Abstract
Introduction
Nonambulatory children with severe cerebral palsy (CP) have an underdeveloped bone architecture, low bone strength and a high degree of fat infiltration in the lower extremity musculature. The present study aims to determine if such a profile exists in ambulatory children with mild CP and if excess fat infiltration extends into the bone marrow.
Materials and methods
Ambulatory children with mild spastic CP and typically developing children (4 to 11 years; 12/group) were tested. Magnetic resonance imaging was used to estimate cortical, medullary and total bone volume and width, bone strength [i.e., section modulus (Z) and polar moment of inertia (J)], and bone marrow fat concentration in the midtibia, and muscle volume, intermuscular, subfascial, and subcutaneous adipose tissue (AT) volume and intramuscular fat concentration in the midleg. Physical activity monitors worn on the ankle were used to assess physical activity.
Results
There were no group differences in age, height, body mass, body mass percentile, BMI, BMI percentile or tibia length, but children with CP had lower height percentile (19th vs. 50th percentile) and total physical activity counts (44 %) than controls (both p < 0.05). Children with CP also had lower cortical volume (30 %), cortical width in the posterior (16 %) and medial (32 %) portion of the shaft, total bone width in the medial-lateral direction (15 %), Z in the medial-lateral direction (34 %), J (39 %) and muscle volume (39 %), and higher bone marrow fat concentration (82.1 ± 1.8 % vs. 80.5 ± 1.9 %), subfascial AT volume (3.3 fold) and intramuscular fat concentration (25.0 ± 8.0 % vs. 16.1 ± 3.3 %) than controls (all p < 0.05). When tibia length was statistically controlled, all group differences in bone architecture, bone strength, muscle volume and fat infiltration estimates, except posterior cortical width, were still present (all p < 0.05). Furthermore, a higher intermuscular AT volume in children with CP compared to controls emerged (p < 0.05).
Conclusions
Ambulatory children with mild CP exhibit an underdeveloped bone architecture and low bone strength in the midtibia and a greater infiltration of fat in the bone marrow and surrounding musculature compared to typically developing children. Whether the deficit in the musculoskeletal system of children with CP is associated with higher chronic disease risk and whether the deficit can be mitigated requires further investigation.
Keywords: bone structure, bone strength, cerebral palsy, unloading, fat depots
1. Introduction
Cerebral palsy (CP) is a neurological condition that is associated with dysfunctional gait and progressive decrements in physical activity from childhood to adulthood [1]. Nonambulatory children with more severe spastic CP present with low bone mass and underdeveloped bone architecture [2–6], as well as small, weak [7] and qualitatively-compromised musculature, as indicated by a high degree of fat infiltration [8]. It is not surprising that this musculoskeletal phenotype is associated with a high incidence of low-energy fractures, primarily occurring in the lower limbs [9, 10]. The latter complication may be due mainly to the lack of physical activity [4, 8] and mechanical loading, which reduces the stimulus for periosteal and endocortical expansion [4] in the lower extremities. To date, it is unclear if the adverse musculoskeletal profile exhibited in nonambulatory children with more severe CP is also present in ambulatory children with a milder form of the disorder.
In addition to possessing an underdeveloped musculoskeletal system, nonambulatory children with severe CP also have elevated adipose tissue (AT) surrounding bone and muscle [8]. Furthermore, human models of reduced mechanical loading show elevated levels of fat infiltration within the bone marrow cavity [11] and muscle [12], which are linked to osteoporosis [13–15], impaired glucose tolerance [12, 16, 17] and cardiometabolic disease [18]. The objective of the present study was to determine if ambulatory children with mild spastic CP have a deficit in bone architecture and elevated fat infiltration within the bone marrow cavity of the midtibia and the surrounding leg musculature. We hypothesized that ambulatory children with mild CP vs. typically developing children would have a thinner shaft with a thinner cortex and lower estimates of strength in the midtibia. We also hypothesized that children with CP vs. typically developing children would have an elevated fat infiltration within the bone marrow cavity and the surrounding leg musculature.
2. Materials and Methods
2.1. Participants and study design
Ambulatory children with mild spastic CP and between the ages of 4 and 11 y were recruited from the AI duPont Hospital for Children in Wilmington, DE and other pediatric hospitals in the Mid-Atlantic region of the U.S. Typically developing children that matched children with CP for age, sex and race were recruited from the Newark and Wilmington, DE areas using flyers and word of mouth. Additional inclusion criteria for controls included falling between the 5th and 95th percentile for height and body mass, no history of chronic medication use, no previous fracture in the nondominant lower extremity and no current or previous regular participation in an activity that involved high loading of the skeleton, such as artistic gymnastics. Participants were recruited from April 2012 through May 2016 and testing was conducted from November 2012 to May 2016. The Institutional Review Boards at the AI duPont Hospital for Children and the University of Delaware approved the study procedures. Prior to testing, written consent and assent was obtained by the parents and the participants, respectively.
2.2. Anthropometrics
Height and body mass were measured while the child was in a t-shirt and shorts. Height was measured to the nearest 0.1 cm using a stadiometer (Seca 217; Seca GmbH & Co. KG., Hamburg, GER). Body mass was measured to the nearest 0.2 kg using a digital scale (Detecto 6550, Cardinal Scale, Webb City, MO). Height, body mass and BMI percentile were calculated from the normative graphs published by the Centers for Disease Control and Prevention [19].
2.3. Tanner Staging
Sexual maturity was assessed by a physician assistant using the Tanner staging technique [20]. The technique is based on a 5-point scale, with I indicating no development and V indicating full development. Pubic hair and breast development were assessed in girls. Pubic hair and testicular/penile development were assessed in boys.
2.4. Gross Motor Function
Gross motor function of children with CP was assessed by a physician assistant using the gross motor function classification system (GMFCS) [21]. Children who were GMFCS I or II were included in the study. In short, a child with the ability of walking indoors and outdoors and gross motor skills of running and jumping, but limited ability of speed, balance and coordination was classified as GMFCS level I. Limitations of walking on uneven surfaces and inclines and minimal gross motor skills of running and jumping was classified as GMFCS level II.
