Abstract
Many basic characteristics of gustatory neurons remain unknown, partly due to the absence of specific markers. Some neurons in the geniculate ganglion project to taste regions in the oral cavity, whereas others innervate the outer ear. We hypothesized that the transcription factor Phox2b would identify oral cavity-projecting neurons in the geniculate ganglion. To test this possibility, we characterized mice in which Phox2b-Cre mediated gene recombination labeled neurons with tdTomato. Nerve labeling revealed that all taste neurons projecting through the chorda tympani (27%) and greater superficial petrosal nerves (15%) expressed Phox2b during development, whereas non-oral somatosensory neurons (58%) in the geniculate ganglion did not. We found tdTomato-positive innervation within all taste buds. Most (57%) of the fungiform papillae had labeled innervation only in taste buds, whereas 43% of the fungiform papillae also had additional labeled innervation to the papilla epithelium. Chorda tympani nerve transection eliminated all labeled innervation to taste buds, but most of the additional innervation in the fungiform papillae remained. Some of these additional fibers also expressed tyrosine hydroxylase, suggesting a sympathetic origin. Consistent with this, both sympathetic and parasympathetic fibers innervating blood vessels and salivary glands contained tdTomato labeling. Phox2b-tdTomato labels nerve fascicles in the tongue of the developing embryo and demonstrates a similar stereotyped branching pattern DiI-labeling.
Keywords: geniculate ganglion, Phox2b, taste, taste bud, taste bud innervation, RRID: MMRRC_034613-UCD, RRID: IMSR_JAX:007914, RRID: AB_531826, RRID: AB_632496, RRID: AB_2314683
1 | INTRODUCTION
Taste receptor cells located on the tongue and palate detect the chemical content of food and respond to various taste qualities such as bitter or sweet (Chaudhari & Roper, 2010). Afferent fibers from neurons in the geniculate and petrosal ganglia innervate these taste receptor cells and carry taste information to the first synaptic relay of the brain, the nucleus of the solitary tract (Krimm, 2007). Although our understanding of taste receptor cells has advanced considerably over the past two decades, the neurons that innervate these cells are still poorly understood, at least in part due to the lack of specific molecular markers for taste neurons.
The geniculate ganglion contains taste neurons that project through the chorda tympani and greater superficial petrosal nerves; however, it also contains non-gustatory neurons, most notably those innervating the external acoustic meatus through the posterior auricular nerve (Folan-Curran & Cooke, 2001; Gomez, 1978; Semba, Sood, Shu, Nagele, & Egger, 1984). Although the exact proportion of geniculate ganglion neurons that project to the tongue and palate is unknown, estimates in the rat range from 50 to 70% (Gomez, 1978; Semba et al., 1984). The quantification of innervation to taste buds is limited by labels that do not primarily label taste fibers (e.g., neurofilaments; Krimm, Miller, Kitzman, Davis, & Albers, 2001), labels that also identify certain subpopulations of taste cells (e.g., NCAM; Nosrat, Margolskee, & Nosrat, 2012; Yee, Yang, Bottger, Finger, & Kinnamon, 2001), or that show a patchy appearance and likely do not label entire nerve fibers (e.g., P2X3; Huang, Ma, & Krimm, 2015).
Taste neurons, similarly to those innervating digestive, respiratory, and cardiovascular end organs, can be described as “visceral” sensory neurons, whereas those innervating the pinna and external ear canal can be classified as somatosensory neurons, because they innervate external structures. Thus, a transcription factor that regulates visceral sensory neuron development would be a useful candidate for identifying gustatory neurons in the geniculate ganglion. Phox2b is a homeodomain transcription factor that is essential for development of the placodally-derived geniculate and petrosal ganglion (Dauger et al., 2003). An early study reports the widespread expression of Phox2b in the geniculate ganglion and the degeneration of the entire ganglion upon Phox2b deficiency (Dauger et al., 2003), implying limited specificity. However, Phox2b was more recently found to be expressed in roughly half of the geniculate ganglion (D’Autreaux, Coppola, Hirsch, Birchmeier, & Brunet, 2011), suggesting that Phox2b might be specifically expressed by gustatory neurons within the ganglion.
Here, our goal was to characterize a genetic label for taste neurons projecting through the chorda tympani and greater superficial petrosal nerves that could be used to further investigate taste bud innervation. To accomplish this goal, we used a Phox2b-Cre-tdTomato mouse line in which all neurons expressing Phox2b (at any time during development) would be genetically labeled. We found that Phox2b was specifically expressed in the 42% of geniculate ganglion neurons that projected through the chorda tympani and the greater superficial petrosal nerves. All tdTomato-positive fibers within taste buds in the fungiform papillae originated from the chorda tympani permitting measurement of total volume of innervation within the taste bud. The Phox2b construct allows for both genetic access and labeling of taste neurons.
2 | MATERIALS AND METHODS
2.1 | Animals
To genetically identify neurons expressing the transcription factor Phox2b, we bred Phox2b-Cre mice (Mutant Mouse Resource Research Centers; MMRRC strain 034613-UCD, NP91Gsat/Mmcd, RRID: MMRRC_034613-UCD) with tdTomato reporter mice (Ai14, The Jackson Laboratory, Stock No: 007914, RRID: IMSR_JAX:007914) that have a loxP-flanked STOP cassette preventing transcription of a CAG promoter-driven red fluorescent protein variant (tdTomato). tdTomato is expressed following Cre-mediated recombination for the lifetime of the cell regardless of the persistence of Phox2b expression. Mice were genotyped according to protocols provided by the MMRRC and Jackson Laboratory. All mice were housed in a central facility and maintained under controlled conditions of normal humidity and temperature, with standard alternating 12-hr periods of light and dark and free access to water and food. All mice were cared for and studied in accordance with guidelines set by the US Public Health Service Policy on the Humane Care and Use of Laboratory Animals and the NIH Guide for the Care and Use of Laboratory Animals. All procedures were approved by the University of Louisville Institutional Animal Care and Use Committee.
2.2 | Chorda tympani nerve transection
Mice were sedated with a 0.32 mg/kg injection of Domitor (medetomidine hydrochloride, I.M.) and anesthetized with 40 mg/kg Ketaset® (ketamine hydrochloride). Mice were placed in a non-traumatic head holder to provide access to the nerve in the neck via a ventral approach (Guagliardo & Hill, 2007). The chorda tympani nerve was located as it bifurcates from the lingual branch of the trigeminal nerve and was cut without damaging the trigeminal nerve. The nerve was cut such that a portion of the nerve was removed. The wound was sutured, and mice recovered on a water-circulating heating pad before being returned to their home cage. Atipamezole hydrochloride (2 mg/kg) was injected intramuscularly immediately after surgery to promote reversal of anesthesia and thus reduce recovery time. Meloxicam was also administered orally through food pellets for the 2 days after surgery. To achieve a dose of 1–2 mg/kg, 0.5 cc of a 0.5 mg/ml meloxicam solution was applied to each of two food pellets, which were then moistened with water and placed in the home cage. After 7 days, mice were euthanized and perfused with 4% paraformaldehyde (PFA).
2.3 | Fluorescent anterograde nerve labeling
Procedures used to label the chorda tympani and greater superficial nerves with fluorescent tracers were previously described (Sun, Dayal, & Hill, 2015; Sun, Hummler, & Hill, 2017). Adult Phox2b-Cre:tdTomato mice were anesthetized in the same manner as with CT-nerve section. A water-circulating heating pad was used to maintain body temperature. Mice were positioned in a non-traumatic head holder, and a ventral approach was used to expose the greater superficial petrosal and chorda tympani nerves within the right tympanic bulla. The chorda tympani and greater superficial petrosal nerves were cut near and peripheral to the geniculate ganglion in the tympanic bulla, and crystals of 3kD fluorescein dextran (D3306, Invitrogen) were applied to either the proximal cut end of both nerves or only the chorda tympani. A small amount of Kwik-Sil (World Precision Instruments, Inc., Sarasota, FL) was then placed over the cut end of the nerves to prevent crystals from diffusing from the intended labeling site. Post-surgical was the same as for nerve labels. After 2 days, mice were euthanized and perfused with 4% PFA. The geniculate ganglia were dissected and immediately mounted and imaged using a confocal microscope.
