Abstract
Introduction
Muscle is strongly related to cortical bone architecture in children; however, the relationship between muscle volume and trabecular bone architecture is poorly studied. The aim of this study was to determine if muscle volume is related to trabecular bone architecture in children and if the relationship is different than the relationship between muscle volume and cortical bone architecture.
Materials and methods
Forty typically developing children (20 boys and 20 girls; 6 to 12 y) were included in the study. Measures of trabecular bone architecture [apparent trabecular bone volume to total volume (appBV/TV), trabecular number (appTb.N), trabecular thickness (appTb.Th), and trabecular separation (appTb.Sp)] in the distal femur, cortical bone architecture [(cortical volume, medullary volume, total volume, polar moment of inertia (J) and section modulus (Z)] in the midfemur, muscle volume in the midthigh and femur length were assessed using magnetic resonance imaging. Total and moderate-to-vigorous physical activity were assessed using an accelerometer-based activity monitor worn around the waist for four days. Calcium intake was assessed using diet records. Relationships among the measures were tested using multiple linear regression analysis.
Results
Muscle volume was moderately-to-strongly related to measures of trabecular bone architecture [appBV/TV (r = 0.81, appTb.N (r = 0.53), appTb.Th (r = 0.67), appTb.Sp (r = −0.71; all p < 0.001] but more strongly related to measures of cortical bone architecture [cortical volume (r = 0.96), total volume (r = 0.94), Z (r = 0.94) and J (r = 0.92; all p < 0.001)]. Similar relationships were observed between femur length and measures of trabecular (p < 0.01) and cortical (p < 0.001) bone architecture. Sex, physical activity and calcium intake were not related to any measure of bone architecture (p > 0.05). Because muscle volume and femur length were strongly related (r = 0.91, p < 0.001), muscle volume was scaled for femur length (muscle volume/femur length2.77). When muscle volume/femur length2.77 was included in a regression model with femur length, sex, physical activity and calcium intake, muscle volume/femur length2.77 was a significant predictor of appBV/TV, appTb.Th and appTb.Sp (partial r = 0.44 to 049, p < 0.05) and all measures of cortical bone architecture (partial r = 0.47 to 054; p < 0.01).
Conclusions
The findings suggest that muscle volume in the midthigh is related to trabecular bone architecture in the distal femur of children. The relationship is weaker than the relationship between muscle volume in the midthigh and cortical bone architecture in the midfemur, but the discrepancy is driven, in large part, by the greater dependence of cortical bone architecture measures on femur length.
Keywords: muscle, bone architecture, trabecular, cortical, femur length, MRI
Introduction
Childhood is viewed as a critical time to maximize the mass, architecture and strength of bone and minimize fracture risk later in life [1, 2]. Areal bone mineral density (aBMD) is the single best surrogate of fracture risk widely available. However, there is a significant overlap in aBMD in those who have and have not fractured [3]. Moreover, using aBMD alone to make clinical decisions in children has been questioned [4, 5]. The development of more sophisticated imaging techniques, such as magnetic resonance imaging and computed tomography, now allow for the separation on bone into different compartments and for the evaluation of different architectural features of trabecular and cortical bone. The architecture of bone is an important contributor to its biomechanical strength [6]. Magnetic resonance imaging is particularly attractive for the study of bone in children because it does not expose them to ionizing radiation.
Due to the importance of bone architecture, identifying factors that contribute to its development could lead to an immediate and long-term reduction in fracture risk. Muscle clearly has a positive influence on bone which has led to the proposal of muscle-bone indexes that reflect the relationship between muscle and bone [7]. In theory, muscle-bone indexes can be used to identify those at greatest risk for fracture or if factors other than muscle are contributing to poor bone development [8]. Although the relationship between muscle and cortical bone architecture in children has been studied [9, 10], the relationship between muscle and trabecular bone architecture remains unclear. Because other factors may influence the development of bone architecture, such as general growth, as indicated by bone length, sex, physical activity and dietary factors, such as calcium intake, these factors must be considered when evaluating the relationship between muscle and trabecular bone architecture.
The primary aim of this study was to determine if muscle volume is related to measures of trabecular bone architecture in typically developing children and if the relationship is different than the relationship between muscle volume and cortical bone architecture. We hypothesized that thigh muscle volume would be related to measures of trabecular and cortical bone architecture in the distal femur of typically developing children while controlling for femur length, sex, physical activity and calcium intake.
Methods
Subjects
Typically developing children 6 to 12 years of age, between the 5th and 95th percentile for height and body mass, with no history of lower extremity fracture, no intramedullary fixation in the femur or tibia, no use of chronic medications known to affect bone or muscle development and not participating in > 3 hours of organized physical activity per week were recruited from the Newark, DE, USA community by distributing flyers. The procedures followed were in accordance with the ethical standards of the institutions on human experimentation. The Institutional Review Boards at the University of Delaware and the AI duPont Hospital for Children approved the study. Written consent from parents and written assent from children, if able, were taken before testing.
Protocol
For each participant, height, body mass, sexual maturity, physical activity, calcium intake, measures of trabecular and cortical bone architecture, muscle volume and femur length were assessed and recorded within a two week period.
Anthropometrics
Children wore shorts, a t-shirt and no shoes during anthropometric assessment. Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer (Seca model 222, Novel products, Rockton, IL). Body mass was rounded to the nearest 0.2 kg using a digital scale (Detecto 6550, Cardinal Scale, MO). Height, body mass and body mass index (BMI) percentiles were calculated from the normative graphs published by the Centers for Disease Control and Prevention [11].
Tanner staging
Sexual maturity was assessed by a physician assistant using the Tanner staging technique [12]. Signs of pubic hair growth were assessed along with testicular/penile development in boys, and breast development in girls. The Tanner stages range from 1 to 5, with 1 indicating no signs of sexual development (pre-pubertal), 2 indicating early sexual development and 5 indicating full sexual development.
Physical activity
Physical activity was assessed using an accelerometer-based activity monitor (Actical; Philips Respironics, Sunriver, OR) that was worn on the non-dominant hip for four days (three weekdays and one weekend day). Four days of data collection was chosen because it yields accurate and reliable estimates of physical activity [13]. The children were asked not to remove the activity monitor at any time (including sleep) except for swimming at a depth greater than three feet or while bathing/showering. Activity counts were registered in 15 second epochs. Total physical activity was reported in average counts/d. Moderate-to-vigorous physical activity was reported in average counts/d, average minutes/d and average counts/minute/d. Physical activity was divided into moderate and vigorous levels based on the work of Puyau et al. [14] with 1500–6500 counts per minute classified as moderate activity and > 6500 counts per minute classified as vigorous activity. Only participants who wore the activity monitors for at least three complete days were included in the final analysis. This was confirmed by reviewing physical activity logs that were kept by the child with the assistance from the parent and by reviewing the graphical output generated by the activity monitor software.
