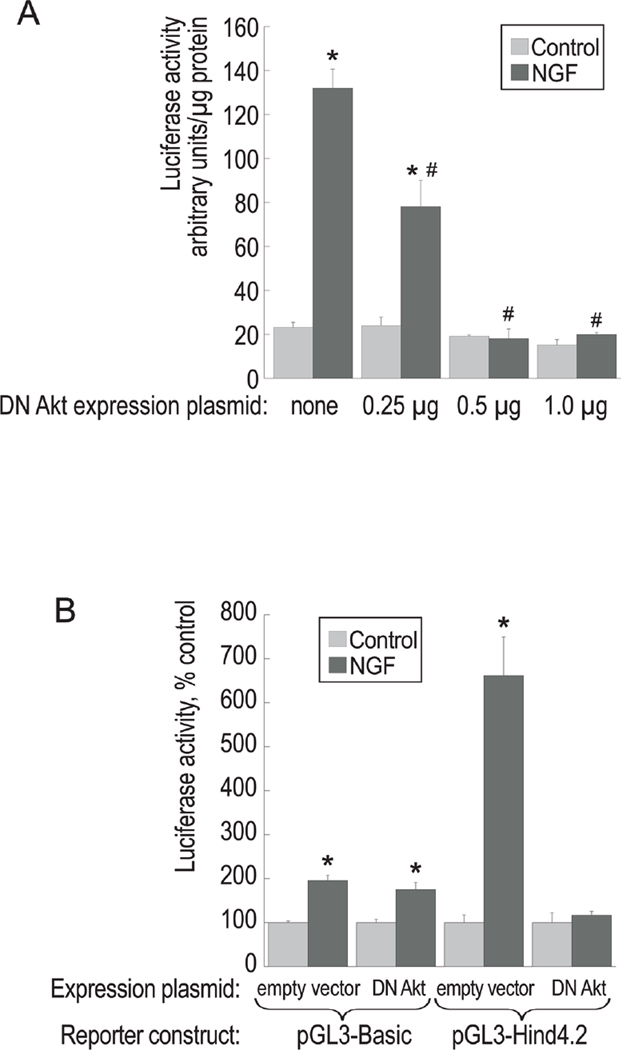
The reporter plasmid pGL3Hind4.2 was also used in the co-transfection experiments with a dominant-negative (DN) Akt construct, kindly provided by Dr. Alex Toker (Beth Israel Deaconess Medical Center and Harvard Medical School). This construct expresses a kinase-dead mutant of Akt (K179M), which has been demonstrated to inhibit the actions of endogenous Akt (Dudek et al. 1997; Cheng et al. 2000). Increasing amounts of DN Akt construct (mixed in with an empty expression vector to maintain constant total DNA input) were co-transfected together with pGL3Hind4.2 into PC12 cells. Following transfection, the cells were cultured in the presence or absence of NGF, and luciferase activity was assayed in cell extracts (Figure 4A). Consistent with our previous results, NGF increased luciferase activity of pGL3Hind4.2 by over 6-fold. This increase was significantly reduced by co-transfection with DN Akt in a concentration-dependent manner, indicating that Akt activity is necessary for the response of the reporter to NGF.
Figure 4. Effect of a dominant-negative Akt construct on the transcriptional activity of the cholinergic promoter-luciferase reporter construct.
(A) PC12 cells were co-transfected with pGL3Hind4.2 (0.5 µg per 35-mm dish) and increasing amounts of DN Akt (K179M) expression plasmid mixed with an empty expression vector for a constant total amount of DNA (1 µg per dish). The transfectants were treated with 100 ng/ml NGF and luciferase activity was measured in cell extracts 60 h post-transfection. The results are presented as means ± S.E.M. of three independent cultures. (*) significantly different from untreated control, p<0.001; (#) significantly different from the sample transfected with the reporter plasmid and the empty expression vector, p<0.01 (ANOVA, followed by Dunnett’s test). The results are representative of two experiments.
(B) PC12 cells were co-transfected with 0.5 µg of a reporter plasmid (pGL3-Basic or pGL3-Hind4.2) and 1 µg of an expression construct (DN Akt or the empty vector) and processed as described in (A). The results are presented as means ± S.E.M. of three independent cultures. (*) significantly different from the corresponding untreated control (p<0.005, ANOVA, followed by Dunnett’s test).
