Abstract
In recent years, drug conjugate vaccines have shown promise as therapeutics for substance use disorder. As a means to improve the efficacy of a heroin conjugate vaccine, we systematically explored 20 vaccine formulations with varying combinations of carrier proteins and adjuvants. In regard to adjuvants, we explored a Toll-like receptor 9 (TLR9) agonist and a TLR3 agonist in the presence of alum. The TLR9 agonist was cytosine-guanine oligodeoxynucleotide 1826 (CpG ODN 1826), while the TLR3 agonist was virus-derived genomic doubled-stranded RNA (dsRNA). The vaccine formulations containing TLR3 or TLR9 agonist alone elicited strong antiheroin antibody titers and blockade of heroin-induced antinociception when formulated with alum; however, a combination of TLR3 and TLR9 adjuvants did not result in improved efficacy. Investigation of month-long stability of the two lead formulations revealed that the TLR9 but not the TLR3 formulation was stable when stored as a lyophilized solid or as a liquid over 30 days. Furthermore, mice immunized with the TLR9 + alum heroin vaccine gained significant protection from lethal heroin doses, suggesting that this vaccine formulation is suitable for mitigating the harmful effects of heroin, even following month-long storage at room temperature.
Keywords: heroin, opioids, vaccine, immunopharmacotherapy, adjuvants
Graphical Abstract
INTRODUCTION
Heroin is a schedule I, highly addictive opioid drug and a significant public health concern. In the United States (US), drug overdose deaths have nearly tripled between 1999 and 2014.1 In 2015, 52 404 overdose deaths were reported, 63% of which involved opioids.1 Recently, there has been a marked increase in prescriptions of synthetic opioid pain relievers (OPRs) for management of chronic pain.2 Evidence suggests that misuse of OPRs is the strongest risk factor for initiating heroin abuse, and OPR users are 40-times more likely to abuse heroin.3,4 This phenomenon is driven by the relatively low cost of heroin and its wide availability.3,4 Current treatments for heroin addiction involve opioid replacement therapy, for example, methadone administration, to promote heroin detoxification.5 Unfortunately, the addictive nature of heroin and other opioids, combined with the adverse effects of withdrawal and high cost of treatment, leads to a high incidence of drug relapse.4 In the face of increasing opioid abuse and overdose, the development of improved therapies that can attenuate the effects of opioids is crucial.
Vaccination is a promising strategy to promote cessation of heroin abuse and prevent relapse. Implementation of this strategy involves active immunization using a small molecule–protein conjugate, which elicits high-affinity, drug-specific antibodies. These polyclonal IgG antibodies sequester free drug in the blood and prevent access to the brain, subsequently reducing the drug compound’s psychoactive effects. This approach has been preclinically validated for vaccines against nicotine,6,7 cocaine,8,9 and methamphetamine.10,11 For heroin specifically, vaccination efficacy has been repeatedly demonstrated in mice, rats, and nonhuman primates.12–18
In general, formulation of a vaccine with an adjuvant is an attractive approach to enhance the magnitude and length of vaccine immunity against the target antigen by stimulating antigen presenting cells, T-cells or B-cells. Historically, Alhydrogel (alum) has been the most commonly used adjuvant, but numerous alternatives have been pursued in recent years.19 Adjuvants can act as pathogen-associated molecular patterns (PAMPs), which activate Toll-like receptors (TLRs) resulting in upregulation of an immune response. Specific PAMPs include lipopolysaccharides (LPS), double-stranded RNA (dsRNA), and unmethylated cytosine-guanine (CpG) motifs.19 However, at this time only a limited number of adjuvants are approved for use in humans. By exploring new adjuvants or combinations of adjuvants, we can rationally design vaccines with enhanced immunogenicity directed toward production of heroin-neutralizing antibodies.
CpG oligodeoxynucleotide (ODN) 1826 is a class-B ODN that stimulates B-cell responses though TLR920,21 and was recently shown to elicit robust titers in antiheroin vaccine studies.13,22 Natural or synthetic dsRNA, for example, polyinosinic:polycytidylic acid (poly I:C), is a molecular pattern associated with viral replication, which elicits an immune response via TLR3 and has been used as an effective adjuvant in several vaccine studies.23–25 Given the potent immunostimulatory capacity of viral or bacterial PAMPs, we were interested in evaluating the efficacy of a yeast-derived viral dsRNA genome relative to CpG ODN using a well-studied dsRNA virus of Saccharomyces cerevisiae, L-A.26 To date, only the L-BC viral dsRNA genome generated from infected S. cerevisiae has been used as an adjuvant, where it increased immunogenicity of a prophylactic viral vaccine in mice.27
Here, we investigate L-A-derived dsRNA in combination with alum or CpG ODN in the context of our drug of abuse vaccine. Although alum is not necessary for TLR activation, it is one of the few adjuvants used in FDA-approved vaccines and has shown promising activity in antidrug vaccines. In comparison to alum, we selected conjugatable adjuvant lipid vesicles (CALV) as an alternative vehicle for vaccine delivery.28 CALVs are nanoparticulate liposomes designed to effectively deliver encapsulated antigens for immune uptake.
Our most successful antiheroin vaccine to date involves a second generation heroin hapten adjuvanted with alum and CpG (7, Figure 1).22 We used this formulation as a benchmark while investigating new adjuvant combinations and formulation conditions in an effort to find a lead vaccine candidate. We then measured the effects of adjuvant dosing on vaccine efficacy, and the vaccines were tested under various storage conditions for stability as a liquid or lyophilized solid after mixing with alum adjuvant and trehalose as a cryoprotectant. Our most successful formulation was then selected for an overdose challenge to see if protection was conferred against a lethal dose of heroin.
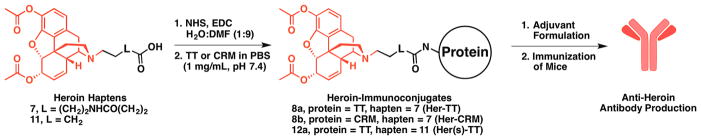
Figure 1.
Structures of the heroin haptens, corresponding immunoconjugates and the general vaccine approach. The structure of heroin is highlighted in red.
EXPERIMENTAL SECTION
Synthesis of Heroin Haptens and Conjugation to Carrier Proteins
The synthesis and characterization of the heroin haptens and immunoconjugates are described in detail in the Supporting Information (Figures S1–S14) along with additional information on animals, formulation conditions, vaccine administration schedule, behavioral testing, ELISA, and other experimental data. Our key hapten design element is a strategically placed linker on the morphinan nitrogen that ultimately presents an immune epitope with high structural congruence to heroin (7, Figure 1 and Scheme S1).22 We also prepared a heroin hapten with a truncated linker at this same position to probe the effect of linker length on vaccine efficacy (11, Figures 1 and S1). The haptens were activated and conjugated to carrier protein tetanus toxoid (TT) or a mutant diphtheria toxoid (CRM), using an EDC-mediated coupling reaction (Figure 1), followed by dialysis against pH 7.4 phosphate buffered saline (PBS). The degree of haptenation was determined by MALDI-ToF mass spectrometry using a heroin-bovine serum albumin (Her-BSA) immunoconjugate as a surrogate for determining hapten density (Figures S9–S14). Immunoconjugates were stored at −80 °C until the day of formulation and vaccination. More information on the synthesis and characterization data are described in the Supporting Information.