2.4 | Tongue injections with CTB-488
Adult Phox2b-Cre:tdTomato mice were anesthetized in the same manner as with CT-nerve section. Once the mouse was anesthetized the tongue was carefully pulled from the oral cavity and secured to a silicone putty surface and visualized under a fluorescent dissecting microscope. A 10 μl Hamilton syringe with a flexifil bevel tip (Cat# 500818, World Precision Instruments) was filled with cholera-toxin B conjugated with Alexa 488 (Cat# C34775, Life Technologies). A micromanipulator was used to control the syringe, such that the bevel was placed just below the epithelial surface. The syringe was slowly depressed and the diffusion of CTB-488 into the tongue could be seen under the fluorescent dissecting microscope. To ensure a large region of the tongue was labeled, this procedure was repeated 2–4 times such that a sizable portion of the tongue surface appeared to be labeled green. Once labeling was complete the tongue surface was cleaned with a cotton-tipped applicator dipped in distilled water and place back in the animal’s mouth. Post-surgical was the same as for nerve labels. After 48-hr, animals were perfused and processed for immunohistochemistry.
2.5 | Immunohistochemistry
Mice were sacrificed by avertin overdose (4 mg/kg) and perfused trans-cardially with 4% PFA. Dissected tissues were post-fixed in 4% PFA for 2 hr (for thin serial sections) or overnight (thick sections and whole mounts). Dissected tissues were then rinsed with PBS and transferred to 30% sucrose at 4°C overnight. A razor blade was used to remove the circumvallate papilla, the tongues were then carefully split in half with a razor blade down the mid-line under a dissection microscope, which was used to verify that the tongue was bi-sectioned at the mid-line. Tongues were frozen the next day in and stored at −80°C until sectioned on a cryostat or processed for whole-mount staining.
To visualize taste buds and their innervation in serial sagittal sections, each tongue-half, was sectioned sagittally from the midline to the lateral edge. Serial sagittal sections of the tongue (20 μm) were mounted on glass slides. Slides containing tongue sections were first dried on a slide warmer (37°C) overnight. The next day, slides were rehydrated, placed into citric acid buffer (10 mM citric acid, 0.05% Tween 20, pH 6.0), heated to 98–100°C for ~15 min in a boiling water bath, and allowed to cool for 20 min on ice for antigen retrieval. Slides were washed in PBS (pH 7.4) and incubated overnight at room temperature with primary antibodies in PBS and 0.5% Triton-X100 (Table 1). Following incubation, slides were rinsed in PBS and incubated for 1.5 hr with Alexa 488 anti-rat secondary antibody (1:500; Invitrogen) and Alexa 555 anti-rabbit secondary antibody (1:500; Invitrogen) in PBS and 0.5% Trition-X100. Slides were rinsed in PBS three times for 5 min and mounted with fluoromount-G (SouthernBiotech, Birmingham, AL). Alternatively, thick (70 μm) sections were blocked at 4°C in 0.1 M PB with 3% donkey serum and 0.5% Triton-X100 overnight, incubated at 4°C with primary antibodies as floating sections (without antigen retrieval) for 5 days, rinsed four times for 15 min each, incubated in secondary antibodies for 2 days, rinsed another four times for 15 min each, and then mounted and cover slipped.
TABLE 1.
Primary antibodies
| Antibody (antibody registry ID) | Antigen | Source (catalog #) | Working dilution |
|---|---|---|---|
| Rat monoclonal anti-TROMA1 (RRID: AB_531826) | Full length protein, TDM1 (Trophoblasoma) cell line intermediate filaments | Developmental Studies Hybridoma Bank, Iowa City, Iowa (TROMA1) | 1:50 |
| Rabbit polyclonal anti-dsRed (RRID: AB_632496) | DsRed-Express | Clontech Laboratories, Inc. (632496) | 1:500 |
| Rat monoclonal anti- Islet-1 and Islet-2 (RRID: AB_2314683) | Recombinant fusion protein containing aa 178–349 of Isl1 protein (C-terminus) | Developmental Studies Hybridoma Bank, Iowa City, Iowa (39.4D5) | 1:500 |
To visualize entire taste buds and geniculate ganglia, we performed whole-mount immunohistochemistry of the lingual epithelium and geniculate ganglia. After dissection, geniculate ganglia were frozen in OCT and thawed three times to improve antibody penetration. The lingual epithelium was prepared by cutting the tongue in half and then cutting the tongue epithelium away from underlying muscle and connective tissue. The epithelium was then divided into several pieces roughly representing the dorsal and ventral tip, mid-region, and back of the tongue. Surgical scissors were used to remove as much tissue from the epithelium as possible under a dissecting scope. The epithelium was then frozen flat (muscle side down) in OCT, and additional muscle and lamina propria were slowly shaved using a cryostat; each section of removed muscle or lamina propria was examined under a microscope to ensure that it did not contain epithelium. After sufficient muscle was removed to reach the underside of the epithelium, the tissue was thawed and processed for whole-mount staining. The lingual epithelium and ganglion were then processed using the same protocol as for thick sections.
2.6 | Antibody characterization
The antibodies used are described in detail in Table 1. Troma1 (RRID: AB_531826) and DsRed (RRID: AB_632496) are used for cell morphology for Keratin-8 containing taste cells and tdTomato nerve fibers, respectively. Both antibodies have been established in the literature for this purpose (Guagliardo & Hill, 2007; Meng, Huang, Sun, Hill, & Krimm, 2017; Meng, Ohman-Gault, Ma, & Krimm, 2015; Patel & Krimm, 2010; Rutlin et al., 2014). Isl-1 (RRID: AB_2314683) was used to label all neuronal nuclei in the geniculate ganglion (Usoskin et al., 2015).
2.7 | Data analysis
The number of innervated taste buds was quantified using a fluorescent microscope, each section was examined and each taste bud was followed through serial sagittal sections starting with the midline and working toward the lateral edge to ensure that each taste bud was counted only once. For each taste bud, the presence or absence of innervation inside the Keratin-8 borders of the taste bud or outside the taste bud but within the associated papillae was recorded.
Whole-mount taste buds were imaged using a confocal microscope at Zoom 3 and 60× magnification with a step size of 0.47 μm along the long axis of the taste bud. This step size was chosen, because the Olympus Fluoview recommends this step size as “optimum” for the magnification used. Deconvolution was performed using AutoQuant X3 software (Media Cybernetics, Maryland). Imaris software (Bitplane, Switzerland) was used for analysis of total taste bud volume and the volume of innervation within taste buds.
To quantify the absolute number of geniculate ganglion neurons, serial optical sections of whole geniculate ganglia were captured using a confocal microscope (FV1200, Olympus). High-resolution images of whole-mount geniculate ganglia were obtained by stitching multiple fields under a 40× lens, yielding a high-magnification image including the entire ganglion in one z-stack. Each labeled signal was collected individually with specific wavelength excitation. Images of whole geniculate ganglia were analyzed using Neurolucida 10 (MBF Bioscience, Williston, VT). All labeled neurons were counted by examining each cell through multiple optical sections; ganglia were imaged with a step size of 1 μm so that each cell could be followed through several sections. To ensure that each neuron was counted only once, markers were added to the center of each counted cell in the z-stack. Data are presented as mean ±SD.