Calcium intake
Children, with the aid of a parent, completed a diet record for two days during the week and one day on the weekend. To facilitate accurate quantification of foods, each participant and their parent received a list of serving size estimates based on comparisons to everyday objects (e.g. 3 oz of meat or poultry is approximately the size of a deck of cards). Calcium intake was estimated using the diet records and the USDA Food and Nutrient Database for Dietary Studies, v. 1.0, as previously done [15]. Only participants who recorded diet for three complete days were included in the final analysis.
Magnetic resonance imaging
Trabecular bone architecture at the distal femur and cortical bone architecture and muscle volume at the level of the middle-third of the femur on the non-dominant side were estimated using MRI (GE 1.5 T; Milwaukee, WI). Children were immobilized from the waist down using the BodyFIX (Medical Intelligence, Inc., Schwabm nchen, GER), as previously described [16]. Different imaging protocols were used to collect images needed for the assessment of trabecular bone architecture and cortical bone architecture.
Trabecular bone architecture
A phased array coil (USA Instruments; Aurora, OH) was secured to the lateral portion of the distal femur using a VacFIX system (PAR Scientific A/S; Sivlandvaenge, Denmark). The lateral distal femur was identified using a three-plane localizer. Then 26 high resolution axial images were collected immediately above the growth plate in the metaphysis using a 3D fast gradient echo sequence with a partial echo acquisition (4.5 ms echo time; 30 ms repetition time; 30° flip angle; 13.89 kHz bandwidth; 9 cm field of view) and an imaging matrix of 512 × 384 reconstructed to a 175 × 175 × 700 μm3 voxel size. Measures of trabecular bone architecture [i.e., appBV/TV, appTb.N (1/mm), appTb.Th (mm), and appTb.Sp (1/mm)] in the 20 most central images were estimated using custom software created with Interactive Data Language (IDL; Research Systems, Inc, Boulder, CO) and a procedure similar to that described by Majumdar et al. [17]. The procedure has been described in detail previously [16]. One investigator oversaw the collection of all images and one research assistant conducted all image analysis. In our laboratory, the reproducibility of the trabecular bone architecture assessment in children using this procedure ranges from 2 to 3 % [16].
Cortical bone architecture, muscle volume and femur length
Axial images (1 cm thick and 0.5 cm spacing) were collected along the length of the nondominant femur using a torso PA coil (750 ms repetition time, 14 ms echo time, 16 cm field of view and a 512 × 512 imaging matrix). Before image collection, the femur was identified using a three-plane localizer. The region of interest box was carefully positioned using the proximal end of the femoral head and the distal end of the femoral condyles to indicate the top and bottom, respectively, of the region of interest box. Using the localizer in the coronal plane, the region of interest box was rotated so its midline was placed alongside the lateral portion of the femoral head and ran through the center of the interchondalar notch between the femoral condyles. Using the localizer in the sagittal plane, the region of interest box was positioned so its midline went through the center of the femoral head and the center of the medial condyle. Images at the level of the middle third of the femur were analyzed for measures of cortical bone architecture and strength and muscle volume using software designed in-house with IDL. Images were first filtered with a median filter to reduce pixel noise. The filtered images were then subjected to automatic segmentation with a fuzzy C-means clustering algorithm. Using the software, voxels representing cortical bone, the medullary cavity and muscle at the level of the mid-third of the femur were identified and summed to determine their cross-sectional areas. Their volumes were quantified by accounting for image thickness and spacing between images. Middle images were multiplied by 1.5 to account for the 1.0 cm slice thickness of each image and the 0.5 cm gap between images. Images that included the proximal and distal ends of the region of interest were multiplied by an appropriate correction factor (< 1.5) so the entire image set represented the middle third of the femur. Volume (cm3) of the total midfemur was determined by summing cortical volume and medullary volume. Femur length was determined by counting the number of images and adjusting for image thickness and spacing. Cross-sectional moment of inertia of the midfemur in the anterior-posterior (CSMIap; cm4) and medial-lateral (CSMIml; cm4) directions were determined using the parallel-axis theorem [18]. Polar moment of inertia (J; cm4) was determined by adding CSMIap and CSMIml. Section modulus (Z; cm3) in the anterior-posterior and medial-lateral directions was calculated by dividing CSMIap and CSMIml by the furthest distance from the neutral axis in the anterior-posterior and medial-lateral directions, respectively. The average Z from the two directions is reported. One investigator oversaw the collection of all images and one research assistant conducted all image analysis. In our laboratory, the reproducibility of the cortical bone architecture and muscle volume estimates ranges from 0.5 to 2.6 % [19, 20]. To assess the reproducibility of femur length measurements by MRI, five children (6 to 12 years) were tested twice within 1 week. Tests were either on the same day or on a separate day. Femur length estimates from the repeat tests were identical.
Statistical analysis
Data were analyzed using SPSS (Version 22.0, Chicago, IL). Descriptive statistics for all variables were conducted to screen for outliers and to assess normality using the Shapiro-Wilk test. Sex differences in physical characteristics, including muscle volume and measures of trabecular and cortical bone architecture, were tested using independent t-tests for normally distributed data and Mann Whitney U tests otherwise. Height, body mass and BMI percentiles were compared to their respective 50th age- and sex-based percentile using a one-sample t-test. Sex by Tanner stage comparisons were conducted using the Chi-square test of independence. Zero-order correlation analysis was used to assess relationships between muscle volume and measures of trabecular and cortical bone architecture and to screen for multicollinearity among the predictors of bone architecture (i.e., muscle volume, age, femur length, sex, physical activity and calcium intake). Multiple linear regression was used to determine the amount of variance in measures of bone architecture explained by muscle volume after controlling for femur length, sex, physical activity and calcium intake. Age was excluded because it was strongly related to muscle volume (r = 0.86) and femur length (r = 0.92) causing a high variance inflation factor when it was introduced into the regression models (> 10). In addition, although all measures of trabecular and cortical bone architecture were related to age (r ranged from −0.57 to 0.66, and 0.78 to 0.85, respectively, all p < 0.01), they were more strongly related to femur length. Muscle volume was also strongly correlated with femur length (r = 0.91, p < 0.001), resulting in a high variance inflation factor when both variables were included in regression models (> 6). Therefore, a scaling factor was created to determine the contribution of muscle volume to variability in measures of trabecular and cortical bone architecture independent of femur length. The scaling factor was created using a log-log regression analysis [21]. Muscle volume and femur length were both natural log transformed and the transformed muscle volume was regressed on transformed femur length. The regression slope (2.77) represents the power by which femur length was raised to reduce collinearity. The resulting index (muscle volume/femur length2.77) was unrelated to femur length (r = 0.03) suggesting that it would be independent of femur length in a regression model. In addition, when it was included in a regression model with femur length, sex, MVPA and calcium intake, the variance inflation factor was low (< 1.5). Standardized (β) coefficients and partial correlations were reported to express the degree of variance in measures of bone architecture explained by a variable while controlling for the other covariates. Alpha level was set at 0.05.