We have shown previously that the empty luciferase vector pGL3-Basic, used to create our reporter plasmid, is, surprisingly, also stimulated by NGF, albeit to a much smaller degree than the cholinergic promoter construct pGL3Hind4.2 (Madziar et al. 2005). Therefore, we examined whether this effect on the vector is modulated by the DN Akt expression plasmid. Figure 4B demonstrates that NGF treatment of the cells transfected with the pGL3-Basic vector increases luciferase activity by approximately 2-fold. However, co-transfection with DN Akt does not change NGF responsiveness of pGL3-Basic. Thus, DN Akt interferes with the transcriptional activity of the cholinergic promoter and not the vector. Therefore, we conclude that the regulation of the distal promoter region of the cholinergic locus by NGF is mediated by Akt.
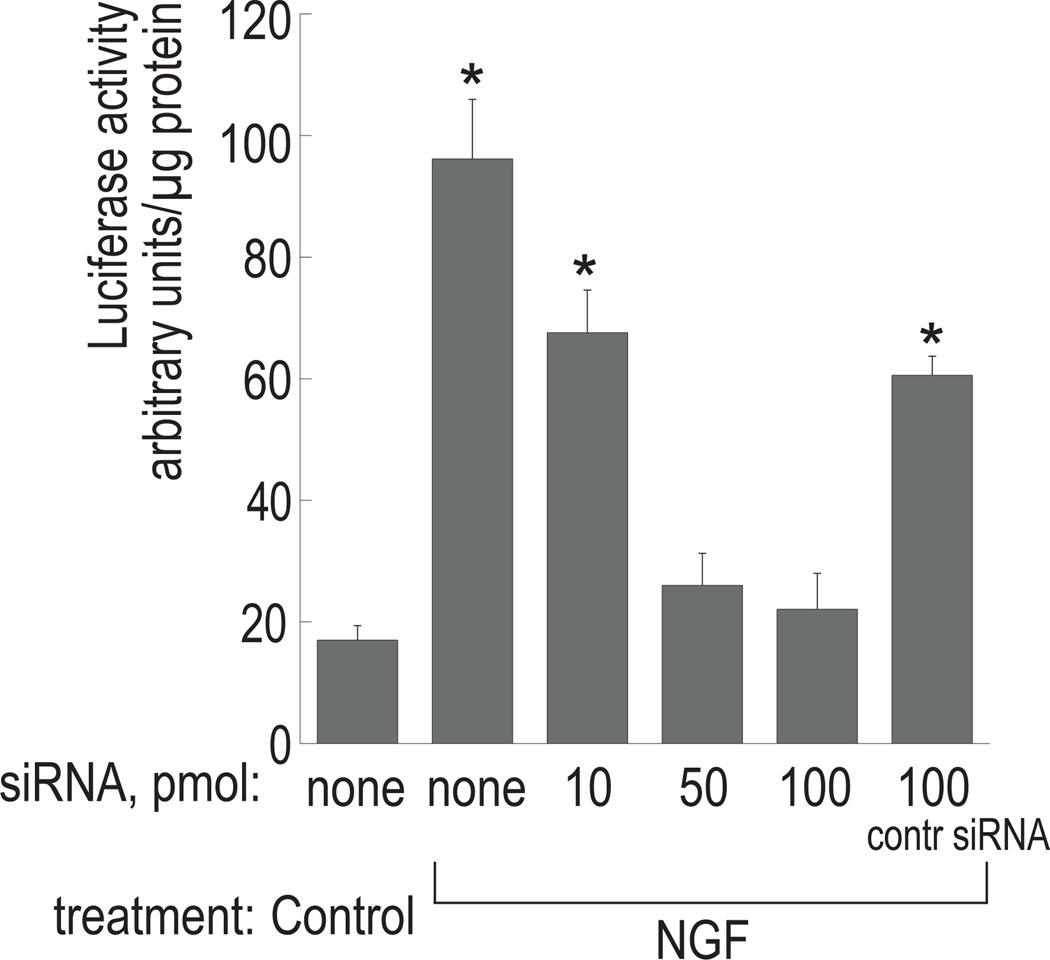
To further examine the involvement of Akt in NGF-mediated cholinergic expression, we used siRNA to target the expression of the Akt isoform Akt1/PKBα in PC12 cells. Cells were co-transfected with varied amounts of Akt1-specific siRNA (10, 50, and 100 pmol per 35-mm dish) together with 1 µg of pGL3-Hind4.2. As shown in Figure 5, cells transfected with 10 pmol siRNA exhibited a decrease in luciferase activity by ~30% in comparison to those treated with NGF alone. Luciferase activity dropped further at higher concentrations of siRNA, bringing activity down to nearly control levels. These findings suggest that inhibition of Akt1 expression decreases cholinergic promoter activity in response to NGF in PC12 cells. The control siRNA, which consists of non-specific sequences, reduced luciferase activity by ~35% when used at 100 pmol. This may possibly be due to nonspecific effects of a large number of small RNA molecules on cellular metabolism post-transfection. However, statistical analysis indicated a significant difference between NGF-treated cells transfected with 100 pmol Akt1-specific siRNA and those transfected with control siRNA at the same concentration, suggesting a specific role of Akt1 in cholinergic gene expression.