3 | RESULTS
3.1 | Taste buds in all regions of the oral cavity are innervated by Phox2b-tdTomato nerve fibers
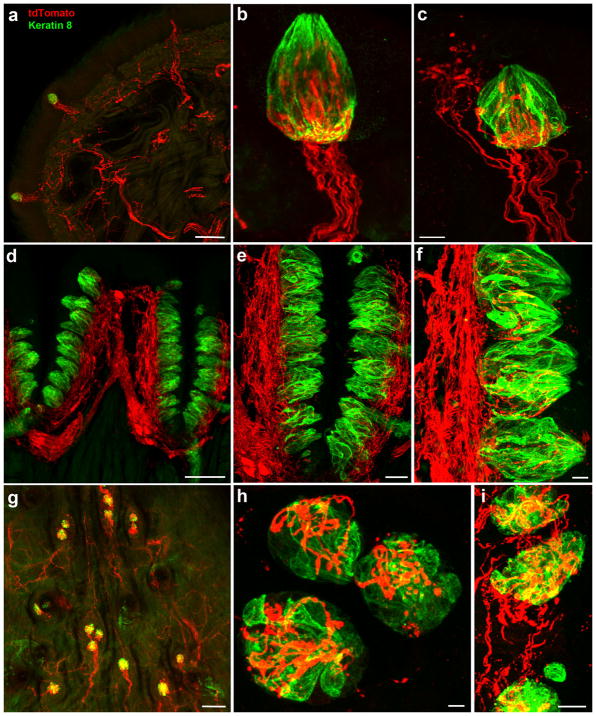
To determine whether neurons innervating taste buds express the transcription factor Phox2b during development, we bred Phox2b-Cre mice with Cre-dependent tdTomato mice and examined taste bud innervation. tdTomato-positive nerve fibers were found to innervate the front of the tongue and all fungiform papillae (Figure 1a). For some (57.5 ±4%, n =6 mice, 42.6 ±13.4 papillae/tongue half) fungiform papillae, tdTomato-positive innervation was specific to taste buds (Figure 1b, Supporting Information Movie S1), whereas in other fungiform papillae (42.5%) fibers also innervated adjacent regions of the fungiform papillae (Figure 1c). Circumvallate papillae had a large tdTomato-positive nerve plexus in their core (Figure 1d), and nerve fibers could be seen penetrating the taste buds (Figure 1e, f). Taste buds on the posterior palatine field of the soft palate are organized into single taste buds or small clusters of two or three taste buds located in eminences (Miller & Spangler, 1982). We observed tdTomato-positive nerve fibers coursing through the posterior palatine field and innervating these taste bud clusters (Figure 1g, h). In addition, taste buds located on the geschmackstreifen (i.e., taste stripe; Miller & Spangler, 1982), which sits above circumvallate papillae and anterior to the posterior palatine field, were also innervated by tdTomato-positive nerve fibers (Figure 1i).
FIGURE 1.
Tongue tip from a Phox2b-tdTomato mouse with labeled nerve fibers projecting to fungiform papillae (a). For many fungiform papillae, Phox2b-tdTomato-positive nerve fibers only innervated taste buds (b), whereas Phox2b-tdTomato-positive fibers also innervated surrounding epithelium in other fungiform papillae (c). These fibers always entered and traveled up the center of papillae and not up the sides. A dense Phox2b-tdTomato-positive plexus was observed in the core of circumvallate papillae (d), with many of these fibers seeming to penetrate into taste buds (e, f). A whole mount of the posterior palatine field (viewed top down) shows taste buds innervated by Phox2b-tdTomato-positive fibers (g), which were also seen in higher magnification images (h) and the geschmackstreifen (i). All images are project z-stacks of 70 μm sections (tongue) and whole mount (soft palate). Scale bars: (a) =100 μm, (c) =10 μm (applies to b), (d) =100 μm, (e) =30 μm, (f) =10 μm, (g) =100 μm, (h) =10 μm, and (i) =10 μm
3.2 | Phox2b-tdTomato expression distinguishes geniculate ganglion neurons innervating the tongue and palate from those innervating non-taste regions
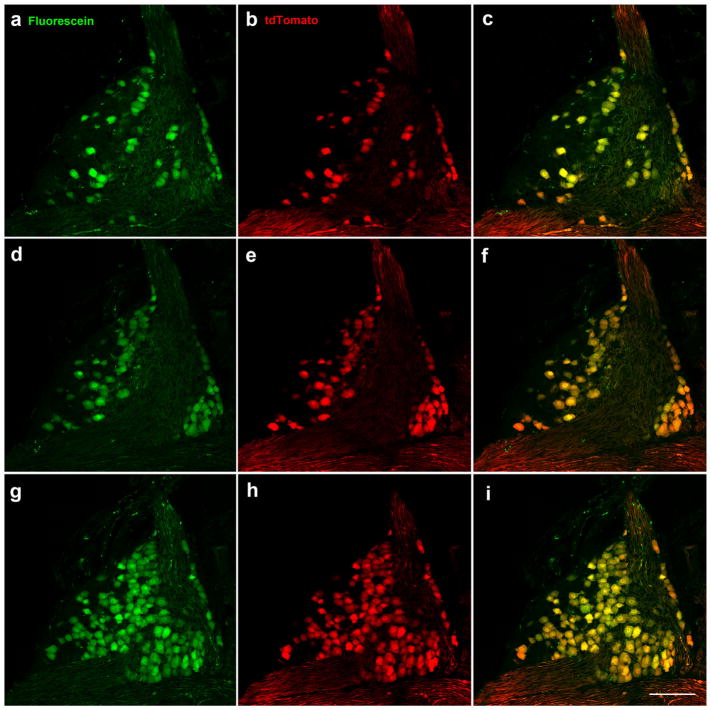
In addition to innervating taste regions of the tongue and palate, geniculate ganglion neurons innervate the ear pinna and external ear canal through multiple branches of the facial nerve including the posterior auricular nerve. To determine whether developmental Phox2b expression could distinguish between these neuron populations, we labeled the chorda tympani nerve, which innervates the tongue, and the greater superficial petrosal nerve, which innervates the palate, of Phox2b-Cre:tdTomato mice with fluorescein dextran. All 624.3 ±4.0 (n =3; individual counts =620, 625, 628) neurons in the geniculate ganglion that were labeled with tdTomato, were co-labeled with fluorescein dextran (Figure 2). In addition, of the neurons that were labeled with fluorescein dextran, all but three neurons in one mouse were also labeled with tdTomato. Together, these findings indicate that Phox2b-tdTomato specifically labels geniculate neurons innervating the tongue and palate.
FIGURE 2.
All Phox2b-tdTomato-positive neurons (red) were retrogradely labeled in the geniculate ganglion following placement of tracer (green) on chorda tympani and greater superficial petrosal nerves. Retrogradely labeled neurons are shown in the ventral (a), middle (d), and dorsal (g) geniculate ganglion in single 1 μm optical slices taken from a whole mount geniculate ganglion. Phox2b-tdTomato-positive neurons (b, e, h). Double-labeled neurons (c, f, i). Scale bar in (i) =100 μm and applies to all images
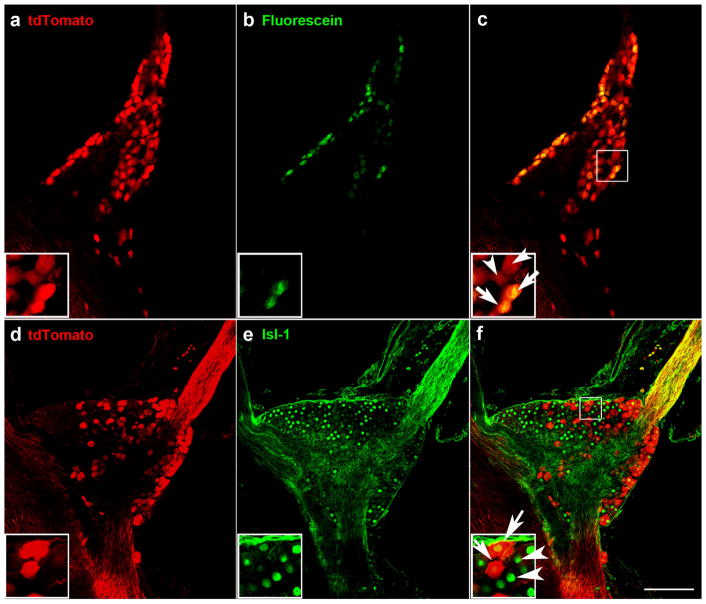
After determining that Phox2b is a genetic identifier of neurons projecting through the chorda tympani and greater superficial petrosal nerves, we then quantified the number of neurons contributing to each of these nerves. We labeled the chorda tympani nerve of Phox2b-Cre: tdTomato mice (n =3) with fluorescein dextran and quantified the double-labeled neurons innervating the tongue and the tdTomato only-labeled neurons innervating the palate (Figure 3a–c). We found that 378 ±11.7 (365, 383, 387) neurons innervated the fungiform taste field, whereas 208 ±32.5 (237, 173, 215) neurons innervated the palate through the greater superficial petrosal nerve. Therefore, of the Phox2b-positive neurons, 64% innervated the tongue via the chorda tympani, and 35% innervated the palate via the greater superficial petrosal nerve.
FIGURE 3.