Results
Fifty one children (n = 25 boys and 26 girls) were enrolled in the study. Four boys were excluded because they did not complete diet records. One boy was excluded because he did not complete MRI testing. Five girls were excluded because they had a Tanner stage > 2. One girl was excluded because she did not complete the MRI testing. Twenty boys and twenty girls were included in the final analysis. Physical characteristics, physical activity and calcium intake are listed in Table 1. All data were normally distributed except the measures of physical activity, which were log transformed before statistical analyses were conducted. However, non-transformed physical activity data are reported in Table 1 for easier comparison with other studies. There were no differences in age, Tanner stages, height, body mass or BMI, or age- and sex-based percentiles for height, body mass or BMI between boys and girls. Percentiles for height, body mass, and BMI were not different from the 50th age- and sex-based percentiles (p > 0.05). There were also no differences in any measure of physical activity or calcium intake between boys and girls (p > 0.05). There were no differences in measures of trabecular and cortical bone architecture between boys and girls (p > 0.05), as shown in Table 2.
Table 1.
Physical characteristics of pre- and early-pubertal typically developing children
| Boys (n = 20) | Girls (n = 20) | p | d | |
|---|---|---|---|---|
| Age (y) | 9.7 ± 1.6 | 10.0 ± 1.8 | 0.451 | 0.017 |
| Tanner stage (1/2) | ||||
| Pubic hair | 17/3 | 16/4 | 0.677 | 0.129 |
| Testicular-penile/Breast | 17/3 | 15/5 | 0.429 | 0.247 |
| Height (m) | 1.38 ± 0.12 | 1.39 ± 0.11 | 0.765 | 0.174 |
| Height percentile | 53 ± 29 | 48 ± 21 | 0.494 | 0.222 |
| Body mass (kg) | 33.2 ± 7.7 | 33.4 ± 7.7 | 0.926 | 0.026 |
| Body mass percentile | 57 ± 26 | 45 ± 24 | 0.195 | 0.437 |
| BMI (kg/m2) | 17.2 ± 1.8 | 17.1 ± 2.3 | 0.837 | 0.069 |
| BMI percentile | 54 ± 27 | 45 ± 28 | 0.283 | 0.345 |
| Midthigh muscle volume (cm3) | 891 ± 267 | 896 ± 250 | 0.945 | 0.019 |
| Femur length (m) | 0.378 ± 0.040 | 0.385 ± 0.037 | 0.500 | 0.181 |
| Total physical activity (counts/d) | 480,370 ± 205,490 | 458,902 ± 197,209 | 0.738 | 0.107 |
| MVPA (counts/d) | 296,220 ± 193,585 | 266,999 ± 167,695 | 0.613 | 0.162 |
| MVPA (min/d) | 92 ± 44 | 88 ± 35 | 0.788 | 0.077 |
| MVPA (counts/min/d) | 3068 ± 612 | 2908 ± 549 | 0.389 | 0.276 |
| Calcium intake (mg/d) | 989 ± 308 | 899 ± 220 | 0.212 | 0.352 |
Values are means ± SD. Height percentile = height relative to age- and sex-based norms; Body mass percentile = body mass relative to age- and sex-based norms; BMI = body mass index; BMI percentile = BMI relative to age- and sex-based norms; MVPA = moderate plus vigorous physical activity (values are not log transformed)
Table 2.
Measures of trabecular and cortical bone architecture
| Boys (n = 20) | Girls (n = 20) | p | d | |
|---|---|---|---|---|
| Trabecular bone architecture | ||||
| appBV/TV | 0.322 ± 0.027 | 0.320 ± 0.024 | 0.838 | 0.078 |
| appTb.N (1/mm) | 1.672 ± .063 | 1.683 ± 0.069 | 0.597 | 0.182 |
| appTb.Th (mm) | 0.192 ± 0.013 | 0.190 ± 0.012 | 0.534 | 0.160 |
| appTb.Sp (mm) | 0.407 ± 0.028 | 0.405 ± 0.030 | 0.856 | 0.069 |
| Cortical bone architecture | ||||
| Cortical volume (cm3) | 26.6 ± 8.8 | 27.0 ± 8.5 | 0.861 | 0.046 |
| Total bone volume (cm3) | 42.0 ± 13.2 | 42.2 ± 12.1 | 0.960 | 0.016 |