Figure 5. Akt1-specific siRNA interferes with cholinergic promoter activity in reporter gene assays.
PC12 cells were co-transfected with pGL3-Hind4.2 and increasing amounts of Akt1-specific siRNA (as indicated, in pmol) or 100 pmol of control siRNA. The cells were treated with 100 ng/ml NGF and luciferase activity was measured in cell extracts 72 h post-transfection. The results are presented as means ± SD of three independent cultures. *Significantly different from control (p<0.0001). All samples were significantly different from that treated with NGF in the absence of siRNA (p<0.0001).
4. NGF-stimulated cholinergic gene expression is Akt-dependent in primary septal cells
We also examined the involvement of Akt signaling on cholinergic gene expression in primary neuronal cultures isolated from E18 mouse septum (Figure 6). After 48 h in culture, the cells were pre-treated with HIMO for 12 h, followed by 3 days of NGF treatment. As was observed for PC12 cells, the Akt inhibitor treatment of primary cultures did not affect cell viability or overall neuronal morphology (Figure 6A). RT-PCR revealed that NGF treatment stimulated ChAT mRNA accumulation, and that this NGF effect was prevented by HIMO (Figure 6B). In addition, we applied real-time RT-PCR using mouse ChAT primers and FAM-labeled TaqMan probes (Applied Biosystems) to evaluate changes in ChAT mRNA expression. Real-time PCR quantification (Figure 6C) agrees with the results of end-point RT-PCR at a limited number of cycles followed by electrophoresis and digital analysis of band intensity.
Figure 6. Blocking the PI3K/Akt pathway inhibits NGF-stimulated cholinergic gene expression in primary septal neurons.
Primary cultures were derived from E18 mouse septum and treated with 10 µM HIMO followed by 100 ng/ml NGF as described in Experimental Procedures.
(A) Phase-contrast photographs were taken after 72 h of treatment. Objective lens 20 ×.
(B) Total RNA was prepared and RT/PCR with ChAT and β-actin primers was carried out as described in Experimental Procedures. The results are representative of two independent experiments.
(C) Total RNA from the samples in (B) was converted into cDNA and subjected to real-time PCR analysis with ChAT primers as described in Experimental Procedures. The results are presented as means ± range of two independent experiments, normalized to β-actin amplification.
(D) Primary cultures from E18 mouse septum were treated with 5 μM LY294002 or 10 μM HIMO followed by 100 ng/ml NGF. RT/PCR with CHT primers was carried out as described in Experimental Procedures. The RNA preparations were digested with RNase-free DNase followed by DNase removal. RT/PCR for VAChT and β-actin was performed on the purified RNA samples. The results are representative of two independent experiments.