Some but not all Phox2b-tdTomato-positive neurons in the geniculate ganglion were retrogradely labeled following placement of fluorescein on the chorda tympani nerve (a–c). A single optical slice through the geniculate ganglion whole mount shows Phox2b-tdTomato-positive neurons (a), retrogradely labeled neurons from the chorda tympani nerve (b), and the overlap between Phox2b-tdTomato and retrograde labeling was observed in some geniculate ganglion neurons (arrows in inset of c). There were also some Phox2b-tdTomato neurons without retrograde labeling (arrowheads). Expression of Phox2b-tdTomato was observed in some but not all geniculate ganglion neurons (d–f). Single optical slices through the geniculate ganglion stained for tdTomato (d), and the neuronal marker, Isl-1 (e). In the inset of (f), arrows indicate overlap between Isl-1 and tdTomato, and arrowheads indicate Isl-1-only labeled neurons. Scale bar in (c) =100 μm and applies to all images
Next, we quantified neurons innervating the oral cavity (chorda tympani and greater superficial petrosal) versus non-oral regions (posterior auricular and facial proprioceptors) by immunolabeling geniculate ganglia from Phox2b-tdTomato mice with anti-Islet-1 (Figure 3d–f), which is a transcription factor important for determining sensory neuronal fate (Sun et al., 2008) and labels all neurons within the geniculate ganglion (Harlow, Yang, Williams, & Barlow, 2011). We found that the geniculate ganglion contained 1,572.3 ±202.5, (1,367, 1,578, 1,772) neurons, of which 658.6 ±18.4 (42%; 643, 654, 679) were Phox2b-positive (i.e., taste neurons). Our results indicate that 58% of neurons in the mouse geniculate ganglion provide somatosensory innervation the ear, 27% project to the tongue via the chorda tympani, and 15% project to the palate through the greater superficial petrosal nerve.
3.3 | Chorda tympani nerve transection eliminates all Phox2b-tdTomato-positive fibers from taste buds but not from all fungiform papillae
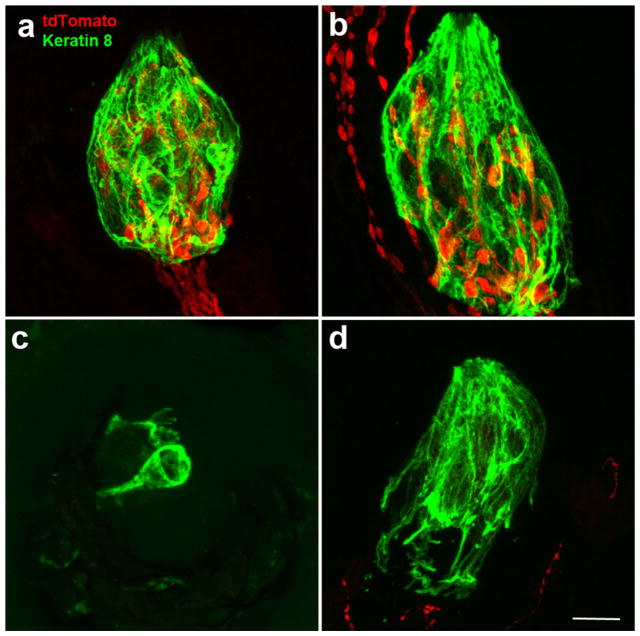
Whereas Phox2b-tdTomato labels all chorda tympani and greater superficial petrosal neurons within the geniculate ganglion, it is unclear whether all the Phox2b-tdTomato-positive innervation to taste buds and papillae originates from these gustatory nerves. To examine this issue, we transected the chorda tympani nerve and compared the remaining innervation to fungiform papillae on the sectioned versus the unsectioned sides of the tongue. In general, disrupting innervation results in a loss of taste buds (Guagliardo & Hill, 2007; Oakley, Wu, Lawton, & deSibour, 1990), although roughly half the fungiform taste buds remain present with an altered morphology following nerve section (Guagliardo & Hill, 2007; Meng et al., 2017; Whitehead, Frank, Hettinger, Hou, & Nah, 1987). On the un-transected side of the tongue, we found that all fungiform papillae and taste buds (identified with keratin-8, green) had tdTomato-positive innervation (Figure 4a). In addition, some fungiform papillae also had labeled innervation within the fungiform papillae but outside the taste bud (Figure 4b). By contrast, on the transected side of the tongue, we found no taste buds with tdTomato-positive innervation within the keratin-8 borders (Figure 4c, d), indicating that all labeled innervation within taste buds was from the chorda tympani nerve. However, some taste buds were surrounded by tdTomato-positive innervation, indicating that at least some of the non-taste innervation within the fungiform papillae is not derived from the chorda tympani nerve. Therefore, the chorda tympani is the only source of Phox2b-positive innervation to the fungiform taste bud but is not the source of the non-taste Phox2b-positive innervation to the fungiform papillae.
FIGURE 4.
Following unilateral chorda tympani transection, most taste buds on the un-transected side of the tongue had nerve fibers specifically innervating taste buds (a), whereas some taste buds also had innervation outside the taste bud but within the fungiform papilla (b). On the transected side of the tongue, most taste buds lacked innervation both inside and outside the keratin-8 border (c), although some taste buds still had Phox2b-tdTomato-positive fibers in the papilla and outside the taste bud (d). All images are a projected z-stack of 20 μm sections. Scale bar in (d) =10 μm and refers to all images
3.4 | Additional innervation to papillae arises from autonomic sources
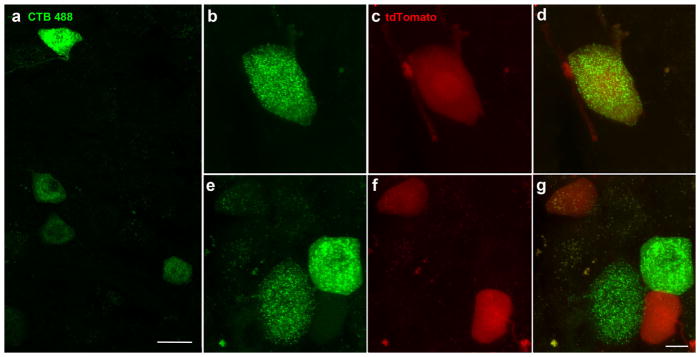
In addition to chorda tympani nerve fibers, 43% of fungiform papillae are also innervated by tdTomato-positive nerve fibers that innervate the fungiform papillae without innervating taste buds. We observed a few scattered tdTomato-labeled neurons in the whole mount trigeminal ganglion from Phox2b-Cre:tdtomato mice. To determine whether these neurons innervated the tongue, we injected the tongues of Phox2b-tdTomato mice with a green neural tracer (CTB-488) and examined serial sections through both trigeminal ganglia for double-labeled neurons (n =3). CTB-labeled neurons innervating the tongue were scattered throughout the mandibular portion of the trigeminal ganglion (Figure 5a). Most sections in this region had at least one and most had several CTB-488–labeled neurons (Figure 5a), while tdTomato-positive neurons were not seen in every section and were more sparsely distributed. We found only one double-labeled neuron from a total of three mice (Figure 5b–d). Although a few tdTomato-positive trigeminal neurons may innervate the tongue, their number seemed insufficient to account for all of the extra innervation to fungiform papillae.
FIGURE 5.