Vaccine Formulation
After conjugation of the proteins, immunoconjugates were formulated with different adjuvants as described in Tables S1–S5. The adjuvants were CpG ODN 1826, dsRNA, Alhydrogel (alum), and VesiVax CALV.28 CpG ODN 1826 is a phosphorothioated oligonucleotide with the following sequence (5′ to 3′): TCCATGACGTTCCTGACGTT. The 4.6 kb viral dsRNA was derived from L-A infected S. cerevisiae (ATCC #22244). The viral dsRNA can be prepared according to literature procedure involving fermentation of killer yeast, Saccharomyces cerevisiae (ATCC 22244), containing the L-A virus grown in Difco YM media (Becton Dickson).29–31 The VesiVax CALV liposomes and dsRNA were obtained from Molecular Express, Inc. Each vaccine was prepared by shaking the mixture for 20 min prior to injection. The delivered dose of each component was 50 μg of immunoconjugate, 50 μg of CpG ODN 1826 or dsRNA, and 1 mg of alum per animal for each injection, unless noted otherwise in Table 1 and Tables S1–S5.
Table 1.
Summary of Vaccine Formulations and Resultsγ
| Group | Vaccine | Immunoconjugate (μg/dose)a |
Alum (mg/dose)a |
Adjuvant (mg/dose)a |
Cryoprotectant (w/v or v/v)b |
Mice (/group) |
Antinociception Assayc | Midpoint Titersd |
|
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Hot Plate (ED50) |
Tail Flick (ED50) |
(×103) | |||||||
| Al | vehicle | – | 1 | – | glycerol | 6 | 0.5 ± 0.1e | 0.4 ± 0.1e | n.d.f |
| A2 | H-CRM-RNA | 50 μg Her-CRM | – | 50 μg dsRNA | glycerol | 4 | 0.6 ± 0.5 | 6.8 ± 0.5 | 6 ± 1 |
| A3 | H-CRM-Alum-RNA | 50 μg Her-CRM | 1 | 50 μg dsRNA | glycerol | 4 | 10.2 ± 1.7 | 12.2 ± 0.9 | 21 ± 5 |
| A4 | H-CRM-CALV-RNA | 50 μg Her-CRM | – | 2.5 mg CALV + 50 μg dsRNA | glycerol | 4 | 3.3 ± 0.8 | 6.7 ± 0.5 | 4 ± 2 |
| A5 | H-CRM-Alum-CpG | 50 μg Her-CRM | 1 | 50 μg CpG | glycerol | 4 | 3.0 ± 0.5 | 8.0 ± 0.3 | 19 ± 3 |
| A6 | H-TT-Alum-CpG | 50 μg Her-TT | 1 | 50 μg CpG | glycerol | 4 | 5.3 ± 1.2 | 8.7 ± 0.8 | 18 ± 10 |
| B1 | H-TT-Alum-RNA | 50 μg Her-TT | 1 | 50 μg dsRNA | glycerol | 4 | 7.9 ± 2.3 | 7.5 ± 0.5 | 28 ± 3 |
| B2 | H-TT-Alum-CpG+RNA | 50 μg Her-TT | 1 | 50 μg CpG + 50 μg dsRNA | glycerol | 4 | 5.1 ± 0.8 | 6.6 ± 0.4 | 55 ± 8 |
| B3 | H-TT-Alum-CpG+RNA-Lyo | 50 μg Her-TT | 1 | 50 μg CpG + 50 μg dsRNA | 15% trehalose | 6 | 5.4 ± 0.3 | 6.5 ± 0.4 | 46 ± 4 |
| B4 | H(s)-TT-Alum-CpG | 50 μg Her(s)-TT | 1 | 50 μg CpG | glycerol | 4 | 5.7 ± 0.7 | 9.6 ± 0.4 | 44 ± 2 |
| B5 | (IP) H-TT-Alum-CpG | 50 μg Her-TT | 1 | 50 μg CpG | glycerol | 4 | 9.6 ± 1.5 | 13.4 ± 1.2 | 103 ± 30 |
| C1 | H-TT-Alum-RNA-CALV | 50 μg Her-TT | 0.2 | 2.5 mg CALV + 50 μg dsRNA | glycerol | 5 | 2.9 ± 0.8 | 3.2 ± 0.6 | 68 ± 9 |
| C2 | H-TT-RNA-CALV | 50 μg Her-TT | – | 2.5 mg CALV + 50 μg dsRNA | glycerol | 5 | 2.2 ± 0.3 | 4.9 ± 0.5 | 28 ± 4 |
| C3 | H-TT-CALV | 50 μg Her-TT | – | 2.5 mg CALV | glycerol | 5 | 1.0 ± 1.5 | 2.5 ± 0.6 | 15 ± 5 |
| D1 | KLH (vehicle) | 50 μg KLH | 1 | 50 μg dsRNA | glycerol | 4 | 0.9 ± 0.7e | 0.0 ± 0.0e | n.d.f |
| D2 | H-TT-Alum-RNA(L) | 50 μg Her-TT | 1 | 10 μg dsRNA | glycerol | 4 | 2.5 ± 1.1 | 2.3 ± 0.6 | 36 ± 5 |
| D3 | H-TT-Alum-RNA(M) | 50 μg Her-TT | 1 | 25 μg dsRNA | glycerol | 4 | 5.4 ± 0.9 | 4.5 ± 0.7 | 42 ± 13 |
| D4 | H-TT-Alum-RNA(H) | 50 μg Her-TT | 1 | 50 μg dsRNA | glycerol | 4 | 3.7 ± 0.7 | 5.9 ± 0.8 | 38 ± 4 |
| D5 | KLH (vehicle) | 50 μg KLH | 0.2 | 50 μg dsRNA | glycerol | 4 | 0.0 ± 0.0e | 1.0 ± 0.6e | n.d.f |
| D6 | H-TT-Alum(L)-RNA | 50 μg Her-TT | 0.2 | 50 μg dsRNA | glycerol | 4 | 2.6 ± 0.5 | 6.4 ± 1.3 | 99 ± 17 |
| D7 | H-TT-Alum(M)-RNA | 50 μg Her-TT | 0.5 | 50 μg dsRNA | glycerol | 4 | 0.2 ± 0.7 | 2.9 ± 0.6 | 57 ± 11 |
| D8 | H-TT-Alum(H)-RNA | 50 μg Her-TT | 1 | 50 μg dsRNA | glycerol | 4 | 3.7 ± 1.2 | 3.9 ± 0.9 | 96 ± 29 |
| E1 | KLH (vehicle) | 50 μg KLH | 0.5 | 50 μg CpG | – | 4 | 0.0 ± 0.0e | 0.0 ± 0.0e | n.d.f |
| E2 | H-TT-Alum(L)-CpG | 50 μg Her-TT | 0.2 | 50 μg CpG | – | 4 | 4.5 ± 0.7 | 3.7 ± 0.6 | 71 ± 12 |
| E3 | H-TT-Alum(M)-CpG | 50 μg Her-TT | 0.5 | 50 μg CpG | – | 4 | 2.9 ± 0.3 | 4.0 ± 0.3 | 67 ± 12 |
| E4 | H-TT-Alum(H)-CpG | 50 μg Her-TT | 1 | 50 μg CpG | – | 4 | 4.2 ± 0.6 | 3.8 ± 0.8 | 94 ± 3 |
| F1 | H-TT-Alum-RNA (1 d) | 50 μg Her-TT | 0.2 | 50 μg dsRNA | 25% trehalose | 5 | 5.7 ± 1.7 | 6.0 ± 0.5 | 75 ± 13 |
| F2 | H-TT-Alum-RNA (30 d) | 50 μg Her-TT | 0.2 | 50 μg dsRNA | 25% trehalose | 5 | 3.1 ± 0.9 | 5.1 ± 0.3 | 49 ± 8 |
| F3 | H-TT-Alum-RNA-Lyo | 50 μg Her-TT | 0.2 | 50 μg dsRNA | 25% trehalose | 5 | 1.3 ± 0.4 | 3.1 ± 0.4 | 81 ± 19 |
| F4 | H-TT-Alum-RNA-Lyo (30 d) | 50 μg Her-TT | 0.2 | 50 μg dsRNA | 25% trehalose | 5 | 1.6 ± 0.6 | 1.9 ± 0.6 | 59 ± 7 |
| G1 | H-TT-Alum-CpG (1 d, 4°C) | 50 μg Her-TT | 1 | 50 μg CpG | >5% trehalose | 5 | 1.7 ± 1.5 | 2.1 ± 0.8 | 93 ± 16 |
| G2 | H-TT-Alum-CpG (30 d, 4°C) | 50 μg Her-TT | 1 | 50 μg CpG | >5% trehalose | 5 | 4.8 ± 1.3 | 4.6 ± 0.6 | 86 ± 21 |
| G3 | H-TT-Alum-CpG-Lyo | 50 μg Her-TT | 1 | 50 μg CpG | >5% trehalose | 5 | 2.9 ± 0.6 | 2.4 ± 0.3 | 37 ± 4 |
| G4 | H-TT-Alum-CpG-Lyo (30 d) | 50 μg Her-TT | 1 | 50 μg CpG | >5% trehalose | 5 | 1.6 ± 0.3 | 0.7 ± 0.6 | 39 ± 6 |
| G5 | H-TT-Alum-CpG (0 d) | 50 μg Her-TT | 1 | 50 μg CpG | 25% trehalose | 5 | 1.7 ± 0.8 | 2.3 ± 0.7 | 75 ± 26 |
| G6 | H-TT-Alum-CpG (30 d, RT) | 50 μg Her-TT | 1 | 50 μg CpG | 25% trehalose | 5 | 6.0 ± 2.1 | 6.4 ± 1.0 | 81 ± 14 |
| G7 | H-TT-Alum-CpG-Lyo | 50 μg Her-TT | 1 | 50 μg CpG | 25% trehalose | 5 | 3.1 ± 1.0 | 4.9 ± 0.6 | 92 ± 22 |
| G8 | H-TT-Alum-CpG-Lyo (30 d) | 50 μg Her-TT | 1 | 50 μg CpG | 25% trehalose | 5 | 4.6 ± 0.8 | 4.0 ± 0.4 | 124 ± 12 |
Amount of each vaccine component is given as the concentration per dose per mouse, respectively.