| Z (cm3) | 0.722 ± 0.234 | 0.716 ± 0.239 | 0.873 | 0.025 |
| J (cm4) | 1.54 ± 0.68 | 1.54 ± 0.68 | 0.978 | 0.001 |
Values are means ± SD.
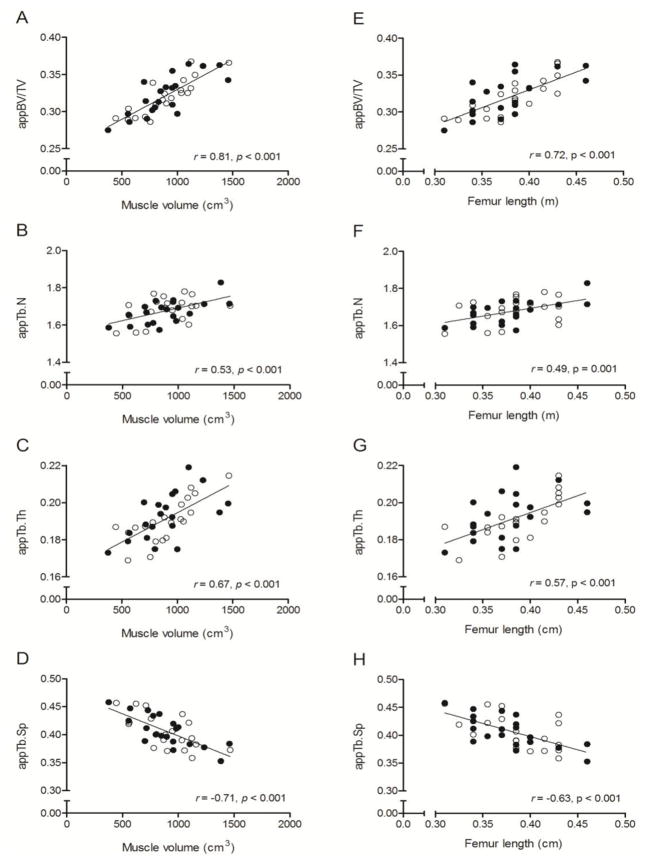
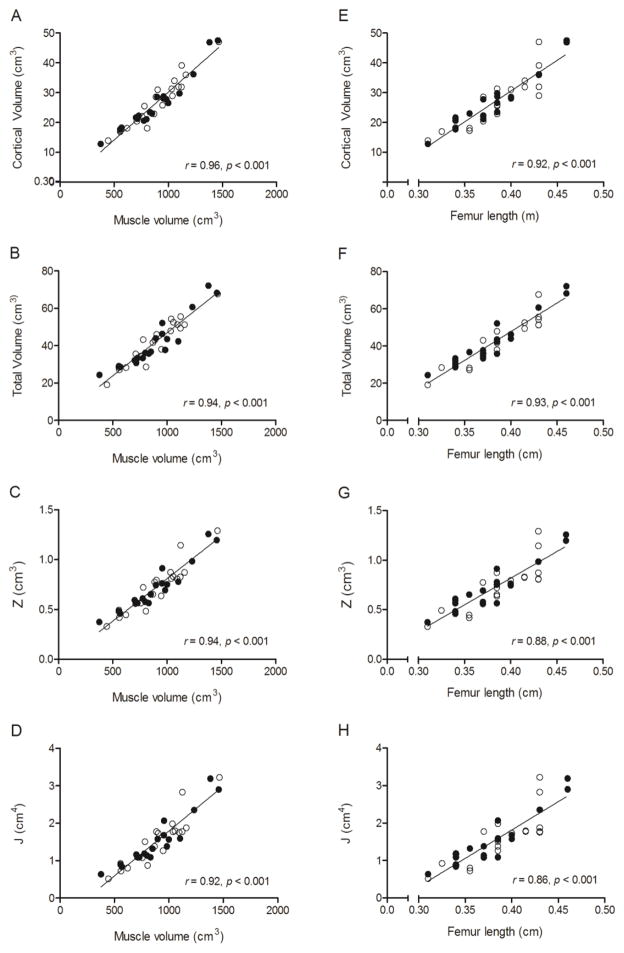
Zero-order correlations between measures of trabecular and cortical bone architecture and muscle volume, femur length, sex, MVPA and calcium intake are in Table 3. Muscle volume was moderately-to-strongly and positively related to appBV/TV, appTb.N and appTb.Th and moderately-to-strongly and negatively related to appTb.Sp (all p < 0.001). Femur length was also moderately-to-strongly and positively related to appBV/TV, appTb.N and appTb.Th and moderately-to-strongly and negatively related to appTb.Sp (all p < 0.01). These relationships are demonstrated by scatter plots in Figure 1. Muscle volume and femur length were strongly and positively related to cortical volume, total volume, Z and J (all p < 0.001). These relationships are demonstrated by scatter plots in Figure 2. No other variables were significantly correlated with measures of trabecular or cortical bone architecture.
Table 3.
Zero-order correlations demonstrating the relationships between bone architecture and muscle volume, femur length, sex, physical activity and calcium intake
| Muscle volume (cm3) | Femur length (m) | Sex | MVPA (min/d) | Calcium Intake (mg/d) | |
|---|---|---|---|---|---|
| Trabecular bone architecture | |||||
| appBV/TV | .81* | .72* | .03 | .17 | −.06 |
| appTb.N (1/mm) | .53* | .49** | −.09 | .23 | −.23 |
| appTb.Th (mm) | .67* | .57* | .10 | .07 | .11 |
| appTb.Sp (mm) | −.71* | −.63* | .03 | −.22 | .16 |
| Cortical bone architecture | |||||
| Cortical vol (cm3) | .96* | .92* | −.03 | .15 | −.16 |
| Total vol (cm3) | .94* | .93* | −.01 | .21 | −.18 |
| Z (cm3) | .94* | .88* | .01 | .15 | . −18 |
| J (cm4) | .92* | .86* | .01 | .14 | −.20 |
Significant correlation,
p < 0.001 and
p = 0.001;
MVPA = moderate to vigorous physical activity
Figure 1.
Scatter plots demonstrating the unadjusted relationships between measures of trabecular bone architecture in the distal femur (i.e., appBV/TV, appTb.N, appTb.Th, and appTb.Sp) and midthigh muscle volume (A-D) and femur length (E-H) in pre- and early-pubertal boys ● and girls ○. Because no sex effect was detected, the solid line represents the regression line for the combined sample.
Figure 2.
Scatter plots demonstrating the unadjusted relationships between measures of cortical bone architecture in the midfemur (i.e., cortical volume, total volume, J, and Z) and midthigh muscle volume (A-D) and femur length (E-H) in pre- and early-pubertal boys ● and girls ○. Because no sex effect was detected, the solid line represents the regression line for the combined sample.
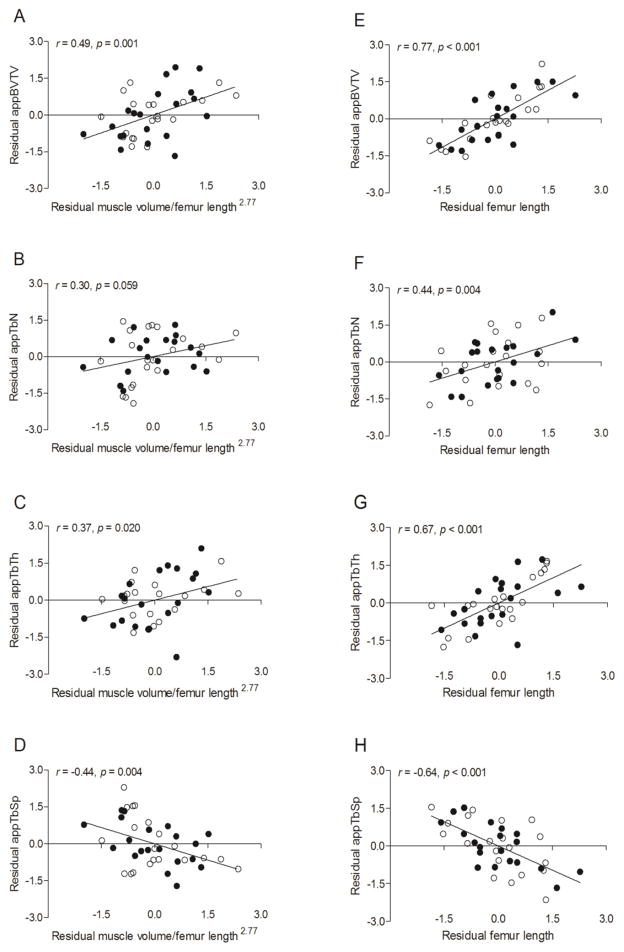
Results from the multiple regression analysis with muscle volume/femur length2.77, femur length, sex, MVPA and calcium intake as independent variables and measures of trabecular bone architecture as dependent variables are reported in Table 4. The only significant predictors of appBV/TV, appTb.Th and appTb.Sp were muscle volume/femur length2.77 (all p < 0.05) and femur length (all p < 0.001). The only significant predictor of appTb.N was femur length (p = 0.004); however, muscle volume/femur length2.77 was only marginally insignificant (p = 0.059). The relationships are demonstrated by scatter plots of residuals in Figure 3.
Table 4.