Although PC12 cells are capable of ACh synthesis, they do not express the high affinity choline transporter (CHT), an important component of the cholinergic phenotype in vivo (Apparsundaram et al. 2001; Ferguson et al. 2003). We had reported previously that the expression of CHT, along with that of the cholinergic locus, is upregulated by NGF in primary septal cells, and that this effect is prevented by the PI3K inhibitor LY294002 (Berse et al. 2005). In order to examine the involvement of PI3K/Akt signaling on CHT expression, we treated E18 primary septal cultures with NGF in the presence and absence of LY294002 or HIMO. RT-PCR for CHT and VAChT and subsequent analysis with the Kodak Image Station revealed that the amounts of both RT-PCR products were markedly increased in reactions with RNA obtained from NGF-treated cells as compared to control (Figure 6D). In samples treated with LY294002, regardless of NGF treatment, CHT and VAChT products remained below or at control levels, respectively, confirming that their expression was PI3K-dependent. Furthermore, pretreatment with the Akt inhibitor HIMO prevented NGF-induced increases in both CHT and VAChT mRNA accumulation (Figure 6D). RT/PCR with β-actin was performed to ensure equal loading and RNA integrity. These experiments demonstrated that the expression of CHT is also regulated by NGF in a PI3K/Akt-dependent manner.
Taken together, the data indicate that the NGF-stimulated induction of both CHT and cholinergic locus expression is mediated through the PI3K/Akt pathway in primary septal neurons.
DISCUSSION
In this paper, we present data suggesting a novel potential role for the signaling molecule Akt/PKB, specifically in regulating cholinergic differentiation. Using PC12 cells and primary septal neurons, we demonstrated that blocking Akt eliminates NGF-dependent cholinergic promoter activity, ChAT, VAChT and CHT mRNA accumulation and ACh production. These findings are consistent with our previous reports, based on pharmacological inhibition of PI3K, that showed PI3K-dependent upregulation of the cholinergic locus and the CHT gene by NGF (Berse et al. 2005; Madziar et al. 2005). Here, we used several parallel methods (pharmacological inhibitors, a dominant-negative construct and siRNA) to interfere with either Akt expression or its biological activity. Akt is the major effector of the PI3K pathway, and it is involved in transmission of PI3K survival signals. Therefore, the viability of the treated cells was of concern. We had observed before that extended exposure to the PI3K inhibitor LY294002 in the absence of NGF reduced cell viability (Madziar et al. 2005). However, three-day treatments with the Akt inhibitors HIMO or TAT-Akt-in did not affect either the general neuronal morphology or viability of primary septal neurons or NGF-differentiated PC12 cells. The cells remained firmly attached to the substratum, their shape and the rate of cell divisions and cell death did not change; they also expressed β-actin at normal levels. In the presence of Akt inhibitors, PC12 cells still formed neurites in response to NGF treatment. Thus, some manifestations of NGF signaling are present even when the activation of Akt is prevented. We demonstrated previously that pharmacological treatments can discriminate between neurite formation and the upregulation of the cholinergic phenotype in response to NGF, as the former was significantly reduced in PC12 cells treated with MEK inhibitors, while the latter was not. Conversely, inhibition of PI3K with LY294002, which completely blocked the expression of the cholinergic genes, had little effect on neurite formation in PC12 cells (Madziar et al. 2005). The experiments with Akt inhibitors, presented here, confirm that neurite formation in response to NGF is regulated independently from cholinergic differentiation. Taken together, our data suggest that the suppression of cholinergic differentiation in the presence of Akt inhibitors is not the result of a general downregulation of neuronal properties, but a more specific effect on cholinergic gene expression.
Our results on ChAT and VAChT expression obtained with the PC12 cell line were confirmed in primary cultures obtained from E18 mouse septum. Additionally, the experiments with primary septal cells demonstrated that the expression of another cholinergic marker, CHT, is also regulated by NGF in an Akt-dependent manner. The CHT gene was cloned relatively recently and there is little information available on its regulation. Our data reveal for the first time the specific signals governing the regulation of CHT expression (Berse et al. 2005; Brock et al. 2007; Mellott et al. 2007, this study). The best-described biological function of CHT is providing choline for ACh production. Initial reports, based on the cellular distribution of CHT mRNA, which was found almost exclusively in cholinergic tissues, suggested that CHT is a cholinergic marker co-regulated with ChAT and VAChT (Kobayashi et al. 2002; Ferguson et al. 2003; Kus et al. 2003). Our current data are in agreement with those observations. However, although CHT is essential for the cholinergic phenotype, there are also differences between the regulation of its expression and that of the cholinergic gene locus (Brandon et al. 2004; Berse et al. 2005; Lecomte et al. 2005; Brock et al. 2007). More studies on CHT expression could reveal other potential biological functions of this protein.