Cryoprotectant amounts are given for trehalose as w/v percentages for the total vaccine volume. In vaccines where glycerol was employed, the immunoconjugates were diluted 50% (v/v) with glycerol before being stored at −80 °C. Therefore glycerol content in total vaccine volumes ranged from 12 to 25% (v/v).
Behavioral assay results are reported as the mean heroin ED50 (mg/kg) ± SEM for each vaccine.
Midpoint titers are reported as the mean antiheroin midpoint titers ± SEM for each vaccine.
Heroin ED50 (mg/kg) for vehicles did not exhibit antinociceptive protection. However, some controls are noted as zero due to the lack of convergence of the data through PRISM. An appropriate ED50 for these controls can be designated ≥1.0 mg/kg.
Not detected. Antiheroin antibody titers were not detected in control serum.
Red section indicates the adjuvant selection studies; the blue section indicates the adjuvant and alum dosing; the green section indicates the stability studies.
Chemical Stability Studies of Individual Vaccine Components
A systematic analysis of individual components under various storage conditions was performed to monitor potential unwanted degradation. Lack of chemical stability may represent potential causal factors for any change in potency that is observed over time. Samples were prepared according to standard vaccine formulation conditions and listed in Table S6. To perform these studies, we ran TBE-Urea gels for CpG, agarose gel, UV–vis analysis and E-Gels for dsRNA, and SDS-PAGE gels for tetanus toxoid (TT) under various storage conditions. Results from stability studies are in the Supporting Information (Table S6 and Figures S22–S28).
Animals and Vaccine Administration
All studies were performed in compliance with the Scripps Institutional Animal Care and Use Committee and all protocols adhered to the National Institute of Health Guide for the Care and Use of Laboratory Animals. Male Swiss Webster mice (Taconic Farms, Germantown, NY; 6–8 weeks old; 25–30 g) were immunized subcutaneously (s.c.) on days 0, 14, and 28, unless noted otherwise (Figure S15–S17; Tables S1–S5). Exact formulation parameters are given for each group in Tables S1–S5. All animals in a given series were run at the same time, except for Series D and G. The bold lines separating the series indicate that the series were run in two sets, instead of simultaneously (Tables S2 and S5). Mice were bled on day 38 using retro-orbital puncture to collect approximately 100–150 μL of whole blood, unless noted otherwise. Groups were composed of 4 to 6 mice. Mice were group-housed in an AAALAC-accredited vivarium containing temperature and humidity controlled rooms and kept on a reverse light cycle (lights on: 9 PM–9 AM). Immunoconjugate 12a with the shortened linker hapten was used in Group B4 and the hapten 11 was termed H(s) in Tables 1 and S1.
Antinociception Assays
On week 6, antinociceptive responses under escalating heroin doses were evaluated to determine vaccine-mediated blockade of heroin psychoactivity.32 A set of mice was tested for spinal (tail immersion) and supraspinal (hot plate) antinociceptive responses to thermal stimuli at 54 °C, according to our laboratory procedure.33 Following administration of the drug, the analgesic effect (represented as maximal possible effect, % MPE) was measured for each test after every dose. The data were then fit using a nonlinear regression in GraphPad PRISM to determine ED50 values. The ED50 is calculated from plotting the %MPE with respect to heroin dose. Since the effect is based on %MPE, the ED50 describes when 50% of the animals within a group experienced the maximum effect of heroin-induced antinociception.
ELISAs
Bleeds were taken on weeks 6 and 10 for Series B, and maximum titer levels occurred at week 6 (Figure S18). Therefore, we opted to perform bleeds on day 38 for Series C–G and perform antinociception assays around week 6. Since heroin is rapidly metabolized to 6-AM before entering the brain,34,35 an ELISA using heroin or 6-monoacetylmorphine (6-AM) as coating antigens was performed for Series E to characterize antigen specificity of the antibody response. The equivalent titer response to coating antigen may suggest that the heroin immunoconjugate hydrolyzes to 6-AM before or during antigen presentation (Figure S19). Additional information on ELISAs is described in the Supporting Information.
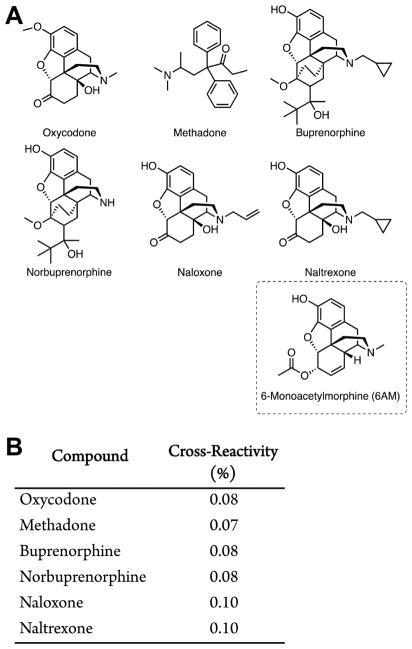
Analyzing Cross-Reactivity of Polyclonal Antiheroin Antibodies by Surface Plasmon Resonance
The binding IC50 for mouse serum IgGs from Group G6 and 6-AM was determined by competitive binding assay via surface plasmon resonance (SPR) using a Biacore 3000 instrument (GE Healthcare) equipped with a research-grade CM5 sensor chip according to literature methods.36 Diluted mouse serum from day 38 was incubated with serial dilutions of heroin, 6-AM, methadone, oxycodone, naloxone, buprenorphine, norbuprenorphine, naltrexone, and morphine and injected into a Biacore 3000 containing a Her-BSA-loaded sensor chip. The heroin–BSA conjugate was immobilized on the sensor chip using a NHS, EDC-mediated coupling reaction. The conjugate was resuspended in 10 mM sodium acetate (pH 4.0) was immobilized at a density of 5000 RU on flow cell 2, whereas flow cell 1 was immobilized with BSA at the same density to serve as a reference surface. All the surfaces were blocked with a 7 min injection of 1.0 M ethanolamine-HCl (pH 8.5). The pooled mouse sera was diluted in running buffer (HBS-EP + buffer) and titrated on both coated flow cells so as to give a response of ~100 RU within 3 min of injection and 2.5 min dissociation at a flow rate of 30 μL/min. The chip surface was regenerated by injection of 10 mM Gly-HCl (pH 1.5) for 30 s before the next round of assays. Signal produced by antibody binding to the SPR chip without drug present was used as a reference for 100% binding. Rapid hydrolysis of heroin interfered with collecting sufficient binding data.