2.5. Physical Activity
Physical activity was estimated using accelerometer-based activity monitors (Actical; Respironics Inc., Bend, OR). The activity monitors contain an omnidirectional accelerometer that is most sensitive to movements in the vertical plane when worn on the ankle and is sensitive to movements in the 0.5 to 3 Hz frequency range [22]. Physical activity counts were registered in 15 second epochs. Each participant wore two monitors on the lateral aspect of the ankle on the more affected side in children with CP and on the nondominant side in controls. Monitors were worn continuously (i.e., 24 hours per day) for four days (three week days and one weekend day). Participants and participant parents were instructed to take the monitors off only when swimming at a depth greater than 0.91 meters and during bathing/showering. This was confirmed by reviewing activity logs kept by the children with assistance from their parent and by visually examining the graphical output generated using software provided by the manufacturer. If participants did not wear the monitors on any of the days, they were asked to re-wear the monitors to make up for the lost day(s). The total physical activity counts per day averaged from the two monitors are reported. The reliability of the total physical activity counts was assessed in 8 ambulatory children with mild CP and 8 typically developing children between 4 and 11 years of age who wore the monitors for four days on two separate occasions approximately one month apart. The intraclass correlation was 0.935 for children with CP and 0.913 for typically developing children indicating excellent reliability.
2.6. Magnetic Resonance Imaging
Magnetic resonance imaging (MRI; GE, 1.5 T, Milwaukee, WI) was used to assess bone architecture and the degree of fat infiltration in the bone marrow at the level of the middle-third of the tibia (i.e., midtibia) and in the surrounding leg musculature (i.e., midleg). The more affected limb in children with CP and in the nondominant limb in controls were tested. Children were immobilized from the waist down using the BodyFIX (Medical Intelligence, Inc., Schwabm nchen, GER), as previously described [3]. A three plane localizer was used to identify the region of interest. Axial images were collected from the tibia plateau to the malleolar articular surface (0.5 cm thick separated by 0.5 cm of spacing) using a semiflex long bone array coil (ScanMed, Omaha, NE) and two different sequences. The first sequence (fast spin echo, TR = 650, TE = 14, FOV = 12, NEX = 3, BW = 15.63, frequency = 512, phase = 256) yielded T1-weighted images. The second sequence (IDEAL: fast-spin-echo, TR = 600, TE = min full, FOV = 12, NEX = 2, BW = 31.25, frequency = 320, phase = 224) yielded fat and water images.
All image collection was overseen by the senior author (CMM). Images at the level of the midtibia were processed blindly by the same technician using software developed with Interactive Data Language (Research Systems, Inc, Boulder, CO) and procedures previously described for the midthigh [8]. A general visual description of the image processing procedure used to quantify bone architecture and strength of the midtibia and the fat infiltration of the bone marrow and surrounding musculature is provided in Figure 1. A more specific description follows. Using an automated procedure, the T1-weighted images at the level of the middle-third of the tibia were filtered using a median filter and voxels were segmented and assigned to cortical bone, bone marrow, muscle and AT using a fuzzy clustering algorithm [23] and their volumes (cm3) were calculated. Widths (cm) of the cortical bone in the anterior, posterior, medial and lateral portions, widths of the medullary cavity and total bone in the anterior-posterior and medial-lateral directions and estimates of bone strength [i.e., polar moment of inertia (J) and section modulus] were also calculated during the same procedure. As described previously [4], the parallel-axis theorem [24] was used to determine the cross-sectional moment of inertia of the midtibia in the anterior-posterior and medial-lateral directions. Polar moment of inertia (cm4) was calculated by averaging the cross-sectional moment of inertia in the two directions. Section modulus (Z; cm3) was calculated by dividing cross-sectional moment of inertia measure by the furthest distance from the neutral axis in the anterior-posterior (Zap) and medial-lateral (Zml) directions. Tibia length was estimated by counting the number of images used to cover the entire midtibia and adjusting for image thickness and spacing. The test-retest reliability for measures of bone architecture, bone strength and muscle size measures in the lower extremity from MRI was assessed previously in a combined sample of children with CP and typically developing children tested twice on separate days or on the same day after repositioning [4, 8]. Intraclass correlation coefficients > 0.99 and coefficients of variation between 0.6 and 2.6 % indicated excellent reliability.
Figure 1.
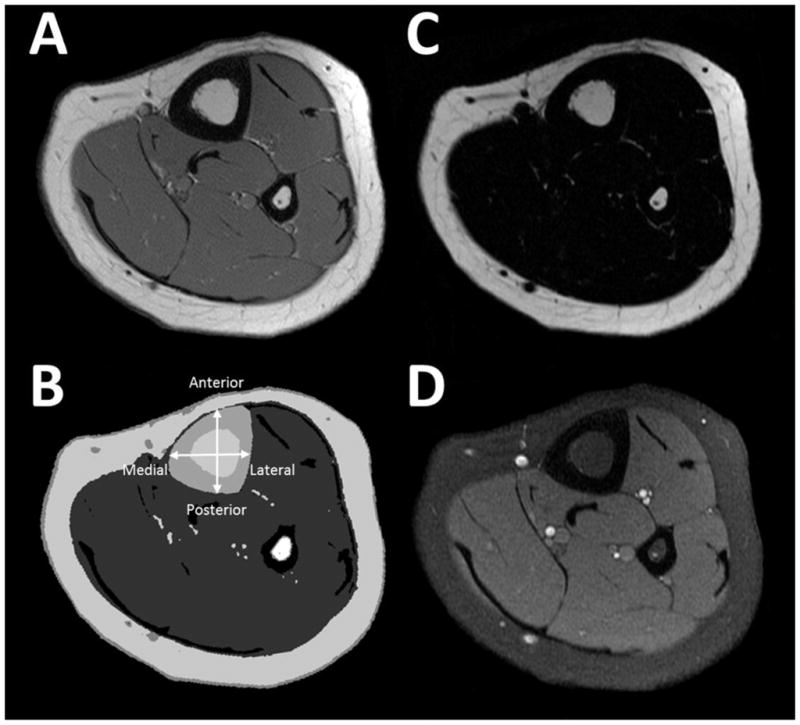
A general description of the image processing procedure used to quantify bone architecture and strength of the midtibia and the fat infiltration of the bone marrow and surrounding musculature. Raw T1-weighted magnetic resonance images (A) were segmented (B) into tibia cortical bone (large black ring; fibula is the small black ring) and bone marrow (white region surrounded by the cortical bone), muscle (gray region surrounding the bone) and adipose tissue (AT; white ring surrounding the muscles and bones and the white voxels interspersed among the musculature). The bone, muscle and AT volumes, bone widths (cortical bone in the anterior, posterior, medial and lateral portions and the bone marrow and total bone in the anterior-posterior and medial-lateral directions) and estimates of bone strength (section modulus in the anterior-posterior and medial-lateral directions and polar moment of inertia) were calculated. Voxels identified as bone marrow and muscle were applied to fat (C) and water (D) images to determine bone marrow and intramuscular fat concentration [25].