The precise mechanism by which the PI3K/Akt pathway regulates cholinergic function is currently unknown. We used FKHRL1 and GSK-3 phosphorylation as indicators of Akt activity, but our experiments did not address the question whether these proteins are the downstream effectors of Akt. However, GSK-3 is a likely and interesting candidate. It is a crucial signaling molecule in several physiological processes and corresponding diseases. It is involved in control of glucose metabolism through insulin actions, type 2 diabetes mellitus (T2DM), apoptosis, Wnt signaling and cancer, and recently it has been linked to Alzheimer’s disease (AD). GSK-3 phosphorylates the protein tau, which leads to neurofibrillary tangles, one of the two key neuropathological features of AD. Also, the anti-apoptotic function of the PI3K/Akt/GSK-3 pathway makes it important for nerve cell survival and our data demonstrate its role in cholinergic function. Thus, a defect in this pathway may contribute to AD in several ways. Furthermore, clinical studies show a higher risk of dementia or significant cognitive decline in diabetic populations, revealing a direct connection between T2DM and AD (for review, see Cole et al. 2007). The results presented here, suggesting that the PI3K/Akt signaling pathway is necessary for the stimulation of ACh synthesis by NGF, are in agreement with those observations. The exact mechanism of insulin resistance in T2DM is not yet known, however the IR seems to function normally in diabetic patients. Thus, it is believed that origin of resistance is downstream from the receptor, and since insulin and NGF share downstream signaling pathways, it can be hypothesized that NGF resistance may also develop in neurons where insulin resistance is present.
Our results with the reporter construct indicate that at least some of the Akt effects on cholinergic gene expression are transcriptional. Akt has been associated mainly with cell survival through interfering with the cytoplasmic mechanisms of apoptosis, but it is also involved in the transcriptional induction of genes related to cell survival, cell cycle, tumor progression, T-cell activation and energy metabolism (Brunet et al. 2001; Kane and Weiss 2003; Liang and Slingerland 2003; Downward 2004; Agarwal et al. 2005; Huang and Chen 2005; Lee et al. 2005; Porstmann et al. 2005). Some of the transcriptional effects of Akt have been attributed to its inhibitory phosphorylation of GSK-3, which in turn can act as a negative regulator of gene expression (Bijur and Jope 2000). Interestingly, in neuroblastoma cells expressing the estrogen receptor (ER), PI3K/Akt/GSK-3 signaling has been shown to modulate estrogen-dependent gene expression (Mendez and Garcia-Segura 2006). The mechanism of the cross-talk between these pathways is still unclear, although there are indications that it involves regulation of the stability of the ER/β-catenin complex by GSK-3. Akt has also been found to regulate gene expression by direct phosphorylation of transcription factors, which leads to their activation (e.g. CREB), or inactivation (e.g. the proteins of the Forkhead family) (Hanada et al. 2004). These biological functions of Akt are conducted both in the cytoplasm and in the nucleus. It has been shown recently that PI3K enhancer (PIKE), a newly identified brain-specific nuclear GTPase, is involved in the anti-apoptotic action of NGF in PC12 cells (Ahn et al. 2004; reviewed in Chan and Ye 2007). It would be interesting to investigate whether nuclear PI3K, nuclear Akt and/or PIKE are also involved in the cholinergic differentiation of neurons by NGF.
ACKNOWLEDGEMENTS
The authors thank Dr. Alex Toker for the generous gift of the dominant-negative Akt construct and Dr. Barbara Slack for helpful comments. This work was supported by National Institute of Neurological Disorders and Stroke grants NS44238 (to B.B.) and NS42793 (to J.K.B.) and by an National Institute on Aging grant AG009525 (to J.K.B.).
Abbreviations
- AD
Alzheimer’s disease
- ACh
acetylcholine
- AraC
cytosine arabinoside
- ChAT
choline acetyltransferase
- CHT
choline transporter
- CREB
cyclic AMP response element-binding protein
- DN
dominant-negative
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- GSK-3
glucose synthase kinase-3
- HIMO
1L-6-hydroxymethyl-chiro-inositol 2(R)-2-O-methyl-3-O-octadecylcarbonate
- HS
horse serum
- MAPK
mitogen-activated protein kinase
- MEK
MAPK kinase
- NGF
nerve growth factor
- nt
nucleotide
- PC12
pheochromocytoma 12
- PDK
phosphoinositide-dependent kinase
- PH
pleckstrin homology
- PI3K
phosphatidylinositol 3’-kinase
- PIKE
PI3K enhancer
- PIP3
phosphatidylinositol-3,4,5-trisphosphate
- PKA
protein kinase A
- PKB
protein kinase B
- RT
reverse transcription
- PCR
polymerase chain reaction
- siRNA
small interfering RNA
- TAT
human immunodeficiency virus transactivator
- TCL1
T-cell leukemia/lymphoma 1
- VAChT
vesicular acetylcholine transporter
Contributor Information
Beata Madziar, Department of Pathology and Laboratory Medicine, Boston University School of Medicine; Boston, MA 02118, USA..