Predictors of trabecular bone architecture in the distal femur of typically developing children determined using multiple regression analysis
| appBV/TV | appTb.N (1/mm) | appTb.Th (mm) | appTb.Sp (mm) | |||||
|---|---|---|---|---|---|---|---|---|
| β | P value | β | P value | β | P value | β | P value | |
| Muscle volume/femur length2.77 | .344 | .001 | .257 | .059 | .286 | .020 | −.348 | .004 |
| Femur length (m) | .758 | .000 | .410 | .004 | .675 | .000 | −.611 | .000 |
| Sex | .037 | .728 | −.045 | .754 | .075 | .563 | .018 | .885 |
| MVPA (min/d) | .025 | .809 | .149 | .302 | −.063 | .625 | −.098 | .426 |
| Calcium intake (mg/d) | .052 | .627 | −.200 | .173 | .187 | .156 | .057 | .645 |
MVPA = moderate to vigorous physical activity log transformed; β = standardized coefficient
Figure 3.
Scatter plots demonstrating the relationships between measures of trabecular bone architecture in the distal femur (i.e., appBV/TV, appTb.N, appTb.Th, and appTb.Sp) and 1) midthigh muscle volume/femur length2.77 while statistically controlling for femur length, sex, MVPA (min/d) and calcium intake (A-D) and 2) femur length while statistically controlling for midthigh muscle volume/femur length2.77, sex, MVPA (min/d) and calcium intake (E-H) in pre- and early-pubertal boys ● and girls ○. Because no sex effect was detected, the solid line represents the regression line for the combined sample.
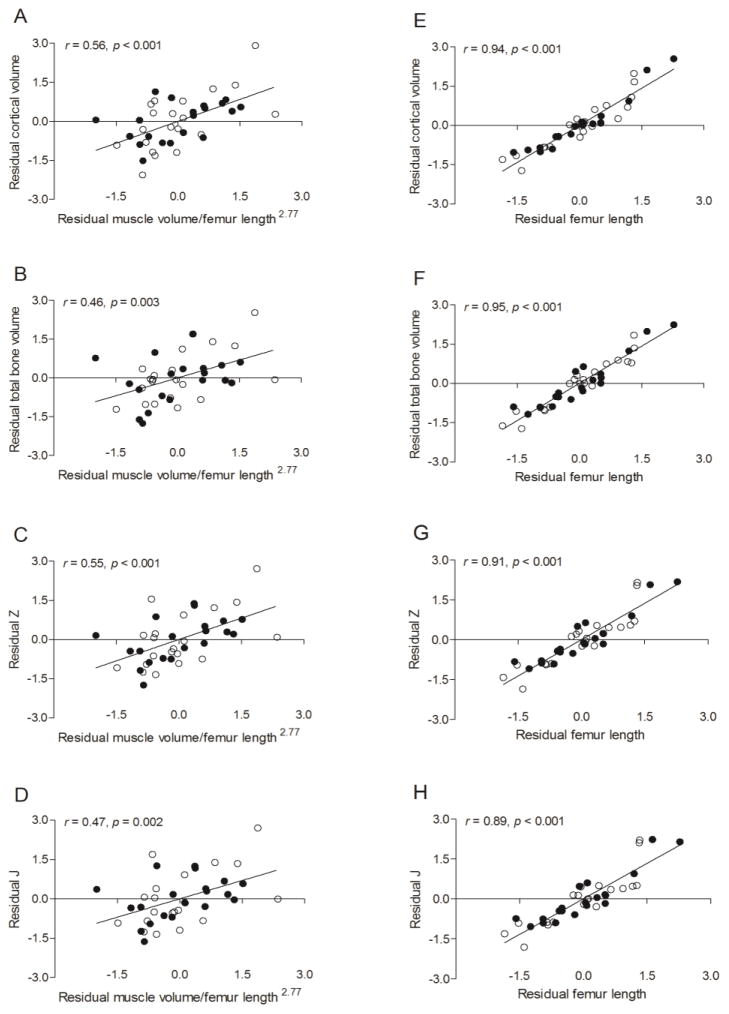
Results from the multiple regression analysis with muscle volume/femur length2.77, femur length, sex, MVPA and calcium intake as independent variables and measures of cortical bone architecture as dependent variables are reported in Table 5. The only significant predictors of measures of cortical bone architecture were muscle volume/femur length2.77 (all p < 0.01) and femur length (all p < 0.001). The relationships are demonstrated by scatter plots of residuals in Figure 4.
Table 5.
Predictors of cortical bone architecture in the midfemur of typically developing children determined using multiple regression analysis
| Cortical bone volume (cm3)
|
Total bone Volume (cm3)
|
Z (cm3)
|
J (cm4)
|
|||||
|---|---|---|---|---|---|---|---|---|
| β | P value | β | P value | β | P value | β | P value | |
| Muscle volume/femur length2.77 | .219 | .000 | .160 | .003 | .260 | .001 | .234 | .002 |
| Femur length (m) | .954 | .000 | .943 | .000 | .905 | .000 | .889 | .000 |
| Sex | .027 | .640 | .075 | .176 | .062 | .384 | .061 | .437 |
| MVPA (min/d) | −.055 | .333 | .008 | .886 | −.037 | .597 | −.045 | .561 |
| Calcium intake (mg/d) | .021 | .714 | −.046 | .400 | −.016 | .816 | −.033 | .671 |
MVPA = moderate to vigorous physical activity log transformed; β = standardized coefficient
Figure 4.
Scatter plots demonstrating the relationships between measures of cortical bone architecture in the midfemur (i.e., cortical volume, total volume, Z and J) and 1) midthigh muscle volume/femur length2.77 while statistically controlling for femur length, sex, MVPA (min/d) and calcium intake (A-D) and 2) femur length while statistically controlling for muscle volume/femur length2.77, sex, MVPA (min/d) and calcium intake (E-H) in pre- and early-pubertal boys ● and girls ○. Because no sex effect was detected, the solid line represents the regression line for the combined sample.
For each of the multiple regression models, the patterns of the relationships were the same irrespective of the type of physical activity (i.e., total physical activity or MVPA) or unit of physical activity (i.e., counts/d, min/d or counts/min/d) included in the model.
Discussion
To our knowledge, this is the first study to examine whether muscle is related to measures of trabecular bone architecture in children. A primary finding was that midthigh muscle volume was positively related to appBV/TV, appTb.N and appTb.Th and negatively related to appTb.Sp in the distal femur of pre and early-pubertal children. The present study is also the first to determine if the relationship between muscle volume and trabecular bone architecture is different than the relationship between muscle volume and cortical bone architecture in children. Midthigh muscle volume was more strongly related to measures of cortical than trabecular bone architecture. However, the magnitude of the disparity in the relationships was much smaller when femur length, sex, physical activity and calcium intake were statistically controlled. The findings suggest that muscle volume is an independent contributor to the development of trabecular and cortical bone architecture in children.