Statistical Analysis
Tests for homogeneity of variance and normal distribution were performed on behavioral observation test scores. If conditions were met, analyses of variance (ANOVAs) were performed. Results were analyzed via one-way ANOVA with Dunnett’s post hoc comparisons for titers and Tukey’s post hoc test for analgesia. Pearson correlation coefficient was used to test the linear relationship between antiheroin midpoint titers to analgesia results for all animals tested (hot plate, P = 0.002, R2 = 0.093, Figure S21A,C; tail immersion, P = 0.009, R2 = 0.047, Figure S21B,D). However, there is no meaningful correlation between titers and antinociception data; titer data are reflective of antibody binding to hapten and not necessarily to free drug.
RESULTS AND DISCUSSION
To evaluate the series of heroin vaccine formulations, mice (n = 4–6/group) were vaccinated subcutaneously (s.c.) with the specific formulations listed in Tables S1–S5. Series A–C were designed to broadly explore the scope of vaccine conditions with the new dsRNA adjuvant in multiple contexts and to compare the adjuvant to our most successful heroin vaccine: Her-TT adjuvanted with 50 ug CpG adjuvanted and 1 mg of alum (Group A6). We used our previously reported second-generation heroin hapten17 in the majority of our formulations (7, Figure 1), although a truncated heroin hapten (11, Figure 1) was compared to 7 and showed no difference in behavioral efficacy (Group B4, Table 1). Moreover, ELISA results revealed that antibody titers for both hapten 7 and 11 vaccination groups were similar regardless of coating antigens (8c and 12b), suggesting that the hapten linker does not noticeably affect immunogenicity or antibody–hapten binding (Figure S19A,B). In moving forward with hapten 7, optimization of vaccine formulation conditions for the dsRNA included varying the carrier protein, the delivery system (i.e., CALV liposomal delivery or alum as a depot), and combining CpG and dsRNA. Findings from the first three series (highlighted in red in Table 1) were used to guide successive series of refinement. The subsequent Series D and E were designed to focus on a specific TLR agonist and observe its response to dose ranging with alum. After establishing an optimal dose with each TLR agonist, the integrity of the vaccine was tested under various storage conditions (Series F and G).
Following behavioral assays and titer measurements of all the series, a one-way ANOVA was performed on the resulting data (Table 1). The ANOVA confirmed a significant effect of formulation conditions in the hot plate assay [F (37, 135) = 5.851; p < 0.001]. A similar result was observed for the ANOVA in the tail flick assay [F (37,135) = 22.92; p < 0.001]. A Dunnett or Tukey post hoc test was then used to confirm significance among the groups. In Series A–C, we observed several interesting trends pertaining to (1) RNA versus DNA-based adjuvants, (2) carrier protein, (3) delivery vehicle, and (4) preliminary vaccine stability (Figure 2).
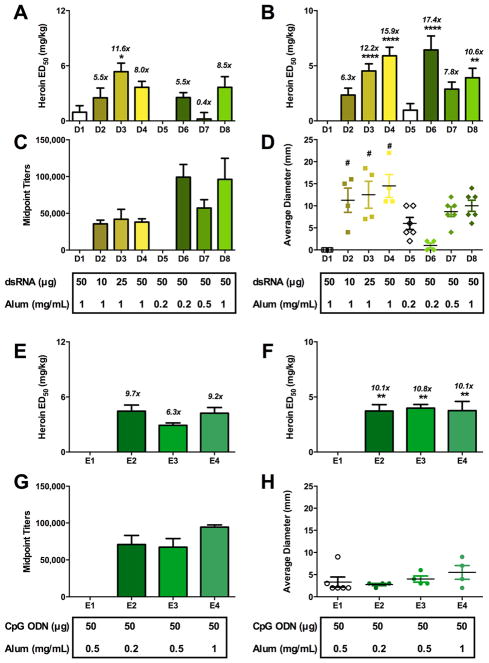
Figure 2.
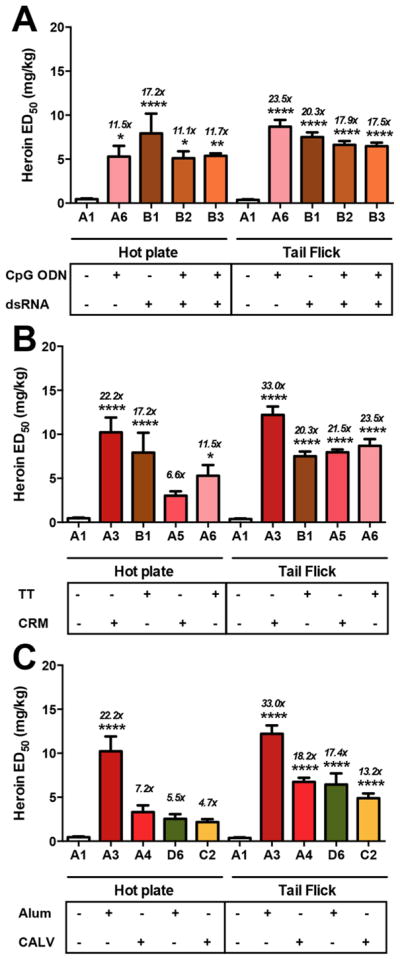
Effects of adjuvants and carrier proteins on heroin vaccine efficacy in antinociception assays. Differences in formulation are shown below the x-axis in each panel. A1 is a vehicle control. Panel A shows the effects of RNA versus DNA. All vaccines contain 50 μg Her-TT and 1 mg of alum. Only B3 used trehalose as a cryoprotectant for lyophilization treatment; all other vaccines contained glycerol. Panel B shows the effect of carrier protein. All vaccines contain 50 μg of immunoconjugate, 1 mg of alum and glycerol. A3 and B1 contain 50 μg of dsRNA, and A5 and A6 contain 50 μg of CpG. Panel C displays the effect of alum versus CALV as delivery vehicles. All vaccines contain 50 μg dsRNA. Groups A3 and A4 contain 50 μg of Her-CRM, and groups D6 and C2 contain 50 μg of Her-TT. Italicized numbers above the bars represent the ED50 ratio versus nonvaccinated control animals from control A1. A one-way ANOVA was performed for each antinociception assay, followed by a Dunnett’s post hoc comparison test, respectively. *P < 0.05, **P < 0.01, ****P < 0.0001 versus control A1.
Comparison of CpG and dsRNA as adjuvants revealed equipotency in the context of TT as the carrier protein coadministered with alum (Figure 2A). Intriguingly, the addition of CpG to the dsRNA/TT/alum formulation did not improve efficacy (Group B2, Figure 2A), indicating that the adjuvants do not act synergistically and may possibly interfere with each other’s adjuvant effects.