Voxels representing muscle and bone marrow in the T1-weighted images were then used to identify the bone marrow and muscle voxels in the corresponding fat and water images. The fat concentration within the bone marrow of the midtibia and the muscles (i.e.,intramuscular fat concentration) at the same level was determined by using the signal intensity (SI) from the fat image, the SI from the water image and the following equation: fat concentration = [fat SI / (fat SI + water SI)] * 100 [25]. Fat content estimated using MRI and the IDEAL sequence is highly correlated with fat content measured using magnetic resonance spectroscopy in phantoms (r2 = 0.985) [26] and human vertebrae (r2 = 0.904) [27].
Intermuscular, subfascial and subcutaneous AT volume were estimated using the same custom software and the T1-weighted images used to assess bone architecture, bone strength, muscle volume and AT volume. Using a procedure similar to the procedure used for the midthigh [8], subcutaneous AT was separated from intermuscular and subfascial AT by manually tracing a line over the crural fascia. Intermuscular AT was separated from subfascial AT and bone marrow by manually tracing a line on the outside border of the cortical bone and another line on the inside border of skeletal muscle. In areas where muscles separated, the line was drawn midway between the muscle borders. Thus, subcutaneous AT was the volume of AT between the skin and the crural fascia. Subfascial AT was considered the volume of AT between the deep crural fascia and the skeletal muscle border. Intermuscular AT was considered the volume of AT between the subfascial AT and the bone marrow.
The test-retest reliability for AT measures in the lower extremity using MRI was assessed previously in a combined sample of children with CP and typically developing children tested twice on separate days or on the same day after repositioning [8]. Intraclass correlation coefficients > 0.99 and coefficients of variation between 0.6 and 3.4 % indicated excellent reliability. The test-retest reliability of midtibia bone marrow fat and midleg intramuscular fat concentrations from MRI were assessed in four typically developing children and four children with CP (5 to 11 years of age) tested on two separate days, six months apart. The intraclass correlations were 0.96 and > 0.99 and the coefficients of variation were 0.04 % and 0.3 % for bone marrow and intramuscular fat concentrations, respectively.
2.7. Statistical Analysis
Using an effect size of 1.2 and setting the alpha at 0.05 and power at 0.8, a minimum sample size of 10 participants per group was estimated to determine if there were group differences in measures of bone architecture and bone marrow and muscle fat infiltration. Data were analyzed by using SPSS version 24.0 (IBM Corp, Armonk, NY). Descriptive statistics were conducted for all variables to screen for outliers and to assess normality. Independent sample t-tests were used to determine if there were group differences. The Mann-Whitney U test was used to assess differences in Tanner stage. To adjust all outcome measures of bone, muscle, AT and fat concentration for tibia length, a one-way analysis of covariance was used with tibia length as a covariate. Due to the equal number of boys and girls in each group, and the small number of girls included in the study (n = 4/group), sex differences were not considered in the statistical analysis. Mean ± SD were reported for all data. The magnitude of the effects were determined using Cohen’s d (d = mean difference between groups/pooled SD), with 0.2, 0.5 and 0.8 representing small, moderate and large effect sizes, respectively [28].
3. Results
Twenty-three children with CP who met the inclusion criteria were invited to participate in the study. Eighteen children with CP enrolled in the study. Two children were unable to complete any MRI testing due to claustrophobia. One child was unable to remain still for the MRI testing in which the fat concentration of the bone marrow and muscle were determined due to attention deficit hyperactivity disorder and an inability to follow directions. Twelve typically developing children who met the inclusion criteria and matched a child with CP for age (± 1.2 y), sex (n = 4 girls and 8 boys per group) and race (n = 1 white Hispanic, 10 white non-Hispanic and 1 Asian per group) were enrolled in the study as controls. All controls completed all testing. Data for the 12 matched pairs are presented. Two of the children with CP had a previous fracture (finger, n = 1 and foot/ankle, n = 1) and 3 were taking antiepileptic medication. None of the controls reported a previous fracture or chronic medication use. All data were normally distributed except subfascial and intermuscular AT volume, which were log transformed before statistical analyses were conducted.
Physical characteristics of the participants are shown in Table 1. Of the 12 children with CP, 8 children were considered GMFCS I and 4 children were considered GMFCS II. There were no group differences in age, pubic hair Tanner stage, testicular-penile/breast Tanner stage, height, body mass, body mass percentile, BMI, BMI percentile or tibia length (all p > 0.05). However, children with CP compared to controls had lower height percentile (p < 0.05). When compared to the 50th age- and sex-based percentiles, height percentile was lower in children with CP (p = 0.003), but not different in controls (p = 0.531). When compared to the 50th age- and sex-based percentiles for body mass and BMI, there were no differences in children with CP or controls (all p > 0.20). Group comparisons of physical activity are also presented in Table 1. Children with CP had 44 % fewer physical activity counts at the ankle compared to controls (p < 0.05).
Table 1.
Characteristics of children with cerebral palsy (CP) and typically developing children (Con)
| CP (n = 12) | Con (n = 12) | p | d | |
|---|---|---|---|---|
| Age (y) | 8.8 ± 2.1 | 8.8 ± 2.0 | 0.971 | 0.015 |
| Tanner Stage (I/II/III) | ||||
| Pubic hair | 8/2/2 | 11/1/0 | 0.291 | 0.767 |
| Testicular-Penile/Breast | 7/5/0 | 10/1/1 | 0.378 | 0.293 |
| Height (m) | 1.24 ± 0.11 | 1.32 ± 0.11 | 0.106 | 0.689 |
| Height (%) | 19 ± 23 | 50 ± 28 | 0.006 | 1.232 |
| Body mass (kg) | 26.6 ± 8.2 | 30.0 ± 7.4 | 0.293 | 0.441 |
| Body mass (%) | 34 ± 33 | 55 ± 25 | 0.094 | 0.720 |
| BMI (kg/m2) | 17.0 ± 3.4 | 17.0 ± 2.6 | 0.966 | 0.018 |
| BMI (%) | 50 ± 36 | 53 ± 28 | 0.872 | 0.067 |
| Tibia length (m) | 0.273 ± 0.036 | 0.293 ± 0.031 | 0.173 | 0.577 |
| GMFCS (I/II) | 8/4 | - | ||
| Physical activity (counts/d) | 348,366 ± 197,001 | 628,044 ± 147,499 | 0.001 | 1.624 |
Values are means ± SD. % reflects the percentile relative to age- and sex-based norms; BMI = body mass index; GMFCS = Gross Motor Function Classification System
Group comparisons of bone architecture at the level of the midtibia are shown in Table 2. Children with CP had 30 % lower cortical volume (p < 0.05) than controls with no differences observed in medullary volume (p > 0.05). Although marginally insignificant (p = 0.063), children with CP had 25 % lower total bone volume than controls. Cortical width was 16 % thinner in the posterior portion and 32 % thinner in the medial portion of the shaft in children with CP compared to controls (p < 0.05). There were no group differences observed in the anterior or lateral portions of the bone (p > 0.05). Although marginally insignificant (p = 0.058), children with CP displayed 17 % lower medullary width in the medial-lateral direction compared to controls. No group difference in medullary width was observed in the anterior-posterior direction (p > 0.05). Children with CP displayed 15 % lower total bone width in the medial-lateral direction compared to controls (p < 0.05), but no difference was observed in the anterior-posterior direction (p > 0.05). When tibia length was used as a covariate, the differences remained statistically significant for all bone architecture measures (p < 0.05), except for cortical width in the posterior portion of the bone (p > 0.05).