Sonia Shah, Department of Pathology and Laboratory Medicine, Boston University School of Medicine; Boston, MA 02118, USA..
Martina Brock, Department of Pathology and Laboratory Medicine, Boston University School of Medicine; Boston, MA 02118, USA..
Rebecca Burke, Department of Pathology and Laboratory Medicine, Boston University School of Medicine; Boston, MA 02118, USA..
Ignacio Lopez-Coviella, Department of Psychiatry, Boston University School of Medicine; Boston, MA 02118, USA..
Ann-Christin Nickel, Department of Pathology and Laboratory Medicine, Boston University School of Medicine; Boston, MA 02118, USA..
Esra Betul Cakal, Department of Pathology and Laboratory Medicine, Boston University School of Medicine; Boston, MA 02118, USA..
Jan Krzysztof Blusztajn, Department of Pathology and Laboratory Medicine, Boston University School of Medicine; Boston, MA 02118, USA..
Brygida Berse, Department of Pathology and Laboratory Medicine, Boston University School of Medicine; Boston, MA 02118, USA..
REFERENCES
- Agarwal A, Das K, Lerner N, Sathe S, Cicek M, Casey G, Sizemore N. The AKT/I kappa B kinase pathway promotes angiogenic/metastatic gene expression in colorectal cancer by activating nuclear factor-kappa B and beta-catenin. Oncogene. 2005;24:1021–1031. doi: 10.1038/sj.onc.1208296. [DOI] [PubMed] [Google Scholar]
- Ahn JY, Rong R, Liu X, Ye K. PIKE/nuclear PI 3-kinase signaling mediates the antiapoptotic actions of NGF in the nucleus. Embo J. 2004;23:3995–4006. doi: 10.1038/sj.emboj.7600392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apparsundaram S, Ferguson SM, Blakely RD. Molecular cloning and characterization of a murine hemicholinium-3-sensitive choline transporter. Biochem Soc Trans. 2001;29:711–716. doi: 10.1042/0300-5127:0290711. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Berse B, Lopez-Coviella I, Blusztajn JK. Activation of TrkA by nerve growth factor upregulates expression of the cholinergic gene locus but attenuates the response to ciliary neurotrophic growth factor. Biochem J. 1999;342(Pt 2):301–308. [PMC free article] [PubMed] [Google Scholar]
- Berse B, Szczecinska W, Lopez-Coviella I, Madziar B, Zemelko V, Kaminski R, Kozar K, Lips KS, Pfeil U, Blusztajn JK. Expression of high affinity choline transporter during mouse development in vivo and its upregulation by NGF and BMP-4 in vitro. Brain Res Dev Brain Res. 2005;157:132–140. doi: 10.1016/j.devbrainres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Bijur GN, Jope RS. Opposing actions of phosphatidylinositol 3-kinase and glycogen synthase kinase-3beta in the regulation of HSF-1 activity. J Neurochem. 2000;75:2401–2408. doi: 10.1046/j.1471-4159.2000.0752401.x. [DOI] [PubMed] [Google Scholar]
- Brandon EP, Mellott T, Pizzo DP, Coufal N, D'Amour KA, Gobeske K, Lortie M, Lopez-Coviella I, Berse B, Thal LJ, Gage FH, Blusztajn JK. Choline transporter 1 maintains cholinergic function in choline acetyltransferase haploinsufficiency. J Neurosci. 2004;24:5459–5466. doi: 10.1523/JNEUROSCI.1106-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Brock M, Nickel AC, Madziar B, Blusztajn JK, Berse B. Differential regulation of the high affinity choline transporter and the cholinergic locus by cAMP signaling pathways. Brain Res. 2007;1145:1–10. doi: 10.1016/j.brainres.2007.01.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Chan CB, Ye K. PIKE GTPase are phosphoinositide-3-kinase enhancers, suppressing programmed cell death. J Cell Mol Med. 2007;11:39–53. doi: 10.1111/j.1582-4934.2007.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HL, Steinway M, Delaney CL, Franke TF, Feldman EL. IGF-I promotes Schwann cell motility and survival via activation of Akt. Mol Cell Endocrinol. 2000;170:211–215. doi: 10.1016/s0303-7207(00)00324-5. [DOI] [PubMed] [Google Scholar]