When a nontoxic mutant of diphtheria toxin, CRM, was employed as a carrier in eliciting an immunogenic response, we found that CRM adjuvanted with dsRNA was superior in both antinociception assays, as compared to TT (p < 0.001, Figure 2B). However, despite this increased efficacy, we opted to perform the rest of the vaccine studies with TT due to the fact that the CRM conjugate had an unfortunate tendency to precipitate upon storage. In comparing CALV liposomes and alum, antiheroin antibody titers were higher in alum formulations than liposomal formulations (Groups D6 and C2 found in Figures 2C and S20F, respectively). Moreover, CALV formulations (Groups A4, C1–C3 Figure 2, Table 1) were not as effective as vaccines containing alum in protecting mice from heroin-induced antinociception (Figure 2C, Table 1). A notable difference in efficacy was observed when CRM was adjuvanted with alum versus CALV liposomes, although this trend was not observed for TT. It is possible that the large disparity between the two delivery conditions may be due to the marked aggregation of Her-CRM during conjugation, which would impede subsequent encapsulation by liposomes. On the other hand, Her-TT’s solubility would theoretically permit encapsulation by CALV liposomes, possibly explaining the fact that CALV Her-TT liposomes gave the same magnitude of protection against heroin compared to Her-TT adjuvanted with a low dose of alum (0.2 mg/dose). On the basis of the finding that CALV was moderately effective as a Her-TT adjuvant, but never superior to alum, we did not move forward with CALV in our DNA and RNA dose-ranging studies.
Series D and E: RNA and DNA Adjuvant Dose-Ranging with Alum
In any vaccine, the beneficial immunopotentiation of adjuvants needs to be balanced against the risk of adverse side effects. Unfortunately, potent adjuvant action is often correlated with increased toxicity, presenting as inflammation at the site of immunization. Even adjuvants used in FDA-approved vaccines like alum are known to produce inflammation at the injection site.37,38 Preliminary assessment of toxicities in Series A–C showed occasional injection site redness and swelling, particularly in formulations containing dsRNA. Although injection site reactions are typical with alum-containing vaccines, we hypothesized that refining adjuvant dosing parameters might reduce the incidence and severity of these reactions.
Initial screenings of candidate formulations suggested that the preparations containing both dsRNA and alum yielded superb antibody and antinociceptive responses (Table 1). We specifically investigated different dsRNA to alum ratios in the mouse antinociception models to further refine the vaccine formulation. We hypothesized that at lower doses of alum or dsRNA, we might be able to lessen the severity of the injection site reactions without an appreciable loss of immunogenicity. Increasing the amount of dsRNA in vaccine formulations with 1 mg of alum (Groups D2–D4, Table 1) increased the size or incidence of injection site reactions. The increased inflammatory effect was also reflected in an increase in vaccine efficacy in the tail immersion response but not in hot plate antinociception test (Figure 3A,B). However, we found that lower doses of alum (0.2 mg) dramatically reduced the injection site reactions without compromising the efficacy of the vaccine for the dsRNA series (Figure 3A,B,D). In terms of the CpG series, we found that decreasing the alum had no effect on efficacy and that CpG formulations with the lowest alum dose were still adequately efficacious (Figure 3E,F). CpG dosing was previously reported and demonstrated a positive correlation between vaccine efficacy and CpG dose with no increase in adverse reactions.22
Figure 3.
Dose-ranging effects of dsRNA or CpG with alum on vaccine efficacy. Differences in vaccine formulation between the groups are shown below the x-axis. All vaccines in the dsRNA series (Panels A–D) contained 50 μg of Her-TT or KLH (for controls) and glycerol. All vaccines in the CpG series (Panels E–H) contained 50 μg of Her-TT or KLH (for controls) and 50 μg of CpG. Panels A and E are hot plate antinociceptive tests. Panels B and F are tail immersion tests. Panels C and G are antiheroin midpoint titers, and D and H are injection site reactions measured on the day of antinociception. Italicized numbers above the bars represent the ED50 ratio versus nonvaccinated control animals from control A1. A one-way ANOVA was performed for each antinociception assay and the titer data, followed by a Dunnett’s or Tukey’s post hoc comparison test, respectively. *P < 0.05, **P < 0.01, ****P < 0.0001 versus control A1. #P < 0.0001 versus control C1.
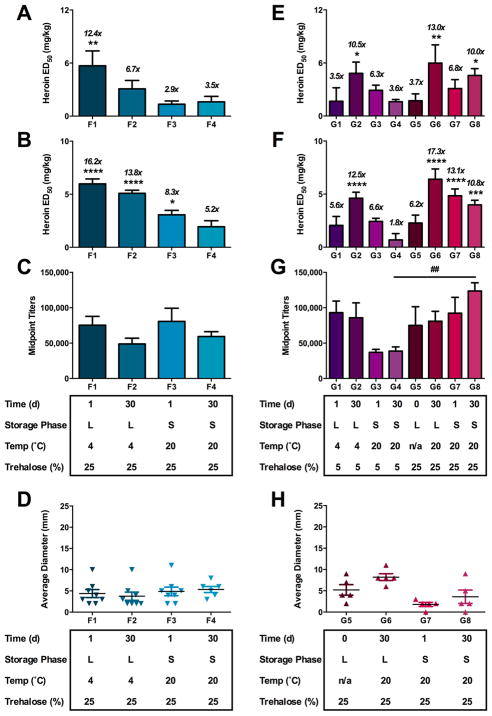
Series F and G: Potency Time-Course Studies of Vaccines under Various Storage Conditions and Time Periods
Another important goal in vaccine design is achieving long-term shelf stability without loss in efficacy, typically via lyophilization. Recently, it was suggested that our hapten was unstable due to the presence of labile ester groups and subsequently was expected to exhibit a “limited shelf-life” due to undesired degradation during storage.16 To examine the clinical viability of our heroin vaccine, we initiated potency studies over various time points and storage conditions to test its efficacy against heroin over time. Consequently, protection of the vaccine components against damage during the freezing and drying process is essential.39 Trehalose can be used as an effective cryoprotectant to prevent alum aggregation during lyophilization,40,41 and therefore, we investigated the stability and efficacy of our heroin vaccines under various storage conditions in the presence of trehalose.
In a preliminary study, we tested a lyophilized vaccine formulation containing 15% w/v trehalose as a cryoprotectant (Group B3, Table 1). When immunized with the reconstituted vaccine, this group demonstrated similar efficacy to the nonlyophilized vaccine Group B2 in antinociceptive assays (Figure 2A, Table 1). This initial result prompted us to explore a broader range of conditions for each nucleotide-based adjuvant and their relative shelf stability over time. In addition, before initiating full 30-day potency time-course studies, we tested a range of trehalose concentrations with three concentrations of alum and qualitatively assessed undesired alum aggregation (Figure S27). Chemical stability studies were also conducted on each individual and combined vaccine component to assess for degradation under conditions such as freezing in liquid nitrogen or lyophilization. The results of the studies are in Figures S22–S28 using dosing parameters of the most promising vaccine conditions for each series. It was determined that each component was found to be relatively stable over time and under different storage conditions.
We also noted that alum was found to bind antigen in phosphate buffered saline (Figures S22–S24, S26, and S28), which was not expected considering that negligible binding was observed both Haemophilus influenza type b and meningococcal group C conjugate vaccines in phosphate buffered saline with alum.42 However, in the case of our heroin conjugate vaccine, alum retains its ability to bind antigen even after lyophilization and resuspension.
For both the dsRNA and CpG series, Her-TT immunoconjugate was formulated with trehalose and dsRNA or CpG, samples were initially stored in the −80 °C freezer, defrosted, mixed with alum, and then subjected to the following storage conditions (Figure S16): (1) formulated with alum 1 day before injection and stored as a liquid at 4 °C (Groups F1 and G1); (2) formulated with alum 30 days before injection and stored as a liquid at 4 °C (Groups F2 and G2) or stored at room temperature (RT, Group G6); (3) formulated with alum 1 day before injection, lyophilized, and stored at RT (Groups F3, G3, and G7); (5) formulated with alum 30 days before injection, lyophilized, and stored at RT (Groups F4, G4, and G8, Table 1). As a negative control in the CpG series, Groups G1–G4, Table 1 were spiked with a lower amount of trehalose (>5%) to measure its effect on protection from lyophilization. On the day of injection, all lyophilized samples were resuspended in water via 20 min of vortex mixing, then administered to mice.