Table 2.
Bone architecture in the midtibia of children with cerebral palsy (CP) and typically developing children (Con)
| CP (n = 12) | Con (n = 12) | p | d | p* | |
|---|---|---|---|---|---|
| Cortical volume (cm3) | 12.3 ± 4.7 | 17.6 ± 5.8 | 0.022 | 1.009 | 0.012 |
| Medullary volume (cm3) | 7.7 ± 3.7 | 9.0 ± 3.0 | 0.343 | 0.398 | 0.705 |
| Total bone volume (cm3) | 20.0 ± 8.2 | 26.6 ± 8.4 | 0.063 | 0.800 | 0.119 |
| Cortical width (cm) | |||||
| Anterior | 0.41 ± 0.10 | 0.43 ± 0.11 | 0.656 | 0.184 | 0.434 |
| Posterior | 0.32 ± 0.07 | 0.38 ± 0.06 | 0.023 | 1.004 | 0.070 |
| Medial | 0.26 ± 0.04 | 0.38 ± 0.10 | 0.002 | 1.770 | 0.002 |
| Lateral | 0.27 ± 0.06 | 0.25 ± 0.07 | 0.586 | 0.227 | 0.397 |
| Medullary width (cm) | |||||
| Anterior-posterior | 0.51 ± 0.10 | 0.51 ± 0.08 | 0.981 | 0.010 | 0.061 |
| Medial-lateral | 0.52 ± 0.07 | 0.59 ± 0.09 | 0.058 | 0.820 | 0.199 |
| Total bone width (cm) | |||||
| Anterior-posterior | 1.24 ± 0.22 | 1.32 ± 0.23 | 0.376 | 0.369 | 0.525 |
| Medial-lateral | 1.04 ± 0.11 | 1.22 ± 0.16 | 0.006 | 1.262 | 0.015 |
Values are means ± SD. d = the effect size of the group differences before tibia length was included as a covariate.
Group difference p value when statistically controlled for tibia length using analysis of covariance.
Group comparisons of bone strength estimates at the level of the midtibia are shown in Figures 2. Children with CP had 34 % lower Zml (d = 1.127, p = 0.012) and 39 % lower J (d = 1.001, p = 0.023) compared to controls. Although marginally insignificant, children with CP had 29 % lower Zap (d = 0.822, p = 0.057) compared to controls. When tibia length was used as a covariate, the significant differences in Zml and J remained statistically significant (p = 0.017 and 0.035, respectively). Figure 3 shows a higher bone marrow fat concentration in children with CP compared to controls (82.1 ± 1.8 % vs. 80.5 ± 1.9 %; d = 0.909, p = 0.037), which remained significantly higher when tibia length was used as a covariate (p = 0.004).
Figure 2.
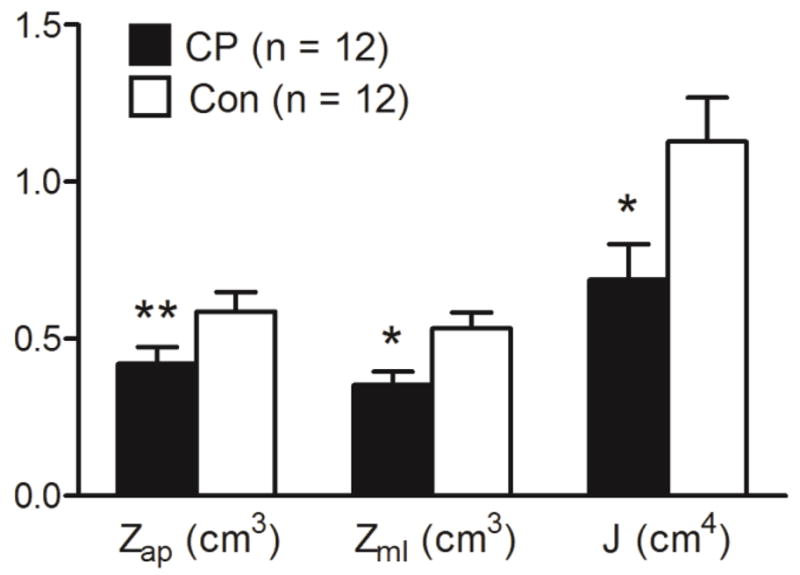
Estimates of bone strength [section modulus in the anterior-posterior (Zap) and medial-lateral (Zml) directions and polar moment of inertia (J)] in the midtibia of children with cerebral palsy (CP) and typically developing children (Con). *Group difference, p < 0.05. **Group difference, p = 0.057.
Figure 3.
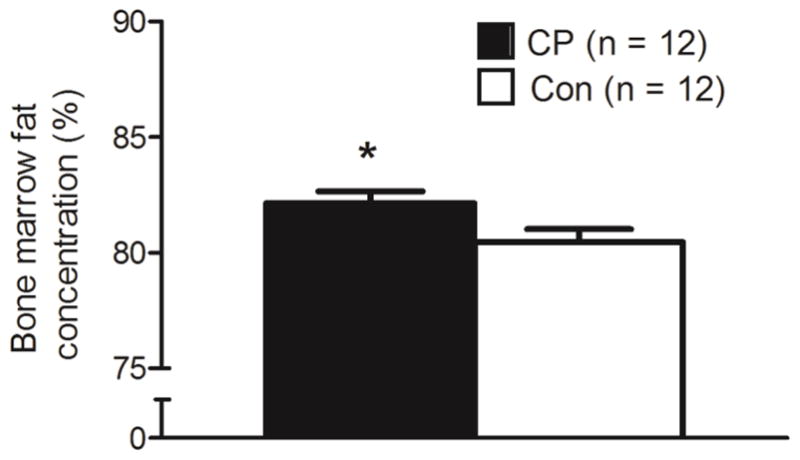
Bone marrow fat concentration (%) in the midtibia of children with cerebral palsy (CP) and typically developing children (Con). *Group difference, p < 0.05.