- Cole AR, Astell A, Green C, Sutherland C. Molecular connexions between dementia and diabetes. Neurosci Biobehav Rev. 2007;31:1046–1063. doi: 10.1016/j.neubiorev.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–1121. [Google Scholar]
- Ferguson SM, Savchenko V, Apparsundaram S, Zwick M, Wright J, Heilman CJ, Yi H, Levey AI, Blakely RD. Vesicular localization and activity-dependent trafficking of presynaptic choline transporters. J Neurosci. 2003;23:9697–9709. doi: 10.1523/JNEUROSCI.23-30-09697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke TF. Intracellular signaling by Akt: bound to be specific. Sci Signal. 2008;1:pe29. doi: 10.1126/scisignal.124pe29. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Ishigami T, Kawano T. Transcriptional regulation of neuronal genes and its effect on neural functions: expression and function of forkhead transcription factors in neurons. J Pharmacol Sci. 2005;98:205–211. doi: 10.1254/jphs.fmj05001x3. [DOI] [PubMed] [Google Scholar]
- Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Hiromura M, Okada F, Obata T, Auguin D, Shibata T, Roumestand C, Noguchi M. Inhibition of Akt kinase activity by a peptide spanning the betaA strand of the proto-oncogene TCL1. J Biol Chem. 2004;279:53407–53418. doi: 10.1074/jbc.M403775200. [DOI] [PubMed] [Google Scholar]
- Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- Hu Y, Qiao L, Wang S, Rong SB, Meuillet EJ, Berggren M, Gallegos A, Powis G, Kozikowski AP. 3-(Hydroxymethyl)-bearing phosphatidylinositol ether lipid analogues and carbonate surrogates block PI3-K, Akt, and cancer cell growth. J Med Chem. 2000;43:3045–3051. doi: 10.1021/jm000117y. [DOI] [PubMed] [Google Scholar]
- Huang WC, Chen CC. Akt phosphorylation of p300 at Ser-1834 is essential for its histone acetyltransferase and transcriptional activity. Mol Cell Biol. 2005;25:6592–6602. doi: 10.1128/MCB.25.15.6592-6602.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Kane LP, Weiss A. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol Rev. 2003;192:7–20. doi: 10.1034/j.1600-065x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Okuda T, Fujioka Y, Matsumura G, Nishimura Y, Haga T. Distribution of the high-affinity choline transporter in the human and macaque monkey spinal cord. Neurosci Lett. 2002;317:25–28. doi: 10.1016/s0304-3940(01)02413-2. [DOI] [PubMed] [Google Scholar]
- Kozikowski AP, Sun H, Brognard J, Dennis PA. Novel PI analogues selectively block activation of the pro-survival serine/threonine kinase Akt. J Am Chem Soc. 2003;125:1144–1145. doi: 10.1021/ja0285159. [DOI] [PubMed] [Google Scholar]
- Kunstle G, Laine J, Pierron G, Kagami Si S, Nakajima H, Hoh F, Roumestand C, Stern MH, Noguchi M. Identification of Akt association and oligomerization domains of the Akt kinase coactivator TCL1. Mol Cell Biol. 2002;22:1513–1525. doi: 10.1128/mcb.22.5.1513-1525.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kus L, Borys E, Ping Chu Y, Ferguson SM, Blakely RD, Emborg ME, Kordower JH, Levey AI, Mufson EJ. Distribution of high affinity choline transporter immunoreactivity in the primate central nervous system. J Comp Neurol. 2003;463:341–357. doi: 10.1002/cne.10759. [DOI] [PubMed] [Google Scholar]
- Lecomte MJ, De Gois S, Guerci A, Ravassard P, Faucon Biguet N, Mallet J, Berrard S. Differential expression and regulation of the high-affinity choline transporter CHT1 and choline acetyltransferase in neurons of superior cervical ganglia. Mol Cell Neurosci. 2005;28:303–313. doi: 10.1016/j.mcn.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Lee SR, Park JH, Park EK, Chung CH, Kang SS, Bang OS. Akt-induced promotion of cell-cycle progression at G(2)/M phase involves upregulation of NF-Y binding activity in PC12 cells. J Cell Physiol. 2005 doi: 10.1002/jcp.20395. [DOI] [PubMed] [Google Scholar]