In interpreting the dsRNA series results, lyophilized vaccines (Groups F3 and F4) were not as effective in tail immersion and hot plate thermal nociception as compared to liquid storage for 1 day (Group F1, Figure 4A,B and Table 1). Samples stored for 30 days also showed modestly lower titer levels (Groups F2 and F4, Figure 4C, Table 1). These results could be explained by the possible instability of the dsRNA genome at room temperature, as cold storage (−20 to −80 °C) is optimal for most extracted DNA samples.43 On the other hand, extended incubation and storage apparently enhanced efficacy for the CpG series (G Series, Figure 4E–H), possibly due to the formation of immunologically active antigen-alum aggregates during storage.44 In assessing the effects of the cryoprotectant, liquid samples with CpG were effective regardless of the presence of trehalose over time (Group G2); however, lyophilized samples without at least 15% trehalose do not survive under storage conditions after 30 days as evidenced by reduced in vivo efficacy (Group G4, Figure 3E–G, Table 1). When a sufficient amount of trehalose was used in the vaccine formulations, lyophilized vaccines performed better at both one and thirty-day time points in thermal nociception assays and titer (G3 vs G7 for 1 day, G4 vs G8 for 30 day lyophilized, Figure 4E–G, Table 1). Promisingly, the efficacy of the vaccine was retained after 30 days as a liquid (G2 and G6) or when lyophilized (G8), and there was no significant difference between the samples that were lyophilized 30 days or 1 day prior to injection (G8 and G7, respectively, Table 1).
Figure 4.
Stability of dsRNA + alum (A–D) and CpG + alum (E–H) vaccines under liquid and solid storage conditions over time. Differences in formulation parameters are shown below the x-axis. Vaccines in the dsRNA stability series contained 50 μg Her-TT, 0.2 mg alum, 50 μg dsRNA, and 25% trehalose. Vaccines in the CpG series contained 50 μg of Her-TT, 1 mg alum, 50 μg CpG, and either 5 or 25% trehalose. Panels A and E are hot plate antinociceptive tests. Panels B and F are tail immersion tests. Panels C and G are antiheroin midpoint titers, and D and H are injection site reactions measured the day of antinociception. Italicized numbers above the bars represent the ED50 ratio versus nonvaccinated control animals from control A1. In the legend, L and S stand for liquid or solid, respectively. A one-way ANOVA was performed for each antinociception assay and the titer data, followed by a Dunnett’s or Tukey’s post hoc comparison test, respectively. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus control A1. ##P < 0.01 Tukey’s comparison test for titer between G4 and G8, which differed only in percentage of trehalose added.
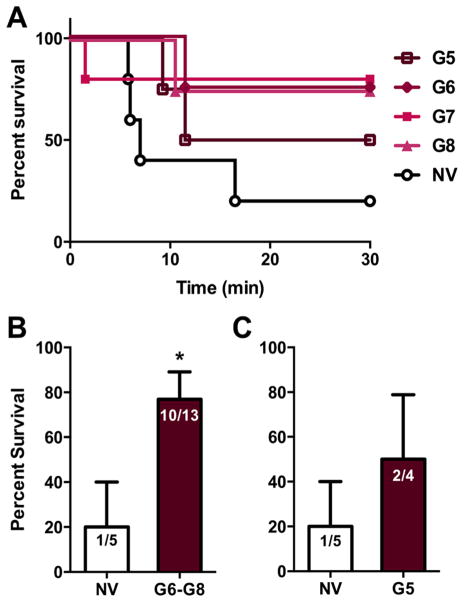
Lethality Challenge with Series G
Upon demonstrating that our vaccine could block substantial doses of heroin in the antinociception assay, we examined the ability of our vaccine to mitigate heroin-induced lethality. On the basis of the antinociceptive data for the stability studies, we defined an efficacious vaccine as a vaccine group having an ED50 ≥ 4.5 mg/kg in at least one measure of thermal nociception. Using this criterion, the CpG series with 25% cryoprotectant were the most successful. Thus, vaccinated mice (n = 17) from the CpG stability studies and nonvaccinated mice (n = 5) were administered a 160 mg/kg dose of heroin and survival was measured (Figure 5A). The survival rate for the pooled efficacious vaccine group was 77% (10 of 13 mice survived), as compared to 20% survival for the nonvaccinated (1 of 5 mice survived, Figure 5B). Taken together, these results clearly indicate that the heroin vaccine is highly effective in diminishing the effects of a lethal heroin challenge in rodents.
Figure 5.
Efficacy of heroin vaccine against a lethal heroin challenge. All vaccines contain 50 μg of Her-TT, 50 μg of CpG, 1 mg of alum, and 25% trehalose. Panel A shows the survival curve of each vaccinated treatment group and nonvaccinated (NV, n = 5) mice challenged with a 160 mg/kg dose (i.p.) and observed for 30 min. Panel B shows the vaccinated mice (n = 13) from the groups that demonstrated efficacious vaccine potency in comparison to the control (n = 5). Panel C shows the vaccinated mice that did not meet our efficacy cutoff criterion (n = 4, G5) versus the nonvaccinated mice (n = 5). Nonvaccinated mice were given a 2 mg/kg dose of heroin the same day the vaccinated mice underwent antinociception assays. The lethal challenge was performed the following week. A nonparametric, unpaired Mann–Whitney U test was performed and revealed survival between the two groups were statistically significant (P < 0.05). Bars represent mean survival percentage ± SEM.
Cross-Reactivity of Antibodies from Group G6
A major benefit of vaccination over traditional pharmacotherapies stems from the increased duration of action of circulating antibodies and decreased side effects. The advancement of a heroin vaccine may benefit from a combination therapy with existing drugs, such as methadone or burprenorphine, to mitigate opioid cravings during cessation therapy. To test whether combination therapy was feasible with our heroin vaccine, we selected Group G6 (Table 1) from the stability series and characterized the polyclonal antibody response by SPR. Sera from Group G6 were pooled to measure the binding affinities of polyclonal antibodies in vaccinated mouse serum G6 for heroin, 6-AM, and morphine using a Biacore 3000 equipped with a Her-BSA-coated chip. Diluted mouse sera was then preincubated with serial dilutions of FDA-approved therapeutic opioids (Figure 6A) to test for potential cross-reactivity that might interfere with combination therapy.
Figure 6.
Cross-reactivity of antiheroin polyclonal antibodies from Group G6 to other therapeutic opioids as determined by surface plasmon resonance (SPR) binding assay. Panel A contains the structures of the relevant opioids. Panel B shows the cross-reactivity of therapeutic opioids (10 μM) compared to 6-AM on a Her-BSA-loaded sensor chip incubated with diluted mouse sera from G6. Surface plasmon resonance revealed the IC50 value of 6-AM for Group G6 was ~100 nM. The IC50 value of heroin could not be determined by SPR due to the rapid hydrolysis of heroin to 6-AM during experimental runs at 37 C for 15 min runs per dose.
By using the SPR competition assay, it was determined that the polyclonal antibodies from G6 had a binding affinity for 6-AM corresponding to ~100 nM. As previously reported,12,22 this hapten is “dynamic” making antibodies against multiple active species, including 6-AM, which is the primary mediator of heroin’s psychoactivity.45
It can also be inferred that the formulation parameters for G6 storage as a liquid at room temperature, presents minimal 6-AM hydrolysis over 30 days in phosphate buffered saline (pH 7.4) with trehalose (25% w/v) suggesting that alum adsorption may inhibit hydrolysis. In addition, it was demonstrated that affinities for FDA-approved opioids were >1000-times lower compared to 6-AM (Figure 6B), indicating minimal cross-reactivity to therapeutic opioids. These data suggest that Her-TT vaccinated subjects may use pharmacotherapies in tandem with vaccination.