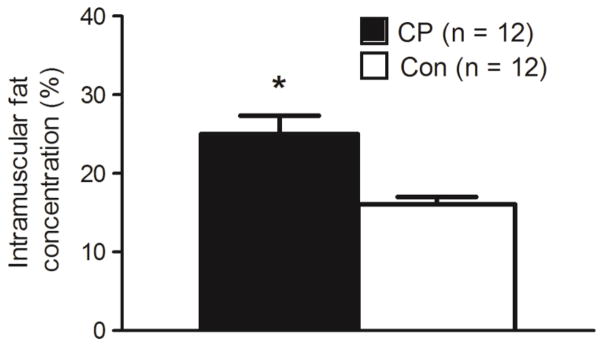
Group comparisons of muscle volume and AT volume in the midleg are shown in Table 3. Children with CP had 39 % lower muscle volume and 3.3 fold higher subfascial AT volume compared to controls (both p < 0.05). There were no group differences in subcutaneous or total AT volume between groups (p > 0.05). Although marginally insignificant (p = 0.077), children with CP had 3.7 fold lower intermuscular AT than controls. The differences in muscle volume and subfascial AT volume remained statistically significant when tibia length was used as a covariate (p < 0.05). Moreover, a significantly higher intermuscular AT in children with CP than controls emerged when tibia length was used as a covariate (p < 0.05). Figure 4 shows a higher intramuscular fat concentration in children with CP compared to controls (25.0 ± 8.0 % vs. 16.1 ± 3.3 %; d = 1.587, p = 0.003), which remained significantly higher when tibia length was used as a covariate (p = 0.002).
Table 3.
Muscle and adipose tissue (AT) volume in the midleg of children with cerebral palsy (CP) and typically developing children (Con)
| CP (n = 12) | Con (n = 12) | p | d | p* | |
|---|---|---|---|---|---|
| Muscle volume (cm3) | 144.8 ± 56.4 | 237.1 ± 70.0 | 0.002 | 1.461 | 0.001 |
| Intermuscular AT volume (cm3)** | 5.1 ± 7.0 | 1.4 ± 1.1 | 0.077 | 0.787 | 0.036 |
| Subfascial AT volume (cm3)** | 4.2 ± 5.4 | 1.3 ± 0.7 | 0.008 | 1.229 | 0.002 |
| Subcutaneous AT volume (cm3) | 119.5 ± 58.3 | 140.6 ± 43.3 | 0.325 | 0.415 | 0.868 |
| Total AT volume (cm3) | 128.8 ± 66.2 | 143.3 ± 44.7 | 0.537 | 0.261 | 0.796 |
Values are means ± SD. d = the effect size of the group differences before tibia length was included as a covariate.
Group difference p value when statistically controlled for tibia length using analysis of covariance.
Group difference p value and d for the log transformed data are reported, but means ± SD for untransformed data are presented for easier comparisons among the adipose tissue depots.
Figure 4.
Intramuscular fat concentration (%) in the midtibia of children with cerebral palsy (CP) and typically developing children (Con). *Group difference, p < 0.05.
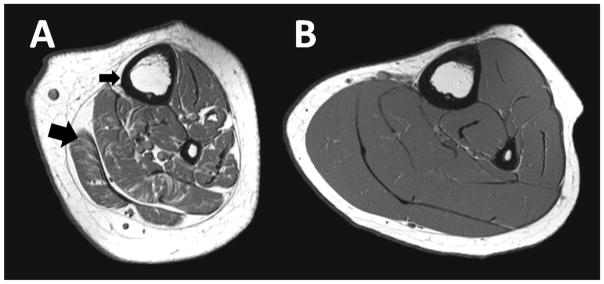
A visual depiction of the bone, muscle and fat contrast between a boy with CP and a typically developing control boy of similar height and the same tibia length is shown in Figure 4.
4. Discussion
This is the first study to report that ambulatory children with mild spastic CP relative to typically developing children have markedly underdeveloped bone architecture and lower estimates of bone strength. It is also the first study to report that ambulatory children with mild spastic CP have elevated fat infiltration of bone marrow and skeletal muscle. The finding that bone marrow is infiltrated with fat in children with mild CP is particularly novel because, to our knowledge, it has not been examined in individuals with CP, irrespective of level of involvement or age. Together the findings suggest that the underdevelopment of bone architecture and fat infiltration of the musculoskeletal system in nonambulatory children with more severe forms of CP is also present in ambulatory children with milder forms of the disorder. The findings are concerning because poor bone architecture and an elevated bone marrow adiposity are associated with an increased risk of fracture [13–15]. Moreover, elevated bone marrow and muscle adiposity are linked to impaired glucose tolerance [12, 16, 17] and cardiometabolic disease [18].
The finding that bone architecture is underdeveloped and bone strength is lower in ambulatory children with mild CP is consistent with previous studies in children [4, 6] and adults [29, 30] with reduced mechanical loading. However, the magnitude of the deficit is surprising. In the current study, cortical volume, Z in the medial-lateral plane and J were ≥ 30 % lower in the midtibia of ambulatory children with mild CP vs. controls. These estimated deficits are approximately half those observed in the midfemur of nonambulatory children with CP [4], but generally worse than the 15–31 % estimated bone deficits reported in the tibial [30] and femoral shaft [29, 30] of adults with complete spinal cord injury (SCI). The smaller deficits in the ambulatory children with CP observed in the present study compared to the deficits observed in a previous study of nonambulatory children with CP [4] is not surprising because ambulatory children with CP experience mechanical loading while they are walking or running. Moreover, although physical activity was 44% lower in ambulatory children with CP compared to typically developing children matched for age, sex and race in the present study, it was not as dramatic as the discrepancy (70 % lower) previously reported for nonambulatory children with CP [4]. The generally larger bone strength deficits observed in ambulatory children with CP than the deficits previously reported for adults with SCI is likely due to the onset of the condition. In children with CP, the restriction of mechanical loading begins at birth or shortly afterward and continues throughout life. Therefore, the mechanical loading needed for optimal cortical expansion at the subperiosteal surface is reduced or absent and the total width of the bone does not reach its capacity. On the other hand, because SCI usually occurs during adulthood, the restriction of mechanical loading usually begins after growth and cortical expansion is complete or nearly complete. Therefore, the total width of the bone reaches its capacity [29, 30]. The deficit in bone strength in adults with SCI results from increased bone remodeling and endocortical resorption, which has a smaller effect on bone strength than restricted subperiosteal expansion [31]. The difference in timing of physical activity restriction highlights the importance of mechanical loading during childhood to optimize cortical expansion and the strength of bone. Together, findings from the current study and previous studies suggest that ambulatory children with mild CP have considerable bone strength deficits that need clinical attention. A handful of studies suggest that treatments, such as high-frequency, low-magnitude vibration [33, 34], may aid in addressing such bone deficits.