- Li Y, Holtzman DM, Kromer LF, Kaplan DR, Chua-Couzens J, Clary DO, Knusel B, Mobley WC. Regulation of TrkA and ChAT expression in developing rat basal forebrain: evidence that both exogenous and endogenous NGF regulate differentiation of cholinergic neurons. J Neurosci. 1995;15:2888–2905. doi: 10.1523/JNEUROSCI.15-04-02888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–345. [PubMed] [Google Scholar]
- Madziar B, Lopez-Coviella I, Zemelko V, Berse B. Regulation of cholinergic gene expression by nerve growth factor depends on the phosphatidylinositol-3'-kinase pathway. J Neurochem. 2005;92:767–779. doi: 10.1111/j.1471-4159.2004.02908.x. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellott TJ, Kowall NW, Lopez-Coviella I, Blusztajn JK. Prenatal choline deficiency increases choline transporter expression in the septum and hippocampus during postnatal development and in adulthood in rats. Brain Res. 2007;1151:1–11. doi: 10.1016/j.brainres.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez P, Garcia-Segura LM. Phosphatidylinositol 3-kinase and glycogen synthase kinase 3 regulate estrogen receptor-mediated transcription in neuronal cells. Endocrinology. 2006;147:3027–3039. doi: 10.1210/en.2005-1224. [DOI] [PubMed] [Google Scholar]
- Oosawa H, Fujii T, Kawashima K. Nerve growth factor increases the synthesis and release of acetylcholine and the expression of vesicular acetylcholine transporter in primary cultured rat embryonic septal cells. J Neurosci Res. 1999;57:381–387. [PubMed] [Google Scholar]
- Pongrac JL, Rylett RJ. NGF-induction of the expression of ChAT mRNA in PC12 cells and primary cultures of embryonic rat basal forebrain. Brain Res Mol Brain Res. 1998;62:25–34. doi: 10.1016/s0169-328x(98)00215-0. [DOI] [PubMed] [Google Scholar]
- Porstmann T, Griffiths B, Chung YL, Delpuech O, Griffiths JR, Downward J, Schulze A. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene. 2005 doi: 10.1038/sj.onc.1208802. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Szutowicz A, Madziar B, Pawelczyk T, Tomaszewicz M, Bielarczyk H. Effects of NGF on acetylcholine, acetyl-CoA metabolism, and viability of differentiated and non-differentiated cholinergic neuroblastoma cells. J Neurochem. 2004;90:952–961. doi: 10.1111/j.1471-4159.2004.02556.x. [DOI] [PubMed] [Google Scholar]
- Tian X, Sun X, Suszkiw JB. Developmental age-dependent upregulation of choline acetyltransferase and vesicular acetylcholine transporter mRNA expression in neonatal rat septum by nerve growth factor. Neurosci Lett. 1996;209:134–136. doi: 10.1016/0304-3940(96)12629-x. [DOI] [PubMed] [Google Scholar]
- Williams B, Granholm AC, Sambamurti K. Age-dependent loss of NGF signaling in the rat basal forebrain is due to disrupted MAPK activation. Neurosci Lett. 2007;413:110–114. doi: 10.1016/j.neulet.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BJ, Bimonte-Nelson HA, Granholm-Bentley AC. ERK-mediated NGF signaling in the rat septo-hippocampal pathway diminishes with age. Psychopharmacology (Berl) 2006;188:605–618. doi: 10.1007/s00213-006-0477-1. [DOI] [PubMed] [Google Scholar]
- Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Yuen EC, Howe CL, Li Y, Holtzman DM, Mobley WC. Nerve growth factor and the neurotrophic factor hypothesis. Brain Dev. 1996;18:362–368. doi: 10.1016/0387-7604(96)00051-4. [DOI] [PubMed] [Google Scholar]