We have examined adjuvant formulation and carrier protein in the context of our heroin vaccine to improve vaccine efficacy. Substituting CRM197 for TT as a carrier protein gave similar efficacy in heroin antinociception tests. Evaluation of an RNA-based adjuvant similar to TLR3 agonist poly(I:C), showed an increase in vaccine efficacy versus our previously used TLR9 adjuvant, CpG, while a combination of the two was not as effective. Furthermore, formulation of the RNA adjuvant without alum or with a liposome (CALV) showed poor vaccine efficacy. Dosing of the adjuvants with alum and dsRNA or CpG was optimized to reduce injection site reactions while maintaining vaccine efficacy. The RNA-based adjuvant in combination with a lower dose of alum was promising, while CpG was unaffected by alum dosing, so both RNA and DNA adjuvant vaccines were further explored in stability studies.
In the dsRNA stability studies, it was determined that vaccines containing dsRNA perform the best 1 day after formulation. Liquid dsRNA and CpG samples stored for 30 days at 4 °C were comparable, but the CpG vaccine stored as a liquid at RT surpassed both adjuvant samples in the measures of vaccine efficacy. In terms of lyophilized treatment, trehalose is essential for lyophilized vaccine performance. Both lyophilized CpG samples with 25% trehalose (w/v) achieved much higher ED50’s than the lyophilized dsRNA samples. Therefore for our lethality challenge, we tested the CpG stability series and found that the vaccine conferred protection against a lethal dose of heroin. On the basis of the results of this systematic formulation assessment for vaccines against heroin abuse, the CpG + alum Her-TT formulation has demonstrated the most promise to move beyond preclinical development.
Supplementary Material
Acknowledgments
This is manuscript no. 29606 from The Scripps Research Institute. This work was supported by National Institutes of Health Grant Nos. UH3DA041146 (K.D.J.), F32AI126628 (C.S.H.), F32DA043323 (C.J.W.), R42DA040422 (G.F.), and R44AI094770 (S.O.H.).
ABBREVIATIONS
- OPR
opioid pain reliever
- 6-AM
6-monoacetylmorphine
- PAMPS
pathogen-associated molecular patterns
- LPS
lipopolysaccharides
- CpG ODN
cytosine-phosphodiester-guanine oligodeoxynucleotide
- dsRNA
double-stranded RNA
- TLR
Toll-like receptor
- TT
tetanus toxoid
- CRM
nontoxic mutant of diphtheria toxin
- KLH
keyhole limpet hemocyanin
- PBS
pho sphate buffered saline
- EDC
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
- MALDI-ToF MS
matrix-assisted laser desorption ionization time-of-flight mass spectrometry
- CALV
conjugatable adjuvant lipid vesicles
- s.c
subcutaneous
- SPR
surface plasmon resonance
- Lyo
lyophilized
Footnotes
Author Contributions
All authors have given approval to the final version of the manuscript.
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.molpharmaceut.7b00933.
Detailed hapten synthetic procedures and characterization data including 1H and 13C NMR spectra; additional data including immunoconjugate characterization by MALDI-ToF MS, titer data, gels, and detailed vaccination tables and corresponding schedules (PDF)
References
- 1.Rudd RA, Seth P, David F, Scholl L. Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 2.Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Internal Medicine. 2015;175(4):608–615. doi: 10.1001/jamainternmed.2014.8071. [DOI] [PubMed] [Google Scholar]
- 3.Mars SG, Bourgois P, Karandinos G, Montero F, Ciccarone D. “Every ’never’ I ever said came true”: transitions from opioid pills to heroin injecting. Int J Drug Policy. 2014;25(2):257–66. doi: 10.1016/j.drugpo.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollini RA, Banta-Green CJ, Cuevas-Mota J, Metzner M, Teshale E, Garfein RS. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Substance Abuse and Rehabilitation. 2011;2:173–180. doi: 10.2147/SAR.S24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Principles of Drug Addiction Treatment: A Research-Based Guide. 3. NIH National Institute on Drug Abuse; U.S. Department of Health and Human Services; 2012. [Google Scholar]
- 6.Hoogsteder PHJ, Kotz D, van Spiegel PI, Viechtbauer W, van Schayck OCP. Efficacy of the nicotine vaccine 3′-AmNic-rEPA (NicVAX) co-administered with varenicline and counselling for smoking cessation: a randomized placebo-controlled trial. Addiction. 2014;109(8):1252–1259. doi: 10.1111/add.12573. [DOI] [PubMed] [Google Scholar]
- 7.Hieda Y, Keyler DE, Ennifar S, Fattom A, Pentel PR. Vaccination against nicotine during continued nicotine administration in rats: immunogenicity of the vaccine and effects on nicotine distribution to brain. Int J Immunopharmacol. 2000;22(10):809–819. doi: 10.1016/s0192-0561(00)00042-4. [DOI] [PubMed] [Google Scholar]
- 8.Kimishima A, Wenthur CJ, Eubanks LM, Sato S, Janda KD. Cocaine Vaccine Development: Evaluation of Carrier and Adjuvant Combinations That Activate Multiple Toll-Like Receptors. Mol Pharmaceutics. 2016;13(11):3884–3890. doi: 10.1021/acs.molpharmaceut.6b00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orson FM, Wang R, Brimijoin S, Kinsey BM, Singh RA, Ramakrishnan M, Wang HY, Kosten TR. The future potential for cocaine vaccines. Expert Opin Biol Ther. 2014;14(9):1271–83. doi: 10.1517/14712598.2014.920319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller ML, Moreno AY, Aarde SM, Creehan KM, Vandewater SA, Vaillancourt BD, Wright MJ, Janda KD, Taffe MA. A methamphetamine vaccine attenuates methamphet-amine-induced disruptions in thermoregulation and activity in rats. Biol Psychiatry. 2013;73(8):721–728. doi: 10.1016/j.biopsych.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gooyit M, Miranda PO, Wenthur CJ, Ducime A, Janda KD. Influencing Antibody-Mediated Attenuation of Methamphetamine CNS Distribution through Vaccine Linker Design. ACS Chem Neurosci. 2017;8:468. doi: 10.1021/acschemneuro.6b00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stowe GN, Vendruscolo LF, Edwards S, Schlosburg JE, Misra KK, Schulteis G, Mayorov AV, Zakhari JS, Koob GF, Janda KD. A vaccine strategy that induces protective immunity against heroin. J Med Chem. 2011;54(14):5195–204. doi: 10.1021/jm200461m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bremer PT, Schlosburg JE, Lively JM, Janda KD. Injection route and TLR9 agonist addition significantly impact heroin vaccine efficacy. Mol Pharmaceutics. 2014;11(3):1075–80. doi: 10.1021/mp400631w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature. 1974;252(5485):708–710. doi: 10.1038/252708a0. [DOI] [PubMed] [Google Scholar]
- 15.Anton B, Salazar A, Florez A, Matus M, Marin R, Hernandez J-A. Vaccines against morphine/heroine and its use as effective medication for preventing relapse to opiate addictive behaviors. Hum Vaccines. 2009;5(4):214–229. doi: 10.4161/hv.5.4.7556. [DOI] [PubMed] [Google Scholar]