Trigeminal ganglion neurons following injection of the neural tracer CTB-488 into the tongue, were scattered throughout the mandibular division of the trigeminal ganglion (a). We found one double-labeled neuron (b, c, d) as well as neurons that were Phox2b-tdTomato-positive or Alexa-488-positive only (e, f, g). Images are the projected z-stack of 20 μm section. The scale bar in (a) =20 μm, the scale bar in (g) =10 μm and applies to (b–g)
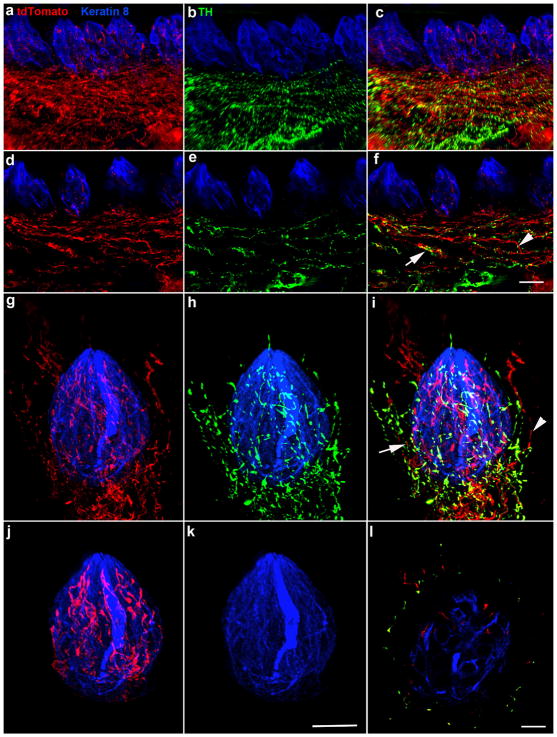
Phox2b is a transcription factor that regulates both sympathetic and parasympathetic neuron development (Pattyn, Morin, Cremer, Goridis, & Brunet, 1997; Stanke et al., 1999), and tyrosine hydroxylase (TH)-positive fibers are characteristic of sympathetic nerve innervation and are present in taste papillae (Dvoryanchikov, Tomchik, & Chaudhari, 2007). Therefore, to determine whether the non-taste fibers in fungiform papillae could be sympathetic fibers, we labeled gustatory regions with TH (green) in Phox2b-tdTomato mice (n =3). We observed TH-positive fibers in circumvallate papillae (Figure 6a–f) and in some fungiform papillae (Figure 6g–l). In both circumvallate and fungiform papillae, most TH-positive fibers were also tdTomato-positive, suggesting that a portion of non-gustatory tdTomato-positive innervation to fungiform papillae is sympathetic innervation (Figure 6a–f). TH-positive innervation was only present in papillae that also contained non-taste TH-negative/tdTomato-positive innervation. TH-positive/tdTomato-positive innervation surrounded taste buds in whole-mount preparations but did not extend into the epithelium (Figure 6g–i), whereas a subset of TH-negative/tdTomato-positive fibers did extend above the pore and into the epithelium (Figure 6g, i). Using software to isolate all label only within the keratin 8 defined taste bud, we observed that only TH-negative/tdTomato-positive innervation was present in taste buds within fungiform papillae (Figure 6j, k; Supporting Information Movie S2). Viewing a single optical section through the taste bud that only TH-negative/tdTomato-positive innervation is present within the taste bud (Figure 6l).
FIGURE 6.
TH-negative/tdTomato-positive and TH-positive/tdTomato-positive innervation was present in circumvallate papilla (a–c, z-stack, 70 μm). Single optical sections show that TH-negative/tdTomato-positive (arrowhead) and TH-positive/tdTomato-positive (arrow) fibers in circumvallate papillae appeared to run together, making it difficult to distinguish whether there was also a population of TH-positive, tdTomato-negative nerve fibers (d–f). Only TH-negative/tdTomato-positive innervation was observed within circumvallate taste buds (a–f). Fungiform papillae demonstrated a similar pattern of innervation in whole-mount preparations. The tdTomato-labeled (red, g), TH-labeled (green, h), and merged innervation is shown on the z axis of a whole mount fungiform taste bud (keratin 8, blue, i). Both TH-negative/tdTomato-positive (arrowhead) and TH-positive/tdTomato-positive (arrow) fibers were present in some fungiform papillae and ran closely alongside each other. Interestingly, TH-negative/tdTomato-positive fibers penetrated the epithelium, whereas double-labeled fibers tended not to extend above taste buds (g–i). We used the keratin 8 border to mask the label within the taste bud and remove the label outside the taste bud. This illustrates that tdTomato-positive innervation (red) is present within the taste bud (j), whereas TH-positive innervation (green) was not (k). This can also be seen in a single optical section through the same taste bud, where green innervation is only outside the keratin 8 border (i). Scale bar in (f) =20 μm and applies to (a–f), scale bar in (k) =15 μm and applies to (g–k), scale bar in (l) =10 μm
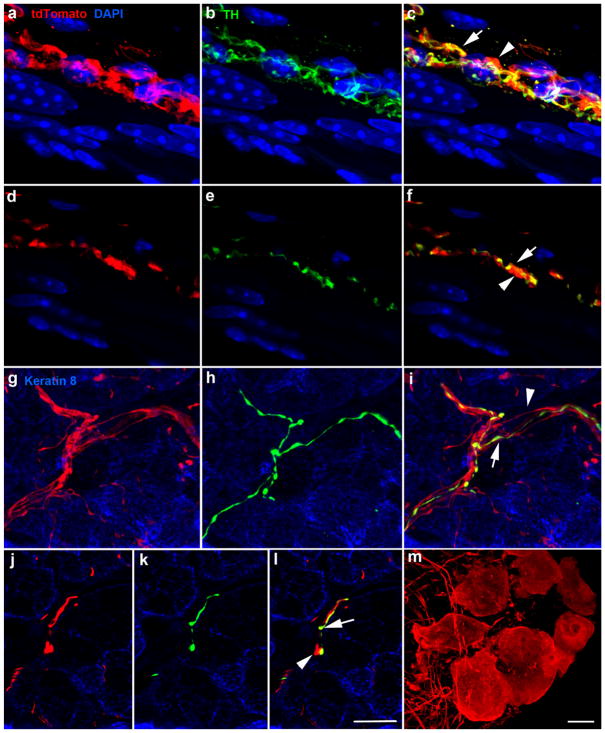
As expected, based on previous studies of sympathetic fibers in the tongue, sympathetic TH-positive/tdTomato-positive innervation was also present encircling blood vessels (Figure 7a–f) and in Von Ebner’s glands (Figure 7g–l). In both locations, TH-negative/tdTomato-positive fibers appeared to run alongside TH-positive/tdTomato-negative fibers (Figure 7c, f, i), suggesting that parasympathetic nerve fibers were tdTomato-positive. Consistent with this, neuron cell bodies of the lingual parasympathetic ganglia were tdTomato-positive (Figure 7m).
FIGURE 7.
Image z-stack (70 μm) of blood vessel innervated by tdTomato-positive fibers (red, a), TH-positive fibers (green, b), and overlay (c). TH-negative/tdTomato-positive and TH-positive/tdTomato-positive fibers run alongside each other. A single optical section from the z-stack illustrates individual TH-negative/tdTomato-positive fibers (arrowhead) and TH-positive/tdTomato-positive (arrow) (d–f). Innervation of Von Ebner’s glands was similar to that of blood vessels in that TH-negative/tdTomato–positive fibers (arrowhead) ran closely alongside TH-positive/tdTomato double-labeled fibers (arrow) (g, i). Single optical slices of von Ebner’s gland illustrating a single TH-negative/tdTomato-positive fiber (arrowhead) and TH-positive/tdTomato-positive fibers (arrow) (j–l). Cell bodies of the lingual parasympathetic ganglia were also tdTomato-positive (m). Scale bar in (l) =10 μm and applies to (a–l). The scale bar in (m) =10 μm
3.5 | Phox2b-tdTomato is a useful marker for examining gustatory innervation to the tongue during embryonic development
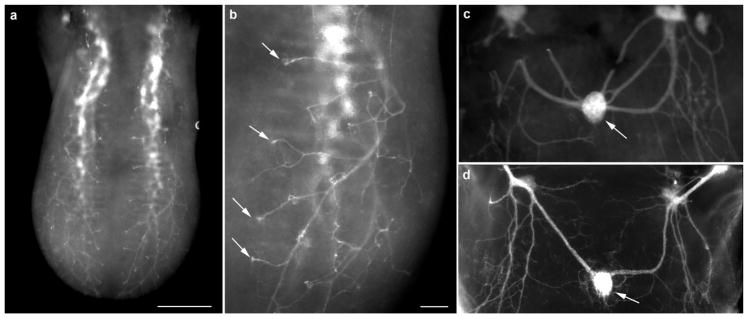
Whole-mount labeling of the tongue is a useful method for examining target innervation (Lopez & Krimm, 2006b; Ma, Lopez, & Krimm, 2009); however, such experiments are typically conducted using DiI-labeling in fixed embryos, which is not a perfect approach. To determine whether genetic labeling could replace DiI-labeling for developmental studies, we examined whole-mount tongues of Phox2b-tdTomato mice immediately after dissection with no additional processing. Similar to previous observations (Lopez & Krimm, 2006b), we observed that nerve fascicles exhibited a clear stereotyped branching pattern (Figure 8a), in which individual fiber bundles reached the tongue surface and branched, forming a small tuft (Figure 8b, arrows). Similarly, the tdTomato-positive innervation to the circumvallate papilla (arrow, Figure 8c) was similar to that observed in DiI-labeled tissue (Figure 8d) Thus, genetic labeling under the control of Phox2b appeared similar and could replace DiI-labeling of fixed embryos for examining nerve fiber innervation to the tongue during development.