The moderate-to-strong relationship between muscle volume and trabecular bone architecture in children that was observed in the present study is consistent with the pattern reported in animals [22, 23] and humans [24, 25]. For example, muscle paralysis induced by botulinum toxin has been shown to decrease trabecular thickness of the mandible in rats and decrease BV/TV, Tb.N and Tb.Th and increase in Tb.Sp in the proximal tibia of mice [23]. Conversely, muscle contraction induced by electrical stimulation has been shown to blunt the loss of BV/TV and Tb.N associated with hindlimb suspension in rats [26]. In humans, conditions that result in a marked loss of muscle volume and strength in adults or poor accretion in children, such as spinal cord injury [25] and cerebral palsy [16], are associated with impaired trabecular bone architecture. Moreover, diminished trabecular bone architecture is associated with low muscle volume and low muscle strength [27]. In addition, relationships between appendicular muscle volume predicted by dual-energy X-ray absorptiometry and measures of trabecular bone architecture have been observed in men and women 20–97 years of age [28].
The positive relationship between muscle volume and measures of cortical bone architecture in the present study are consistent with previous reports [7, 9, 10, 29–31]. A positive moderate correlation between muscle cross sectional area and tibial cortical area was observed in 13 year olds with different ethnicities (r ranged from 0.45–0.60, p < 0.001) [29]. A moderate-to-strong relationship between muscle (or lean mass) and cortical bone architecture has also been reported in adult athletes and non-athletes [7], chronic stroke patients [30], patients with spinal cord injury [31], and children, adolescents and young adults [9, 10]. Despite the differences in research designs, the results of previous studies are generally consistent with ours. In the present study, although midthigh muscle volume was not as strongly related to measures of trabecular bone architecture (r ranged from 0.53 to 0.81) as it was to measures of cortical bone architecture (r ranged from 0.92 to 0.96), the relationships were still moderate-to-strong. The disparity suggests that muscle volume may be more closely linked to cortical than trabecular bone. However, the degree of the discrepancy was reduced substantially when femur length was statistically controlled (partial r ranged from −0.44 to 0.49 for trabecular bone architecture and −0.47 to 0.56 for cortical bone architecture). Interestingly, when femur length was statistically controlled, the standardized β values representing the relationship between midthigh muscle volume and measures of bone architecture were generally larger for measures of trabecular bone architecture (Table 4) than cortical bone architecture (Table 5). Therefore, it appears that the discrepancy in muscle’s relationship with trabecular vs. cortical bone is driven, in large part, by the dependence of the cortical bone architecture measures on femur length.
To our knowledge, this is also the first study to examine the relationship between a measure of bone size (i.e., bone length) and measures of trabecular bone architecture in children. Femur length, like midthigh muscle volume, was moderately-to-strongly and positively related to appBV/TV, appTb.N and appTb.Th and negatively related to appTb.Sp in the distal femur of pre and early-pubertal children. Also, similar to muscle volume, femur length was not as strongly related to measures of trabecular bone architecture as it was to measures of cortical bone architecture. Because femur length was highly correlated with muscle volume, the unique variance in trabecular bone architecture and cortical bone architecture explained by muscle volume vs. femur length is difficult to separate. In an attempt to resolve the issue of collinearity, muscle volume was scaled for femur length (i.e., muscle volume/femur length2.77). When femur length was included in a multiple regression model with muscle volume/femur length2.77, sex, physical activity and calcium intake, femur length was a significant predictor of appBV/TV, appTb.N and appTb.Sp. This finding suggests that femur length is related to measures of trabecular and cortical bone architecture independent of muscle volume. Future studies that determine if measures of trabecular and cortical bone architecture track changes in muscle due to intervention, such as resistance training, or unloading due to a neurological insult, such as spinal cord injury, would provide valuable insight into their relationship with muscle independent of bone size or general growth.
Another important finding in the present study was that there was no detectable sex-effect on the relationship between muscle and bone architecture. Although boys are stronger than girls [32], previous studies have reported no sex disparity in the relationship between muscle and bone in pre- and early-pubertal children [33, 34]. Sex hormones have been shown to affect the muscle-bone relationship, but this influence may not be observed until puberty. In a study of 318 boys and girls 6 to 22 years of age, Schoenau et al. [33] reported that girls have higher cortical area than boys at the onset of puberty due to the surge in estrogen. On the other hand, another cross-sectional comparison of children through young adults (6 to 21 years of age) suggests that appBV/TV and appTb.Th become higher and appTb.Sp becomes lower in boys during late puberty and this may be related to the surge in testosterone in boys [35].
One of the strengths of the present study was that the independent relationship between muscle volume and bone architecture was examined while muscle volume was scaled for femur length. This allowed us to control muscle volume for femur length while avoiding the collinearity associated with the two variables. Evaluating bone architecture’s relationship with muscle volume while statistically controlling for physical activity was another strength of the study. High levels of physical activity, especially MVPA, have a positive influence on muscle [36] and bone [37–39]. Therefore, it is plausible that the relationship between muscle and bone architecture is driven, at least in part, by physical activity. However, the relationship existed even when total physical activity or MVPA was used as a covariate in a regression model. Statistically controlling for calcium intake is another strength of the study because higher bone mineral content is seen in children with a regular dietary intake of calcium [40]. Moreover, there is evidence that adolescent boys retain more calcium than girls [41]. On the other hand, calcium intake is not associated with increases in lean mass [42]. Although it is plausible that calcium intake can cause discord in the relationship between muscle and bone, the relationships between muscle volume and measures of bone architecture remained even when calcium intake was used as a covariate in a regression model.
We acknowledge that this study has limitations not already mentioned. One is the cross-sectional design which does not allow us to make inferences about cause and effect. Although cortical bone was strongly related to muscle volume in the present study, some studies suggest that cortical bone is more strongly related to impact loading than the bone strain generated by muscle forces [43]. There is also evidence that changes in cortical bone architecture occur before changes in muscle area during growth [44]. Therefore, additional studies are needed to determine if changes in bone architecture track changes in muscle volume that occur with growth or with intervention. A second limitation is that the sample size was small and restricted to Caucasians which prevents us from generalizing our findings to children of other origins. The small sample also limits our confidence in the finding that sex did not affect bone architecture’s relationship with muscle volume and femur length. However, only children who were pre- or early-pubertal were included in the study and the finding of no sex-effect on the muscle-bone relationship at this stage of maturity is consistent with previous studies [33, 34]. A third limitation is related to the physical activity data. Only children who participated in three or fewer hours of organized physical activity per week participated in the study. Furthermore, assessment of physical activity was limited to four days. Although we quantified MVPA, and MVPA for the boys and girls in the present study (92 and 88 min/d) were similar to values previously reported for a sample of 6–11 year-old Caucasian boys and girls (average MVPA = 95 and 75 min/d, respectively)[45], appropriate thresholds used to classify physical activity into different categories have not been agreed upon [46], especially for the activity monitors used in the present study. Nonetheless, the results were the same irrespective of the physical activity measure used (i.e., MVPA or total physical activity) or how it was expressed (i.e., counts/d, min/d or counts/min/d).