- 16.Sulima A, Jalah R, Antoline JFG, Torres OB, Imler GH, Deschamps JR, Beck Z, Alving CR, Jacobson AE, Rice KC, Matyas GR. A Stable Heroin Analog That Can Serve as a Vaccine Hapten to Induce Antibodies that Block the Effects of Heroin and its Metabolites in Rodents and that Cross-React Immunologically with Related Drugs of Abuse. J Med Chem. 2018;61:329. doi: 10.1021/acs.jmedchem.7b01427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jalah R, Torres OB, Mayorov AV, Li F, Antoline JF, Jacobson AE, Rice KC, Deschamps JR, Beck Z, Alving CR, et al. Efficacy, but not antibody titer or affinity, of a heroin hapten conjugate vaccine correlates with increasing hapten densities on tetanus toxoid, but not on CRM197 carriers. Bioconjugate Chem. 2015;26(6):1041–1053. doi: 10.1021/acs.bioconjchem.5b00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li QQ, Luo YX, Sun CY, Xue YX, Zhu WL, Shi HS, Zhai HF, Shi J, Lu L. A morphine/heroin vaccine with new hapten design attenuates behavioral effects in rats. J Neurochem. 2011;119(6):1271–1281. doi: 10.1111/j.1471-4159.2011.07502.x. [DOI] [PubMed] [Google Scholar]
- 19.Aguilar JC, Rodriguez EG. Vaccine adjuvants revisited. Vaccine. 2007;25(19):3752–62. doi: 10.1016/j.vaccine.2007.01.111. [DOI] [PubMed] [Google Scholar]
- 20.Verthelyi D, Ishii KJ, Gursel M, Takeshita F, Klinman DM. Human Peripheral Blood Cells Differentially Recognize and Respond to Two Distinct CpG Motifs. J Immunol. 2001;166(4):2372–2377. doi: 10.4049/jimmunol.166.4.2372. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann G, Krieg AM. Mechanism and Function of a Newly Identified CpG DNA Motif in Human Primary B Cells. J Immunol. 2000;164(2):944–953. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 22.Bremer PT, Schlosburg JE, Banks ML, Steele FF, Zhou B, Poklis JL, Janda KD. Development of a Clinically-Viable Heroin Vaccine. J Am Chem Soc. 2017;139:8601. doi: 10.1021/jacs.7b03334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stahl-Hennig C, Eisenblatter M, Jasny E, Rzehak T, Tenner-Racz K, Trumpfheller C, Salazar AM, Uberla K, Nieto K, Kleinschmidt J, Schulte R, Gissmann L, Muller M, Sacher A, Racz P, Steinman RM, Uguccioni M, Ignatius R. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog. 2009;5(4):e1000373. doi: 10.1371/journal.ppat.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S, Nguyen MT. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 2015;15(2):51–7. doi: 10.4110/in.2015.15.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peine KJ, Bachelder EM, Vangundy Z, Papenfuss T, Brackman DJ, Gallovic MD, Schully K, Pesce J, Keane-Myers A, Ainslie KM. Efficient delivery of the toll-like receptor agonists polyinosinic:polycytidylic acid and CpG to macrophages by acetalated dextran microparticles. Mol Pharmaceutics. 2013;10(8):2849–57. doi: 10.1021/mp300643d. [DOI] [PubMed] [Google Scholar]
- 26.Wickner RB. Double-stranded RNA viruses of Saccharomyces cerevisiae. Microbiological Reviews. 1996;60(1):250–265. doi: 10.1128/mr.60.1.250-265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claudepierre MC, Hortelano J, Schaedler E, Kleinpeter P, Geist M, Remy-Ziller C, Brandely R, Tosch C, Laruelle L, Jawhari A, et al. Yeast virus-derived stimulator of the innate immune system augments the efficacy of virus vector-based immunotherapy. J Virol. 2014;88:5242–55. doi: 10.1128/JVI.03819-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lockner JW, Ho SO, McCague KC, Chiang SM, Do TQ, Fujii G, Janda KD. Enhancing nicotine vaccine immunogenicity with liposomes. Bioorg Med Chem Lett. 2013;23(4):975–8. doi: 10.1016/j.bmcl.2012.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adler J, Wood H, Bozarth R. Virus-like particles from killer, neutral, and sensitive strains of Saccharomyces cerevisiae. J Virol. 1976;17(2):472–476. doi: 10.1128/jvi.17.2.472-476.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hewitt CW, Adler J. Murine immunosuppression with mycoviral dsRNA. Immunopharmacology. 1982;5(2):103–109. doi: 10.1016/0162-3109(82)90041-8. [DOI] [PubMed] [Google Scholar]
- 31.Poteet E, Lewis P, Chen C, Ho SO, Do T, Chiang S, Labranche C, Montefiori D, Fujii G, Yao Q. Toll-like receptor 3 adjuvant in combination with virus-like particles elicit a humoral response against HIV. Vaccine. 2016;34(48):5886–5894. doi: 10.1016/j.vaccine.2016.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlosburg JE, Vendruscolo LF, Bremer PT, Lockner JW, Wade CL, Nunes AAK, Stowe GN, Edwards S, Janda KD, Koob GF. Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Natl Acad Sci U S A. 2013;110(22):9036–9041. doi: 10.1073/pnas.1219159110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bremer PT, Janda KD. Investigating the effects of a hydrolytically stable hapten and a Th1 adjuvant on heroin vaccine performance. J Med Chem. 2012;55(23):10776–80. doi: 10.1021/jm301262z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gottas A, Boix F, Oiestad EL, Vindenes V, Morland J. Role of 6-monoacetylmorphine in the acute release of striatal dopamine induced by intravenous heroin. Int J Neuropsychopharmacol. 2014;17(9):1357–65. doi: 10.1017/S1461145714000169. [DOI] [PubMed] [Google Scholar]
- 35.Kamendulis LM, Brzezinski MR, Pindel EV, Bosron WF, Dean RA. Metabolism of cocaine and heroin is catalyzed by the same human liver carboxylesterases. J Pharmacol Exp Ther. 1996;279(2):713–717. [PubMed] [Google Scholar]
- 36.Bremer PT, Kimishima A, Schlosburg JE, Zhou B, Collins KC, Janda KD. Combatting Synthetic Designer Opioids: A Conjugate Vaccine Ablates Lethal Doses of Fentanyl Class Drugs. Angew Chem, Int Ed. 2016;55(11):3772–5. doi: 10.1002/anie.201511654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noe SM, Green MA, HogenEsch H, Hem SL. Mechanism of immunopotentiation by aluminum-containing adjuvants elucidated by the relationship between antigen retention at the inoculation site and the immune response. Vaccine. 2010;28(20):3588–94. doi: 10.1016/j.vaccine.2010.02.085. [DOI] [PubMed] [Google Scholar]
- 38.Kool M, Fierens K, Lambrecht BN. Alum adjuvant: some of the tricks of the oldest adjuvant. J Med Microbiol. 2012;61(7):927–34. doi: 10.1099/jmm.0.038943-0. [DOI] [PubMed] [Google Scholar]
- 39.Clapp T, Siebert P, Chen D, Jones Braun L. Vaccines with aluminum-containing adjuvants: optimizing vaccine efficacy and thermal stability. J Pharm Sci. 2011;100(2):388–401. doi: 10.1002/jps.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clausi AL, Merkley SA, Carpenter JF, Randolph TW. Inhibition of aggregation of aluminum hydroxide adjuvant during freezing and drying. J Pharm Sci. 2008;97(6):2049–61. doi: 10.1002/jps.21143. [DOI] [PubMed] [Google Scholar]
- 41.Smallshaw JE, Vitetta ES. A lyophilized formulation of RiVax, a recombinant ricin subunit vaccine, retains immunogenicity. Vaccine. 2010;28(12):2428–35. doi: 10.1016/j.vaccine.2009.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otto RB, Burkin K, Amir SE, Crane DT, Bolgiano B. Patterns of binding of aluminum-containing adjuvants to Haemophilus influenzae type b and meningococcal group C conjugate vaccines and components. Biologicals. 2015;43(5):355–62. doi: 10.1016/j.biologicals.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SB, Crouse CA, Kline MC. Optimizing Storage and Handling of DNA Extracts. Forensic Sci Rev. 2010;22(2):131–44. [PubMed] [Google Scholar]
- 44.Romero Méndez IZ, Shi Y, HogenEsch H, Hem SL. Potentiation of the immune response to non-adsorbed antigens by aluminum-containing adjuvants. Vaccine. 2007;25(5):825–833. doi: 10.1016/j.vaccine.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 45.Raleigh MD, Pentel PR, LeSage MG. Pharmacokinetic Correlates of the Effects of a Heroin Vaccine on Heroin Self-Administration in Rats. PLoS One. 2014;9(12):e115696. doi: 10.1371/journal.pone.0115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.