FIGURE 8.
Whole tongue from an embryonic day 14.5 Phox2b-Cre:tdTomato mouse. Without any additional labeling, fiber bundles could be seen entering the base of the tongue and branching near the surface (a). Fibers could be seen projecting to specific locations on the tongue surface where fungiform papillae were located (arrows) (b). tdTomato-positive innervation was also seen innervating the circumvallate papilla (arrow) in the back of the same tongue (c) and innervation has a similar pattern as seen in fixed DiI-labeled tissue (d). Scale bar: (a) =200 μm, (b) =250 μm (applies to c and d)
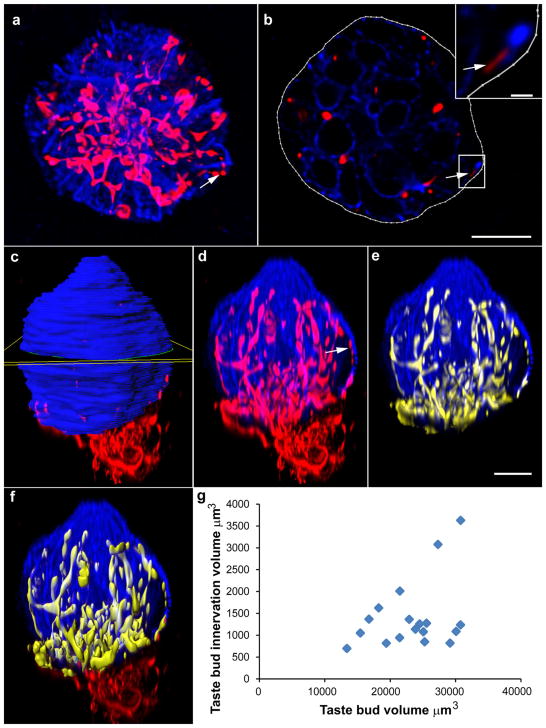
Methods for accurately measuring taste bud innervation are important for assessing the effects of various manipulations on taste bud structure and function. Previous studies rely on immunohistochemical labels that are not uniformly expressed throughout nerve fibers (Castillo et al., 2014; Meng et al., 2015), and antibodies may not sufficiently penetrate thick tissue or whole mounts. Here, we quantified tdTomato-positive innervation in whole mounts to allow for three-dimensional examination of entire taste buds without sampling bias (Figure 9). We found bright and reliable labels in the whole mounts, permitting easy visualization, and measurement of innervation. Next, we deconvoluted and analyzed images of whole-mount taste buds to measure total taste bud volume and the volume of innervation within taste buds. The contour function was used to outline the taste bud in each optical slice along the axis of highest resolution. These outlines were used to generate a volume which was representative of the total volume of the taste bud (Figure 9a–c). Masking the fluorescent channels within the taste bud allowed for creation of a duplicate fluorescent channels each representing only the taste bud (blue; Figure 9d) and then eliminating innervation outside the taste bud (Figure 9e). This new channel was used to generate a surface representing the total volume of innervation within the taste bud (Figure 9f). Whole mount staining of taste buds revealed a distribution of both taste buds and innervation volumes. We found that total taste bud volume ranged from 12,000 to 32,000 μm3, and the volume of innervation within taste buds ranged from 600 to 3,600 μm3. As taste bud volume increases some taste buds exhibit additional innervation while others do not, such that the variation in taste bud innervation increases as taste bud volume increases (Figure 9g; see also Supporting Information Movie S1).
FIGURE 9.
Image oriented in the plane of highest resolution (viewed from dorsal surface) (a). The contour function was used to manually draw the border of keratin 8-marked taste buds in each optical section (b). Arrows in (a), (b), and (d) indicate a location where two red dots do not appear to be connected by a filamentous red fiber. The single optical slice in (b) is taken from this location where there appears to be a break in fluorescence demonstrating that fibers can still be observed when the taste bud is viewed section-by-section in the plane of highest resolution. Insert shows a magnified image of this fiber. After a taste bud was outlined in all slices, a surface was created that represented total taste bud volume (c, yellow square indicating one slice). Masking the fluorescent channel corresponding to keratin 8 inside the taste bud surface allowed for duplication of the fluorescent signal from that channel within the taste bud only, generating a new fluorescent channel representing the taste bud but excluding any blue label outside the taste bud (d) or all of the label outside the taste bud (e). Generating a surface for red fluorescence inside the taste bud allowed quantification of the volume of innervation (f). Scale bar in (b) =10 μm (applies to a), scale bar in inset =1 μm, scale bar in (e) =10 μm (applies to c–f). Measurements of taste bud volume and volume of innervation within the taste bud revealed that there is not a correlation between the volume of the taste bud and the volume of innervation (g)
4 | DISCUSSION
The geniculate, nodose, and petrosal ganglia originate in epibranchial placodes and are considered primarily visceral sensory neurons (Baker & Bronner-Fraser, 2001; Krimm, 2007; Schlosser, 2010). All of these ganglia express the transcription factor Phox2b (D’Autreaux et al., 2011; Dauger et al., 2003). Also, Phox2b is expressed in the central target for these ganglia, the nucleus of the solitary tract (Stornetta et al., 2006). Phox2b is required for the normal development of both gustatory ganglia and the nucleus of the solitary tract (Dauger et al., 2003) and mice lacking Phox2b do not develop a chorda tympani nerve (Coppola et al., 2010). It was originally reported that the entire geniculate ganglion was lost in the absence of Phox2b (Dauger et al., 2003), which is consistent with findings that very few geniculate neurons are derived from neural crest in mammals (Harlow & Barlow, 2007). However, more recently Phox2b has been reported to be important for visceral sensory neuron target innervation and is only expressed in half the geniculate ganglion neurons (D’Autreaux et al., 2011). Consistent with this, we found that within the geniculate ganglion only neurons projecting through gustatory nerves ever express Phox2b. Since non-taste neurons of the geniculate ganglion are not visceral sensory, they are likely regulated by a different set of transcription factors during development (Quina, Tempest, Hsu, Cox, & Turner, 2012). These data suggest that Phox2b could be useful for identifying oral cavity-projecting neurons in the geniculate ganglion during early development, for expression analysis, and in vitro physiology. Also, as most non-chorda tympani tongue afferents lack Phox2b, this transcription factor could be a useful genetic label for studying tongue innervation and/or conditional gene removal during development. Furthermore, Phox2b can be used to limit labeling to neuron subsets using intersectional genetics (Hirsch, d’Autreaux, Dymecki, Brunet, & Goridis, 2013).
Here, we took advantage of the genetic Phox2b label to determine the proportion of geniculate neurons that project via the two gustatory nerves compared with the posterior auricular and other branches of the facial nerve. Our data were consistent with previous findings in rats, where ~28% of geniculate neurons project through the chorda tympani, 20% through the greater superficial petrosal, and 44% through the facial nerve (Gomez, 1978; Semba et al., 1984). It is interesting that fewer taste neurons project to the palate in both species, even though there are substantially more taste buds in the palate than in the tongue (Miller & Spangler, 1982; Ohtubo & Yoshii, 2011). As many of these neurons are gustatory, this implies that neurons in the palate may innervate a greater number of taste receptor cells than neurons in the tongue. However, as some tongue afferents are somatosensory in function (Yokota & Bradley, 2016), another possibility is that there are more somatosensory neurons innervating the tongue via the chorda tympani compared with the palate via the greater superficial petrosal.
Although the relative number of neurons projecting through each nerve are similar to those described previously, we found more neurons in the geniculate ganglion than were previously estimated. Specifically, the 378 neurons we found projecting through the chorda tympani was greater than the 213 (mouse) or 244 (hamster) neurons that have been estimated to innervate tongue based on single papilla injections (Whitehead, Ganchrow, Ganchrow, & Yao, 1999; Zaidi & Whitehead, 2006). These earlier estimates could be low because they are based on sampling the number of neurons innervating only a subset of papillae, which may not be representative of the full population. In addition, they could be low because they were estimated from a formula using the value of 52 taste buds per half tongue in the denominator (Zaidi & Whitehead, 2006), which is high for an adult mouse (Patel & Krimm, 2012). We also found more neurons projecting through the mouse chorda tympani nerve (Ishida et al., 2009) and more total neurons (1,572) in the geniculate ganglion than previously estimated in sectioned ganglia. However, given that we followed each cell in serial optical sections of a whole mount ganglia, our neuron counts are more accurate than in previous studies in which neuron number was estimated (Coggeshall, La Forte, & Klein, 1990).