In summary, the findings from the present study suggest that midthigh muscle volume is related to measures of trabecular bone architecture in the distal femur and measures of cortical bone architecture in the midfemur. The findings also suggest that the relationship of bone architecture with midthigh muscle volume is not different in pre and early-pubertal boys and girls. The relationship remained after controlling for femur length, suggesting that it may not be driven solely by general growth. Although midthigh muscle volume is more strongly related to cortical bone architecture in the midfemur than trabecular bone architecture in the distal femur, the reason for the discrepancy is due, in large part, to the greater dependence of cortical bone architecture measures on femur length. Longitudinal studies that track changes in trabecular and cortical bone architecture, muscle volume and bone length and intervention studies that facilitate increases in muscle size and strength would help us confirm whether muscle has a unique relationship with trabecular and cortical bone architecture in children.
Highlights.
Muscle volume is related to trabecular and cortical bone architecture in children
The relationship between muscle volume and bone architecture is not different in boys vs. girls
Midthigh muscle volume is more strongly related to cortical bone architecture in the midfemur than trabecular bone architecture in the distal femur of children
Muscle volume’s stronger relationship with cortical than trabecular bone architecture is driven mainly by the dependence of cortical bone architecture measures on bone length
Acknowledgments
We are thankful to the children who participated in our study and their families for their time and cooperation. This study was funded by the National Institutes of Health (HD071397 and HD505030) and the National Osteoporosis Foundation.
Abbreviations
- appBV/TV
apparent bone volume to total volume
- appTb.N
apparent trabecular number
- appTb.Th
apparent trabecular thickness
- appTb.Sp
apparent trabecular separation
- Z
section modulus
- J
polar moment of inertia
Footnotes
Conflicts of interest: The authors declare that they have no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, Weaver C. Peak Bone Mass. Osteoporos Int. 2000;11:985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 2.Modlesky CM, Lewis RD. Does exercise during growth have a long-term effect on bone health? Exerc Sport Sci Rev. 2002;30:171–6. doi: 10.1097/00003677-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Ciarelli TE, Fyhrie DP, Schaffler MB, Goldstein SA. Variations in three-dimensional cancellous bone architecture of the proximal femur in female hip fractures and in controls. J Bone Miner Res. 2000;15:32–40. doi: 10.1359/jbmr.2000.15.1.32. [DOI] [PubMed] [Google Scholar]
- 4.Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, Jaworski M, Gordon CM International Society for Clinical D. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17:225–42. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Adams JE, Engelke K, Zemel BS, Ward KA International Society of Clinical D. Quantitative computer tomography in children and adolescents: the 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17:258–74. doi: 10.1016/j.jocd.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Y, Zhao J, Augat P, Ouyang X, Lu Y, Majumdar S, Genant HK. Trabecular bone mineral and calculated structure of human bone specimens scanned by peripheral quantitative computed tomography: relation to biomechanical properties. J Bone Miner Res. 1998;13:1783–90. doi: 10.1359/jbmr.1998.13.11.1783. [DOI] [PubMed] [Google Scholar]
- 7.Rittweger J, Beller G, Ehrig J, Jung C, Koch U, Ramolla J, Schmidt F, Newitt D, Majumdar S, Schiessl H, Felsenberg D. Bone-muscle strength indices for the human lower leg. Bone. 2000;27:319–26. doi: 10.1016/s8756-3282(00)00327-6. [DOI] [PubMed] [Google Scholar]
- 8.Schoenau E. The “functional muscle-bone unit”: a two-step diagnostic algorithm in pediatric bone disease. Pediatr Nephrol. 2005;20:356–9. doi: 10.1007/s00467-004-1744-1. [DOI] [PubMed] [Google Scholar]
- 9.Leonard MB, Elmi A, Mostoufi-Moab S, Shults J, Burnham JM, Thayu M, Kibe L, Wetzsteon RJ, Zemel BS. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95:1681–9. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogler W, Blimkie CJ, Cowell CT, Inglis D, Rauch F, Kemp AF, Wiebe P, Duncan CS, Farpour-Lambert N, Woodhead HJ. Sex-specific developmental changes in muscle size and bone geometry at the femoral shaft. Bone. 2008;42:982–9. doi: 10.1016/j.bone.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei RMZ, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 12.Tanner JA. Growth and Adolescence. Oxford: Blackwell Scientific Publications; 1962. [Google Scholar]
- 13.Trost SG, Pate RR, Freedson PS, Sallis JF, Taylor WC. Using objective physical activity measures with youth: how many days of monitoring are needed? Med Sci Sports Exerc. 2000;32:426–31. doi: 10.1097/00005768-200002000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Puyau MR, Adolph AL, Vohra FA, Zakeri I, Butte NF. Prediction of activity energy expenditure using accelerometers in children. Med Sci Sports Exerc. 2004;36:1625–1631. [PubMed] [Google Scholar]
- 15.Johnson DL, Miller F, Subramanian P, Modlesky CM. Adipose tissue infiltration of skeletal muscle in children with cerebral palsy. J Pediatr. 2009;154:715–720. doi: 10.1016/j.jpeds.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modlesky CM, Subramanian P, Miller F. Underdeveloped trabecular bone microarchitecture is detected in children with cerebral palsy using high-resolution magnetic resonance imaging. Osteoporos Int. 2008;19:169–76. doi: 10.1007/s00198-007-0433-x. [DOI] [PubMed] [Google Scholar]
- 17.Majumdar S, Genant HK, Grampp S, Newitt DC, Truong VH, Lin JC, Mathur A. Correlation of trabecular bone structure with age, bone mineral density, and osteoporotic status: in vivo studies in the distal radius using high resolution magnetic resonance imaging. J Bone Miner Res. 1997;12:111–8. doi: 10.1359/jbmr.1997.12.1.111. [DOI] [PubMed] [Google Scholar]
- 18.Turner CH, Burr DB. Experimental techniques for bone mechanics. In: Cowen SC, editor. Bone Mechanics Handbook. Boca Raton: CRC Press; 2001. pp. 1–35. [Google Scholar]