The observation that ambulatory children with mild CP have elevated fat infiltration of muscle in the midleg, as indicated by elevated subfascial AT and intramuscular fat concentration, is consistent with the observation in nonambulatory children with moderate to severe CP [8] and young adults with mild to moderate CP [35]. Using magnetic resonance imaging, Johnson et al. [8] reported a 2.3-fold and 1.7-fold higher intermuscular and subfascial AT area in the midthigh of nonambulatory children with CP compared to typically developing children. These AT depots were negatively correlated with physical activity (r = -0.76 and r = -0.63, respectively). Furthermore, Noble and colleagues [35] reported greater intramuscular fat in the leg muscles in young adults with CP compared to controls. In the present study, physical activity assessed using physical activity monitors was 44 % lower in children with mild CP than controls. There is also evidence that gait declines in 25 % of children with CP as they become adults [1]. The sedentary behavior and reduced mechanical loading observed with CP is also observed with SCI. Individuals with SCI, concomitant with the altered skeletal metabolism, experience vast muscular atrophy, increased muscular adiposity, insulin resistance and an increased prevalence of type 2 diabetes mellitus [12, 36]. On the other hand, the sedentary behavior and reduced mechanical loading in individuals with CP occurs at or near the time of birth, rather than it being acquired later in life. Therefore, the musculoskeletal metabolic dysregulation in children with CP, resulting in losses of normal muscle function, likely leads to an enhanced risk for cardiometabolic disease [18]. This idea is supported by the 2-to-3 fold greater mortality rate from coronary heart disease in individuals with CP compared to the general population [37]. Therefore, ameliorating regressive behavior in physical activity, which is apparent even in children with a mild form of the disorder, is imperative to the health of the musculature, and minimizing the risk of cardiometabolic disease in individuals with CP.
The novel finding of elevated fat concentration in the bone marrow of children with CP in the present study could be viewed as somewhat surprising because the children were ambulatory with a mild form of CP, the children averaged only 8.8 years of age and their BMI was not different from the 50th age-based percentile; however, the finding is consistent with their underdeveloped bone architecture and low estimates of bone strength relative to controls. Moreover, the finding is consistent with the elevated fat concentration in the bone marrow of other groups with limited mobility [11, 14, 38]. Bone marrow-derived mesenchymal progenitor cells have a multipotency to differentiate into an osteogenic or adipogenic lineage while concomitantly suppressing the opposed lineage [39]. Recently, it has been reported that bone marrow fat concentration is inversely related to cortical bone area in adolescents 15 to 20 years of age [40] and adults [40, 41]. There is evidence suggesting that physical activity governs the extent of bone marrow AT. Rantalainen et al. [42] reported that female athletes had significantly greater cortical area, strength strain index and bone marrow density (indicative of lower marrow adiposity) in the midtibia compared to healthy controls. On the other hand, it has been suggested that adults with SCI have a higher amount of yellow vs. red bone marrow AT compared to healthy controls [11]. The higher proportion of yellow marrow may be explained by the metabolic shift in mesenchymal progenitor cell differentiation away from osteogenesis and towards adipogenesis at the endocortical surface due to the reduced mechanical loading observed after an SCI. In the current study, children with mild CP had a deficit in cortical volume and bone strength, but they had no significant difference in the volume of the medullary cavity. Therefore, the connection between the higher concentration of bone marrow adiposity and the deficit in bone macroarchitecture in ambulatory children with mild CP relative to typically developing children and the clinical implications of the relationship requires further investigation.
The present study has several strengths. First, there was very careful attention paid to the selection of the research participants which limited the within group variability and the need for a large sample size. Specifically, all children with CP had spasticity, were ambulatory and had a mild form of the disorder. The typically developing children were not different from the children with CP in age, sex, race or sexual maturity. Furthermore, the typically developing children were not different from the 50th age- and sex-based percentiles for height, body mass and BMI. A second strength of the present study was that most of the deficits in bone architecture and bone strength and all of the elevated estimates of fat infiltration of bone marrow and muscle in the children with CP remained when small, insignificant differences in tibia length were statistically controlled. A third strength of the study was the use of MRI to assess different AT and fat depositions. Magnetic resonance imaging is considered the gold standard in vivo imaging modality for assessing soft tissue [44], it provides valid estimates of bone architecture [45] and fat concentration [26] and it does not expose participants to ionizing radiation, as does computed tomography. Furthermore, the reliability of the measures in the present study was excellent, with intraclass correlations for repeat testing ≥ 0.96 for all MRI measures of bone architecture, bone marrow and muscle fat concentration and muscle volume. Lastly, physical activity was estimated using accelerometer-based activity monitors that have been validated with a wide range of both gross and fine motor physical activities [43] and have good reliability for assessment in children with CP, as reported in the present study. The assessment of physical activity allowed for a broader mechanistic approach to understanding the underdeveloped musculoskeletal system and increased fat infiltration within muscle and bone in a group of ambulatory children with mild spastic CP.
The limitations of the study must also be discussed. One limitation is the small sample size. Therefore, the results should be interpreted with caution. Studies of children with CP using MRI are challenging because the children often have difficulty holding still due to spasticity and/or behavioral issues, and they often have noise sensitivity and cognitive issues. Moreover, the cost of MRI studies limits the number of participants that can be enrolled. However, even with these challenges, significant differences in cortical volume, bone widths, estimates of bone strength, bone marrow fat concentration, muscle volume, subfascial AT volume and intramuscular fat concentration were observed. This is attributed to the magnitude of the differences as well as the robustness of the measures. As previously noted, the reliability of the MRI assessment of bone architecture, muscle volume and fat infiltration measures used in the present study is extremely good. Furthermore, a set of identical twins was included in the study and their data reflect the group differences. For example, cortical volume, muscle volume, and bone marrow and intramuscular fat concentration were 30 % lower, 39 % lower, 2 percentage points higher, and 9 percentage points higher, respectively, in the children with CP compared to the typically developing children. The same measurements were 18 % lower, 39 % lower, 2 percentage points higher and 7 percentage points higher, respectively, in the twin with CP (5.5 years of age, GMFCS I) compared to the typically developing twin. A second study limitation is the absence of glucose tolerance, insulin sensitivity and inflammatory markers. It has been established that elevated AT surrounding muscle and fat within muscle are associated with a disturbance of euglycemic conditions [12] and insulin resistance [16], as well as promoting a pro-inflammatory environment [46]; whether this relationship exists in children with mild CP and if elevated bone marrow fat is associated with a disturbance in these metabolic markers warrants further investigation.