Approximately 43% of fungiform papillae contained Phox2b-tdTomato-positive fibers innervating fungiform papillae outside the taste bud. Some of these fibers may be somatosensory innervation from the chorda tympani (Yokota & Bradley, 2016). However, because some tdTomato-positive innervation still remains in fungiform papillae following chorda tympani nerve transection, some is also from another source. As we only observed a few scattered trigeminal neurons that were tdTomato-positive and these did not co-localize with tongue injections these additional neurons are not likely sensory. Instead, it is more likely that at least some of these additional fibers are sympathetic in nature since (a) Phox2b is expressed in and regulates sympathetic neuron development (Pattyn et al., 1997) and noradrenergic phenotype (Stanke et al., 1999), (b) taste papillae receive sympathetic innervation (Dvoryanchikov et al., 2007; Hua, Yang, Yang, Liu, & Tang, 2013), and (c) many tdTomato-positive fibers that innervate papillae also express TH. While it is also the case the some somatosensory subpopulations are also TH-positive (Li et al., 2011; Usoskin et al., 2015), most of these fibers would originate from the trigeminal ganglion and we ruled out this possibility as a source of extra innervation. Therefore, Phox2b-tdTomato-positive/TH-positive innervation is likely sympathetic in nature. We also found some, non-taste tdTomato-positive innervation to the papilla that was TH-negative, and therefore not sympathetic. Since we observed tdTomato-positive parasympathetic ganglia and these fibers consistently innervated the same papillae as putative sympathetic fibers, some of these fibers may be of parasympathetic origin. In conclusion, the non-taste Phox2b fibers in the fungiform papillae are likely a mixture of mechanoreceptors from the geniculate ganglion and autonomic fibers from the parasympathetic and sympathetic ganglia.
Our findings differ from a previous published Phox2b-Cre mouse line in which Cre expression is not present in sympathetic or parasympathetic neurons or described in the trigeminal ganglion (Scott, Williams, Rossi, Lee, & Elmquist, 2011). The transgenic line described here mimics endogenous expression patterns of Phox2b in sympathetic and parasympathetic ganglion (Pattyn et al., 1997). While previous studies have not described Phox2b expression in the trigeminal ganglion, it is not clear if previous studies had sufficient sensitivity to detect the small number of scattered Phox2b cells that may be present in the trigeminal. In addition, Phox2b may be decreased during development in the trigeminal. Therefore, Phox2b expression in these neurons may simply have been missed, particularly since the trigeminal ganglion is a complex ganglion with mixed embryonic origins (Park & Saint-Jeannet, 2010), which includes a visceral sensory subpopulation (Horgan & van der Kooy, 1992). However, it is also possible that this represents miss-expression of the transgene. In conclusion, there are two transgenic Phox2b-Cre mouse lines available with slightly different distributions of Cre expression (Scott et al., 2011), but they may both express Cre recombinase in visceral sensory neurons including gustatory neurons.
Accurate measures of tongue and taste bud innervation are critical for understanding the development and maintenance of this innervation. DiI-labeling has been used to examine innervation to the tongue during development (Lopez & Krimm, 2006a, 2006b; Ma et al., 2009), but this technique has limitations. Specifically, if the nerve is pulled or damaged during dissection then DiI-label is not transported in a subset of animals. Also, when a manipulation eliminates most innervation to the tongue, it can be difficult to know whether lack of innervation is the effect of the knockout or failure of the DiI to be transported. In Phox2b-tdTomato mice, gustatory fibers can be seen innervating the tongue at embryonic day 14.5, when targeting first occurs in every animal. Thus, we propose that the use of Phox2b-tdTomato mice is superior to DiI-labeling for examining target innervation. Genetic labeling also provides an even distribution of label in nerve fibers of the adult taste bud, permitting more accurate representation of taste bud innervation.
Supplementary Material
Acknowledgments
Funding information
NIH, Grant/Award Number: DC007176
This work was supported by NIH grant DC007176.
Footnotes
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Developmental Biology. 2001;232(1):1–61. doi: 10.1006/dbio.2001.0156. https://doi.org/10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Castillo D, Seidel K, Salcedo E, Ahn C, de Sauvage FJ, Klein OD, Barlow LA. Induction of ectopic taste buds by SHH reveals the competency and plasticity of adult lingual epithelium. Development. 2014;141(15):2993–3002. doi: 10.1242/dev.107631. https://doi.org/10.1242/dev.107631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD. The cell biology of taste. The Journal of Cell Biology. 2010;190(3):285–296. doi: 10.1083/jcb.201003144. https://doi.org/10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE, La Forte R, Klein CM. Calibration of methods for determining numbers of dorsal root ganglion cells. Journal of Neuroscience Methods. 1990;35(3):187–194. doi: 10.1016/0165-0270(90)90123-w. [DOI] [PubMed] [Google Scholar]
- Coppola E, Rallu M, Richard J, Dufour S, Riethmacher D, Guillemot F, … Brunet JF. Epibranchial ganglia orchestrate the development of the cranial neurogenic crest. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(5):2066–2071. doi: 10.1073/pnas.0910213107. https://doi.org/10.1073/pnas.0910213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Autreaux F, Coppola E, Hirsch MR, Birchmeier C, Brunet JF. Homeoprotein Phox2b commands a somatic-to-visceral switch in cranial sensory pathways. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(50):20018–20023. doi: 10.1073/pnas.1110416108. https://doi.org/10.1073/pnas.1110416108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, Gallego J, Brunet JF. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130(26):6635–6642. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- Dvoryanchikov G, Tomchik SM, Chaudhari N. Biogenic amine synthesis and uptake in rodent taste buds. Journal of Comparative Neurology. 2007;505(3):302–313. doi: 10.1002/cne.21494. https://doi.org/10.1002/cne.21494. [DOI] [PubMed] [Google Scholar]
- Folan-Curran J, Cooke FJ. Contribution of cranial nerve ganglia to innervation of the walls of the rat external acoustic meatus. Journal of the Peripheral Nervous System. 2001;6(1):28–32. doi: 10.1046/j.1529-8027.2001.006001028.x. [DOI] [PubMed] [Google Scholar]
- Gomez MM. Doctor of Philosophy. Wake Forest University; Winston-Salem, NC: 1978. The peripheral distribution of the rat geniculate ganglion. [Google Scholar]
- Guagliardo NA, Hill DL. Fungiform taste bud degeneration in C57BL/6J mice following chorda-lingual nerve transection. Journal of Comparative Neurology. 2007;504(2):206–216. doi: 10.1002/cne.21436. https://doi.org/10.1002/cne.21436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow DE, Barlow LA. Embryonic origin of gustatory cranial sensory neurons. Developmental Biology. 2007;310(2):317–328. doi: 10.1016/j.ydbio.2007.07.042. https://doi.org/10.1016/j.ydbio.2007.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow DE, Yang H, Williams T, Barlow LA. Epibranchial placode-derived neurons produce BDNF required for early sensory neuron development. Developmental Dynamics. 2011;240(2):309–323. doi: 10.1002/dvdy.22527. https://doi.org/10.1002/dvdy.22527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch MR, d’Autreaux F, Dymecki SM, Brunet JF, Goridis C. A Phox2b::FLPo transgenic mouse line suitable for intersectional genetics. Genesis. 2013;51(7):506–514. doi: 10.1002/dvg.22393. https://doi.org/10.1002/dvg.22393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan K, van der Kooy D. Visceral targets specify calcitonin gene-related peptide and substance P enrichment in trigeminal afferent projections. Journal of Neuroscience. 1992;12(4):1135–1143. doi: 10.1523/JNEUROSCI.12-04-01135.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua TE, Yang TL, Yang WC, Liu KJ, Tang SC. 3-D neurohistology of transparent tongue in health and injury with optical clearing. Frontiers in Neuroanatomy. 2013;7:36. doi: 10.3389/fnana.2013.00036. https://doi.org/10.3389/fnana.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Ma L, Krimm RF. Postnatal reduction of BDNF regulates the developmental remodeling of taste bud innervation. Developmental Biology. 2015;405(2):225–236. doi: 10.1016/j.ydbio.2015.07.006. https://doi.org/10.1016/j.ydbio.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Ugawa S, Ueda T, Yamada T, Shibata Y, Hondoh A, … Shimada S. P2X(2)- and P2X(3)-positive fibers in fungiform papillae originate from the chorda tympani but not the trigeminal nerve in rats and mice. Journal of Comparative Neurology. 2009;514(2):131–144. doi: 10.1002/cne.22000. https://doi.org/10.1002/cne.22000. [DOI] [PubMed] [Google Scholar]