- 19.Modlesky CM, Kanoff SA, Johnson DL, Subramanian P, Miller F. Evaluation of the femoral midshaft in children with cerebral palsy using magnetic resonance imaging. Osteoporos Int. 2009;20:609–15. doi: 10.1007/s00198-008-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Modlesky CM, Cavaiola ML, Smith JJ, Rowe DA, Johnson DL, Miller F. A DXA-based mathematical model predicts midthigh muscle mass from magnetic resonance imaging in typically developing children but not in those with quadriplegic cerebral palsy. J Nutr. 2010;140:2260–5. doi: 10.3945/jn.110.126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells JC, Cole TJ. Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obes Relat Metab Disord. 2002;26:947–52. doi: 10.1038/sj.ijo.0802027. [DOI] [PubMed] [Google Scholar]
- 22.Tsai CY, Huang RY, Lee CM, Hsiao WT, Yang LY. Morphologic and bony structural changes in the mandible after a unilateral injection of botulinum neurotoxin in adult rats. J Oral Maxillofac Surg. 2010;68:1081–7. doi: 10.1016/j.joms.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Poliachik SL, Bain SD, Threet D, Huber P, Gross TS. Transient muscle paralysis disrupts bone homeostasis by rapid degradation of bone morphology. Bone. 2010;46:18–23. doi: 10.1016/j.bone.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollock NK, Laing EM, Taylor RG, Baile CA, Hamrick MW, Hall DB, Lewis RD. Comparisons of trabecular and cortical bone in late adolescent black and white females. J Bone Miner Metab. 2011;29:44–53. doi: 10.1007/s00774-010-0186-z. [DOI] [PubMed] [Google Scholar]
- 25.Slade JM, Bickel CS, Modlesky CM, Majumdar S, Dudley GA. Trabecular bone is more deteriorated in spinal cord injured versus estrogen-free postmenopausal women. Osteoporos Int. 2005;16:263–72. doi: 10.1007/s00198-004-1665-7. [DOI] [PubMed] [Google Scholar]
- 26.Lam H, Qin YX. The effects of frequency-dependent dynamic muscle stimulation on inhibition of trabecular bone loss in a disuse model. Bone. 2008;43:1093–100. doi: 10.1016/j.bone.2008.07.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szulc P, Blaizot S, Boutroy S, Vilayphiou N, Boonen S, Chapurlat R. Impaired bone microarchitecture at the distal radius in older men with low muscle mass and grip strength: the STRAMBO study. J Bone Miner Res. 2013;28:169–78. doi: 10.1002/jbmr.1726. [DOI] [PubMed] [Google Scholar]
- 28.Lebrasseur NK, Achenbach SJ, Melton LJ, 3rd, Amin S, Khosla S. Skeletal muscle mass is associated with bone geometry and microstructure and serum insulin-like growth factor binding protein-2 levels in adult women and men. J Bone Miner Res. 2012;27:2159–69. doi: 10.1002/jbmr.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Micklesfield LK, Norris SA, Pettifor JM. Determinants of bone size and strength in 13-year-old South African children: The influence of ethnicity, sex and pubertal maturation. Bone. 2011;48:777–85. doi: 10.1016/j.bone.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 30.Pang MY, Ashe MC, Eng JJ. Tibial bone geometry in chronic stroke patients: influence of sex, cardiovascular health, and muscle mass. J Bone Miner Res. 2008;23:1023–30. doi: 10.1359/jbmr.080224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modlesky CM, Slade JM, Bickel CS, Meyer RA, Dudley GA. Deteriorated geometric structure and strength of the midfemur in men with complete spinal cord injury. Bone. 2005;36:331–9. doi: 10.1016/j.bone.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Newman DG, Pearn J, Barnes A, Young CM, Kehoe M, Newman J. Norms for hand grip strength. Arch Dis Child. 1984;59:453–9. doi: 10.1136/adc.59.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoenau E, Neu CM, Mokov E, Wassmer G, Manz F. Influence of puberty on muscle area and cortical bone area of the forearm in boys and girls. J Clin Endocrinol Metab. 2000;85:1095–8. doi: 10.1210/jcem.85.3.6451. [DOI] [PubMed] [Google Scholar]
- 34.Schoenau E, Neu CM, Beck B, Manz F, Rauch F. Bone mineral content per muscle cross-sectional area as an index of the functional muscle-bone unit. J Bone Miner Res. 2002;17:1095–101. doi: 10.1359/jbmr.2002.17.6.1095. [DOI] [PubMed] [Google Scholar]
- 35.Kirmani S, Christen D, van Lenthe GH, Fischer PR, Bouxsein ML, McCready LK, Melton LJ, 3rd, Riggs BL, Amin S, Muller R, Khosla S. Bone structure at the distal radius during adolescent growth. J Bone Miner Res. 2009;24:1033–42. doi: 10.1359/JBMR.081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacDougall JD, Ward GR, Sale DG, Sutton JR. Biochemical adaptation of human skeletal muscle to heavy resistance training and immobilization. J Appl Physiol Respir Environ Exerc Physiol. 1977;43:700–3. doi: 10.1152/jappl.1977.43.4.700. [DOI] [PubMed] [Google Scholar]
- 37.Fuchs RK, Bauer JJ, Snow CM. Jumping improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial. J Bone Miner Res. 2001;16:148–56. doi: 10.1359/jbmr.2001.16.1.148. [DOI] [PubMed] [Google Scholar]
- 38.Janz KF, Burns TL, Levy SM, Torner JC, Willing MC, Beck TJ, Gilmore JM, Marshall TA. Everyday activity predicts bone geometry in children: the iowa bone development study. Med Sci Sports Exerc. 2004;36:1124–31. doi: 10.1249/01.mss.0000132275.65378.9d. [DOI] [PubMed] [Google Scholar]
- 39.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the university of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999;14:1672–9. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 40.Lee WTK, Leung SSF, Lui SSH, Lau J. Relationship between long-term calcium intake and bone mineral content of children aged from birth to 5 years. Br J Nutr. 1993;70:235–248. doi: 10.1079/bjn19930120. [DOI] [PubMed] [Google Scholar]
- 41.Braun M, Martin BR, Kern M, McCabe GP, Peacock M, Jiang Z, Weaver CM. Calcium retention in adolescent boys on a range of controlled calcium intakes. Am J Clin Nutr. 2006;84:414–8. doi: 10.1093/ajcn/84.1.414. [DOI] [PubMed] [Google Scholar]
- 42.Smilowitz JT, Wiest MM, Teegarden D, Zemel MB, German JB, Van Loan MD. Dietary fat and not calcium supplementation or dairy product consumption is associated with changes in anthropometrics during a randomized, placebo-controlled energy-restriction trial. Nutr Metab (Lond) 2011;8:67. doi: 10.1186/1743-7075-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schipilow JD, Macdonald HM, Liphardt AM, Kan M, Boyd SK. Bone microarchitecture, estimated bone strength, and the muscle-bone interaction in elite athletes: an HR-pQCT study. Bone. 2013;56:281–9. doi: 10.1016/j.bone.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Xu L, Nicholson P, Wang Q, Alen M, Cheng S. Bone and muscle development during puberty in girls: a seven-year longitudinal study. J Bone Miner Res. 2009;24:1693–8. doi: 10.1359/jbmr.090405. [DOI] [PubMed] [Google Scholar]
- 45.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 46.Guinhouya BC, Samouda H, de Beaufort C. Level of physical activity among children and adolescents in Europe: a review of physical activity assessed objectively by accelerometry. Public Health. 2013;127:301–11. doi: 10.1016/j.puhe.2013.01.020. [DOI] [PubMed] [Google Scholar]