Taken together, the findings from the present study suggest that ambulatory children with mild spastic CP have an underdeveloped bone architecture, low bone strength and a greater infiltration of fat within the bone marrow cavity of the midtibia and the surrounding leg musculature. The underdeveloped bone architecture and fat infiltration may be related to the low level of physical activity potentially promoting an intramuscular pro-inflammatory environment and blunting osteogenesis while favoring adipogenesis within the bone marrow cavity. Additional studies that determine whether the adverse musculoskeletal fat profile is associated with a disturbance in glucose homeostasis or insulin function are needed. Studies that examine whether increased physical activity or other interventions would enhance the bone architecture while reducing the observed infiltration of fat in the musculoskeletal system of children with mild CP are also needed.
Figure 5.
Raw T1-weighted magnetic resonance images from the midtibia demonstrate the marked deficit in bone architecture and muscle volume and the high infiltration of fat within and around the musculature in an ambulatory boy with mild CP (A) compared to a typically developing boy with the same tibia length (B). In the image of the child with CP (A), the small black arrow highlights the thin cortical shell and the large arrow highlights the fat infiltration of muscle.
Highlights.
Children with mild cerebral palsy (CP) vs. typically developing children had a marked deficit in cortical bone architecture and estimated bone strength.
Bone marrow in the midtibia was more infiltrated with fat in children with mild CP vs. typically developing children.
Muscle in the midleg was more infiltrated with fat in children with mild CP compared to typically developing children.
Acknowledgments
The study was supported by the NIH (HD071397). We thank all research participants and their families. We thank Keri DiAlessandro for assistance with testing and Nancy Lennon for assistance with recruitment.
Abbreviations
- CP
cerebral palsy
- Zap
section modulus in the anterior-posterior direction
- Zml
section modulus in the medial-lateral direction
- J
polar moment of inertia
- AT
adipose tissue
- GMFCS
gross motor function classification system
- SI
signal intensity
- d
Cohen’s d
Footnotes
Conflicts of Interest: Daniel G. Whitney, Harshvardhan Singh, Freeman Miller, Mary F. Barbe, Jill M. Slade and Christopher M. Modlesky declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morgan P, McGinley J. Gait function and decline in adults with cerebral palsy: a systematic review. Disabil Rehabil. 2014;36:1–9. doi: 10.3109/09638288.2013.775359. [DOI] [PubMed] [Google Scholar]
- 2.Modlesky CM, Whitney DG, Singh H, Barbe MF, Kirby JT, Miller F. Underdevelopment of trabecular bone microarchitecture in the distal femur of nonambulatory children with cerebral palsy becomes more pronounced with distance from the growth plate. Osteoporos Int. 2015;26:505–12. doi: 10.1007/s00198-014-2873-4. [DOI] [PubMed] [Google Scholar]
- 3.Modlesky CM, Subramanian P, Miller F. Underdeveloped trabecular bone microarchitecture is detected in children with cerebral palsy using high-resolution magnetic resonance imaging. Osteoporos Int. 2008;19:169–76. doi: 10.1007/s00198-007-0433-x. [DOI] [PubMed] [Google Scholar]
- 4.Modlesky CM, Kanoff SA, Johnson DL, Subramanian P, Miller F. Evaluation of the femoral midshaft in children with cerebral palsy using magnetic resonance imaging. Osteoporos Int. 2009;20:609–15. doi: 10.1007/s00198-008-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson RC, Lark RK, Gurka MJ, Worley G, Fung EB, Conaway M, Stallings VA, Stevenson RD. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics. 2002;110:e5. doi: 10.1542/peds.110.1.e5. [DOI] [PubMed] [Google Scholar]
- 6.Binkley T, Johnson J, Vogel L, Kecskemethy H, Henderson R, Specker B. Bone measurements by peripheral quantitative computed tomography (pQCT) in children with cerebral palsy. J Pediatr. 2005;147:791–6. doi: 10.1016/j.jpeds.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Elder GC, Kirk J, Stewart G, Cook K, Weir D, Marshall A, Leahey L. Contributing factors to muscle weakness in children with cerebral palsy. Dev Med Child Neurol. 2003;45:542–50. doi: 10.1017/s0012162203000999. [DOI] [PubMed] [Google Scholar]
- 8.Johnson DL, Miller F, Subramanian P, Modlesky CM. Adipose tissue infiltration of skeletal muscle in children with cerebral palsy. J Pediatr. 2009;154:715–20. doi: 10.1016/j.jpeds.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McIvor WC, Samilson RL. Fractures in patients with cerebral palsy. J Bone Joint Surg Am. 1966;48:858–66. [PubMed] [Google Scholar]
- 10.Presedo A, Dabney KW, Miller F. Fractures in patients with cerebral palsy. J Pediatr Orthop. 2007;27:147–53. doi: 10.1097/BPO.0b013e3180317403. [DOI] [PubMed] [Google Scholar]
- 11.Gorgey AS, Poarch HJ, Adler RA, Khalil RE, Gater DR. Femoral bone marrow adiposity and cortical bone cross-sectional areas in men with motor complete spinal cord injury. PM R. 2013;5:939–48. doi: 10.1016/j.pmrj.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA. Intramuscular fat and glucose tolerance after spinal cord injury--a cross-sectional study. Spinal Cord. 2004;42:711–6. doi: 10.1038/sj.sc.3101652. [DOI] [PubMed] [Google Scholar]
- 13.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–54. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Minaire P, Edouard C, Arlot M, Meunier PJ. Marrow changes in paraplegic patients. Calcif Tissue Int. 1984;36:338–40. doi: 10.1007/BF02405340. [DOI] [PubMed] [Google Scholar]
- 15.Cohen A, Recker RR, Lappe J, Dempster DW, Cremers S, McMahon DJ, Stein EM, Fleischer J, Rosen CJ, Rogers H, Staron RB, Lemaster J, Shane E. Premenopausal women with idiopathic low-trauma fractures and/or low bone mineral density. Osteoporos Int. 2012;23:171–82. doi: 10.1007/s00198-011-1560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–92. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 17.Baum T, Yap SP, Karampinos DC, Nardo L, Kuo D, Burghardt AJ, Masharani UB, Schwartz AV, Li X, Link TM. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J Magn Reson Imaging. 2012;35:117–24. doi: 10.1002/jmri.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson MD, Gordon PM, Hurvitz EA, Burant CF. Secondary muscle pathology and metabolic dysregulation in adults with cerebral palsy. Am J Physiol Endocrinol Metab. 2012;303:E1085–93. doi: 10.1152/ajpendo.00338.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 20.Tanner J. Growth and Adolescence. 2. Oxford: Blackwell Scientific Publications; 1962. [Google Scholar]