- Krimm RF. Factors that regulate embryonic gustatory development. BMC Neuroscience. 2007;8(Suppl 3):S4. doi: 10.1186/1471-2202-8-S3-S4. https://doi.org/10.1186/1471-2202-8-S3-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimm RF, Miller KK, Kitzman PH, Davis BM, Albers KM. Epithelial overexpression of BDNF or NT4 disrupts targeting of taste neurons that innervate the anterior tongue. Developmental Biology. 2001;232(2):508–521. doi: 10.1006/dbio.2001.0190. [DOI] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, … Ginty DD. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147(7):1615–1627. doi: 10.1016/j.cell.2011.11.027. https://doi.org/10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez GF, Krimm RF. Epithelial overexpression of BDNF and NT4 produces distinct gustatory axon morphologies that disrupt initial targeting. Developmental Biology. 2006a;292(2):457–468. doi: 10.1016/j.ydbio.2006.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez GF, Krimm RF. Refinement of innervation accuracy following initial targeting of peripheral gustatory fibers. Journal of Neurobiology. 2006b;66(10):1033–1043. doi: 10.1002/neu.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Lopez GF, Krimm RF. Epithelial-derived brain-derived neurotrophic factor is required for gustatory neuron targeting during a critical developmental period. Journal of Neuroscience. 2009;29(11):3354–3364. doi: 10.1523/JNEUROSCI.3970-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Huang T, Sun C, Hill DL, Krimm R. BDNF is required for taste axon regeneration following unilateral chorda tympani nerve section. Experimental Neurology. 2017;293:27–42. doi: 10.1016/j.expneurol.2017.03.016. https://doi.org/10.1016/j.expneurol.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Ohman-Gault L, Ma L, Krimm RF. Taste bud-derived BDNF is required to maintain normal amounts of innervation to adult taste buds(1,2,3) eNeuro. 2015;2(6) doi: 10.1523/ENEURO.0097-15.2015. https://doi.org/10.1523/ENEURO.0097-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller IJ, Jr, Spangler K. Taste bud distribution and innervation on the palate of the rat. Chemical Senses. 1982;7:99–108. [Google Scholar]
- Nosrat IV, Margolskee RF, Nosrat CA. Targeted taste cell-specific overexpression of brain-derived neurotrophic factor in adult taste buds elevates phosphorylated TrkB protein levels in taste cells, increases taste bud size, and promotes gustatory innervation. The Journal of Biological Chemistry. 2012;287(20):16791–16800. doi: 10.1074/jbc.M111.328476. https://doi.org/10.1074/jbc.M111.328476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B, Wu LH, Lawton A, deSibour C. Neural control of ectopic filiform spines in adult tongue. Neuroscience. 1990;36(3):831–838. doi: 10.1016/0306-4522(90)90026-z. [DOI] [PubMed] [Google Scholar]
- Ohtubo Y, Yoshii K. Quantitative analysis of taste bud cell numbers in fungiform and soft palate taste buds of mice. Brain Research. 2011;1367:13–21. doi: 10.1016/j.brainres.2010.10.060. https://doi.org/10.1016/j.brainres.2010.10.060. [DOI] [PubMed] [Google Scholar]
- Park B-Y, Saint-Jeannet J-P. Induction and segregation of the vertebrate cranial placodes. Vol. 1. Morgan and Claypool Life Sciences; San Rafael, California: 2010. [PubMed] [Google Scholar]
- Patel AV, Krimm RF. BDNF is required for the survival of differentiated geniculate ganglion neurons. Developmental Biology. 2010;340(2):419–429. doi: 10.1016/j.ydbio.2010.01.024. https://doi.org/10.1016/j.ydbio.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AV, Krimm RF. Neurotrophin-4 regulates the survival of gustatory neurons earlier in development using a different mechanism than brain-derived neurotrophic factor. Developmental Biology. 2012;365(1):50–60. doi: 10.1016/j.ydbio.2012.02.008. https://doi.org/10.1016/j.ydbio.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124(20):4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- Quina LA, Tempest L, Hsu YW, Cox TC, Turner EE. Hmx1 is required for the normal development of somatosensory neurons in the geniculate ganglion. Developmental Biology. 2012;365(1):152–163. doi: 10.1016/j.ydbio.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutlin M, Ho CY, Abraira VE, Cassidy C, Bai L, Woodbury CJ, Ginty DD. The cellular and molecular basis of direction selectivity of Adelta-LTMRs. Cell. 2014;159(7):1640–1651. doi: 10.1016/j.cell.2014.11.038. https://doi.org/10.1016/j.cell.2014.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser G. Making senses development of vertebrate cranial placodes. International Review of Cell and Molecular Biology. 2010;283:129–234. doi: 10.1016/S1937-6448(10)83004-7. https://doi.org/10.1016/S1937-6448(10)83004-7. [DOI] [PubMed] [Google Scholar]
- Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. The Journal of Clinical Investigation. 2011;121(6):2413–2421. doi: 10.1172/JCI43703. https://doi.org/10.1172/JCI43703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K, Sood V, Shu NY, Nagele RG, Egger MD. Examination of geniculate ganglion cells contributing sensory fibers to the rat facial ‘motor’ nerve. Brain Research. 1984;308(2):354–359. doi: 10.1016/0006-8993(84)91077-1. [DOI] [PubMed] [Google Scholar]
- Stanke M, Junghans D, Geissen M, Goridis C, Ernsberger U, Rohrer H. The Phox2 homeodomain proteins are sufficient to promote the development of sympathetic neurons. Development. 1999;126(18):4087–4094. doi: 10.1242/dev.126.18.4087. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, … Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. Journal of Neuroscience. 2006;26(40):10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. https://doi.org/10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Dayal A, Hill DL. Expanded terminal fields of gustatory nerves accompany embryonic BDNF overexpression in mouse oral epithelia. Journal of Neuroscience. 2015;35(1):409–421. doi: 10.1523/JNEUROSCI.2381-14.2015. https://doi.org/10.1523/JNEUROSCI.2381-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Hummler E, Hill DL. Selective deletion of sodium salt taste during development leads to expanded terminal fields of gustatory nerves in the adult mouse nucleus of the solitary tract. Journal of Neuroscience. 2017;37(3):660–672. doi: 10.1523/JNEUROSCI.2913-16.2016. https://doi.org/10.1523/JNEUROSCI.2913-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Dykes IM, Liang X, Eng SR, Evans SM, Turner EE. A central role for Islet1 in sensory neuron development linking sensory and spinal gene regulatory programs. Nature Neuroscience. 2008;11(11):1283–1293. doi: 10.1038/nn.2209. https://doi.org/10.1038/nn.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, … Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nature Neuroscience. 2015;18(1):145–153. doi: 10.1038/nn.3881. https://doi.org/10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Frank ME, Hettinger TP, Hou LT, Nah HD. Persistence of taste buds in denervated fungiform papillae. Brain Research. 1987;405(1):192–195. doi: 10.1016/0006-8993(87)91008-0. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Ganchrow JR, Ganchrow D, Yao B. Organization of geniculate and trigeminal ganglion cells innervating single fungiform taste papillae: A study with tetramethylrhodamine dextran amine labeling. Neuroscience. 1999;93(3):931–941. doi: 10.1016/s0306-4522(99)00115-3. [DOI] [PubMed] [Google Scholar]
- Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: Immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. Journal of Comparative Neurology. 2001;440(1):97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- Yokota Y, Bradley RM. Receptive field size, chemical and thermal responses, and fiber conduction velocity of rat chorda tympani geniculate ganglion neurons. Journal of Neurophysiology. 2016;115(6):3062–3072. doi: 10.1152/jn.00045.2016. https://doi.org/10.1152/jn.00045.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi FN, Whitehead MC. Discrete innervation of murine taste buds by peripheral taste neurons. Journal of Neuroscience. 2006;26(32):8243–8253. doi: 10.1523/JNEUROSCI.5142-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.