- 21.Wood E, Rosenbaum P. The gross motor function classification system for cerebral palsy: a study of reliability and stability over time. Dev Med Child Neurol. 2000;42:292–6. doi: 10.1017/s0012162200000529. [DOI] [PubMed] [Google Scholar]
- 22.John D, Freedson P. ActiGraph and Actical physical activity monitors: a peek under the hood. Med Sci Sports Exerc. 2012;44:S86–9. doi: 10.1249/MSS.0b013e3182399f5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suckling J, Sigmundsson T, Greenwood K, Bullmore ET. A modified fuzzy clustering algorithm for operator independent brain tissue classification of dual echo MR images. Magn Reson Imaging. 1999;17:1065–76. doi: 10.1016/s0730-725x(99)00055-7. [DOI] [PubMed] [Google Scholar]
- 24.Turner CH, Burr DB. Experimental techniques for bone mechanics. In: Cowen SC, editor. Bone Mechanics Handbook. Boca Raton: CRC Press; 2001. pp. 1–35. [Google Scholar]
- 25.Slade JM, Coe LM, Meyer RA, McCabe LR. Human bone marrow adiposity is linked with serum lipid levels not T1-diabetes. J Diabetes Complications. 2012;26:1–9. doi: 10.1016/j.jdiacomp.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Bernard CP, Liney GP, Manton DJ, Turnbull LW, Langton CM. Comparison of fat quantification methods: a phantom study at 3. 0T. J Magn Reson Imaging. 2008;27:192–7. doi: 10.1002/jmri.21201. [DOI] [PubMed] [Google Scholar]
- 27.Liney GP, Bernard CP, Manton DJ, Turnbull LW, Langton CM. Age, gender, and skeletal variation in bone marrow composition: a preliminary study at 3. 0 Tesla. J Magn Reson Imaging. 2007;26:787–93. doi: 10.1002/jmri.21072. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J. Statistical Power for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 29.Modlesky CM, Slade JM, Bickel CS, Meyer RA, Dudley GA. Deteriorated geometric structure and strength of the mid-femur in men with complete spinal cord injury. Bone. 2005;36:331–339. doi: 10.1016/j.bone.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H. Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone. 2004;34:869–80. doi: 10.1016/j.bone.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Cole JH, van der Meulen MC. Whole bone mechanics and bone quality. Clin Orthop Relat Res. 2011;469:2139–49. doi: 10.1007/s11999-011-1784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henderson RC, Lark RK, Kecskemethy HH, Miller F, Harcke HT, Bachrach SJ. Bisphosphonates to treat osteopenia in children with quadriplegic cerebral palsy: a randomized, placebo-controlled clinical trial. J Pediatr. 2002;141:644–51. doi: 10.1067/mpd.2002.128207. [DOI] [PubMed] [Google Scholar]
- 33.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004 doi: 10.1359/JBMR.040129. Published online on January 27, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Wren TA, Lee DC, Hara R, Rethlefsen SA, Kay RM, Dorey FJ, Gilsanz V. Effect of high-frequency, low-magnitude vibration on bone and muscle in children with cerebral palsy. J Pediatr Orthop. 2010;30:732–8. doi: 10.1097/BPO.0b013e3181efbabc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noble JJ, Charles-Edwards GD, Keevil SF, Lewis AP, Gough M, Shortland AP. Intramuscular fat in ambulant young adults with bilateral spastic cerebral palsy. BMC Musculoskelet Disord. 2014;15:236. doi: 10.1186/1471-2474-15-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001;24:266–77. doi: 10.1080/10790268.2001.11753584. [DOI] [PubMed] [Google Scholar]
- 37.Strauss D, Cable W, Shavelle R. Causes of excess mortality in cerebral palsy. Dev Med Child Neurol. 1999;41:580–5. doi: 10.1017/s001216229900122x. [DOI] [PubMed] [Google Scholar]
- 38.Trudel G, Payne M, Madler B, Ramachandran N, Lecompte M, Wade C, Biolo G, Blanc S, Hughson R, Bear L, Uhthoff HK. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. J Appl Physiol. 2009;107:540–8. doi: 10.1152/japplphysiol.91530.2008. [DOI] [PubMed] [Google Scholar]
- 39.David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, Vico L, Guignandon A. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148:2553–62. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- 40.Di Iorgi N, Mo AO, Grimm K, Wren TA, Dorey F, Gilsanz V. Bone acquisition in healthy young females is reciprocally related to marrow adiposity. J Clin Endocrinol Metab. 2010;95:2977–82. doi: 10.1210/jc.2009-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wren TA, Chung SA, Dorey FJ, Bluml S, Adams GB, Gilsanz V. Bone marrow fat is inversely related to cortical bone in young and old subjects. J Clin Endocrinol Metab. 2011;96:782–6. doi: 10.1210/jc.2010-1922. [DOI] [PubMed] [Google Scholar]
- 42.Rantalainen T, Nikander R, Heinonen A, Cervinka T, Sievanen H, Daly RM. Differential effects of exercise on tibial shaft marrow density in young female athletes. J Clin Endocrinol Metab. 2013;98:2037–44. doi: 10.1210/jc.2012-3748. [DOI] [PubMed] [Google Scholar]
- 43.Puyau MR, Adolph AL, Vohra FA, Zakeri I, Butte NF. Prediction of activity energy expenditure using accelerometers in children. Med Sci Sports Exerc. 2004;36:1625–1631. [PubMed] [Google Scholar]
- 44.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–22. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 45.Woodhead HJ, Kemp AF, Blimkie CJR, Briody JN, Duncan CS, Thompson M, Lam A, Howman-Giles R, Cowell CT. Measurement of midfemoral shaft geometry: repeatability and accuracy using magnetic resonance imaging and dual-energy X-ray absorptiometry. J Bone Miner Res. 2001;16:2251–9. doi: 10.1359/jbmr.2001.16.12.2251. [DOI] [PubMed] [Google Scholar]
- 46.Addison O, Drummond MJ, LaStayo PC, Dibble LE, Wende AR, McClain DA, Marcus RL. Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. J Nutr Health Aging. 2014;18:532–8. doi: 10.1007/s12603-014-0019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]